Abstract
A T60M mutation in the thiazide-sensitive sodium chloride cotransporter (NCC) is common in patients with Gitelman’s syndrome (GS). This mutation prevents Ste20-related proline and alanine-rich kinase (SPAK)/oxidative stress responsive kinase-1 (OSR1)–mediated phosphorylation of NCC and alters NCC transporter activity in vitro. Here, we examined the physiologic effects of NCC phosphorylation in vivo using a novel Ncc T58M (human T60M) knock-in mouse model. NccT58M/T58M mice exhibited typical features of GS with a blunted response to thiazide diuretics. Despite expressing normal levels of Ncc mRNA, these mice had lower levels of total Ncc and p-Ncc protein that did not change with a low-salt diet that increased p-Spak. In contrast to wild-type Ncc, which localized to the apical membrane of distal convoluted tubule cells, T58M Ncc localized primarily to the cytosolic region and caused an increase in late distal convoluted tubule volume. In MDCK cells, exogenous expression of phosphorylation-defective NCC mutants reduced total protein expression levels and membrane stability. Furthermore, our analysis found diminished total urine NCC excretion in a cohort of GS patients with homozygous NCC T60M mutations. When Wnk4D561A/+ mice, a model of pseudohypoaldosteronism type II expressing an activated Spak/Osr1-Ncc, were crossed with NccT58M/T58M mice, total Ncc and p-Ncc protein levels decreased and the GS phenotype persisted over the hypertensive phenotype. Overall, these data suggest that SPAK-mediated phosphorylation of NCC at T60 regulates NCC stability and function, and defective phosphorylation at this residue corrects the phenotype of pseudohypoaldosteronism type II.
The thiazide-sensitive sodium (Na+)-chloride (Cl−) cotransporter (NCC) is a member of the cation/Cl− cotransporter gene family and has 12 transmembrane domains with both termini and two loops located intracellularly and six loops located extracellularly.1 It is the major NaCl transport pathway in the apical membrane of the distal convoluted tubules (DCTs) and accounts for the reabsorption of 5%–10% of filtered Na+.2 It is also the molecular target of thiazide diuretics and modulates magnesium and calcium reabsorption in the DCT.3–5 Inactivating mutations in SLC12A3 gene encoding NCC lead to Gitelman’s syndrome (GS), an autosomal recessive renal tubular salt-losing disorder characterized by hypokalemia, metabolic alkalosis, hypomagnesemia, and hypocalciuria.6–8 Most patients with GS may manifest fatigue, cramps, nocturia, salt carving, muscle weakness, and even paralysis during early childhood or adolescence.9 To date, >180 different mutations have been identified in patients with GS.1,10–13
Of note, a missense threonine (T) to methionine (M) mutation at T60 residue is the most common NCC mutation in Asian patients with GS.9,14–16 T60 in NCC has been well known as a phosphoacceptor site for Ste20-related proline and alanine-rich kinase (SPAK)/oxidative stress responsive kinase-1 (OSR1)17,18 and is very important in triggering the activation of NCC because defective phosphorylation-mimicking mutations not only abolish intrinsic activity without affecting its membrane expression but also decrease phosphorylation of the adjacent T46 and T55 sites in heterologous expression studies.19–22 In contrast, constitutive phosphorylation of Ncc T53, T58, and Serine (S)71 (corresponding to human NCC T55, T60, and S73) in Wnk4D561A/+ knock-in mice exhibit pseudohypoaldosteronism type II (PAHII; Gordon syndrome) as the mirror image of GS, with increased total and apical membrane-expressed Ncc.23,24 Nevertheless, it remains unclear whether the NCC T60M mutation causes GS in vivo by reducing NCC transporter activity, decreasing cell surface expression, or both.
To further explore the molecular mechanism(s) of the NCC T60M mutation in vivo, we created and analyzed Ncc T58M (corresponding to human T60M) knock-in mice. These homozygous T58M knock-in mice were also crossed with Wnk4D561A/+ knock-in mice. Results to be reported indicate that defective phosphorylation of Ncc at the T58 residue alone contributes to reduced cell surface membrane expression and reverses the phenotype of PHAII caused by the Wnk4 mutation.
Results
Phenotype of the Ncc T58M Knock-In Mice
Ncc T58M knock-in mice were generated by genetic engineering targeting techniques (Supplemental Figure 1) and phenotype was evaluated at the age of 12–14 weeks. On a normal rodent chow diet, BP, serum, and urine electrolytes were not significantly different between NccT58M/+ mice and wild-type (WT) mice (data not shown). Compared with WT mice, the NccT58M/T58M mice, however, exhibited relative hypotension (P<0.05), high aldosterone levels (P<0.05), hypokalemia (P<0.05) with increased fractional excretion of K+ (P<0.05), hypomagnesemia (P<0.01) with increased fractional excretion of Mg2+ (P<0.05), hypocalciuria (P<0.01), and metabolic alkalosis (P<0.01) (Table 1), consistent with typical features of GS patients.
Table 1.
BP, blood, and urine biochemistries in Ncc T58M mice
| Genotyping (n) | WT (8) | Homozygous (8) |
|---|---|---|
| BP (mmHg) | ||
| Systolic | 104.5±4.0 | 90.7±8.2a |
| Diastolic | 52.5±4.9 | 53.7±6.4 |
| Mean | 69.7±3.5 | 62.1±4.5a |
| Weight (g) | 24.6±2.8 | 24.3±3.2 |
| Plasma | ||
| Aldosterone (pg/ml) | 367±111 | 837±212a |
| pH | 7.35±0.03 | 7.40±0.04a |
| Na+ (mmol/L) | 152±1 | 151±2 |
| K+ (mmol/L) | 4.2±0.2 | 3.6±0.3a |
| Cl− (mmol/L) | 111±1 | 106±2a |
| HCO3− (mmol/L) | 22.5±0.6 | 25.9±0.4a |
| Total Ca2+ (mg/dl) | 9.6±0.2 | 9.5±0.3 |
| Mg2+ (mg/dl) | 2.4±0.1 | 1.9±0.1a |
| Cr (mg/dl) | 0.18±0.07 | 0.17±0.06 |
| Urine (ml/d) | 1.2±0.5 | 1.2±0.4 |
| CCr (μl/min per gram) | 7.8±3.6 | 7.9±3.2 |
| FENa (%) | 0.31±0.07 | 0.30±0.07 |
| FEK (%) | 15.8±3.2 | 23.4±4.4a |
| FEMg (%) | 3.2±0.5 | 5.3±0.4a |
| Ca2+/Cr (mg/mg) | 0.22±0.08 | 0.13±0.03a |
Cr, creatinine; CCr, clearance of creatinine; FENa, FEK, and FEMg represent the fractional excretion of Na+, K+, and Mg2+, respectively.
