This study examined the impact of laser-assisted liposuction on the quality and differentiation potential of adipose-derived stromal cells (ASCs). It was found that laser-assisted liposuction negatively impacts the biology of ASCs, and therefore cell harvest using suction-assisted liposuction is preferable for tissue-engineering purposes.
Keywords: Adipose, Adult stem cells, Stromal cells, Stem cell transplantation, Laser lipoplasty, Liposuction
Abstract
Harvesting adipose-derived stromal cells (ASCs) for tissue engineering is frequently done through liposuction. However, several different techniques exist. Although third-generation ultrasound-assisted liposuction has been shown to not have a negative effect on ASCs, the impact of laser-assisted liposuction on the quality and differentiation potential of ASCs has not been studied. Therefore, ASCs were harvested from laser-assisted lipoaspirate and suction-assisted lipoaspirate. Next, in vitro parameters of cell yield, cell viability and proliferation, surface marker phenotype, osteogenic differentiation, and adipogenic differentiation were performed. Finally, in vivo bone formation was assessed using a critical-sized cranial defect in athymic nude mice. Although ASCs isolated from suction-assisted lipoaspirate and laser-assisted lipoaspirate both successfully underwent osteogenic and adipogenic differentiation, the cell yield, viability, proliferation, and frequency of ASCs (CD34+CD31−CD45−) in the stromal vascular fraction were all significantly less with laser-assisted liposuction in vitro (p < .05). In vivo, quantification of osseous healing by micro-computed tomography revealed significantly more healing with ASCs isolated from suction-assisted lipoaspirate relative to laser-assisted lipoaspirate at the 4-, 6-, and 8-week time points (p < .05). Therefore, as laser-assisted liposuction appears to negatively impact the biology of ASCs, cell harvest using suction-assisted liposuction is preferable for tissue-engineering purposes.
Introduction
Approximately 205,000 liposuction surgeries are performed in the United States each year, and the growing popularity of liposuction has been associated with an evolution of techniques and equipment for fat removal and body contouring [1]. In addition to traditional suction-assisted liposuction, other options now include ultrasound-assisted liposuction and laser-assisted liposuction. These alternative techniques have been developed to reduce down time, operator effort for the surgeon, and bleeding, and to promote skin tightening [2].
Although it is normally discarded, adipose tissue also contains an easily accessible source of adipose-derived stromal cells (ASCs) that may be used for tissue engineering purposes [3]. Unlike human bone marrow-derived mesenchymal stromal cells, ASCs can be easily and safely harvested in large quantities with minimal morbidity. The abundance of stem cells in adipose tissue is 100-fold higher than that in the bone marrow [3–5]. In addition, traditional bone marrow harvesting procedures typically necessitate an ex vivo expansion step to obtain clinically significant cell numbers. All these points of comparison make adipose tissue an attractive cell source for tissue engineering. Nevertheless, the specific liposuction technique used to isolate ASCs may potentially diminish the therapeutic use of these cells for reconstruction of both hard and soft tissues.
Until recently, research investigating ASCs has primarily been performed on specimens obtained principally by means of suction-assisted liposuction, as this has been the gold standard since first introduced by Arpad and Giorgio Fischer in the 1970s [6, 7]. Ultrasound-assisted liposuction, introduced by Scuderi and Zocchi in 1987, uses ultrasonic energy to allow selective destruction of subcutaneous adipose tissue [8–10]. A previous study in our laboratory found that exposure of ASCs to ultrasound energy during tissue harvest by means of third-generation ultrasound-assisted liposuction (VASER Lipo System; Solta Medical, Hayward, CA, http://www.solta.com) does not have a negative consequence on their proliferative capacity or osteogenic potential [11]. However, the effects of laser-assisted liposuction on the quality and differentiation potential of ASCs have yet to be elucidated. Thus, in this study, we investigated whether differences in yield, proliferative capacity, and differentiation potential exist between ASCs obtained by means of suction-assisted liposuction versus laser-assisted liposuction.
Materials and Methods
Tissue Procurement
Lipoaspiration specimens were obtained after acquiring informed consent from patients in accordance with Stanford University Institutional Review Board guidelines. To perform suction-assisted liposuction, aspiration was performed using 3.0- to 5.0-mm hollow cannulas. Laser-assisted liposuction was performed using a pulsed 1,064-nm Nd:YAG laser (Smart-Lipo; Deka, Florence, Italy, http://www.dekalaser.com) with the following parameters: 100 microsecond pulse, 150 mJ per pulse, 40 Hz (peak power, 1.5 kW; average power, 6 W) [2]. The laser was coupled to a 600-μm optical fiber within a 1.0-mm-diameter cannula. ASCs were harvested from the adipose tissue of 12 female patients with no medical comorbidities between the ages of 33 and 55 who were undergoing elective lipoaspiration of the abdomen. All liposuction procedures in both groups were performed by the same plastic surgeon (D.V.). Each patient undergoing laser-assisted liposuction (n = 6) was matched for age (within 2 years) with a patient undergoing suction-assisted liposuction (n = 6). Age-matched patients underwent liposuction procedures on the same day. As all liposuction procedures were elective cases performed in a private practice setting, all patients were within the normal range regarding body mass index (20–25 kg/m2). Because of the nature of the liposuction procedure, laser-assisted lipoaspirate and suction-assisted lipoaspirate could not be harvested from the same anatomical location. However, Schipper et al. have demonstrated that although there is an age-related difference, there is no statistically significant evidence that depot has an effect on proliferation or lipid accumulation [12].
Isolation of Adipose-Derived Stromal Cells
ASCs were isolated as described previously by Zuk [3] Briefly, raw lipoaspirates were washed and treated with 0.075% collagenase type I (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) in Hanks' balanced salt solution (Cellgro, Manassas, VA, http://www.cellgro.com) for 1 hour at 37°C in a water bath with gentle agitation at 125 rpm. The collagenase digest was then inactivated by adding an equal volume of standard cell culture growth medium (Dulbecco's modified Eagle's medium plus GlutaMAX [Invitrogen, Carlsbad, CA, http://www.invitrogen.com],10% fetal bovine serum, and 1% penicillin/streptomycin). The stromal vascular fraction (SVF) was pelleted by means of centrifugation at 1,200g for 5 minutes. The supernatant was then discarded, and the cell pellet was resuspended and filtered through a 100-μm cell strainer to remove undigested tissue fragments. The cells were pelleted and resuspended in standard cell culture growth medium at 37°C in an atmosphere of 5% carbon dioxide. ASCs were grown to confluence and passaged with 0.05% trypsin. Cells were used only up to passage 2 for all in vitro culture assays.
