Abstract
Peritoneal fibrosis (PF) causes ultrafiltration failure (UFF) and is a complicating factor in long-term peritoneal dialysis. Lymphatic reabsorption also may contribute to UFF, but little is known about lymphangiogenesis in patients with UFF and peritonitis. We studied the role of the lymphangiogenesis mediator vascular endothelial growth factor-C (VEGF-C) in human dialysate effluents, peritoneal tissues, and peritoneal mesothelial cells (HPMCs). Dialysate VEGF-C concentration correlated positively with the dialysate-to-plasma ratio of creatinine (D/P Cr) and the dialysate TGF-β1 concentration. Peritoneal tissue from patients with UFF expressed higher levels of VEGF-C, lymphatic endothelial hyaluronan receptor-1 (LYVE-1), and podoplanin mRNA and contained more lymphatic vessels than tissue from patients without UFF. Furthermore, mesothelial cell and macrophage expression of VEGF-C increased in the peritoneal membranes of patients with UFF and peritonitis. In cultured mesothelial cells, TGF-β1 upregulated the expression of VEGF-C mRNA and protein, and this upregulation was suppressed by a TGF-β type I receptor (TGFβR-I) inhibitor. TGF-β1–induced upregulation of VEGF-C mRNA expression in cultured HPMCs correlated with the D/P Cr of the patient from whom the HPMCs were derived (P<0.001). Moreover, treatment with a TGFβR-I inhibitor suppressed the enhanced lymphangiogenesis and VEGF-C expression associated with fibrosis in a rat model of PF. These results suggest that lymphangiogenesis associates with fibrosis through the TGF-β–VEGF-C pathway.
The decrease in ultrafiltration capacity that is associated with the high peritoneal solute transport that is observed after prolonged peritoneal dialysis (PD) treatment is a major reason for its discontinuation.1–4 Several studies have shown that a higher peritoneal solute transport rate is associated with reduced survival of PD patients.1,2,5 The characteristic features of chronic peritoneal damage in PD treatment are associated with submesothelial fibrosis and neoangiogenesis.6,7 Analyses of the surface peritoneum showed no significant changes in vessel density with duration of PD.6,8 In addition, the vessel density in patients with ultrafiltration failure (UFF) was significantly higher than the vessel density in normal individuals or non-PD patients, but it was not higher than the vessel density in patients undergoing PD.6 These findings suggest that factors other than increased vascular density may be involved in disease states associated with increased transport of peritoneal membranes. In addition, the relationship between peritoneal fibrosis and UFF remains obscure.
Blood capillaries have a continuous basal lamina with tight interendothelial junctions and are supported by pericytes and smooth muscle cells. In contrast, lymphatic capillaries are thin-walled with a wide lumen and do not contain pericytes or basement membrane. The structures of lymphatic vessels are suitable for the removal of tissue fluid, cells, and macromolecules from the interstitium.9–11 If lymphangiogenesis develops in the peritoneal membrane, absorption of the PD fluid could be increased and lead to UFF. An increase in the number of lymphatic vessels has recently been reported in several disease conditions, including tumor metastasis,12–15 chronic respiratory inflammatory diseases,16–18 wound healing,19 and renal transplant rejection.20,21 We recently reported that lymphangiogenesis had developed in tubulointerstitial fibrosis of human renal biopsy specimens,22 and we also reported the mechanisms of lymphangiogenesis in rat unilateral ureteral obstruction models.23
The lymphatic absorption rate, which is measured by the rate at which intraperitoneally administered radioactive serum albumin or macromolecule dextran 70 disappears, is significantly higher in patients with UFF, and lymphatic reabsorption is considered to be one of the causes of UFF.24–27 However, the results from these clinical approaches have been controversial.28,29 In addition, little is known about the pathology and the process of lymphangiogenesis in patients with UFF and peritonitis.
In this study, we investigated lymphangiogenesis and the expression of vascular endothelial growth factor-C (VEGF-C), which is a potentially important mediator of lymphangiogenesis, in human peritoneal tissues, PD effluent, and peritoneal mesothelial cells. We also explored VEGF-C induction by TGF-β1 in the human mesothelial cell line (Met-5A) and cultured human peritoneal mesothelial cells (HPMCs) from the spent PD effluent of patients with varying rates of peritoneal transport. Finally, we explored the relationship between peritoneal fibrosis and lymphangiogenesis in rats that were administered chlorhexidine gluconate (CG) into the abdominal cavity, which provides a model of chemically induced peritoneal inflammation/fibrosis.30–32 This work is the first report to show that lymphangiogenesis is linked to the peritoneal fibrosis that is often associated with a high peritoneal transport rate.
Results
VEGF-C Concentration in the Peritoneal Effluent Correlated with the Peritoneal Transport Rate
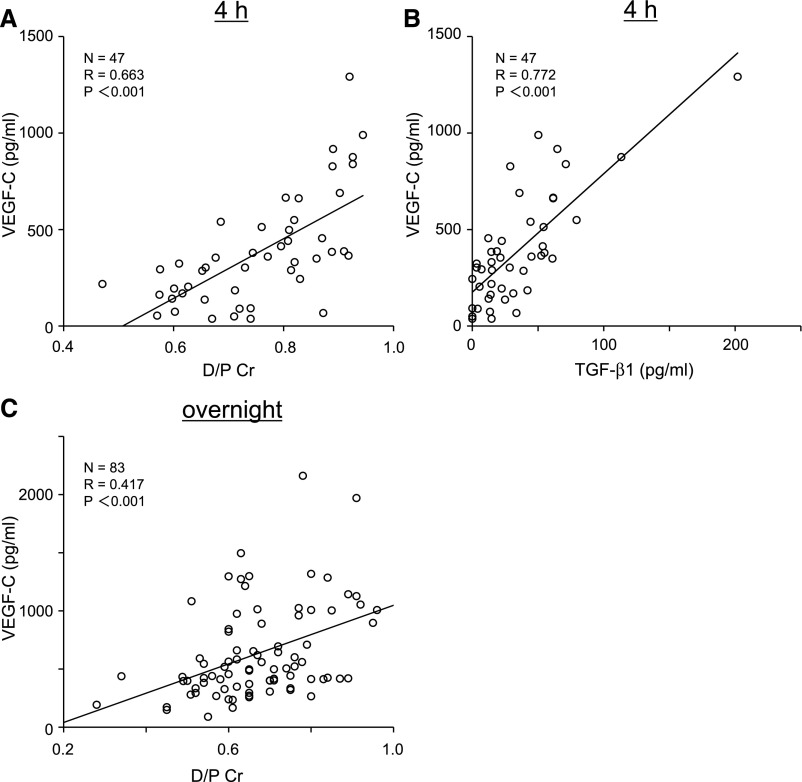
We found a positive correlation between VEGF-C concentration in the PD effluent of 4-hour dwelled samples and dialysate-to-plasma ratio of creatinine (D/P Cr; R=0.663, P<0.001) (Figure 1A). We also measured dialysate TGF-β1 levels. There was a positive correlation between VEGF-C and TGF-β1 concentration in the PD effluent of 4-hour dwelled samples (R=0.772, P<0.001) (Figure 1B). We also assessed VEGF-C concentration in the overnight dwelled PD effluent of 83 patients; there was a positive correlation between the dialysate VEGF-C concentration and the D/P Cr ratio (R=0.417, P<0.001) (Figure 1C).
Figure 1.
VEGF-C concentration in human PD effluent correlates with TGF-β1 concentration in PD effluent and the peritoneal transport rate (D/P Cr). (A) Positive correlation between the VEGF-C concentration in the PD effluent of 4-hour dwelled samples and the D/P Cr. (B) Positive correlation between VEGF-C and TGF-β1 concentrations in the PD effluent of 4-hour dwelled samples. (C) Positive correlation between the VEGF-C concentration in overnight dwelled PD effluent samples and D/P Cr.
VEGF-C, Lymphatic Endothelial Hyaluronan Receptor-1, and Podoplanin mRNA Expression Were Correlated with Peritoneal Thickness in Human Peritoneal Biopsy Samples
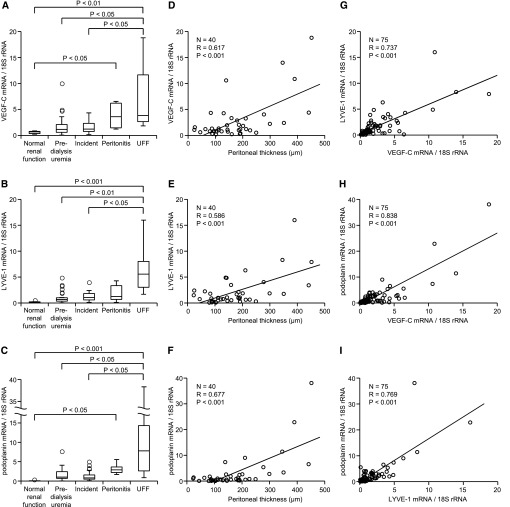
We next investigated the mRNA expression of lymphatic markers (lymphatic endothelial hyaluronan receptor-1 [LYVE-1] and podoplanin) and VEGF-C in the peritoneal membrane of human biopsy samples (Table 1). The peritoneal membrane in the predialysis uremia group (160.0±51.8 μm) was thicker than the peritoneal membrane in living kidney donors with normal renal function (82.0±22.9 μm). The peritoneum from patients with UFF and peritonitis conditions (referred to hereafter as UFF peritoneum and peritonitis peritoneum, respectively) was extremely thick (295.2±125.5 and 311.2±169.6 μm, respectively). The mRNA expression of VEGF-C, LYVE-1, and podoplanin was significantly higher in the UFF peritoneum than the membranes of peritoneum from patients with normal renal function, patients with predialysis uremia, or patients undergoing PD without UFF (Figure 2, A–C). There were correlations between VEGF-C, LYVE-1, and podoplanin mRNA expression and the thickness of the submesothelial compact zone of peritoneal membranes in all patients other than peritonitis patients (Figure 2, D–F). Cases with peritonitis were excluded from this analysis, because as described previously, peritoneum can be thickened by acute inflammatory changes with edema.33 Moreover, there were positive correlations between VEGF-C, LYVE-1, and podoplanin mRNA expression in peritoneal membranes (Figure 2, G–I).