P<0.05 when homozygous versus WT.
Ncc T58M Expression and Localization in the Kidney
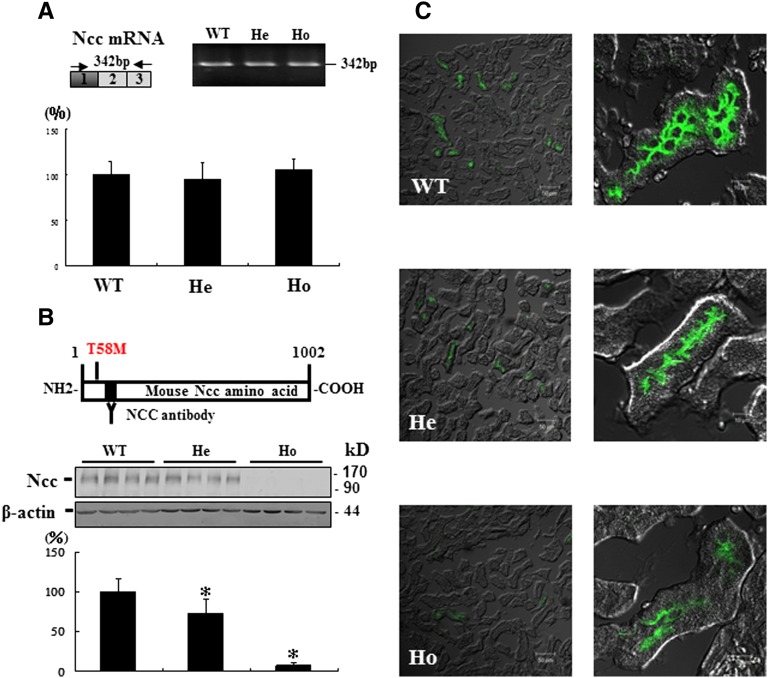
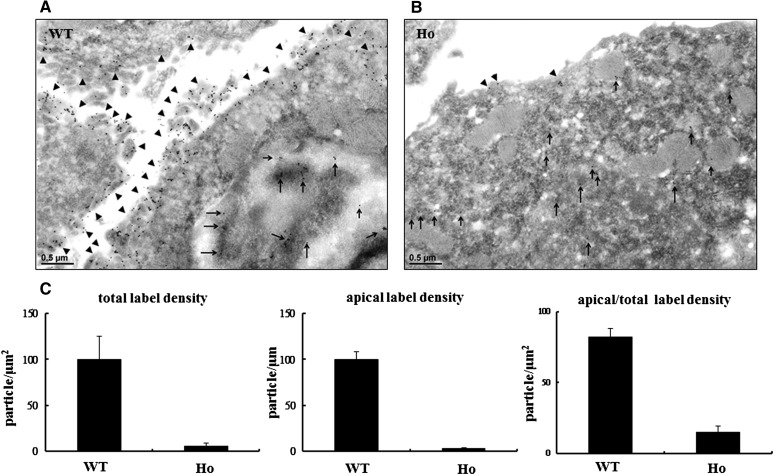
Compared with WT mice, the expression of Ncc mRNA measured by quantitative RT-PCR in the kidneys of Ncc T58M/+and NccT58M/T58M mice was not significantly different (Figure 1A). However, the expression of Ncc protein was significantly reduced in NccT58M/+ mice (73%±12%, n=4; P<0.01) and almost absent in NccT58M/T58M mice (7%±3% n=4; P<0.01) (Figure 1B). In NccT58M/T58M mice, immunofluorescence also showed dramatically decreased total and less condensed NCC expression in the apical membrane of DCT cells (Figure 1C). To observe the cellular distribution and localization of the T58M-Ncc protein in detail, immunogold electronic microscopy was performed. In WT mice, most of the WT-Ncc was located in the apical membrane of DCT cells (Figure 2A). However, in T58M mice, the sparse T58M-Ncc was primarily located in the cytosol (Figure 2B). Similar to the total Ncc findings, p-Ncc expression (T53, 8%±2%; T58, 2%±0.5%; S71, 9%±4%; P<0.01) was also significantly reduced in NccT58M/T58M mice (Supplemental Figure 2).
Figure 1.
Preserved Ncc mRNA transcripts but diminished Ncc protein expression in the kidneys of Ncc T58M knock-in mice. (A) RT-PCR analysis of Ncc mRNA using primers flanking mutation sites (exon 1) (upper panels). Quantitative gene expression of Ncc in WT, heterozygous (He), and homozygous (Ho) Ncc T58M knock-in mice (n=6/group) are presented as percentage of control (control = 100%) (lower panel). (B) An antibody recognizing the amino terminal portion (amino acids 73–88) of Ncc is applied for detecting Ncc expression (upper panel). Semiquantitative immunoblot of Ncc in crude membrane fractions from WT, He, and Ho mice (middle panels) and its semi-quantification by densitometry analysis (lower panel) (n=4/group). (C) Immunofluorescence staining for Ncc. Representative immunofluorescence micrographs of Ncc in the cortex of WT, He, and Ho mice. *P<0.05 versus WT comparison. Original magnification, ×200 and ×1000 in C.
Figure 2.
Total and apically expressed Ncc protein was dramatically attenuated in the distal convoluted tubules (DCTs) of Ncc T58M knock-in mice. Representative immunogold labeling of Ncc in DCT cells of (A) WT and (B) homozygous Ncc T58M knock-in mice (Ho), respectively. Arrows (→) and arrowheads (►) indicate the apical and cytosol located Ncc (x22.5k), respectively, in DCTs. (C) Semi-quantification of immunogold labeling of Ncc. Total and apical Ncc signals in WT and Ho mice are shown in the figures (n=6/group).
Morphometric Analyses of DCT Cells
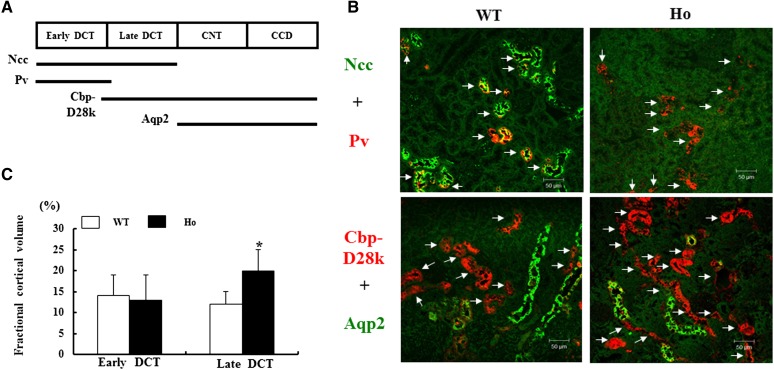
We further examined the change in DCT cellular volume in these Ncc T58M knock-in mice by light microscopy. Strong positive parvalbumin-stained segments were considered early DCT. Because Calbindin-D28k is strongly expressed in the late DCT and cortical connecting tubule and aquaporin 2 (Aqp2) is expressed from the cortical connecting tubule to downstream renal tubules in mice, strong positive Calbindin-D28k but negative Aqp2-stained segments were considered the late DCT (Figure 3A).25,26 In NccT58M/T58M mice, early DCT volume in the cortical tubules was not significantly affected (13%±3% versus 14%±5% in WT) but late DCT volume was significantly increased (20%±5% versus 12%±6% in WT; P<0.05) (Figure 3, B and C).