Cell Viability and Proliferative Capacity
The viability of the cells was assessed using trypan blue exclusion assay. Viable, large cells were counted using light microscopy. After the cells were harvested, they were seeded into 96-well plates for proliferation assays. Cell proliferation was measured with an XTT-based assay (Cell Proliferation Kit II XTT; Roche Applied Science, Indianapolis, IN, http://www.roche.com) according to the manufacturer's instructions. Briefly, cells were seeded in 96-well plates at a density of 20,000 cells per well in culture medium. Plates were incubated at 37°C and 5% CO2, and proliferation was evaluated by XTT assay over a 7-day period. The absorbance of each well was determined using a microplate reader at 492 nm and 690 nm (SpectraMAX 384 Plus; Molecular Devices Ltd., Sunnyvale, CA, http://www.moleculardevices.com). All assays were done in triplicate.
Flow Cytometry Analysis
Freshly isolated cells were examined for surface molecule expression using flow cytometry. The following fluorochrome-conjugated monoclonal antibodies were purchased from BD Biosciences/Pharmingen (San Jose, CA, http://www.bdbiosciences.com): CD31-allophycocyanin (APC), CD34-phycoerythrin (PE), and CD45-Pacific Blue. The analyses were performed on a FACSAria II instrument (BD Biosciences). Briefly, cells were lifted using Accutase (StemCell Technologies, Vancouver, BC, Canada, http://www.stemcell.com) and centrifuged for 5 minutes at 1,000 rpm. The supernatant was discarded by aspiration, and the cells were incubated for 30 minutes in flow cytometry buffer (phosphate-buffered saline, 2% fetal bovine serum [FBS]) containing directly conjugated monoclonal antibodies at the concentrations recommended by the manufacturer. Nonspecific fluorescence was determined by incubating cells with irrelevant control monoclonal antibodies. Propidium iodide was added to the tubes before analysis for dead cell exclusion.
In Vitro Osteogenic Differentiation
For osteogenic differentiation, all assays were performed in triplicate wells. Cells were seeded at equal densities (100,000 cells per well) in side-by-side, six-well culture plates. After attachment, cells were grown to at least 80% confluence before being cultured in osteogenic differentiation medium (ODM), which consisted of Dulbecco's modified Eagle's medium supplemented with 10% FBS, 1% penicillin/streptomycin, 100 μg/ml ascorbic acid, and 10 mM β-glycerophosphate. Alkaline phosphatase staining and quantification were performed at 7 days. Photometric quantification of alizarin red stain was performed at 14 days to assay extracellular mineralization, as previously described [11, 13–18]. Briefly, alizarin red cells were incubated with 2 ml of a solution of 20% methanol, 10% acetic acid under gentle shaking for 15 minutes at room temperature. Supernatants were collected and optical density was measured at 450 nm. Finally, gene expression was analyzed after 7 and 14 days of differentiation by quantitative real-time polymerase chain reaction (qRT-PCR).
In Vitro Adipogenic Differentiation
For adipogenic differentiation, all assays were performed in triplicate wells. Cells were seeded onto six-well plates (150,000 cells per well). Adipogenic differentiation medium consisting of Dulbecco's modified Eagle's medium, 10% FBS, 1% penicillin/streptomycin, 10 μg/ml insulin, 1 μM dexamethasone, 0.5 mM methylxanthine, and 200 μM indomethacin was added after cell attachment. Oil Red O staining was performed at 7 days of differentiation. Finally, specific gene expression was examined after 7 days by qRT-PCR.
Reverse Transcription and Quantitative Real-Time Polymerase Chain Reaction
RNA from cultivated cells was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, http://www.qiagen.com) according to the manufacturer's protocol. Reverse transcription was performed and osteogenic and adipogenic gene expression was examined by qRT-PCR using the Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com) and SYBR Green PCR Master Mix (Applied Biosystems). The amount of PCR product was calculated using an external glyceraldehyde-3-phosphate dehydrogenase (GAPDH) standard curve and LightCycler software. All values were normalized on the basis of the GAPDH expression in the corresponding samples. Specific primers for the genes examined were based on their PrimerBank sequences [11, 15–19].
In Vivo Bone Formation
For evaluation of in vivo osteogenesis, nonhealing, critical-sized (4-mm) calvarial defects were created in the right parietal bone of 60-day-old male Crl:CD-1-Foxn1nu mice (Charles River Laboratories, Wilmington, MA, http://www.criver.com). The dura mater, sagittal, coronal, and lambdoid sutures were left undisturbed. All research involving vertebrate animals was performed in accordance with approved protocols by the Stanford Administrative Panel on Laboratory Animal Care. Hydroxyapatite-coated poly(lactic-co-glycolic acid) (HA-PLGA) scaffolds were fabricated from 85/15 poly(lactic-co-glycolic acid) by solvent casting and a particulate leaching process as previously described [20]. Each scaffold was implanted alone or was seeded with 1 × 106 uncultured, freshly isolated ASCs resuspended in 10 μl of culture medium. Animals were divided equally into four treatment groups: (a) empty defects, in which a 4-mm defect was created but left empty; (b) scaffold only, in which a scaffold without cells was placed in the defect site; (c) suction-assisted liposuction-derived ASCs; and (d) laser-assisted liposuction-derived ASCs.
For micro-computed tomography (micro-CT) scans, the mice were anesthetized with isoflurane. Imaging was performed using an Inveon MicroPET/CT scanner (Siemens Medical Solutions Inc., Malvern, PA, http://www.medical.siemens.com). Using our scan protocol parameters, each high-resolution 103-μm image was acquired in a total scan time of 10 minutes. Mice were scanned immediately postoperatively and at 2, 4, 6, and 8 weeks after surgery. Data were reconstructed into three-dimensional surfaces using the Inveon Research Workplace 4.0 software (Siemens Medical Solutions). The three-dimensional reconstructed images were then analyzed using NIH ImageJ software. The area of the calvarial defects was evaluated by quantifying pixels in the defect. The percentage of healing was then determined by dividing the defect area by the defect size immediately postoperatively.