Table 1.
Peritoneal biopsy cases evaluated for VEGF-C, LYVE-1, and podoplanin mRNA expression
| Normal Renal Function | Predialysis Uremia | Incident | Peritonitis | UFF | |
|---|---|---|---|---|---|
| N | 6 | 32 | 23 | 6 | 8 |
| Men | 2 | 23 | 13 | 5 | 4 |
| Women | 4 | 9 | 10 | 1 | 4 |
| Age, yr | 56.5±14.1 | 62.3±12.4 | 60.8±13.2 | 70.8±11.1 | 57.8±12.0 |
| Duration of treatment, yr | 0 | 0 | 3.6±3.0 | 3.2±1.5 | 9.2±6.1 |
| Average thickness of peritoneum, μm | 82.0±22.9 | 160.0±51.8 | 155.7±93.1 | 311.2±169.6 | 295.2±125.5 |
Values are means ± SD. Normal renal function indicates living kidney donors with normal renal function. Predialysis uremia indicates peritoneal tissues that were taken at the time when a PD catheter was inserted because of advanced renal failure. Incident indicates that peritoneal tissues were taken when the catheter was removed because of reasons other than UFF.
Figure 2.
VEGF-C, LYVE-1, and podoplanin mRNA levels increase in UFF and correlate with the thickness of the peritoneum in human peritoneal biopsy samples. (A–C) VEGF-C, LYVE-1, and podoplanin mRNA levels were significantly higher in patients with UFF than patients with normal renal function, patients with predialysis uremia, or patients in the incident group. (D–F) The expression of VEGF-C, LYVE-1, and podoplanin mRNA significantly correlated with the thickness of the peritoneum. (G–I) Positive correlation among VEGF-C, LYVE-1, and podoplanin mRNA expression in peritoneal membranes.
Lymphatic Vessels and VEGF-C Expression Increased in UFF Human Peritoneum Analyzed by Immunohistochemistry
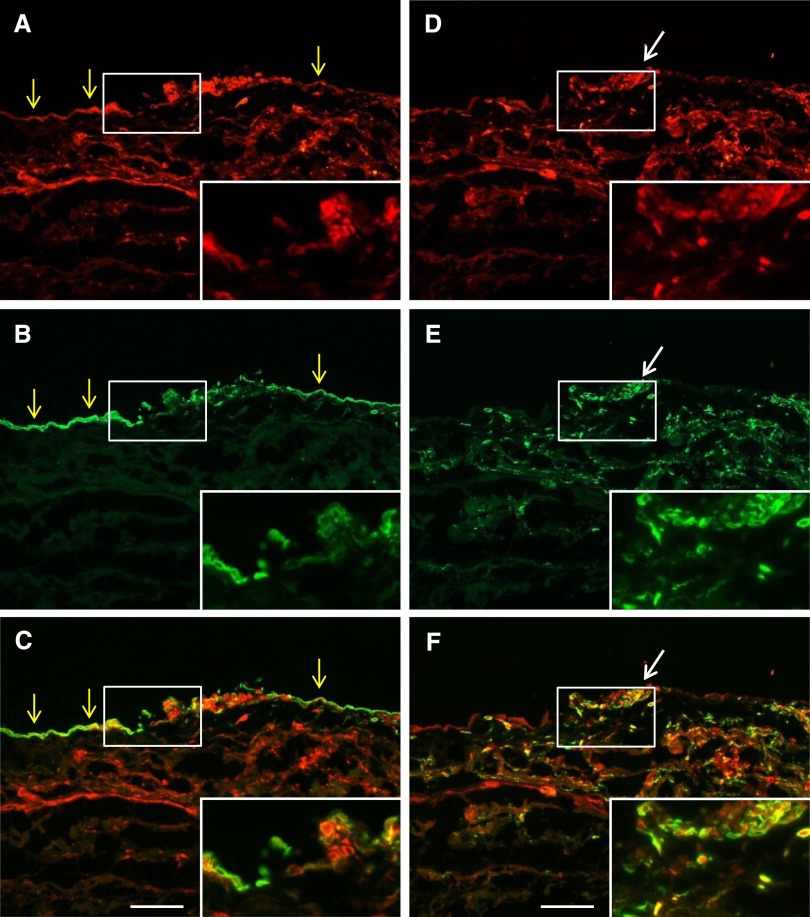
We evaluated lymphatic vessels, blood vessels, and expression of VEGF-C in both UFF and predialysis peritoneum by immunohistochemistry (IHC). LYVE-1–positive lymphatic vessels, pathologische anatomie leiden endothelium-positive blood vessels, and VEGF-C expression were widely observed in UFF peritoneum, but in contrast, they were barely detected in predialysis peritoneum (Supplemental Figure 1). VEGF-C was expressed in cytokeratin-positive mesothelial cells and CD68-positive macrophages, and its expression was enhanced in UFF and peritonitis peritoneal membranes (Figure 3). These findings are consistent with the quantitative PCR data (Figure 2).
Figure 3.
Double immunofluorescent analysis shows VEGF-C expression by mesothelial cells and macrophages in human peritoneal biopsy specimens of bacterial peritonitis. Biopsy specimens were (A and D) stained with VEGF-C and costained with (B) the mesothelial cell marker cytokeratin or (E) the macrophage marker CD68. Respective merged images are shown in C and F. Cytokeratin-positive mesothelial cells (yellow arrows) and CD68-positive macrophages (white arrows) both expressed VEGF-C. (Insets) Magnification of the white boxed area. Scale bars, 100 μm.
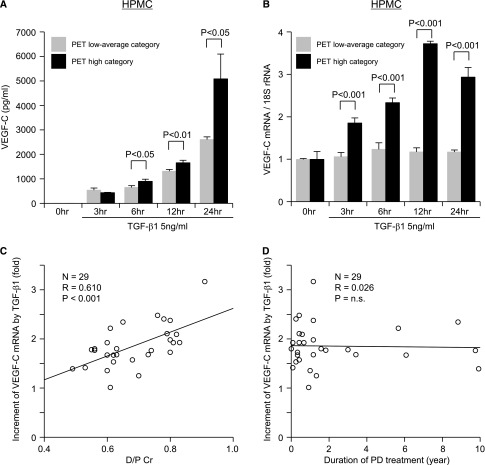
TGF-β1–Induced Upregulation of VEGF-C in Cultured Met-5A Mesothelial Cell Line and HPMCs
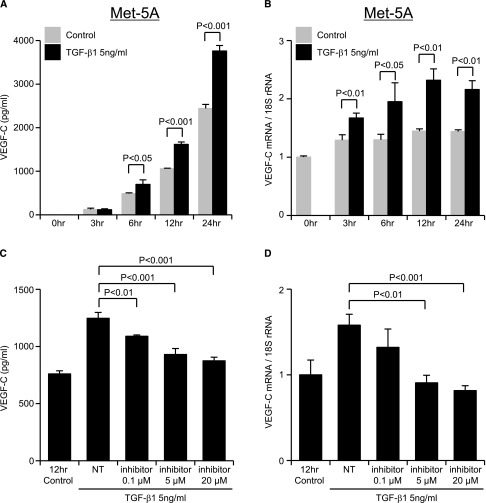
The time course of VEGF-C expression in response to TGF-β1 treatment was studied in both HPMCs and Met-5A cells. Samples were taken after 3, 6, 12, and 24 hours of exposure to TGF-β1 (5 ng/ml). In the Met-5A cells, VEGF-C protein was secreted into the culture supernatants under serum-free conditions without TGF-β1 incubation. The amount of VEGF-C protein secreted was significantly increased by TGF-β1 incubation for 6 (P<0.05), 12, and 24 hours (P<0.001) (Figure 4A). VEGF-C mRNA induction by TGF-β1 also increased over time and peaked at 12 hours (Figure 4B). Both VEGF-C protein and mRNA induction by TGF-β1 in Met-5A cells were suppressed by a TGF-β type I receptor inhibitor (TGFβR-I inhibitor; LY364947) in a dose-dependent manner (Figure 4, C and D). Incubation of HPMCs derived from 29 patients with variable peritoneal membrane transport (Table 2) with TGF-β1 increased VEGF-C protein and mRNA expression (Figure 5, A and B). VEGF-C mRNA expression peaked at 12 hours in 8 of the HPMCs and 24 hours in 21 of the HPMCs. Peak values for VEGF-C mRNA at 12 or 24 hours in all 29 HPMCs showed a correlation with the D/P Cr values of the patients from whom they were derived (R=0.610, P<0.001) (Figure 5C). No significant correlation was found between the extent of the TGF-β1–induced increase in VEGF-C mRNA in the HPMCs and the duration of PD treatment of the patients from whom the HPMCs were derived (Figure 5D).
Figure 4.
VEGF-C expression in a cultured mesothelial cell line (Met-5A cells) increases after treatment with TGF-β1. Met-5A cells that were preincubated for 24 hours in serum-free medium were treated with 5 ng/ml TGF-β1. (A and C) VEGF-C protein levels in the supernatant were determined by ELISA, and (B and D) VEGF-C mRNA levels were determined by real-time PCR. (A) VEGF-C protein levels were significantly increased at 6, 12, and 24 hours after incubation with TGF-β1. (B) VEGF-C mRNA expression was significantly elevated by TGF-β1 compared with the control at each time point. The effect of TGF-β inhibition on VEGF-C expression in cultured Met-5A cells was assayed by incubation of Met-5A cells with 5 ng/ml TGF-β1 in the presence or absence of the TGFβR-I inhibitor LY364947 (inhibitor) for 12 hours. (C) TGF-β–induced VEGF-C protein and (D) mRNA levels were suppressed by the inhibitor in a dose-dependent manner compared with no inhibitor treatment (NT; TGF-β1 stimulation only) at 12 hours. Results are means ± SD of three independent experiments (n=3).