Figure 3.
Hypertrophic late DCTs cellular volume in Ncc T58M knock-in mice. (A) Distribution of Ncc, parvalbumin (Pv), Calbindin (Cbp)-D28k, and Aqp2 in the distal renal tubules. (B) Representative early and late DCT segments by double immunofluorescence of Ncc (green) and Pv (red) or Cbp-D28k (red) and Aqp2 (green) (arrows indicate early DCT [upper panels] and late DCT [lower panels] segments) and (C) their semi-quantification of fractional cortical volume in the cortex of WT and Ho mice (n=5/group). *P<0.05 in WT versus Ho comparison. CNT, cortical connecting tubule; CCD, cortical collecting duct. Scale bar, 50 μm.
Nkcc2 and Epithelium Na+ Channel Expression in the Kidneys
We also semi-quantified the abundance of Na+-K+-2Cl− cotransporter isoform 2 (Nkcc2) and epithelium Na+ channel (ENaC) (β) expressed in the renal tubules upstream and downstream from the DCT, respectively. Whereas total Nkcc2 abundance was not significantly altered in the NccT58M/T58M mice (108%±12%, n=4), p-Nkcc2 (T96) was significantly increased (146%± 12%, n=4; P<0.05) along with ENaC (β) (168%±17%, n=4; P<0.05) (Supplemental Figure 3).
Response to Hydrochlorothiazide, Furosemide, and Amiloride
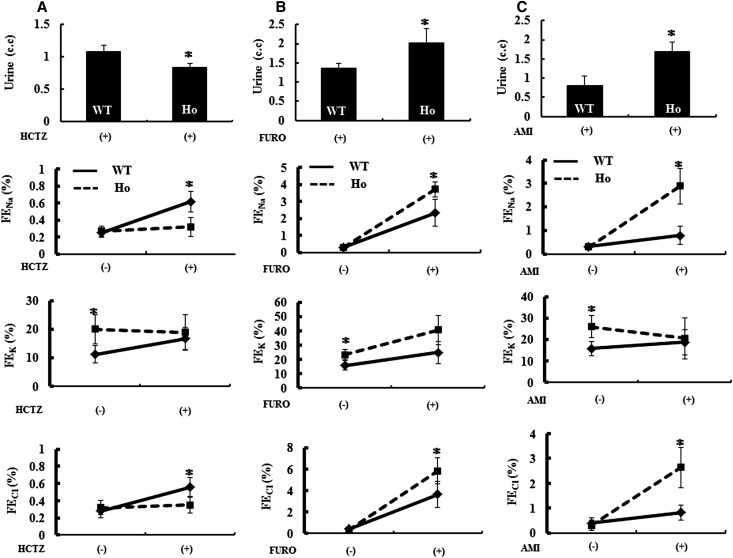
To assess Ncc, Nkcc2, and ENaC function in the NccT58M/T58M mice, we treated the WT mice and NccT58M/T58M littermates with hydrochlorothiazide (a NCC inhibitor), furosemide (a NKCC2 inhibitor), and amiloride (an ENaC inhibitor). The resulting urine amount and Na+, K+, and Cl− excretion rates were used as indices of Ncc, Nkcc2, and ENaC function. Urine excretion of Na+ and Cl− was markedly increased after a single dose of hydrochlorothiazide, furosemide, or amiloride treatment in WT mice. However, the urine amount and excretion of Na+ and Cl− were not significantly affected in NccT58M/T58M mice after hydrochlorothiazide treatment, indicating that Ncc function was strikingly reduced in NccT58M/T58M mice (Figure 4A). An exaggerated response of the urine amount and excretion of Na+ and Cl− after furosemide and amiloride challenge suggested that Nkcc2 and ENaC function had increased in the NccT58M/T58M mice (Figure 4, B and C).
Figure 4.
Blunted Ncc but augmented Nkcc2 and ENaC function in the Ncc T58M knock-in mice. Effect of hydrochlorothiazide (HCTZ), furosemide (FURO), and amiloride (AMI) administration on urine amount and Na+, K+, and Cl− excretion. HCTZ (12.5 mg/kg), FURO (15 mg/kg), or AMI (5mg/kg) is intraperitoneally administered to WT and NccT58M/T58M (Ho, ▪) littermates (n=6/group) and urine samples for the 4 hours after a single dose is collected for analysis. FENa, FEK, and FECl represent the fractional excretion of Na+, K+, and Cl−, respectively. (A) In response to HCTZ, significantly increased urine amount, FENa, FEK, and FECl are observed in WT but blunted in Ho mice. (B) In the case of FURO (15 mg/kg), exaggerated responses of urine amount, FENa, FEK, and FECl are observed in Ho mice. (C) In the case of AMI, exaggerated responses of urine amount, FENa, and FECl but not FEK are observed in Ho mice.
T60M-NCC Urinary Excretion in GS Patients and its Expression in MDCK Cells
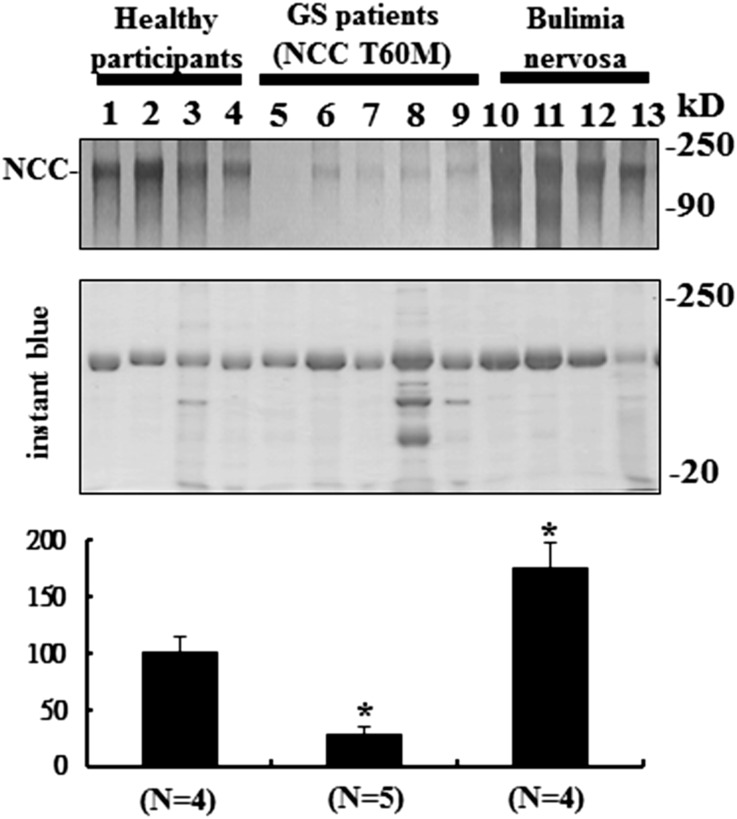
Because urinary NCC excretion reportedly correlates with membrane-expressed NCC in the DCT,27 we also examined urinary NCC excretion in normal healthy participants, GS patients with homozygous NCC T60M mutation, and disease controls (bulimia nervosa) (Supplemental Figure 4). Compared with healthy and bulimic participants, urinary NCC excretion in GS patients with homozygous NCC T60M mutation was markedly decreased (32%±8%; P<0.01) (Figure 5).