Histological Analysis
At 8 weeks postoperatively, calvaria were harvested, immediately fixed in 10% formalin overnight, decalcified in 19% EDTA, dehydrated through an ethanol series, and embedded in paraffin as previously described. Deparaffinized sections were stained with Movat's pentachrome to detect bone matrix formation. Bright-field images were obtained with a ×20 objective at room temperature using a DM5000 microscope (Leica Microsystems Inc., Wetzlar, Germany, http://www.leica.com) equipped with a DFC300FX camera. The images were analyzed using the IM1000 image acquisition software, version 4.0 (Leica Microsystems).
Statistical Analysis
Numerical data are presented as means ± SDs. In all figures, bar graphs represent means, and error bars represent 1 SD. Unless otherwise stated, statistical analysis was performed using a one-way analysis of variance for multiple group comparisons followed by a Newman-Keuls post hoc test. A value of p < .05 was considered significant.
Results
Cell Viability and Proliferative Capacity
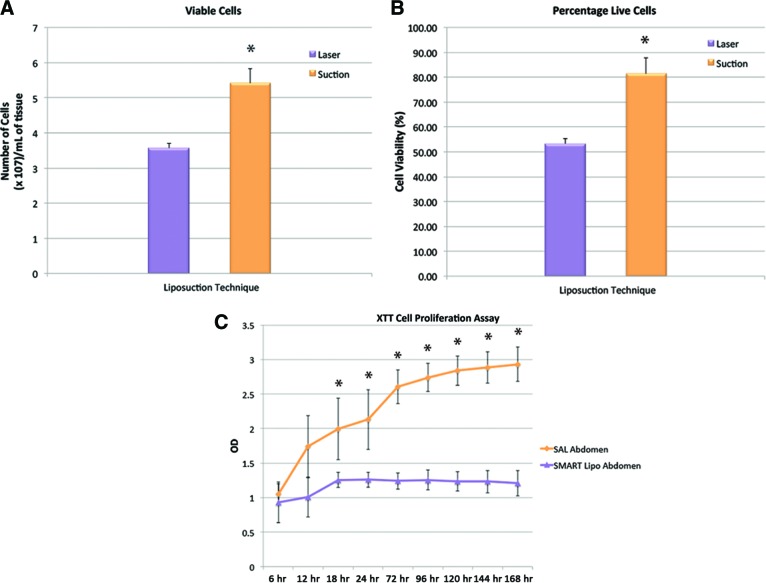
The effects of the liposuction technique on the yield and viability of cells was initially investigated. Laser-assisted liposuction resulted in a significantly lower cell yield than traditional suction-assisted liposuction (p < .05). The total number of cells counted using a hemocytometer was 3.5 × 105 ± 0.1 × 105 cells per milliliter of adipose tissue for laser-assisted liposuction and 5.4 × 105 ± 0.4 × 105 cells per milliliter of adipose tissue for suction-assisted liposuction (Fig. 1A). Laser-assisted liposuction was also associated with a significantly lower percentage of cell viability (p < .05), as determined by trypan blue exclusion. For laser-assisted liposuction aspirates, the percentage of viable ASCs was recorded as 53.3 ± 2.1%. For suction-assisted liposuction aspirates, the percentage of viable cells amounted to 81.4 ± 6.4% (Fig. 1B).
Figure 1.
Cell yield, viability, and proliferation. (A): Overall number of cells isolated from adipose tissue harvested via laser-assisted liposuction and suction-assisted liposuction. Laser-assisted liposuction resulted in a lower viable cell yield than suction-assisted liposuction (*, p < .05). Viable cells were identified by trypan blue exclusion. (B): Laser-assisted liposuction was also associated with a lower percentage of cell viability (*, p < .05). (C): Effect of the liposuction technique on the proliferation of adipose-derived stromal cells in vitro was determined by the XTT cell proliferation assay. There were significant differences in absorption values between the proliferation of cells obtained by laser-assisted liposuction and suction-assisted liposuction at 18, 24, 72, 96, 120, 144, and 168 hours (*, p < .05). Abbreviations: OD, optical density; SAL, suction-assisted liposuction.
The XTT assay displayed significant differences between the proliferation of cells obtained by laser-assisted liposuction and suction-assisted liposuction at 18, 24, 72, 96, 120, 144, and 196 hours (p < .05). The absorption was 1.3 ± 0.1 in the laser-assisted liposuction group and 2.0 ± 0.4 in the suction-assisted liposuction group at 18 hours; 1.3 ± 0.1 in the laser-assisted liposuction group and 2.1 ± 0.4 in the suction-assisted liposuction group at 24 hours; 1.2 ± 0.1 in the laser-assisted liposuction group and 2.6 ± 0.2 in the suction-assisted liposuction group at 72 hours; 1.3 ± 0.1 in the laser-assisted liposuction group and 2.7 ± 0.2 in the suction-assisted liposuction group at 96 hours; 1.2 ± 0.1 in the laser-assisted liposuction group and 2.8 ± 0.2 in the suction-assisted liposuction group at 120 hours; 1.2 ± 0.2 in the laser-assisted liposuction group and 2.9 ± 0.2 in the suction-assisted liposuction group at 144 hours; and 1.2 ± 0.2 in the laser-assisted liposuction group and 2.9 ± 0.2 in the suction-assisted liposuction group at 168 hours (Fig. 1C).