Table 2.
Profiles of patients from whom peritoneal mesothelial cells were isolated for culture studies
| D/P Cr | Men | Women | All Patients | |
|---|---|---|---|---|
| Low | ∼0.49 | 10 | 4 | 14 |
| Low average | 0.5–0.64 | |||
| High average | 0.65–0.8 | 9 | 6 | 15 |
| High | ∼0.81 |
D/P Cr is an index of the peritoneal transport.
Figure 5.
The increase in VEGF-C expression by TGF-β1 in HPMCs correlates with D/P Cr. HPMCs from the spent PD effluent of patients with high peritoneal permeability (PET high category) and low permeability (PET low average category) were stimulated with TGF-β1. (A) VEGF-C protein levels in the supernatant were determined by ELISA, and (B) VEGF-C mRNA levels were determined by real-time PCR. The figures show representative cases from each category. VEGF-C mRNA was increased by TGF-β1, and it peaked at 12 hours in 8 of the HPMCs and 24 hours in 21 of the HPMCs derived from 29 PD patients. (C) Positive correlation between peritoneal permeability (D/P Cr) and the peak values of enhanced VEGF-C mRNA expression at 12 or 24 hours after stimulation with TGF-β1 (5 ng/ml). (D) No significant correlation was observed between the duration of PD treatment and the peak values of increased VEGF-C mRNA expression at 12 or 24 hours after stimulation with TGF-β1 (5 ng/ml).
Lymphangiogenesis Developed in a Rat CG Model of Peritoneal Fibrosis
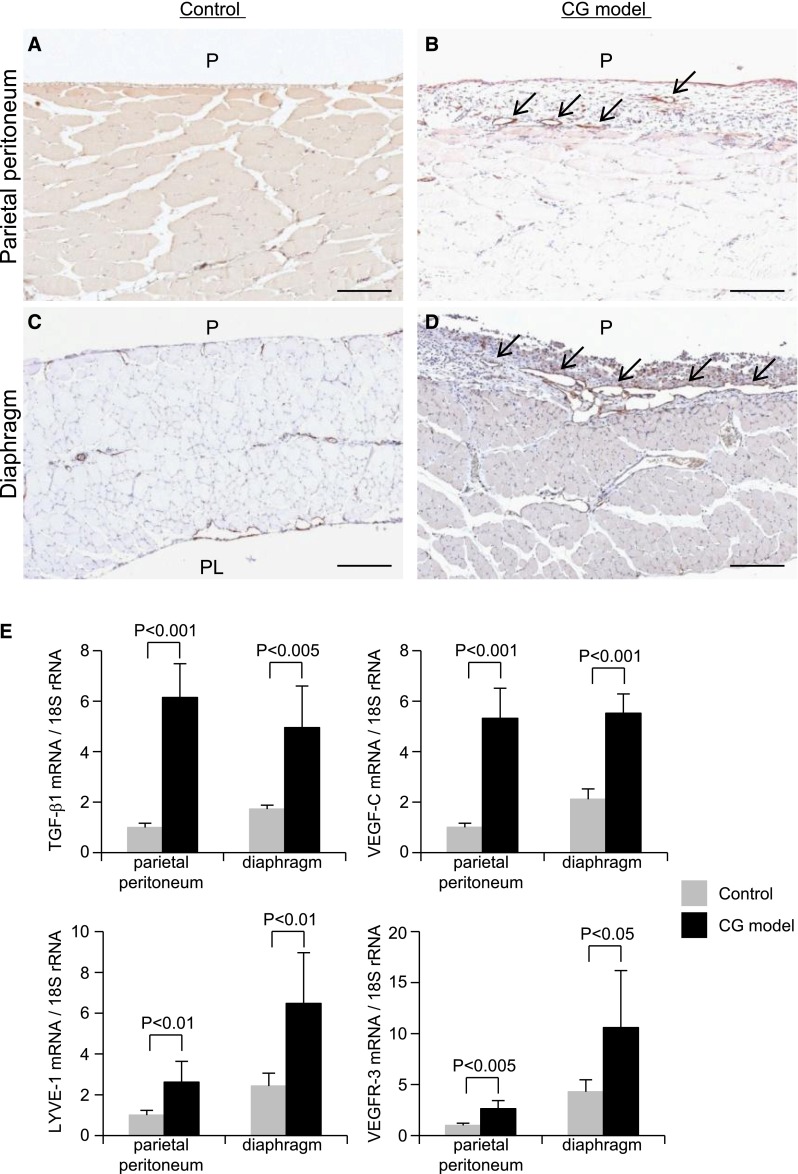
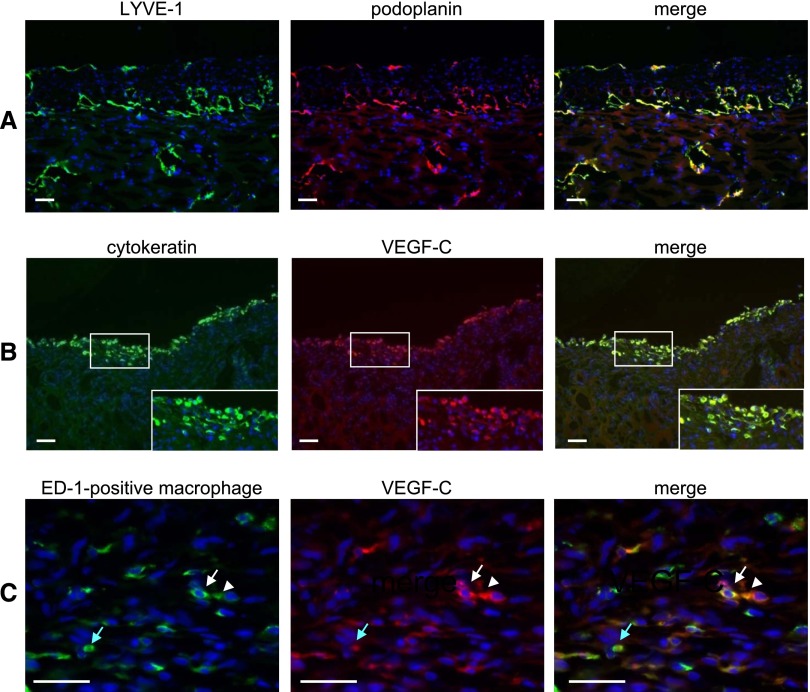
Immunohistochemical analysis indicated that the expression of LYVE-1–positive lymphatic vessels, with a pattern of expression that is similar to the pattern of podoplanin-positive vessels, was increased in both the parietal peritoneum and the diaphragm of CG models compared with controls (Figures 6 and 7A). Furthermore, lymphangiogenesis was more pronounced in the diaphragm than in the parietal peritoneal wall (Figure 6). TGF-β1, VEGF-C, LYVE-1, and VEGF receptor-3 (VEGFR-3) mRNA expression were also increased in both the parietal peritoneum and diaphragm in the CG model compared with controls (Figure 6). VEGFR-3 expression, which was detected in the lymphatics, was also high in the CG model, especially in the diaphragm (Figure 6 and Supplemental Figure 2). Double staining indicated that VEGF-C was expressed by cytokeratin-positive mesothelial cells and ED-1–positive macrophages (Figure 7).
Figure 6.
Expression of LYVE-1 increases in the parietal peritoneum and diaphragm of CG model rats. Staining of (A and B) the parietal peritoneum and (C and D) the diaphragm of (A and C) control and (B and D) CG model rats showed an increased expression of LYVE-1–positive lymphatic vessels (arrows) in the CG model. Strong expression of lymphatic vessels was seen in the diaphragm of the CG rats. Scale bars, 200 μm. (E) TGF-β1, VEGF-C, LYVE-1, and VEGFR-3 mRNA expressions in both the parietal peritoneum and the diaphragm were increased in the CG model compared with the control. Control, n=5; CG models, n=7 for each group. P, peritoneal side; PL, pleural side.
Figure 7.
Double immunofluorescent staining of frozen sections of a CG rat diaphragm shows that VEGF-C is expressed by cytokeratin-positive mesothelial cells and ED-1–positive macrophages. (A) The expression pattern of LYVE-1 (green) was similar to the expression pattern of podoplanin (red). (B) VEGF-C (red) was expressed in cytokeratin-positive mesothelial cells (green). (C) VEGF-C (red) was expressed in ED-1–positive macrophages (green). Arrows and arrowheads of the same color indicate the same cells. Nuclei were counterstained with 4',6-diamino-2-phenylindole (blue). (Insets) Magnification of the white boxed area. Scale bars, 50 μm.