Figure 5.
Reduced T60M-NCC urinary excretion in humans. Urine immunoblot for NCC normalized by urinary creatinine (upper panel) with instant blue stain (middle panel) and semiquantitation (lower panel) from healthy participants, GS patients with NCC T60M homozygous mutation, and controls with bulimia nervosa.
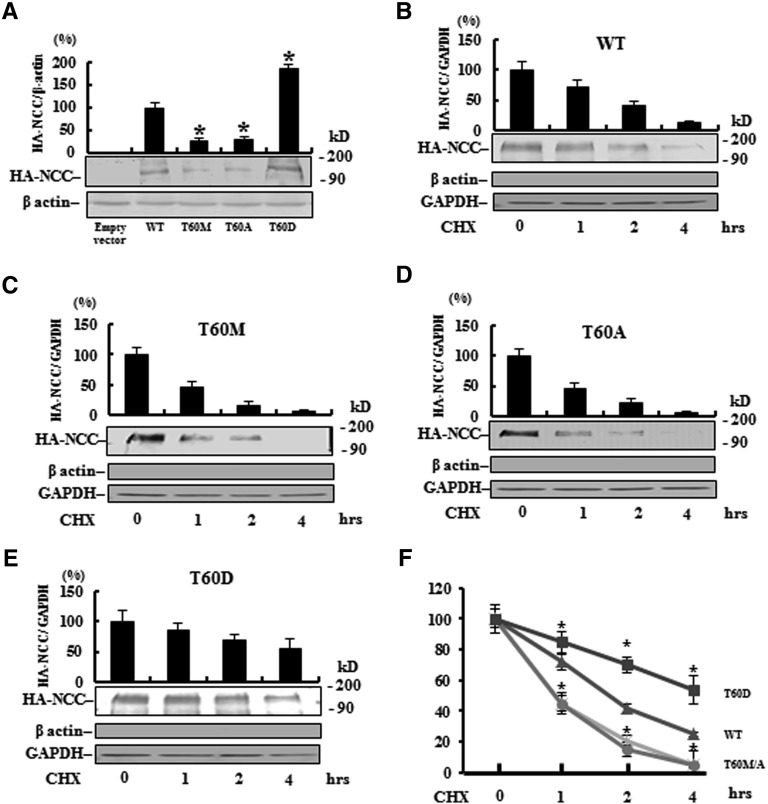
To evaluate whether the reduced NCC expression in knock-in mice and GS patients was due to a change in translation or inability to phosphorylate the site, exogenous T60M-NCC, T60A-NCC (phosphorylation-defective), and T60D-NCC (phosphorylation-mimicking) were overexpressed in the polarized renal tubule MDCK cell. Decreased total protein abundance of T60M-NCC (25%±9%, P<0.01) and T60A-NCC (28%±4%, P<0.01) but increased abundance of T60D (185%±12%, P<0.01) (Figure 6A) were observed, indicating that reduced NCC expression by the T60M mutation was primarily mediated through post-translational phosphorylation rather than translational change. To further verify whether phosphorylation status of the T60 residue could affect protein stability, we conducted a cycloheximide run-down experiment,28 which blocks protein synthesis and thus prevents the trafficking of new NCC to the cell membrane. Compared with WT-NCC (t1/2 1.7±0.5 hours), both membrane-expressed T60M-NCC (t1/2 1.0±0.3 hours; P<0.05) and T60A-NCC (t1/2 1.1±0.4 hours; P<0.05) exhibited significantly more rapid degradation, whereas T60D-NCC (t1/2 4.2±0.8 hours; P<0.01) was significantly more stable (Figure 6, B–F).
Figure 6.
Phosphorylation on T60 affects total NCC expression and membrane stability in MDCK cells. (A) Semiquantitative (n=3) (upper panel) and a representative immunoblot (lower panel) of total NCC protein abundance and (B–E) change in membrane abundance of WT, T60M, T60A, and T60D NCC, respectively, after cycloheximide (CHX) treatment in MDCK cells. The lack of β-actin but positive glyceraldehyde 3-phosphate dehydrogenase (GAPDH) suggests that the loaded protein is obtained from the membrane fraction. (F) Compared with WT, T60A/M NCC exhibits more rapid degradation but T60D NCC has greater membrane stability. *P<0.05 versus WT.