Flow Cytometry Analysis
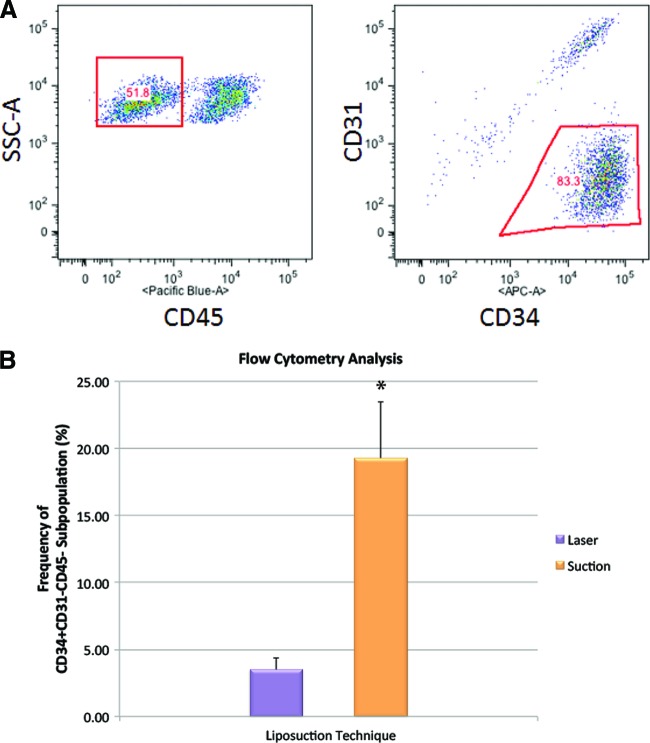
In addition to ASCs, the stromal vascular fraction of adipose tissue also contains a variety of other cells, such as endothelial cells, smooth muscle cells, pericytes, fibroblasts, and blood-derived cells, including erythrocytes and leukocytes [21]. Although an extensive body of work exists pertaining to the phenotypic characterization of mesenchymal stem cells from bone marrow, the phenotypic characterization of ASCs is still in its infancy. However, it is widely accepted that freshly isolated ASCs can be identified as CD34+CD31−CD45− cells in the SVF [22]. To distinguish ASCs from other cells in the SVF, fluorescence-activated cell sorting plots were analyzed after gating on CD34+CD31−CD45− (Fig. 2A). The difference in ASC frequency between adipose tissue harvested using laser-assisted liposuction and suction-assisted liposuction was significant (p < .05). Whereas the percentage of ASCs in the SVF of adipose tissue harvested using suction-assisted liposuction was 19.3 ± 4.2%, the percentage of ASCs in the SVF of adipose tissue harvested using laser-assisted liposuction was only 3.5 ± 0.9% (Fig. 2B).
Figure 2.
Measuring frequency of adipose-derived stromal cell phenotype via flow cytometry. (A): Triple staining was performed using monoclonal antibodies against CD34, CD31, and CD45 to interrogate stromal vascular fraction cells exhibiting an adipose-derived stromal cell (ASC) phenotype. (B): The frequency of cells exhibiting a CD34+CD31−CD45− phenotype was determined. The difference in ASC frequency between adipose tissue harvested using laser-assisted liposuction and suction-assisted liposuction was significantly different (*, p < .05). Abbreviation: SSC, side scatter.
In Vitro Osteogenic Differentiation Assay
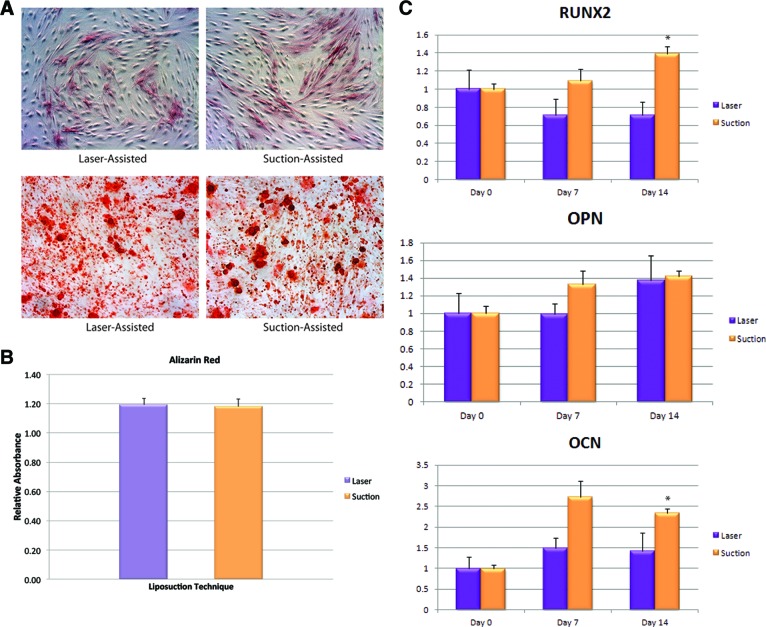
To characterize osteogenic potential of ASCs derived from either laser-assisted liposuction or suction-assisted liposuction, cells were cultured in ODM for 14 days. Alkaline phosphatase (ALP) activity in the ASCs was measured after 7 days of osteogenic stimulation. Interestingly, both laser-assisted liposuction and suction-assisted liposuction (SAL)-derived ASCs stained equally well for ALP activity (Fig. 3A, top). Mineralization of the extracellular matrix was confirmed by using alizarin red staining at 14 days and ASCs derived from laser-assisted liposuction and ASCs derived from suction-assisted liposuction both stained positive by alizarin red (Fig. 3A, bottom). Quantification of ALP (data not shown) and alizarin red staining (Fig. 3B) showed no difference in osteogenic potential between ASCs derived from laser-assisted liposuction and ASCs derived from suction-assisted liposuction.
Figure 3.
Differentiation toward the osteogenic lineage. (A): Osteogenic differentiation of adipose-derived stromal cells (ASCs) isolated from adipose tissue harvested via laser-assisted liposuction and suction-assisted liposuction was demonstrated by alkaline phosphatase staining (top) and alizarin red staining (bottom). Magnification, ×10. (B): Photometric quantification of alizarin red staining showed no significant difference between laser-assisted and suction-assisted liposuction-derived ASCs. (C): Gene expression of early (RUNX2), intermediate (OPN), and late (OCN) osteogenic markers (*, p < 0.05). Abbreviations: OCN, osteocalcin; OPN, osteopontin; RUNX2, runt-related transcription factor 2.