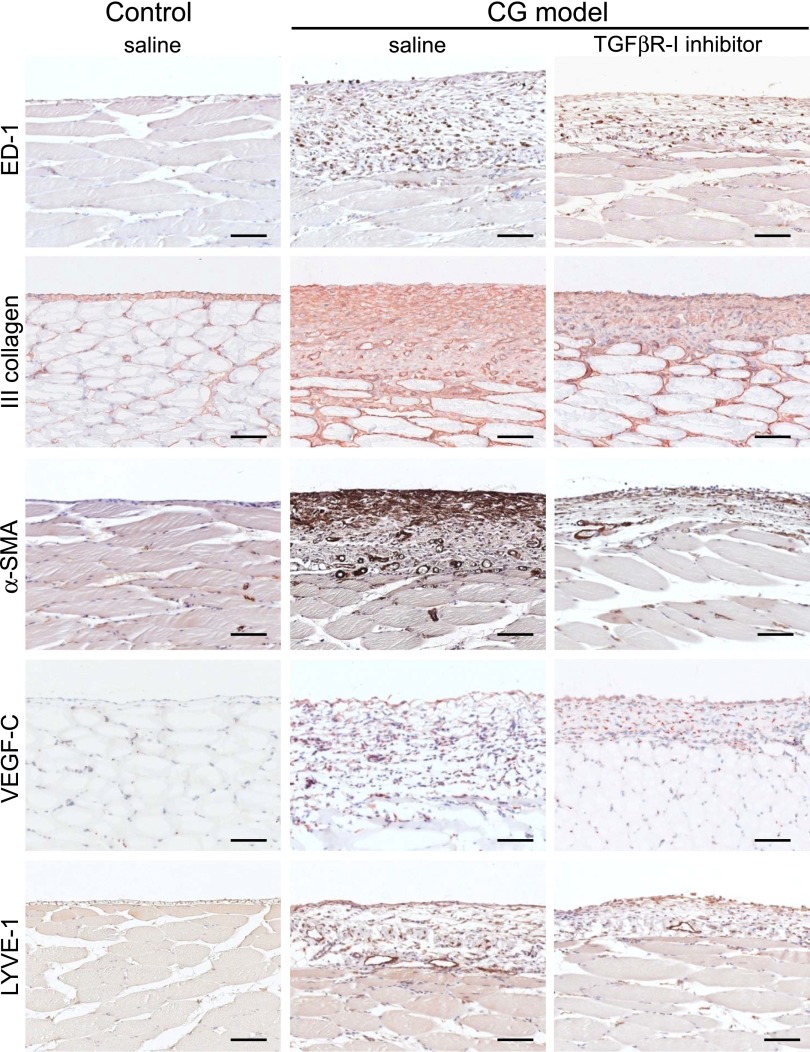
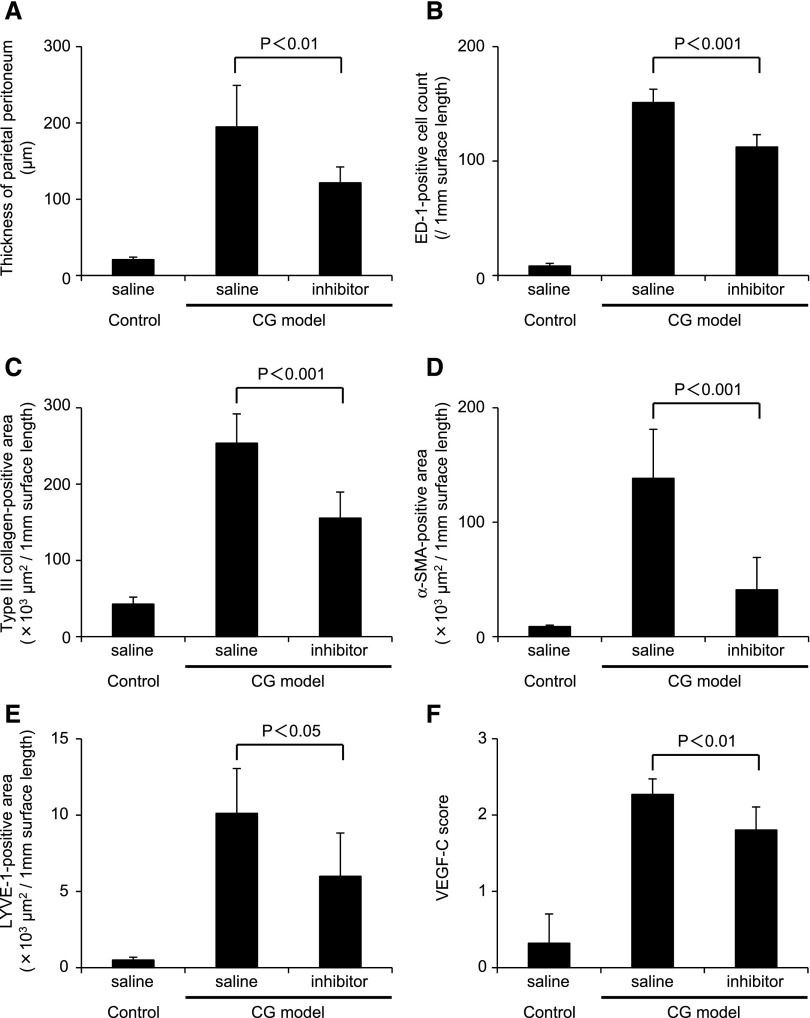
TGFβR-I Treatment Suppressed Lymphangiogenesis in the CG Model
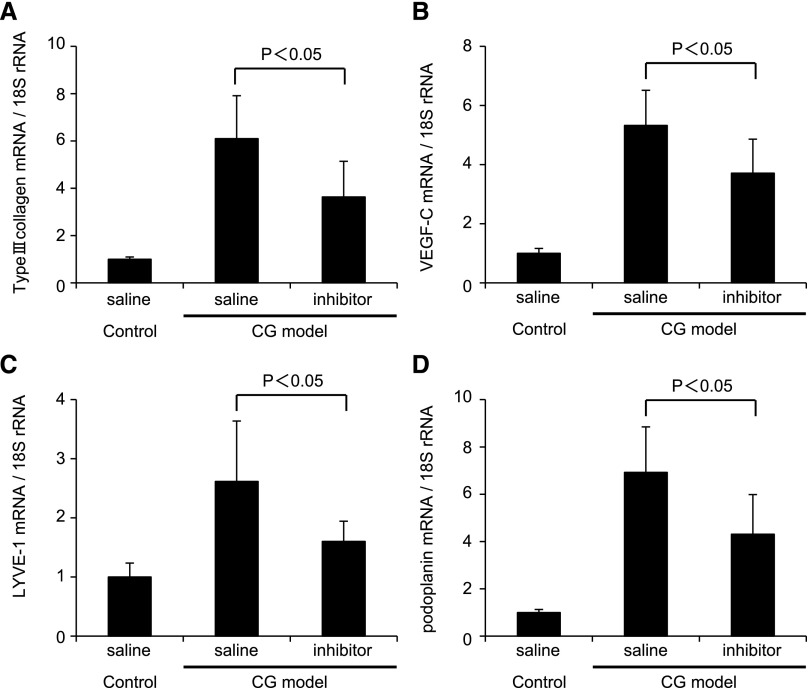
We investigated the effects of a TGFβR-I inhibitor in the rat CG model (Figures 8–10). Analysis of the parietal peritoneum indicated that the thickness of the peritoneum was significantly reduced by TGFβR-I inhibitor treatment. Quantitative immunohistochemical assessment showed that α-smooth muscle actin (α-SMA) expression, type III collagen deposition, and the number of ED-1–positive macrophages were suppressed by TGFβR-I inhibitor treatment (Figures 8 and 9). In addition, VEGF-C expression and LYVE-1–positive areas were significantly suppressed by TGFβR-I inhibitor treatment (Figures 8 and 9). Upregulation of type III collagen, VEGF-C, LYVE-1, and podoplanin mRNA was significantly inhibited by TGFβR-I inhibitor (P<0.05) (Figure 10). Moreover, diaphragm thickness (P<0.01) as well as the number of LYVE-1–positive areas (P<0.05) and VEGF-C (P<0.001) and LYVE-1 (P<0.05) mRNA expression in the diaphragm were all significantly suppressed by TGFβR-I inhibitor treatment (Supplemental Figure 3).
Figure 8.
Under immunohistochemical analysis of the parietal peritoneum, TGFβR-I inhibitor suppresses fibrosis and lymphangiogenesis. Histochemical staining of ED-1–positive macrophages, type III collagen, α-SMA, VEGF-C, and LYVE-1 in TGFβR-I inhibitor-treated (daily intraperitoneal injection of 3 μg/g body wt) and untreated CG model rats and control rats. Scale bars, 100 μm.
Figure 10.
Quantitative real-time PCR analysis of specific mRNA expression in the rat parietal peritoneum of the CG model rat indicates that TGFβR-I inhibitor suppressed lymphangiogenesis. Real-time PCR analysis indicated that increased expression of (A) type III collagen, (B) VEGF-C, (C) LYVE-1, and (D) podoplanin mRNA was significantly suppressed in CG rats compared with controls by TGFβR-I inhibitor treatment. The mRNA expression of the indicated proteins is expressed relative to 18S ribosomal RNA. Control saline treatment, n=5; CG models treated with saline or inhibitor, n=7 for each group.
Figure 9.
Quantitative immunohistochemical parameters in the rat parietal peritoneum of the CG model indicate that TGF βR-I inhibitor suppresses fibrosis and lymphangiogenesis. The histochemical parameters of the parietal peritoneum that were assayed were quantified. Compared with untreated CG rats, TGFβR-I inhibitor treatment of CG rats significantly reduced (A) the thickness of the peritoneum, (B) the number of ED-1–positive cells, (C) type III collagen deposition, (D) α-SMA expression, (E) LYVE-1–positive areas, and (F) VEGF-C expression. VEGF-C expression was analyzed and semiquantitatively scored as follows: 0, no staining; 1, mild staining; 2, moderate staining; 3, pronounced staining. Control saline treatment, n=5; CG models treated with saline or inhibitor, n=7 for each group.