Ncc T58M Mutation Impaired its Phosphorylation Despite Activated Spak and Reversed the PHAII Phenotype
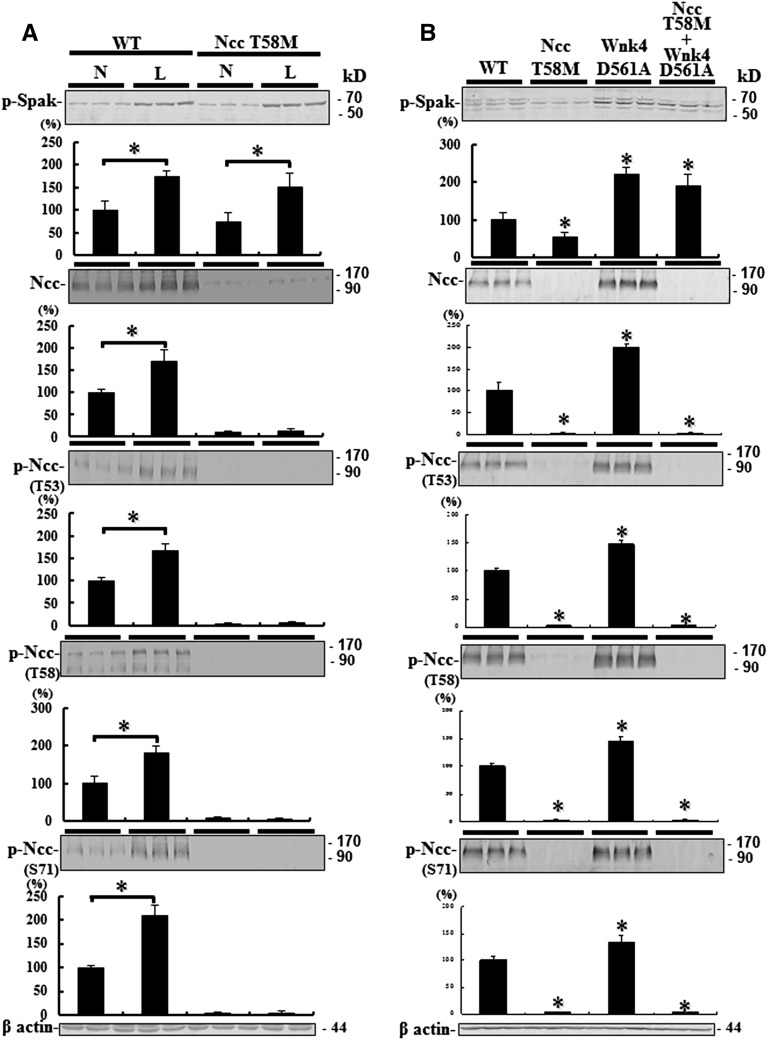
Because the upstream kinase of Ncc, Spak, is activated on a low-salt diet, phosphorylation of Ncc on a low-salt diet would inform whether the reduction in activated Ncc was due to impaired function or decreased protein abundance alone. Despite increased upstream p-Spak, NccT58M/T58M mice on a low Na+ diet (0.05%) could not increase p-Ncc, whereas WT mice on the same low Na+ diet did (Figure 7A).
Figure 7.
Ncc T58M mutation resistant to activated Spak stimulation in vivo. Semiquantitative immunoblotting of p-Spak, total Ncc, and p-Ncc expression. (A) Low Na+ (L) diet enhances Spak phosphorylation in both WT and Ncc T58M homozygous knock-in mice. However, enhanced total and p-Ncc abundance are only observed in WT rather than Ncc T58M homozygous knock-in mice. *P<0.05 L diet versus normal Na+ (N) diet. (B) Constitutive activation of Wnk4-Spak signaling cannot rescue the expression of Ncc in Ncc T58M homozygous with Wnk4 D561A heterozygous double knock-in mice (n=3/group). *P<0.05 versus WT.
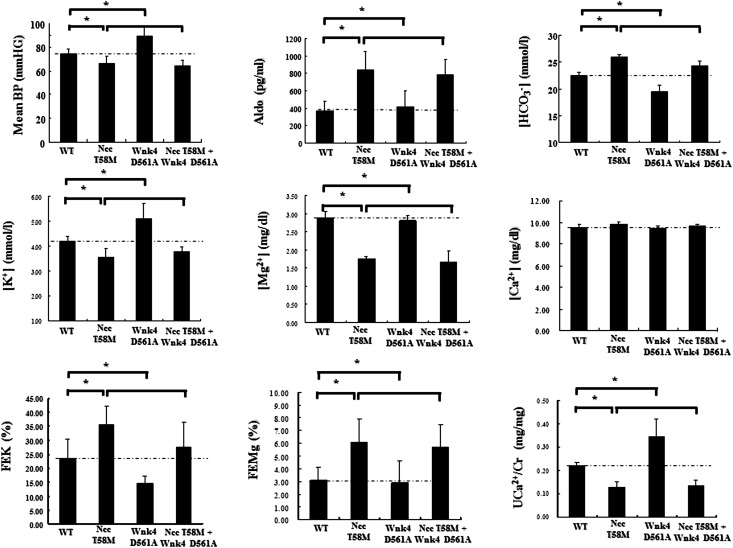
To assess whether defective phosphorylation of NCC at the T60 residue could correct or reverse the phenotype of PHAII with WNK4 mutation, we crossed the NccT58M/T58M mice with Wnk4D561A/+ mice. Although Wnk4-Spak signaling was constitutively activated in double NccT58M/T58M/Wnk4D561A/+ knock-in mice, total and p-Ncc (T53, T58, and S71) expression were still markedly decreased (Figure 7B). Compared with WT and Wnk4D561A/+ knock-in mice, NccT58M/T58M/Wnk4D561A/+ knock-in mice still exhibited the typical GS phenotype with secondary hyperaldosteronism and hypotension, hypokalemic metabolic alkalosis, hypomagnesemia, and hypocalciuria just like the NccT58M/T58M mice (Figure 8). These findings further suggest that the T58 residue of Ncc is the most important phosphoacceptor site for Spak.
Figure 8.
Ncc T58M mutation reversed the phenotype of the PHAII with Wnk4 D561A mutation. Representative phenotype including mean BP, plasma aldosterone (Aldo), bicarbonate [HCO3−], potassium [K+], magnesium [Mg2+], total calcium [Ca2+] concentration, and urinary fraction excretion of potassium [FEK], magnesium [FEMg], and urine Ca2+ to creatinine ratio (UCa2+/Cr) in WT, Ncc T58M homozygous knock-in, Wnk4 D561A heterozygous knock-in, and Ncc T58M homozygous with Wnk4 D561A heterozygous double knock-in mice (n=6/group).
Discussion
In this study, we first created defectively phosphorylated NccT58M/T58M mice to model human GS caused by homozygous NCC T60M mutation. NccT58M/T58M mice exhibited typical features of GS and a blunted response to thiazide, confirming that Ncc function was markedly diminished. Despite intact transcription of Ncc mRNA, total and p-Ncc (T53, T58, and S71) abundance in the kidneys of NccT58M/T58M mice were remarkably reduced and could not be rescued by the activated Spak of a low-salt diet. The impaired phosphorylation of NCC at T60, per se, also caused the decreased NCC membrane stability in MDCK cells. That the PHAII phenotype was overwhelmed by the persistently diminished T58M-Ncc protein abundance in NccT58M/T58M/Wnk4D561A/+ mice further indicates that T58 of Ncc is the most important Spak/Osr1 phosphoacceptor site involved in Ncc protein expression and function.