Osteogenic Gene Expression
To correlate osteogenic differentiation in vitro with changes in osteogenic gene expression, we examined transcript levels for markers of bone differentiation at baseline and after 7 and 14 days of ODM treatment. No significant differences were detected in osteogenic gene expression of Runt-related transcription factor-2 (RUNX2), osteopontin (OPN), and osteocalcin (OCN) between ASCs derived from laser-assisted liposuction and ASCs derived from suction-assisted liposuction at day 7. However, statistically significant enhanced expression of RUNX2 and OCN was seen in ASCs derived from suction-assisted liposuction at day 14 compared with ASCs derived from laser-assisted liposuction (p < 0.05; Fig. 3C).
In Vitro Adipogenic Differentiation
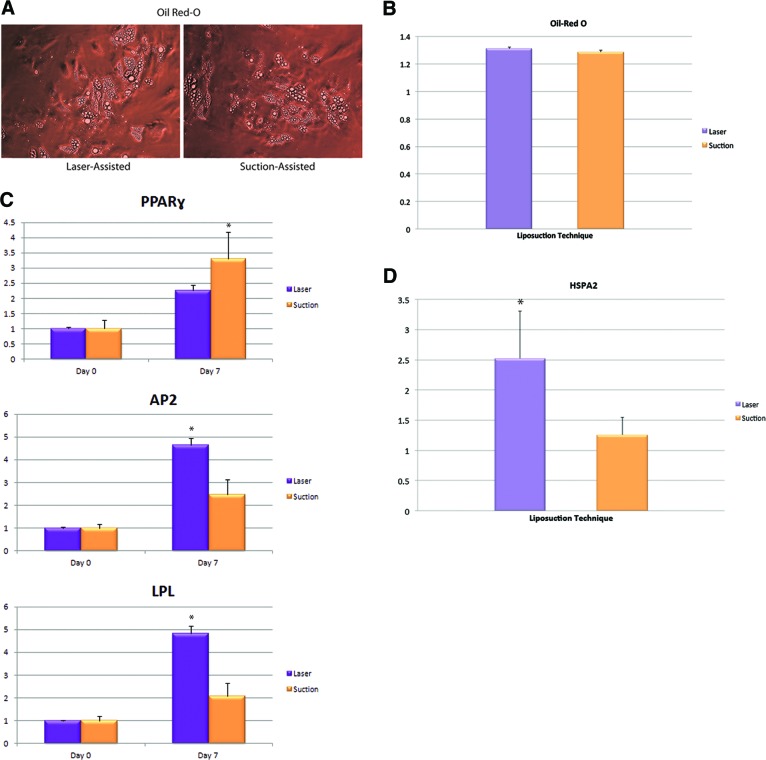
To characterize the adipogenic potential of ASCs from either laser-assisted liposuction or suction-assisted liposuction, cells were cultured in adipogenic medium for 7 days. Similar to osteogenic differentiation, quantification of Oil Red O staining showed no difference in adipogenic potential between ASCs derived from laser-assisted liposuction and ASCs derived from suction-assisted liposuction (Fig. 4A, 4B). To determine whether adipogenic differentiation in vitro correlated with an increase in adipogenic gene expression, we examined transcript levels for markers of fat differentiation at baseline and after 7 days of adipogenic stimulation. Interestingly, ASCs derived from laser-assisted liposuction exhibited reduced expression of the early adipogenic marker peroxisome proliferator-activated receptor γ (PPAR-γ) but enhanced expression of adipogenic markers AP2/FABP4I and LPL compared with ASCs derived from suction-assisted liposuction (p < 0.05; Fig. 4C). To investigate the effects of heat stimulation on adipogenesis, we examined gene expression levels of heat shock protein A2 (HSPA2) in freshly isolated ASCs immediately after harvesting the cells from adipose tissue harvested via laser-assisted liposuction and suction-assisted liposuction. Interestingly, ASCs derived from laser-assisted liposuction had higher levels of HSPA2 activity than ASCs derived from suction-assisted liposuction (p < 0.05; Fig. 4C).
Figure 4.
Differentiation toward the adipogenic lineage. (A): Adipogenic differentiation of adipose-derived stromal cells (ASCs) isolated from adipose tissue harvested via laser-assisted liposuction and suction-assisted liposuction was demonstrated by Oil Red O staining, which demonstrated lipid droplet formation. (B): Photometric quantification of Oil Red O staining showed no significant difference between laser-assisted and suction-assisted liposuction-derived ASCs. (C): Gene expression of PPAR-γ, AP2/FABP4, and LPL (*, p < 0.05). (D): Gene expression of heat shock protein HSPA2 (*, p < 0.05). Abbreviations: AP2, adipocyte protein 2; FABP4, fatty acid binding protein 4; HSPA2, heat shock-related 70-kDa protein 2; LPL, lipoprotein lipase; PPAR-γ, peroxisome proliferator-activated receptor γ.
In Vivo Bone Formation
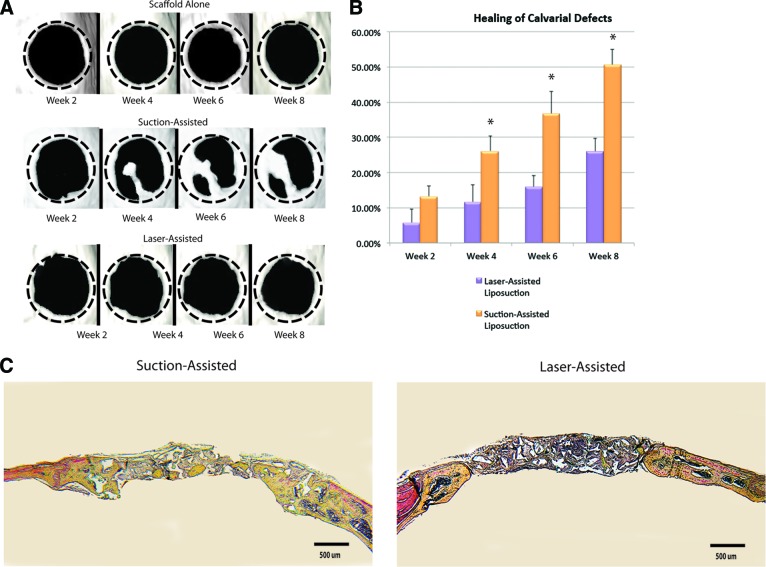
To evaluate the in vivo osteogenic capacity of these ASC populations, repair was performed using uncultured, freshly isolated cells seeded onto HA-PLGA scaffolds. Radiographic analysis was performed from baseline to 8 weeks using micro-CT. ASCs derived from suction-assisted liposuction demonstrated increased de novo bone regeneration when compared with defects treated with ASCs derived from laser-assisted liposuction (Fig. 5A). Quantification of healing postoperatively demonstrated significantly increased bone formation at 4, 6, and 8 weeks in defects treated with ASCs derived from suction-assisted liposuction when compared with ASCs derived from laser-assisted liposuction (p < .05; Fig. 5B).