Function of Lymphatic Vessels
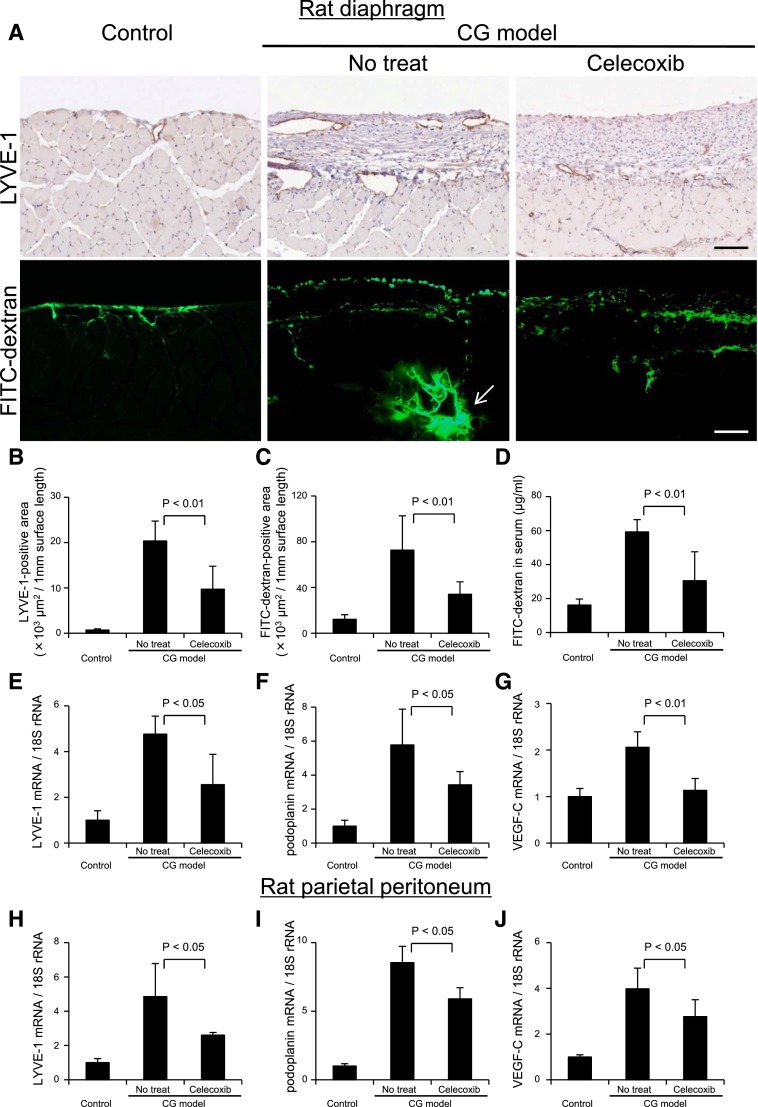
We analyzed lymphatic vessel function using the modified method of lymphangiography as described previously.34 We detected passage of FITC dextran (molecular weight=2,000,000), which can only be absorbed through the lymphatic vessels in the diaphragm, by immunofluorescence microscopy (Figure 11A), and we detected positive levels of FITC in the serum, which were especially high in the rats without treatment in the CG model (Figure 11D). The presence of FITC levels in the blood and analysis of microscopic findings including serial sections (Supplemental Figures 4D and 5) indicated that FITC dextran was absorbed by the lymphatic vessels and drained into the venous circulation by the lymphatic and thoracic ducts. Passage through the parietal lymphatic vessels was not prominent in the parietal peritoneum in the CG rats (Supplemental Figure 6). Inhibition of lymphangiogenesis by celecoxib35,36 reduced the serum FITC dextran levels, suggesting the reduction of the absorption volume.
Figure 11.
Drainage of FITC dextran administered into the abdominal cavity is suppressed by inhibition of lymphangiogenesis with cyclooxygenase-2 inhibitor. (A, upper panels) Immunohistochemical staining of LYVE-1 in the diaphragm of control rats, celecoxib-treated (daily oral administration of 50 mg/kg body wt) CG rats, and untreated CG rats. (A, lower panels) Rats were given intraperitoneal injections of 50 mg FITC dextran (molecular weight=2,000,000). Twenty minutes later, blood, peritoneal, and diaphragmatic samples were obtained. Immunohistochemical findings in the diaphragm of control, untreated, and celecoxib-treated rats were recorded. Scale bars, 100 μm. Arrow indicates the accumulation of FITC dextran in the central collector of lymphatic vessel. Quantification of positive area determined by MetaMorph 6.3 image software (Universal Imaging, West Chester, PA) in the diaphragm for (B) LYVE-1 and (C) FITC dextran showed that celecoxib treatment significantly reduced both positive areas compared with the untreated CG rats. (D) To assess the amount of FITC dextran in blood drawn at euthanization, absorbance of serum samples was measured at 493 nm by spectrophotometry. For preparation of the standard curve, FITC dextran was reconstituted and diluted with normal rat serum. Concentrations of FITC dextran in the serum were significantly decreased in celecoxib-treated CG rats compared with untreated CG rats. Real-time PCR analysis of (E and H) LYVE-1, (F and I) podoplanin, and (G and J) VEGF-C mRNA in (E–G) the rat diaphragm and (H–J) the parietal peritoneum indicated that increased expression of LYVE-1, podoplanin, and VEGF-C mRNA in CG rats was significantly suppressed by celecoxib treatment. Control, n=5; CG models with no treat, n=6; CG models treated with celecoxib, n=6.
Discussion
Ultrafiltration dysfunction often results from a combination of increased vascular surface area and decreased osmotic conductance.37,38 Dysfunction of the water channel aquaporine-1 (so-called ultrasmall pore39,40) is an important cause of UFF, in that this water channel alters osmotic conductance. Hypopermeable peritoneum with a loss of peritoneal surface area is a rare cause of UFF. Lymphatic absorption was reported to be important in patients on short-term PD with UFF.38 However, little is known about the pathology, process, and mechanisms of lymphangiogenesis in patients with UFF and peritonitis.
In the present study, we first found that the VEGF-C content in the PD effluent of 4-hour dwelled samples and overnight dwelled samples correlated with the peritoneal membrane transport rate. Our data are consistent with the recent report by Yang et al.,41 which showed that VEGF-C could possibly be a biomarker for UFF; however, unlike our study, they did not investigate lymphangiogenesis. Our studies of human peritoneal membranes showed that VEGF-C, LYVE-1, and podoplanin mRNA expression were higher in UFF peritoneum than control membranes and that the expression level of VEGF-C and the number of lymphatic vessels correlated with one another. These findings suggest that lymphangiogenesis develops in patients with a high peritoneal transport rate. Markers of lymphatics as well as VEGF-C mRNA levels tended to be increased in the state of peritonitis, which is an important factor for the induction of peritoneal damage and fibrosis (Figure 2). The extent of lymphangiogenesis may be related to the duration of infection and inflammation, which may also be the case in acute and chronic tubulointerstitial nephritis.22 Thus, we reported that lymphangiogenesis developed in chronic but not acute tubulointerstitial nephritis, which indicates that the duration of inflammation together with fibrotic process may be important for lymphangiogenesis.22 We propose that lymphangiogenesis is linked with the fibrotic process in the peritoneal membrane, because it is in other disorders, including renal fibrosis22,23,42 and lung fibrosis.18 Our experiments clearly showed that TGF-β1 functions as an inducer of lymphatic growth in peritoneum as well as an inducer of inflammatory cytokines.16,17,43–46 Lymphangiogenic induction by TGF-β1 may be one of the key mechanisms of the UFF that is associated with peritoneal fibrosis.
Using immunohistochemical and cell culture studies, we clearly showed that mesothelial cells and macrophages expressed VEGF-C, which indicates that these cells are at least the sources of growth factors for lymphangiogenesis in the peritoneum (Figures 3, 4, and 7). VEGF-C was induced by TGF-β1 in HPMCs and a cultured mesothelial cell line; the latter induction was suppressed by the TGFβR-I inhibitor in a dose-dependent manner. In addition, we recently showed that TGF-β induces VEGF-C production by macrophages.23 These findings suggest that TGF-β1 is an important inducer of VEGF-C, leading to the lymphangiogenesis that is associated with peritoneal fibrosis in PD patients. Interestingly, TGF-β1–induced VEGF-C expression in mesothelial cells was enhanced in the mesothelial cells obtained from patients with a high peritoneal transport rate. We recently reported that connective tissue growth factor induction by TGF-β was higher in HPMCs derived from patients with high peritoneal transport.33 In these previous experiments, we confirmed that there was no difference in the level of TGF-β type II receptor expression on HPMCs of patients with high peritoneal transport compared with patients with low peritoneal transport33 and that an imbalance of TGF-β signaling altered connective tissue growth factor and bone morphogenic protein-4 expression.33 Similar to the previous study, VEGF-C induction was also enhanced in HPMCs of patients with a higher peritoneal transport rate, which suggests that the function of HPMCs from patients with higher peritoneal transport rates had changed.33 In contrast, VEGF-C is not upregulated by TGF-β1 in fibroblasts, despite the presence of basal VEGF-C expression.23 TGF-β1 in the peritoneal dialysate was reported to be elevated in the PD effluent and correlate with D/P Cr,47 which we confirmed in our cohort (R=0.487, P<0.01) (Supplemental Figure 7). Furthermore, we found a significant correlation between the content of TGF-β1 and VEGF-C in the PD effluent (Figure 1B), which was consistent with the observed relationship between peritoneal thickness and the expression of VEGF-C and lymphatic vessels (Figure 2). There are several mechanisms by which TGF-β levels can be increased in dialysate.48–51 In this regard, prevention of TGF-β induction may reduce fibrosis and lymphangiogenesis, resulting in the prevention of peritoneal membrane failure.