Protein phosphorylation is a common post-translational modification that may alter intrinsic protein function, cellular localization, and stability.29 The T60 residue of NCC is a phosphoacceptor site for SPAK/OSR117,18 and phosphorylation of T60 has been shown to be crucial in triggering NCC activation because its phosphorylation-defective mutations (T60A or M) alter its intrinsic activity without affecting total and membrane abundance in the heterologous Oocyte expression system.19,20 However, impaired T60A NCC membrane expression in nonpolarized HEK cells after hypotonic stress stimulation was found, suggesting that defective phosphorylation of the T60 residue may also affect its membrane expression.21,22 These in vitro findings in nonrenal tubular cells should be further validated in renal tubular cells such as polarized MDCK cells, genetically modulated animal models, or GS patients with homozygous T60M mutation. Reduced T60M-NCC expression in MDCK cells, markedly diminished Ncc abundance in the DCT cells of NccT58M/T58M mice, and decreased urinary NCC excretion, an index of membrane-expressed NCC, in GS patients all indicated that T60M led to reduced NCC expression primarily through post-translational modification (phosphorylation) rather than translational change. Because increased late DCT volume was found in NccT58M/T58M mice, similar to our previously reported NccS707× knock-in mice,30 the reduced total T58M-Ncc abundance may also be independent of the altered DCT cellular volume. Reminiscent of the markedly reduced total and phosphorylated Ncc expression in Wnk431 and Spak knockout32 as well as kinase-dead Spak knock-in mice,33 the constitutive inactivation of the Wnk4-Spak cascade may not only decrease Ncc phosphorylation but also reduce its accumulation in apical membranes.31–33
Several hormones such as angiotensin II and aldosterone have been shown to affect Ncc phosphorylation through regulation of the Wnk4-Spak signal cascade. Acute infusion of angiotensin II enhances Ncc phosphorylation (at T53 and T58) and translocates Ncc from subapical areas to the apical membrane without affecting its total abundance.34 However, chronic infusion of angiotensin II and hyperaldosteronism induced by low Na+ diet or aldosterone infusion increased both total and p-Ncc abundance through Wnk4-Spak signaling in WT24,35 but not Wnk4 knockout mice.31 In addition, NccT58M/T58M/Wnk4D561A/+ mice still exhibited the GS phenotype with decreased total and p-Ncc expression, implying that even the constitutive activation of the Wnk4-Spak signal cascade could not rescue the expression of Ncc caused by defective phosphorylation at T58. In the cycloheximide run-down experiments, the constitutively phosphorylation-mimicking T60 mutant had much longer t1/2 than the defectively phosphorylated mutants, including T60M. Taken together, these results provide indirect and direct evidence that phosphorylation of NCC on T60 by SPAK affects its membrane stability and expression, akin to the phosphorylation of Aqp2 on S256 by protein kinase A.36–38
The mechanisms underlying reduced NCC expression from defective phosphorylation on T60 residue remain incompletely clear. GS-causing missense mutations might reduce NCC activity through impaired protein processing or sorting, altering protein activity, or accelerating protein removal or degradation.39 Intact membrane expression of T60M and T60A NCC in the Xenopus Oocyte19,20 suggest that reduced anterograde endoplasmic reticulum export from enhanced endoplasmic reticulum–associated degradation, which has been implicated in several GS-causing NCC missense mutations,39–42 is less likely. Adaptor protein (AP)-343 and sortilin-related44 lysosomal degradation have also been reported to regulate NCC stability. The T60 residue of NCC, of note, is located in one of the putative canonical YXXθ (YNT58I) binding motifs of the μ subunit of AP complexes43,45 that are involved in the sophisticated post-Golgi network of protein sorting.46 It also remains unknown whether NCC phosphorylation participates in protein endocytosis through the NEDD4-2 ubiquitination pathway.47
NccT58M/T58M mice also exhibited an exaggerated response to furosemide, which can be caused by the elimination of the compensatory response due to the marked diminution NCC in the DCT and by the enhanced NKCC2 phosphorylation and function in the upstream thick ascending limbs. Similarly, increased compensatory ENaC expression and function in the downstream distal tubules could account for the exaggerated response to amiloride, comparable to the finding in Wnk4 knockout mice.31 These findings would also predict that Na+ reabsorption should be enhanced in the cortical thick ascending limbs and downstream distal tubules in patients with GS.48,49
In summary, Ncc T58M homozygous knock-in mice exhibit the typical phenotype of GS patients and defective Ncc phosphorylation at T58 prevents the development of PHAII in the Wnk4 knock-in mice. Ncc T58M knock-in mice provide a good animal model to decipher the molecular mechanisms of Ncc phosphorylation, protein stability, and membrane expression. Blocking NCC phosphorylation at T60 may be a promising strategy for antihypertensive drug design, especially in conditions with activated WNK4-SPAK/OSR1 signaling.
Concise Methods
Blood and Urine Analyses and BP Measurement
The mice were raised in a 12-hour day and night cycle, fed with normal rodent chow diet (Na+: 0.4% [w/w]; K+: 1.0% [w/w]; Ca2+: 0.9% [w/w]) and plain drinking water ad libitum for 12–14 weeks. For the low Na+ diet challenge test, low Na+ rodent chow diet (Na+ 0.05% [w/w]) was fed for 6 days. Mice were placed in metabolic cages for urine collection.
Blood was drawn from the submandibular venous plexus under light ether anesthesia. Serum and urine biochemistry analysis was performed as previously described.50 The BPs of restrained conscious mice were measured with a programmable tail-cuff sphygmomanometer (MK-2000A; Muromachi, Tokyo, Japan).23,30,51
Hydrochlorothiazide, Furosemide, and Amiloride Challenge Studies
After a single dose of intraperitoneal hydrochlorothiazide (12.5 mg/kg),32,51 furosemide (15 mg/kg), 32,51 or amiloride (5 mg/kg)31 was administered to WT and NccT58M/T58M littermates, urine samples (0–4 hours) were collected for analysis. Fractional excretion rate of a specific ion particle (X) (FEx) is defined as: urine X concentration * plasma Cr concentration/urine Cr concentration* plasma X concentration.
Quantitative Real-Time RT-PCR
Total kidney RNA was isolated by standard RT protocol and the gene expression of Ncc was assessed by real-time RT-PCR as previously described.52 The primers used for mouse Ncc and β-actin gene RT-PCR were as follows: Ncc: forward 5′-AGC CCA GCC ACT TAA CAC AC-3′ (on exon 1), and reverse 5′-GGA TCA CTC CCC AGA TGT TG-3′ (on exon 3); and β-actin: forward 5′-ACC ACA CCT TCT ACA ATG AGC-3′, and reverse 5′-GGC ACA GTG TGG GTG ACC-3′.