Figure 5.
Application of adipose-derived stromal cells in calvarial defects. (A): Three-dimensional reconstruction of calvarial defects. Mice were scanned at 2, 4, 6, and 8 weeks following surgery. (B): Quantification of osseous healing by micro-computed tomography revealed significantly more healing with adipose-derived stromal cells isolated from adipose tissue harvested via suction-assisted liposuction relative to laser-assisted liposuction (*, p < .05) at the 4-, 6-, and 8-week time points. (C): Calvarial defects 4 mm in size were allowed to heal for 8 weeks before histological analysis by Movat's pentachrome staining. Pictures were taken in the middle of the defect site. In pentachrome stains, bone appears yellow.
To evaluate why ASCs derived from suction-assisted liposuction may result in enhanced bone regeneration relative to laser-assisted liposuction, we investigated cell adhesion to HA-PLGA scaffolds. Attached cells were lifted and counted using a hemacytometer. Relative to laser-assisted liposuction, suction-assisted liposuction yielded 12.7-fold more adherent ASCs (data not shown).
Histological Analysis
Histological analysis with Movat's pentachrome staining (in which mature bone stains yellow) of calvarial defects at 8 weeks after surgery correlated with micro-CT findings. Defects treated with ASCs derived from suction-assisted liposuction showed robust and thick bony regeneration throughout the defect. Defects treated with ASCs derived from laser-assisted liposuction demonstrated some bone formation, but to a lesser degree when compared with the suction-assisted liposuction group (Fig. 5C).
Discussion
Recent advances in tissue engineering have expanded the opportunities for use of adipose-derived stromal cells for reconstruction of complex defects. The ultimate goal is to take a patient to the operating room, harvest lipoaspirate, and immediately place the derived ASCs on a biomimetic scaffold to offer a single-step, definitive reconstruction without leaving the operating room. In most studies investigating ASCs, the cells were isolated from adipose tissue obtained via suction-assisted liposuction. However, in daily practice, adipose tissue is harvested via different surgical procedures, including resection, ultrasound-assisted liposuction, water jet-assisted liposuction, power-assisted liposuction, and laser-assisted liposuction [23]. Despite many studies comparing the safety and efficacy of these different liposuction techniques, evaluation of the quality and differentiation potential of ASCs recovered using various liposuction methods is limited.
Although each of these different liposuction techniques has various benefits, all ultimately aim to facilitate fat extraction. Ultrasound-assisted liposuction fundamentally differs from traditional suction-assisted liposuction in that it uses the application of ultrasound energy to selectively emulsify subcutaneous adipose tissue through micromechanical, thermal, and microcavitation effects [7]. A previous study in our laboratory investigated whether differences in proliferative capacity and osteogenic potential existed between ASCs obtained by means of suction-assisted liposuction versus third-generation ultrasound-assisted liposuction (VASER Lipo System) [11]. The data demonstrated that exposure to ultrasound energy by means of ultrasound-assisted liposuction did not impair the osteogenic potential of ASCs relative to ASCs obtained by means of suction-assisted liposuction in vitro. Oedayrajsingh-Varma et al. compared the yield and growth characteristics of ASCs, which were isolated from adipose tissue harvested via three different types of surgical procedures (i.e., resection, tumescent liposuction, and ultrasound-assisted liposuction) [24]. Although they found that the surgical procedure used did not affect the yield of viable stromal vascular fraction cells obtained from adipose tissue, they demonstrated that the surgical procedure did affect the number and functional properties of the ASCs. Ultrasound-assisted liposuction decreased the mean frequency of ASCs (defined as CD34+CD31−CD105+CD166+CD45−CD90+) in the SVF by 16-fold compared with resection, and it increased the population doubling time of cultured ASCs by more than 10-fold [24].
The purpose of the present study was to compare the yield, proliferative capacity, and differentiation potential of ASCs, which were isolated from adipose tissue harvested via two different types of surgical procedures, suction-assisted liposuction and laser-assisted liposuction. We found that adipose tissue obtained by suction-assisted liposuction provided high frequencies of rapidly growing ASCs, which could be used for tissue-engineering purposes, whereas adipose tissue obtained by laser-assisted liposuction provided lower amounts of ASCs. The number of viable ASCs harvested from laser-assisted lipoaspirate was approximately 1.9 × 105 cells per milliliter of adipose tissue, which was lower than the yields obtained from suction-assisted lipoaspirate. Laser-assisted liposuction is designed to provide more selective adipose damage, facilitate fat removal, enhance hemostasis, and increase tissue tightening. Although multiple laser systems using different wavelengths exist for laser-assisted liposuction, the effects of laser-assisted lipolysis are caused by photothermal energy. The laser system emits light in the form of a beam that is converted to heat energy in the fat, collagenous tissue, and hemoglobin. Appropriate laser selection allows preferential targeting of tissues, since the different wavelengths have different absorption coefficients for fat, water, and hemoglobin [25].
With claims that laser lipolysis is less traumatic than traditional liposuction methods because of the laser-tissue effects, plastic surgeons are beginning to expand the application of this technology. Although the location of ASCs in the adipose tissue is not clearly understood, some ASCs reside in the connective tissues, whereas others are located between adipocytes or around microvasculature [26]. Since the stromal compartment of adipose tissue is thought to harbor ASCs, the heating of collagenous fibrous septae may reduce the viability of ASCs, as well as mature adipocytes.