In the normal abdominal cavity, lymphatics in the diaphragm form a specialized system that drains fluid from the peritoneal cavity and returns it to the vascular system.52 The net ultrafiltration volume at the end of a PD exchange equals the cumulative net transcapillary water transport minus lymphatic absorption during the exchange.53 We showed that lymphatic vessels are present in both the peritoneal and pleural sides of the normal rat diaphragm but are rarely detectable in the normal rat parietal peritoneum (Figure 6). Interestingly, LYVE-1 and VEGFR-3 mRNA levels were higher in the diaphragm than the parietal peritoneal wall. In the CG model, lymphangiogenesis developed in association with the upregulation of TGF-β1 and VEGF-C. VEGFR-3 was mainly expressed by lymphatic vessels, and its expression was also increased after intraperitoneal administration of CG (Figure 6 and Supplemental Figure 2). Consistent with the human studies, VEGF-C was mainly expressed by mesothelial cells and macrophages (Figure 7). Indeed, fibrotic processes, reflected by peritoneal thickness and α-SMA and type III collagen expression, were suppressed by the TGFβR-I inhibitor. In addition, VEGF-C expression and lymphangiogenesis in both the parietal peritoneum and the diaphragm were also suppressed by the TGFβR-I inhibitor (Figure 8 and Supplemental Figure 3). These findings indicate that peritoneal lymphangiogenesis is linked with TGF-β1 and VEGF-C expression. The inhibition of lymphangiogenesis by the TGFβR-I inhibitor suggests that TGF-β1 is a key mediator in the development of lymphangiogenesis in the CG rat model. The limitation in the CG model is the difficulty in assessing peritoneal permeability because of severe inflammation, such as in bacterial peritonitis in humans.54 Therefore, we performed FITC dextran lymphangiography to evaluate the function of the lymphatic vessels and found that these lymphatics were functional (Figure 11). Reduction of FITC dextran levels in the serum by inhibition of lymphangiogenesis indicates that the lymphatic vessels might be involved in the control of effluent volume in PD.
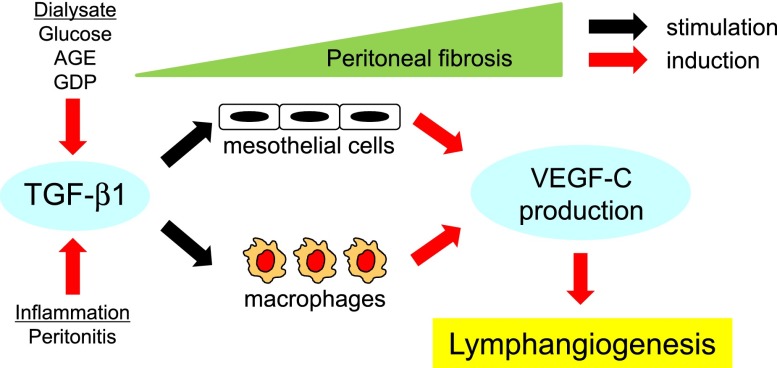
In clinical settings, TGF-β1, with levels in dialysate that are known to be increased by a glucose-based dialysis solution and episodes of peritonitis,48–51,55 may induce VEGF-C production by mesothelial cells and macrophages, thereby leading to lymphangiogenesis in the peritoneal membrane in PD patients, especially after long-term PD treatment (Figure 12). Future studies are required to determine whether specific inhibition of lymphatic vessels can ameliorate ultrafiltration failure in patients undergoing PD.
Figure 12.
Lymphangiogenesis develops associated with peritoneal fibrosis through the TGF-β-VEGF-C pathway. There are several mechanisms by which TGF-β1 levels can be increased in dialysate, including exposure of the dialysate to glucose49 or presence of advanced glycation end products,50 glucose degradation products,51 or bacterial peritonitis.55 TGF-β1 induces VEGF-C expression by mesothelial cells and macrophages, leading to lymphangiogenesis in the peritoneum of the PD patients who have undergone prolonged PD treatment.
Concise Methods
Patient Profiles
All of the studies were approved by the Ethics Committee for Human Research of the Faculty of Medicine, Nagoya University (approval #298: peritoneal fluid experiment; approval #299: peritoneal tissue experiments), and all patients provided informed consent before participation in the study.
Peritoneal Transport of VEGF-C in PD Patients
The VEGF-C concentration in peritoneal effluent was measured in overnight dwelled (8.95±1.63 h) samples collected from 83 PD patients (27 women and 56 men) who were treated between July of 2005 and April of 2008 at the Department of Nephrology and Renal Replacement Therapy of Nagoya University Hospital (Nagoya, Japan) and affiliated hospitals including Handa Municipal Hospital (Handa, Japan), Chubu Rosai Hospital (Nagoya, Japan), Yokkaichi Municipal Hospital (Yokkaichi, Japan), Kounan-Kousei Hospital (Kounan, Japan), Daiyukai-Daiichi Hospital (Ichinomiya, Japan), and Nagoya Kyoritsu Hospital (Nagoya, Japan). The mean age of all patients was 55.9±13.5 (range=28–89) years, and the mean duration of PD treatment was 31.9±32.0 (range=1–132) months. Diabetic nephropathy was the cause of ESRD in 27 PD patients (32.5%). All patients were free from peritonitis for at least 1 month before the study, and patients with other diseases, such as liver or lung diseases and malignancy, were excluded. Patients undergoing combination therapy (hemodialysis + PD) were not included in this study. Peritoneal transport was assessed based on D/P Cr, and the average value was 0.67±0.14 (range=0.28–0.96). The correlation between VEGF-C concentration in the PD effluent and D/P Cr was analyzed. In addition, we measured VEGF-C and TGF-β1 concentration in dialysate samples at 4 hours of peritoneal equilibration tests (PETs) collected from 47 PD patients (16 women and 31 men) treated between November of 2008 and June of 2009 at the Nagoya University Hospital and Handa Municipal Hospital. Fast PET was performed using 2.27% glucose-based dialysis solutions (Dianeal-N PD-4; Baxter) as described in the work by Twardowski.56 The mean age of all patients was 52.3±10.9 (range=25–70) years, and the mean duration of continuous ambulatory peritoneal dialysis (CAPD) treatment was 25.0±23.7 (range=1–103) months.33
VEGF-C, LYVE-1, and Podoplanin mRNA Expression in the Human Peritoneum
A total of 75 peritoneal tissue samples were obtained from 37 PD patients, 32 predialysis chronic renal failure patients at the time of PD catheter insertion, and 6 living kidney donors with normal renal function. Among 37 PD patients, 8 patients were regarded as having impaired UFF, which was defined by the use of more than four hypertonic bags (2.27% glucose and 3.86% glucose or icodextrin) per 24 hours to maintain fluid balance.57 Six patients were peritonitis-positive; 23 patients (incident) had their catheters removed because of transplantation, mental disorders, severe exit site infection, or difficulty in carrying out the bag exchanges (Table 1). The correlations between VEGF-C, LYVE-1, and podoplanin mRNA expression and peritoneal membrane thickness were evaluated.
VEGF-C Production in Human Mesothelial Cells
HPMCs were isolated from spent PD effluent taken from 29 clinically stable patients (Table 2) and cultured by use of a modified method as described previously.33 Basal and TGF-β1–induced VEGF-C mRNA expressions were studied.33
Processing of Biopsy Samples and Morphologic Analysis
Samples of parietal peritoneum were biopsied in the standard manner and processed as reported previously.6,33,57,58 The tissue samples were fixed with 10% buffered formalin overnight, routinely processed for light microscopy, and embedded in paraffin; 4-μm-thick sections were cut and stained with hematoxylin and eosin and Masson’s trichrome. Before analysis of peritoneal thickness, each specimen was assessed for size and the site and direction of the peritoneum. The adequacy of the samples was then judged as described by Honda et al.,57 in which less than 50% of the samples were considered to be appropriate. It was possible to measure the thickness of 45 of 75 samples in our study. To assess the extent of peritoneal thickening, the submesothelial compact zone, which was the zone of peritoneal fibrosis, was defined as the zone between the basal border of the surface of the mesothelial cells and the upper border of the peritoneal adipose tissues.6,57 We measured peritoneal thickness at five random points using a Zeiss Z1 microscope and AxioVision Windows software version 4.4 (Carl Zeiss, Oberkochen, Germany), and mean thickness was calculated.33
Cell Culture Study
A human mesothelial cell line (Met-5A) was purchased from the American Type Culture Collection (Manassas, VA) and maintained as reported previously.33 HPMCs from spent PD effluent were obtained by centrifugation of dialysis fluid taken randomly from clinically stable patients who had a variety of peritoneal permeabilities and were undergoing nocturnal exchanges using modified methods as described previously.33 Cellular components were isolated using low-speed (200×g) centrifugation, washed with RPMI 1640 (Sigma, Tokyo, Japan), and then cultured in RPMI 1640 containing l-glutamine (Sigma) supplemented with 15% FBS (Sigma), insulin/transferrin/selenium A (Invitrogen, Tokyo, Japan), 10 μM 2-mercaptoethanol (Wako, Osaka, Japan), 3.3 nM EGF (R&D Systems, Minneapolis, MN), and 400 μg/L hydrocortisone (Sigma) in humidified air with 5% CO2 at 37°C. Nonadherent material was removed the next day with two brief washes with RPMI 1640, and the adherent population was incubated in fresh culture medium. The cells reached confluence in 7–10 days, and they were then split two to three times and cultured. Subconfluent HPMCs and Met-5A were washed two times with PBS, and the culture medium was replaced with serum-free medium for 24 hours to render the cells quiescent. Subsequently, the cultures were incubated with 5 ng/ml recombinant human TGF-β1 (R&D), which was diluted in serum-free medium. Cells were harvested at 0 (basal condition), 3, 6, 12, and 24 hours (n=3 dishes of cells from each patient at each time point). All experiments were performed during the second to seventh passage. To explore the correlation between the enhancement of VEGF-C expression by TGF-β and D/P Cr, we assessed the increase in VEGF-C mRNA after 12- or 24-hour incubation with TGF-β1 as described previously.33 TGF-β1 inhibition studies were performed by incubating Met-5A cells with 5 ng/ml TGF-β1 combined with a selective inhibitor of TGFβR-I (Calbiochem, La Jolla, CA) for 12 hours.