Immunoblotting, Immunofluorescence, and Immunogold Stain
The preparation of kidney tissue for semiquantitative immunoblotting, immunofluorescence, and the analysis of the densitometry data was performed as previously described.30,32,51 The primary antibodies, which have been verified and used by us or other groups, were as follows: rabbit anti-Ncc (×200; Millipore), Nkcc2 (×200; Alpha Diagnostic), ENaC (β) (×200; Alomone), self-generated p-Ncc (T53, T58, and S71) and p-Nkcc2 (T96) (×200), mouse anti-calbindin-D28k (CBP-D28k) (×10,000; Swant), parvalbumin (×10,000; Swant), and goat anti-Aqp2 (×200; Santa Cruz).30,32,51 Immunogold labeling was performed as previously described 23 and electron microscopic images were obtained using H7000 Hitachi electron microscopy. The semi-quantification of immunogold signals was performed as previously described.23,53
HA-NCC Expression and Cell Surface Stability in the MDCK Cells
In brief, cells seeded in a six-well plate with 70%–90% confluence were transfected with pHM6 empty vector or HA-NCC (WT, T60M, T60A, or T60D) by Lipofectamine 2000 Transfection Reagent (Invitrogen) and cells were harvested 24 hours after transfection. To assess the cell surface stability of WT, T60M, T60A, and T60D HA-NCC in MDCK cells, fresh induction medium supplemented with 20 μg/ml cycloheximide was applied for 1, 2, and 4 hours before harvest of the cells. Whole cell lysate without the nuclear portion prepared as reported previously54 and membrane fraction prepared with the ProteoExtract native membrane protein extraction kit (Merck-Millipore)55 were applied for semiquantitative immunoblot. Rat anti-HA mAb (9Y10; Roche Diagnostics), mouse anti-β actin (SC-47778; Sigma), and mouse anti-glyceraldehyde 3-phosphate dehydrogenase (MAB374; Millipore) were used.
NCC Mutation Analyses and Immunoblotting for NCC in Urine
The study protocol was approved by the Ethics Committee on Human Studies at Tri-Service General Hospital, National Defense Medical Center, Taiwan. The participants were given a detailed description of the study before they provided informed consent. Genomic DNA was isolated and purified from peripheral blood of the healthy participants, disease controls, and patients and family members for PCR amplification of individual exons of the SLC12A3 and RFLP analysis of the mutated exon of SLC12A3 was performed as previously described.9,15
Fresh first morning voided urine from controls and patients was collected in a bottle that contained a collection solution 25× complete protease inhibitor cocktail (Roche). After measuring the volume, the sample was spun at 1300×g for 15 minutes at 4°C to remove cells, nuclei, and large fragments. The supernatant was then transferred to six 8-ml high-speed tubes for ultracentrifugation (200,000×g for 12 hours at 4°C). Urine creatinine was measured in this supernatant. The resultant pellets were suspended with 120 μl of isolation solution (0.25 mol/L of sucrose, 10 mmol/L of triethanolamine, 1 mM of sodium orthovanadate, 50 mM of sodium fluoride, 1 mM of EGTA, 1 mM of EDTA, pH 7.6 and 1× complete protease inhibitor cocktail [Roche]). Samples were then stabilized by adding 30 μl of 5× Laemmli sample buffer (SDS, 3.75 g; glycerol; 15 ml; 1 mol/L of Tris, pH 6.8, 2.5 ml, bromophenol blue dab, ddH2O to 50 ml), heated to 70°C for 15 minutes, and stored at −80°C until analysis. Just before being loaded for electrophoresis, samples were warmed to 37°C.27
Five milligrams of urine creatinine equivalent of each sample was loaded into each lane of gel and electrophoresed in 7.5% (for NCC) polyacrylamide-SDS minigels. Immunoblotting and instant blue (Genemark) staining were performed as described previously.23
Statistical Analyses
All results are expressed as mean ± SD. We used one-way analysis of covariance to compare the difference among three groups (WT, NccT58M/+, and NccT58M/T58M knock-in littermates). The Mann–Whitney U test was used when the variables between two groups were not normally distributed. A P value <0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the technical services provided by the Transgenic Mouse Model Core Facility of the National Research Program for Genomic Medicine, NSC. This study was supported in part by grants from the National Science Council, Taiwan (NSC-100-2314-B-016-019-MY3, NSC-100-2314-B-016-018-MY3, NSC-101-2628-B-016-001-MY3), the Research Fund of Tri-Service General Hospital (TSGH-C-101-113, TSGH-C-102-106), the Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan (SKH-8302-99-DR-07), and the Japan-Taiwan Joint Research Program, Interchange Association, Japan.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012070742/-/DCSupplemental
References
- 1.Gamba G: Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Ellison DH, Velázquez H, Wright FS: Thiazide-sensitive sodium chloride cotransport in early distal tubule. Am J Physiol 253: F546–F554, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Hoenderop JG, Nilius B, Bindels RJ: Calcium absorption across epithelia. Physiol Rev 85: 373–422, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Glaudemans B, Knoers NV, Hoenderop JG, Bindels RJ: New molecular players facilitating Mg(2+) reabsorption in the distal convoluted tubule. Kidney Int 77: 17–22, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Reilly RF, Huang CL: The mechanism of hypocalciuria with NaCl cotransporter inhibition. Nat Rev Nephrol 7: 669–674, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP: Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Gitelman HJ, Graham JB, Welt LG: A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians 79: 221–235, 1966 [PubMed] [Google Scholar]
- 8.Knoers NV, Levtchenko EN: Gitelman syndrome. Orphanet J Rare Dis 3: 22, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin SH, Shiang JC, Huang CC, Yang SS, Hsu YJ, Cheng CJ: Phenotype and genotype analysis in Chinese patients with Gitelman’s syndrome. J Clin Endocrinol Metab 90: 2500–2507, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Riveira-Munoz E, Chang Q, Godefroid N, Hoenderop JG, Bindels RJ, Dahan K, Devuyst O, Belgian Network for Study of Gitelman Syndrome : Transcriptional and functional analyses of SLC12A3 mutations: New clues for the pathogenesis of Gitelman syndrome. J Am Soc Nephrol 18: 1271–1283, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Vargas-Poussou R, Dahan K, Kahila D, Venisse A, Riveira-Munoz E, Debaix H, Grisart B, Bridoux F, Unwin R, Moulin B, Haymann JP, Vantyghem MC, Rigothier C, Dussol B, Godin M, Nivet H, Dubourg L, Tack I, Gimenez-Roqueplo AP, Houillier P, Blanchard A, Devuyst O, Jeunemaitre X: Spectrum of mutations in Gitelman syndrome. J Am Soc Nephrol 22: 693–703, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo YF, Nozu K, Iijima K, Morishita T, Huang CC, Yang SS, Sytwu HK, Fang YW, Tseng MH, Lin SH: Recurrent deep intronic mutations in the SLC12A3 gene responsible for Gitelman’s syndrome. Clin J Am Soc Nephrol 6: 630–639, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glaudemans B, Yntema HG, San-Cristobal P, Schoots J, Pfundt R, Kamsteeg EJ, Bindels RJ, Knoers NV, Hoenderop JG, Hoefsloot LH: Novel NCC mutants and functional analysis in a new cohort of patients with Gitelman