The present study demonstrated that human adipose-derived stromal cells derived from laser-assisted liposuction have different osteogenic and adipogenic potentials compared with those derived from suction-assisted liposuction. Although both groups undergo differentiation, gene analysis suggested terminal acquisition of osteogenic cell fate to be more robust with SAL. Interestingly, although ASCs derived from suction-assisted liposuction had an increased expression of the early-stage adipokine AP2/FABP4, the converse was true for the late stage adipokine LPL. Although these findings differ from our expectations, it should be noted that heat stimulation has been reported to reduce adipogenesis time dependently. Ezure and Amano investigated the effect of heat stimulation on adipogenesis using 3T3-L1 preadipocytes as a model and found that heat downregulated the expression of transcription factors involved in the early phase of adipogenesis [27]. Strikingly, heat shock has been reported to induce expression of late adipocyte markers and has been correlated with increased adipocyte differentiation [28, 29]. Therefore, it is possible that the heat generated by laser-assisted liposuction may trigger adipogenesis in the late phase of differentiation. Consistent with these prior studies, we demonstrated an inverse correlation between the early adipogenic marker PPAR-γ and heat shock protein HSPA2. However, it is important to note that these prior studies used different laser parameters.
With the exception of a small number of studies, most in vivo studies have been performed on cultured adipose-derived stromal cells [30]. If these cells were to be used clinically, prohibitively expensive good manufacturing practice production facilities and Food and Drug Administration approval would be required, which would involve considerable expense and time. These limitations make it difficult to envisage using cultured adipose-derived stromal cells for clinical use. Therefore, in the present study, we first compared the yield, proliferative capacity, and differentiation potential of ASCs in vitro, which were isolated from adipose tissue harvested via two different types of surgical procedures, suction-assisted liposuction and laser-assisted liposuction. To mimic the clinical setting as close as possible, we then evaluated the osteogenic potential of uncultured freshly isolated adipose-derived stromal cells in a critical-sized mouse calvarial defect model and found that laser-assisted liposuction may have a deleterious effect on the osteogenic differentiation of ASCs in vivo. This may in part be attributable to differences in cell adhesion to the scaffolds. Although both ASCs derived from suction-assisted liposuction and ASCs derived from laser-assisted liposuction exhibited similar osteogenic differentiation potential in vitro, quantification of bone healing in vivo revealed striking differences in the amount of new bone formation, which may be attributable to the increased heterogeneity in uncultured ASCs. The SVF of adipose tissue is a heterogeneous mixture of cells, and when SVF cells are seeded in plastic tissue culture dishes, all these cell populations may potentially adhere. Previous studies have demonstrated notable changes between fresh and culture states, including decreased expression of CD31 and CD34 and increased expression of CD29 and CD105 [31, 32]. These changes suggest that cells other than ASCs, such as vascular endothelial cells and blood-derived cells, are selectively excluded when culturing cells on plastic plates in vitro. Therefore, the inconsistency between the in vitro data and the in vivo outcomes may be due to the different immunophenotype exhibited by cultured ASCs and freshly isolated ASCs.
Finally, we acknowledge that several limitations to the present study exist. To minimize potential heterogeneity, all ASCs were consistently derived from female patients from the abdomen. However, patient-to-patient variability may still exist, as laser-assisted liposuction and SAL were performed on different patients. Although we anticipate that ASCs derived from dissimilar donors would have a similar cellular response to thermal laser injury, we cannot exclude that ASCs from a different demographic would have varying reactions to laser-mediated thermal stress/injury. Future studies must verify whether these data are generalizable to ASCs as a whole.
Conclusion
Much of the current work in the field of tissue engineering and regenerative medicine is focused on the development of materials to replace those lost through trauma and aging. Adipose-derived stromal cells may eventually serve as a readily available source of autologous stem cells to combine with biomimetic materials for the engineering of bone and fat. With the advent of new bioengineering and stem cell technologies, the use of autologous ASCs may soon be a clinical reality in the United States. However, a primary problem with such strategies is populating the replacement material with viable cells. Although it has been recently reported that fat tissue has the highest percentage of adult stem cells of any tissue in the body, the method of harvesting adipose tissue can have a significant impact on the yield and viability of isolated cells [33, 34]. Whereas ASCs harvested with laser and suction both undergo osteogenic and adipogenic differentiation, the impact on cellular yield and ASC biology make suction-assisted liposuction more advantageous for clinical applications where large numbers of viable cells are necessary for tissue repair and reconstruction.
Acknowledgments
This study was supported by National Institutes of Health Research Grants R01-DE021683-01, R01-AR052294-01, R01-DE13194, and RC2-DE020771-01; the Oak Foundation; and the Hagey Laboratory for Pediatric Regenerative Medicine (to M.T.L.) and by Howard Hughes Medical Institute Research Fellowships (to M.T.C. and S.D.M.).
Author Contributions
M.T.C. and A.S.Z.: project design, collection and assembly of data, manuscript writing, manuscript revision, final approval of manuscript; K.J.P., S.D.M., J.S.H., D.D.L., A.M., D.T.M, G.G.W., K.S.-Y., M.S., R.R., H.-H.C., and A.S.C.: acquisition of data, provision of study material, manuscript revision, final approval of manuscript; D.V.: provision of patients; G.C.G., M.T.L., and D.C.W.: conception and design, analysis and interpretation of data, manuscript revision, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.American Society of Plastic Surgeons 2011 Plastic Surgery Statistics Report. [Accessed December 8, 2012]. Available at http://www.plasticsurgery.org/Documents/news-resources/statistics/2011-statistics/2011_Stats_Full_Report.pdf.