Animal Model
All animal studies were carried out in accordance with the Animal Experimentation Guidelines of Nagoya University Graduate School of Medicine (Nagoya, Japan). Eight-week-old male Sprague–Dawley rats (Japan SLC, Hamamatsu, Japan) that initially weighed 240–260 g were used throughout the study. The animals were maintained under conventional laboratory conditions and given free access to food and water. Rats were given an intraperitoneal injection of 3 ml/200 g body wt of 0.04% CG (Wako, Japan) and 10% ethanol (Wako) dissolved in saline every other day. These rats were randomly assigned to a treatment or saline group. The rats in the treatment group (n=7) were given a daily intraperitoneal injection of 3 μg/g body wt TGFβR-I inhibitor dissolved in saline and DMSO (140 mg/ml; Wako). On the day of CG injection, the rats were injected with the TGFβR-I inhibitor 2 hours before injection of CG. The rats in the saline group (n=7) were given a daily intraperitoneal injection of saline and DMSO in a similar manner. Five control rats were injected daily with the same dosage of saline without CG or TGFβR-I inhibitor. All rats were euthanized on day 16. All injections and euthanizations were performed under anesthesia with diethyl ether (Wako). Parietal peritoneal and diaphragmatic samples were procured, and the harvested samples were used for analysis of peritoneal thickness, immunohistochemical analysis of VEGF-C, VEGFR-3, LYVE-1, podoplanin, ED-1, type III collagen, α-SMA, and cytokeratin, and analysis of the mRNA expression of VEGF-C, VEGFR-3, LYVE-1, podoplanin, and type III collagen.
Analysis of Lymphatic Vessel Function
We analyzed lymphatic vessel function using the modified method of lymphangiography as described previously.34 We intraperitoneally administered a total of 5 ml FITC-labeled dextran 2,000 (10 mg/ml concentration; molecular weight=2,000,000; Sigma). Twenty minutes later, blood was drawn, and the animals were euthanized. Serum samples were immediately separated from the blood, and absorbance was measured at 493 nm by spectrophotometry; blood from nonadministered rats was used as a normal control. The required time for all procedures by measurement with the spectrophotometer was fixed to 3 hours. The diaphragm and parietal peritoneum were harvested and snap frozen in OCT compound (Sakura Fine Technical, Tokyo, Japan). FITC dextran was mainly taken up by the lymphatic vessels in the diaphragm and passed to the central collectors of lymphatics. The detection of the FITC signal in the blood taken from the rats administered intraperitoneally with FITC dextran of molecular weight of 2,000,000 indicates that the FITC dextran was absorbed through the lymphatic vessels and drained into the blood circulation through the thoracic duct.59
Histology and IHC
Routine histologic and IHC analyses of human and rat tissues were performed and analyzed as described previously.22,23,33,58,60 VEGF-C expression was analyzed and semiquantitatively classified as follows: 0, no staining; 1, mild staining; 2, moderate staining; 3, pronounced staining. The antibodies used are listed in Supplemental Table 1.
ELISA
VEGF-C and total (both active and latent forms) TGF-β1 protein levels in peritoneal dialysate (PD fluid) samples and cell culture supernatant were measured using the Human VEGF-C (IBL, Takasaki, Japan) and the TGF-β1 (R&D) ELISA Kit, respectively, according to the manufacturers’ instructions. Samples were frozen at the time of collection and stored at −80°C. Samples were not subjected to freeze–thaw cycles.
RNA Preparation from Peritoneal Tissues and Cultured Mesothelial Cells and PCR Analysis
Human and rat peritoneal tissues were immersed in RNA later (Ambion, Austin, TX) for 1 day or more. RNA preparation and the synthesis of first-strand cDNA were performed as described previously.23,33,58 Total RNA (1 μg) was then reverse transcribed. Real-time PCR analysis was performed with an Applied Biosystems Prism 7500HT sequence detection system using TaqMan gene expression assays as described previously.58 The TaqMan Gene Expression Assays (Applied Biosystems Inc.) used are described in Supplemental Table 2; 18S ribosomal RNA was used as an endogenous control.23,33,58,60
Statistical Analyses
Values are expressed as means ± SDs. Differences between two groups were analyzed by t test or Mann–Whitney test. Comparisons among groups were performed by one-way ANOVA followed by Dunnett or Kruskal–Wallis multiple comparison test. Pearson correlation coefficient was used to analyze the correlations. Differences were considered to be statistically significant if P<0.05. All analyses were performed using SPSS software (SPSS, Chicago, IL).
Disclosures
None.
Supplementary Material
Acknowledgments
The technical assistance of Mr. Norihiko Suzuki, Ms. Keiko Higashide, Ms. Naoko Asano, and Ms. Yuriko Sawa (Department of Nephrology, Nagoya University, Nagoya, Japan) is gratefully acknowledged. We thank Drs. Isao Ito and Susumu Toda (Nagoya University Hospital, Nagoya, Japan), Drs. Midoriko Watanabe and Makoto Mizutani (Handa Municipal Hospital, Handa, Japan), Dr. Hirotake Kasuga (Nagoya Kyoritsu Hospital, Nagoya, Japan), Dr. Masanobu Horie (Daiyukai-Daiichi Hospital, Ichinomiya, Japan), and Dr. Takeyuki Hiramatsu (Kounan-Kousei Hospital, Kounan, Japan) for collecting peritoneum from the patients.
This work was supported in part by Ministry Education, Science, and Culture, Japan Grant-in-Aid for Scientific Research 20590972 (to Y.I.), a 2011 research grant from the Aichi Kidney Foundation (to H.K. and Y.I.), and the Japanese Association of Dialysis Physicians Grant 2011-13 (to Y.I.). This study was also supported in part by a Ministry of Health, Labor, and Welfare of Japan Grant-in-Aid for Progressive Renal Diseases Research, Research on Rare and Intractable Disease.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012030226/-/DCSupplemental.
References
- 1.Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Pagé D, The Canada-USA (CANUSA) Peritoneal Dialysis Study Group : Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. J Am Soc Nephrol 9: 1285–1292, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Rumpsfeld M, McDonald SP, Johnson DW: Higher peritoneal transport status is associated with higher mortality and technique failure in the Australian and New Zealand peritoneal dialysis patient populations. J Am Soc Nephrol 17: 271–278, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi Y, Ishizaki T, Imada A, Oohira S, Kuriyama S, Nakamoto H, Nakamoto M, Hiramatu M, Maeda K, Ota K, Study Group for Withdrawal from PD in Japan : Searching for the reasons for drop-out from peritoneal dialysis: A nationwide survey in Japan. Perit Dial Int 23[Suppl 2]: S175–S177, 2003 [PubMed] [Google Scholar]
- 4.Mizuno M, Ito Y, Tanaka A, Suzuki Y, Hiramatsu H, Watanabe M, Tsuruta Y, Matsuoka T, Ito I, Tamai H, Kasuga H, Shimizu H, Kurata H, Inaguma D, Hiramatsu T, Horie M, Naruse T, Maruyama S, Imai E, Yuzawa Y, Matsuo S: Peritonitis is still an important factor for withdrawal from peritoneal dialysis therapy in the Tokai area of Japan. Clin Exp Nephrol 15: 727–737, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Brimble KS, Walker M, Margetts PJ, Kundhal KK, Rabbat CG: Meta-analysis: Peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol 17: 2591–2598, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, Mackenzie RK, Williams GT, Peritoneal Biopsy Study Group : Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 13: 470–479, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Mateijsen MA, van der Wal AC, Hendriks PM, Zweers MM, Mulder J, Struijk DG, Krediet RT: Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit Dial Int 19: 517–525, 1999 [PubMed] [Google Scholar]
- 8.Sherif AM, Nakayama M, Maruyama Y, Yoshida H, Yamamoto H, Yokoyama K, Kawakami M: Quantitative assessment of the peritoneal vessel density and vasculopathy in CAPD patients. Nephrol Dial Transplant 21: 1675–1681, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Tammela T, Alitalo K: Lymphangiogenesis: Molecular mechanisms and future promise. Cell 140: 460–476, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Alitalo K, Tammela T, Petrova TV: Lymphangiogenesis in development and human disease. Nature 438: 946–953, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Jones N, Iljin K, Dumont DJ, Alitalo K: Tie receptors: New modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol 2: 257–267, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D: Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol 161: 947–956, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kodama M, Kitadai Y, Tanaka M, Kuwai T, Tanaka S, Oue N, Yasui W, Chayama K: Vascular endothelial growth factor C stimulates progression of human gastric cancer via both autocrine and paracrine mechanisms. Clin Cancer Res 14: 7205–7214, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Kitadai Y, Kodama M, Cho S, Kuroda T, Ochiumi T, Kimura S, Tanaka S, Matsumura S, Yasui W, Chayama K: Quantitative analysis of lymphangiogenic markers for predicting metastasis of human gastric carcinoma to lymph nodes. Int J Cancer 115: 388–392, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Onogawa S, Kitadai Y, Tanaka S, Kuwai T, Kimura S, Chayama K: Expression of VEGF-C and VEGF-D at the invasive edge correlates with lymph node metastasis and prognosis of patients with colorectal carcinoma. Cancer Sci 95: 32–39, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Ylä-Herttuala S, Jackson DG, Alitalo K, McDonald DM: Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest 115: 247–257, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, Yan L, Shealy DJ, McDonald DM: TNF-α drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest 119: 2954–2964, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Chemaly S, Malide D, Zudaire E, Ikeda Y, Weinberg BA, Pacheco-Rodriguez G, Rosas IO, Aparicio M, Ren P, MacDonald SD, Wu HP, Nathan SD, Cuttitta F, McCoy JP, Gochuico BR, Moss J: Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. Proc Natl Acad Sci U S A 106: 3958–3963, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paavonen K, Puolakkainen P, Jussila L, Jahkola T, Alitalo K: Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am J Pathol 156: 1499–1504, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerjaschki D, Huttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G, Kröber SM, Greinix H, Rosenmaier A, Karlhofer F, Wick N, Mazal PR: Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med 12: 230–234, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, Birner P, Krieger S, Hovorka A, Silberhumer G, Laakkonen P, Petrova T, Langer B, Raab I: Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol 15: 603–612, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto I, Ito Y, Mizuno M, Suzuki Y, Sawai A, Tanaka A, Maruyama S, Takei Y, Yuzawa Y, Matsuo S: Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney Int 75: 828–838, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y, Ito Y, Mizuno M, Kinashi H, Sawai A, Noda Y, Mizuno T, Shimizu H, Fujita Y, Matsui K, Maruyama S, Imai E, Matsuo S, Takei Y: Transforming growth factor-β induces vascular endothelial growth factor-C expression leading to lymphangiogenesis in rat unilateral ureteral obstruction. Kidney Int 81: 865–879, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Smit W, Schouten N, van den Berg N, Langedijk MJ, Struijk DG, Krediet RT, Netherlands Ultrafiltration Failure Study Group : Analysis of the prevalence and causes of ultrafiltration failure during long-term peritoneal dialysis: A cross-sectional study. Perit Dial Int 24: 562–570, 2004 [PubMed] [Google Scholar]
- 25.Sampimon DE, Coester AM, Struijk DG, Krediet RT: The time course of peritoneal transport parameters in peritoneal dialysis patients who develop encapsulating peritoneal sclerosis. Nephrol Dial Transplant 26: 291–298, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Heimbürger O, Waniewski J, Werynski A, Tranaeus A, Lindholm B: Peritoneal transport in CAPD patients with permanent loss of ultrafiltration capacity. Kidney Int 38: 495–506, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Fusshöller A, zur Nieden S, Grabensee B, Plum J: Peritoneal fluid and solute transport: Influence of treatment time, peritoneal dialysis modality, and peritonitis incidence. J Am Soc Nephrol 13: 1055–1060, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Krediet RT: The effective lymphatic absorption rate is an accurate and useful concept in the physiology of peritoneal dialysis. Perit Dial Int 24: 309–313, 2004 [PubMed] [Google Scholar]
- 29.Flessner M: Effective lymphatic absorption rate is not a useful or accurate term to use in the physiology of peritoneal dialysis. Perit Dial Int 24: 313–316, 2004 [PubMed] [Google Scholar]
- 30.Nishino T, Miyazaki M, Abe K, Furusu A, Mishima Y, Harada T, Ozono Y, Koji T, Kohno S: Antisense oligonucleotides against collagen-binding stress protein HSP47 suppress peritoneal fibrosis in rats. Kidney Int 64: 887–896, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Ro Y, Hamada C, Inaba M, Io H, Kaneko K, Tomino Y: Inhibitory effects of matrix metalloproteinase inhibitor ONO-4817 on morphological alterations in chlorhexidine gluconate-induced peritoneal sclerosis rats. Nephrol Dial Transplant 22: 2838–2848, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Kushiyama T, Oda T, Yamada M, Higashi K, Yamamoto K, Oshima N, Sakurai Y, Miura S, Kumagai H: Effects of liposome-encapsulated clodronate on chlorhexidine gluconate-induced peritoneal fibrosis in rats. Nephrol Dial Transplant 26: 3143–3154, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Mizutani M, Ito Y, Mizuno M, Nishimura H, Suzuki Y, Hattori R, Matsukawa Y, Imai M, Oliver N, Goldschmeding R, Aten J, Krediet RT, Yuzawa Y, Matsuo S: Connective tissue growth factor (CTGF/CCN2) is increased in peritoneal dialysis patients with high peritoneal solute transport rate. Am J Physiol Renal Physiol 298: F721–F733, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Tammela T, Saaristo A, Holopainen T, Lyytikkä J, Kotronen A, Pitkonen M, Abo-Ramadan U, Ylä-Herttuala S, Petrova TV, Alitalo K: Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med 13: 1458–1466, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, Zhang YF, Williams SP, Farnsworth RH, Chai MG, Rupasinghe TW, Tull DL, Baldwin ME, Sloan EK, Fox SB, Achen MG, Stacker SA: VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell 21: 181–195, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Iwata C, Kano MR, Komuro A, Oka M, Kiyono K, Johansson E, Morishita Y, Yashiro M, Hirakawa K, Kaminishi M, Miyazono K: Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer Res 67: 10181–10189, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Kim YL: Update on mechanisms of ultrafiltration failure. Perit Dial Int 29[Suppl 2]: S123–S127, 2009 [PubMed] [Google Scholar]
- 38.Parikova A, Smit W, Struijk DG, Krediet RT: Analysis of fluid transport pathways and their determinants in peritoneal dialysis patients with ultrafiltration failure. Kidney Int 70: 1988–1994, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Ni J, Verbavatz JM, Rippe A, Boisdé I, Moulin P, Rippe B, Verkman AS, Devuyst O: Aquaporin-1 plays an essential role in water permeability and ultrafiltration during peritoneal dialysis. Kidney Int 69: 1518–1525, 2006 [DOI] [PubMed] [Google Scholar]
- 40.de Arteaga J, Ledesma F, Garay G, Chiurchiu C, de la Fuente J, Douthat W, Massari P, Terryn S, Devuyst O: High-dose steroid treatment increases free water transport in peritoneal dialysis patients. Nephrol Dial Transplant 26: 4142–4145, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Yang WS, Tsai TJ, Shih CL, Huang JW, Chuang HF, Chen MH, Fang CC: Intraperitoneal vascular endothelial growth factor C level is related to peritoneal dialysis ultrafiltration. Blood Purif 28: 69–74, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Matsui K, Nagy-Bojarsky K, Laakkonen P, Krieger S, Mechtler K, Uchida S, Geleff S, Kang DH, Johnson RJ, Kerjaschki D: Lymphatic microvessels in the rat remnant kidney model of renal fibrosis: Aminopeptidase p and podoplanin are discriminatory markers for endothelial cells of blood and lymphatic vessels. J Am Soc Nephrol 14: 1981–1989, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Kerjaschki D: The crucial role of macrophages in lymphangiogenesis. J Clin Invest 115: 2316–2319, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim KE, Koh YJ, Jeon BH, Jang C, Han J, Kataru RP, Schwendener RA, Kim JM, Koh GY: Role of CD11b+ macrophages in intraperitoneal lipopolysaccharide-induced aberrant lymphangiogenesis and lymphatic function in the diaphragm. Am J Pathol 175: 1733–1745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D’Amore PA, Stein-Streilein J, Losordo DW, Streilein JW: Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest 115: 2363–2372, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ristimäki A, Narko K, Enholm B, Joukov V, Alitalo K: Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J Biol Chem 273: 8413–8418, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Lai KN, Lai KB, Szeto CC, Lam CW, Leung JC: Growth factors in continuous ambulatory peritoneal dialysis effluent. Their relation with peritoneal transport of small solutes. Am J Nephrol 19: 416–422, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Zweers MM, de Waart DR, Smit W, Struijk DG, Krediet RT: Growth factors VEGF and TGF-beta1 in peritoneal dialysis. J Lab Clin Med 134: 124–132, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Kang DH, Hong YS, Lim HJ, Choi JH, Han DS, Yoon KI: High glucose solution and spent dialysate stimulate the synthesis of transforming growth factor-beta1 of human peritoneal mesothelial cells: Effect of cytokine costimulation. Perit Dial Int 19: 221–230, 1999 [PubMed] [Google Scholar]
- 50.De Vriese AS, Tilton RG, Mortier S, Lameire NH: Myofibroblast transdifferentiation of mesothelial cells is mediated by RAGE and contributes to peritoneal fibrosis in uraemia. Nephrol Dial Transplant 21: 2549–2555, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Leung JC, Chan LY, Tam KY, Tang SC, Lam MF, Cheng AS, Chu KM, Lai KN: Regulation of CCN2/CTGF and related cytokines in cultured peritoneal cells under conditions simulating peritoneal dialysis. Nephrol Dial Transplant 24: 458–469, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Abu-Hijleh MF, Habbal OA, Moqattash ST: The role of the diaphragm in lymphatic absorption from the peritoneal cavity. J Anat 186: 453–467, 1995 [PMC free article] [PubMed] [Google Scholar]
- 53.Mactier RA, Khanna R, Twardowski Z, Moore H, Nolph KD: Contribution of lymphatic absorption to loss of ultrafiltration and solute clearances in continuous ambulatory peritoneal dialysis. J Clin Invest 80: 1311–1316, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peritoneal Dialysis Adequacy 2006 Work Group : Clinical practice guidelines for peritoneal adequacy, update 2006. Am J Kidney Dis 48[Suppl 1]: S91–S175, 2006. 16813997 [Google Scholar]
- 55.Lai KN, Lai KB, Lam CW, Chan TM, Li FK, Leung JC: Changes of cytokine profiles during peritonitis in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 35: 644–652, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Twardowski ZJ: The fast peritoneal equilibration test. Semin Dial 3: 141–142, 1990 [Google Scholar]
- 57.Honda K, Hamada C, Nakayama M, Miyazaki M, Sherif AM, Harada T, Hirano H, Peritoneal Biopsy Study Group of the Japanese Society for Peritoneal Dialysis : Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: A quantitative study of peritoneal membrane morphology. Clin J Am Soc Nephrol 3: 720–728, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishimura H, Ito Y, Mizuno M, Tanaka A, Morita Y, Maruyama S, Yuzawa Y, Matsuo S: Mineralocorticoid receptor blockade ameliorates peritoneal fibrosis in new rat peritonitis model. Am J Physiol Renal Physiol 294: F1084–F1093, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Grimaldi A, Moriondo A, Sciacca L, Guidali ML, Tettamanti G, Negrini D: Functional arrangement of rat diaphragmatic initial lymphatic network. Am J Physiol Heart Circ Physiol 291: H876–H885, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Kato H, Mizuno T, Mizuno M, Sawai A, Suzuki Y, Kinashi H, Nagura F, Maruyama S, Noda Y, Yamada K, Matsuo S, Ito Y: Atrial natriuretic peptide ameliorates peritoneal fibrosis in rat peritonitis model. Nephrol Dial Transplant 27: 526–536, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.