- 2.Mordon S, Eymard-Maurin AF, Wassmer B, et al. Histologic evaluation of laser lipolysis: Pulsed 1064-nm Nd:YAG laser versus cw 980-nm diode laser. Aesthet Surg J. 2007;27:263–268. doi: 10.1016/j.asj.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Zuk PA. Stem cell research has only just begun. Science. 2001;293:211–212. [PubMed] [Google Scholar]
- 4.Aust L, Devlin B, Foster SJ, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto T, Gotoh M, Hattori R, et al. Periurethral injection of autologous adipose-derived stem cells for the treatment of stress urinary incontinence in patients undergoing radical prostatectomy: Report of two initial cases. Int J Urol. 2010;17:75–82. doi: 10.1111/j.1442-2042.2009.02429.x. [DOI] [PubMed] [Google Scholar]
- 6.Collins PC, Field LM, Narins RS. Liposuction surgery and autologous fat transplantation. Clin Dermatol. 1992;10:365–372. doi: 10.1016/0738-081x(92)90080-i. [DOI] [PubMed] [Google Scholar]
- 7.de Souza Pinto EB, Abdala PC, Maciel CM, et al. Liposuction and VASER. Clin Plast Surg. 2006;33:107–115. vii. doi: 10.1016/j.cps.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Suslick KS, Doktycz SJ, Flint EB. On the origin of sonoluminescence and sonochemistry. Ultrasonics. 1990;28:280–290. doi: 10.1016/0041-624x(90)90033-k. [DOI] [PubMed] [Google Scholar]
- 9.Topaz M, Motiei M, Gedanken A, et al. EPR analysis of radicals generated in ultrasound-assisted lipoplasty simulated environment. Ultrasound Med Biol. 2001;27:851–859. doi: 10.1016/s0301-5629(01)00366-0. [DOI] [PubMed] [Google Scholar]
- 10.Zocchi ML. Ultrasonic assisted lipoplasty: Technical refinements and clinical evaluations. Clin Plast Surg. 1996;23:575–598. [PubMed] [Google Scholar]
- 11.Panetta NJ, Gupta DM, Kwan MD, et al. Tissue harvest by means of suction-assisted or third-generation ultrasound-assisted lipoaspiration has no effect on osteogenic potential of human adipose-derived stromal cells. Plast Reconstr Surg. 2009;124:65–73. doi: 10.1097/PRS.0b013e3181ab10cd. [DOI] [PubMed] [Google Scholar]
- 12.Schipper BM, Marra KG, Zhang W, et al. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg. 2008;60:538–544. doi: 10.1097/SAP.0b013e3181723bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Zhao L, Hantash BM. Support of human adipose-derived mesenchymal stem cell multipotency by a poloxamer-octapeptide hybrid hydrogel. Biomaterials. 2010;31:5122–5130. doi: 10.1016/j.biomaterials.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Wang YH, Ho ML, Chang JK, et al. Microporation is a valuable transfection method for gene expression in human adipose tissue-derived stem cells. Mol Ther. 2009;17:302–308. doi: 10.1038/mt.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta DM, Panetta NJ, Longaker MT. Osteogenic differentiation of human multipotent mesenchymal stromal cells. Methods Mol Biol. 2011;698:201–214. doi: 10.1007/978-1-60761-999-4_16. [DOI] [PubMed] [Google Scholar]
- 16.Levi B, James AW, Glotzbach JP, et al. Depot-specific variation in the osteogenic and adipogenic potential of human adipose-derived stromal cells. Plast Reconstr Surg. 2010;126:822–834. doi: 10.1097/PRS.0b013e3181e5f892. [DOI] [PubMed] [Google Scholar]
- 17.Levi B, Longaker MT. Osteogenic differentiation of adipose-derived stromal cells in mouse and human: In vitro and in vivo methods. J Craniofac Surg. 2011;22:388–391. doi: 10.1097/SCS.0b013e318207b72b. [DOI] [PubMed] [Google Scholar]
- 18.Levi B, Nelson ER, Brown K, et al. Differences in osteogenic differentiation of adipose-derived stromal cells from murine, canine, and human sources in vitro and in vivo. Plast Reconstr Surg. 2011;128:373–386. doi: 10.1097/PRS.0b013e31821e6e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003;31:e154. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowan CM, Shi YY, Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 21.Cherubino M, Marra KG. Adipose-derived stem cells for soft tissue reconstruction. Regen Med. 2009;4:109–117. doi: 10.2217/17460751.4.1.109. [DOI] [PubMed] [Google Scholar]
- 22.Suga H, Matsumoto D, Eto H, et al. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009;18:1201–1210. doi: 10.1089/scd.2009.0003. [DOI] [PubMed] [Google Scholar]
- 23.Berry MG, Davies D. Liposuction: A review of principles and techniques. J Plast Reconstr Aesthet Surg. 2011;64:985–992. doi: 10.1016/j.bjps.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, et al. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8:166–177. doi: 10.1080/14653240600621125. [DOI] [PubMed] [Google Scholar]
- 25.Parlette EC, Kaminer ME. Laser-assisted liposuction: Here's the skinny. Semin Cutan Med Surg. 2008;27:259–263. doi: 10.1016/j.sder.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipotransfer: Supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375–3382. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- 27.Ezure T, Amano S. Heat stimulation reduces early adipogenesis in 3T3–L1 preadipocytes. Endocrine. 2009;35:402–408. doi: 10.1007/s12020-009-9164-4. [DOI] [PubMed] [Google Scholar]
- 28.Aguilar V, Annicotte JS, Escote X, et al. Cyclin G2 regulates adipogenesis through PPAR gamma coactivation. Endocrinology. 2010;151:5247–5254. doi: 10.1210/en.2010-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernabucci U, Basirico L, Morera P, et al. Heat shock modulates adipokines expression in 3T3–L1 adipocytes. J Mol Endocrinol. 2009;42:139–147. doi: 10.1677/JME-08-0068. [DOI] [PubMed] [Google Scholar]
- 30.Rhee SC, Ji YH, Gharibjanian NA, et al. In vivo evaluation of mixtures of uncultured freshly isolated adipose-derived stem cells and demineralized bone matrix for bone regeneration in a rat critically sized calvarial defect model. Stem Cells Dev. 2011;20:233–242. doi: 10.1089/scd.2009.0525. [DOI] [PubMed] [Google Scholar]
- 31.Varma MJ, Breuls RG, Schouten TE, et al. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;16:91–104. doi: 10.1089/scd.2006.0026. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 33.Coleman SR. Structural fat grafting: More than a permanent filler. Plast Reconstr Surg. 2006;118:108S–120S. doi: 10.1097/01.prs.0000234610.81672.e7. [DOI] [PubMed] [Google Scholar]
- 34.Hodgkinson T, Yuan XF, Bayat A. Adult stem cells in tissue engineering. Expert Rev Med Devices. 2009;6:621–640. doi: 10.1586/erd.09.48. [DOI] [PubMed] [Google Scholar]