syndrome. Eur J Hum Genet 20: 263–270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maki N, Komatsuda A, Wakui H, Ohtani H, Kigawa A, Aiba N, Hamai K, Motegi M, Yamaguchi A, Imai H, Sawada K: Four novel mutations in the thiazide-sensitive Na-Cl co-transporter gene in Japanese patients with Gitelman’s syndrome. Nephrol Dial Transplant 19: 1761–1766, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Tseng MH, Yang SS, Hsu YJ, Fang YW, Wu CJ, Tsai JD, Hwang DY, Lin SH: Genotype, phenotype, and follow-up in Taiwanese patients with salt-losing tubulopathy associated with SLC12A3 mutation. J Clin Endocrinol Metab 97: E1478–E1482, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Shao L, Lang Y, Wang Y, Gao Y, Zhang W, Niu H, Liu S, Chen N: High-frequency variant p.T60M in NaCl cotransporter and blood pressure variability in Han Chinese. Am J Nephrol 35: 515–519, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H: WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 280: 42685–42693, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Huang CL, Yang SS, Lin SH: Mechanism of regulation of renal ion transport by WNK kinases. Curr Opin Nephrol Hypertens 17: 519–525, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Pacheco-Alvarez D, Cristóbal PS, Meade P, Moreno E, Vazquez N, Muñoz E, Díaz A, Juárez ME, Giménez I, Gamba G: The Na+:Cl- cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem 281: 28755–28763, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Miao Z, Gao Y, Bindels RJ, Yu W, Lang Y, Chen N, Ren H, Sun F, Li Y, Wang X, Shao L: Coexistence of normotensive primary aldosteronism in two patients with Gitelman’s syndrome and novel thiazide-sensitive Na-Cl cotransporter mutations. Eur J Endocrinol 161: 275–283, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR: Activation of the thiazide-sensitive Na+-Cl- cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci 121: 675–684, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Richardson C, Sakamoto K, de los Heros P, Deak M, Campbell DG, Prescott AR, Alessi DR: Regulation of the NKCC2 ion cotransporter by SPAK-OSR1-dependent and -independent pathways. J Cell Sci 124: 789–800, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S: Molecular pathogenesis of pseudohypoaldosteronism type II: Generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab 5: 331–344, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S: Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int 74: 1403–1409, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Schultheis PJ, Lorenz JN, Meneton P, Nieman ML, Riddle TM, Flagella M, Duffy JJ, Doetschman T, Miller ML, Shull GE: Phenotype resembling Gitelman’s syndrome in mice lacking the apical Na+-Cl- cotransporter of the distal convoluted tubule. J Biol Chem 273: 29150–29155, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Loffing J, Vallon V, Loffing-Cueni D, Aregger F, Richter K, Pietri L, Bloch-Faure M, Hoenderop JG, Shull GE, Meneton P, Kaissling B: Altered renal distal tubule structure and renal Na(+) and Ca(2+) handling in a mouse model for Gitelman’s syndrome. J Am Soc Nephrol 15: 2276–2288, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Joo KW, Lee JW, Jang HR, Heo NJ, Jeon US, Oh YK, Lim CS, Na KY, Kim J, Cheong HI, Han JS: Reduced urinary excretion of thiazide-sensitive Na-Cl cotransporter in Gitelman syndrome: Preliminary data. Am J Kidney Dis 50: 765–773, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Hanwell D, Ishikawa T, Saleki R, Rotin D: Trafficking and cell surface stability of the epithelial Na+ channel expressed in epithelial Madin-Darby canine kidney cells. J Biol Chem 277: 9772–9779, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Deribe YL, Pawson T, Dikic I: Post-translational modifications in signal integration. Nat Struct Mol Biol 17: 666–672, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Yang SS, Lo YF, Yu IS, Lin SW, Chang TH, Hsu YJ, Chao TK, Sytwu HK, Uchida S, Sasaki S, Lin SH: Generation and analysis of the thiazide-sensitive Na+ -Cl- cotransporter (Ncc/Slc12a3) Ser707X knockin mouse as a model of Gitelman syndrome. Hum Mutat 31: 1304–1315, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Castañeda-Bueno M, Cervantes-Pérez LG, Vázquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G: Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci U S A 109: 7929–7934, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH: SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 21: 1868–1877, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanović S, Jovanović A, O’Shaughnessy KM, Alessi DR: Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med 2: 63–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB: ANG II provokes acute trafficking of distal tubule Na+-Cl(-) cotransporter to apical membrane. Am J Physiol Renal Physiol 293: F662–F669, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Talati G, Ohta A, Rai T, Sohara E, Naito S, Vandewalle A, Sasaki S, Uchida S: Effect of angiotensin II on the WNK-OSR1/SPAK-NCC phosphorylation cascade in cultured mpkDCT cells and in vivo mouse kidney. Biochem Biophys Res Commun 393: 844–848, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Noda Y, Sasaki S: Regulation of aquaporin-2 trafficking and its binding protein complex. Biochim Biophys Acta 1758: 1117–1125, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Moeller HB, Praetorius J, Rützler MR, Fenton RA: Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci U S A 107: 424–429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moeller HB, Olesen ET, Fenton RA: Regulation of the water channel aquaporin-2 by posttranslational modification. Am J Physiol Renal Physiol 300: F1062–F1073, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Sabath E, Meade P, Berkman J, de los Heros P, Moreno E, Bobadilla NA, Vázquez N, Ellison DH, Gamba G: Pathophysiology of functional mutations of the thiazide-sensitive Na-Cl cotransporter in Gitelman disease. Am J Physiol Renal Physiol 287: F195–F203, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Kunchaparty S, Palcso M, Berkman J, Velázquez H, Desir GV, Bernstein P, Reilly RF, Ellison DH: Defective processing and expression of thiazide-sensitive Na-Cl cotransporter as a cause of Gitelman’s syndrome. Am J Physiol 277: F643–F649, 1999 [DOI] [PubMed] [Google Scholar]
- 41.De Jong JC, Van Der Vliet WA, Van Den Heuvel LP, Willems PH, Knoers NV, Bindels RJ: Functional expression of mutations in the human NaCl cotransporter: Evidence for impaired routing mechanisms in Gitelman’s syndrome. J Am Soc Nephrol 13: 1442–1448, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Needham PG, Mikoluk K, Dhakarwal P, Khadem S, Snyder AC, Subramanya AR, Brodsky JL: The thiazide-sensitive NaCl cotransporter is targeted for chaperone-dependent endoplasmic reticulum-associated degradation. J Biol Chem 286: 43611–43621, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subramanya AR, Liu J, Ellison DH, Wade JB, Welling PA: WNK4 diverts the thiazide-sensitive NaCl cotransporter to the lysosome and stimulates AP-3 interaction. J Biol Chem 284: 18471–18480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou B, Zhuang J, Gu D, Wang H, Cebotaru L, Guggino WB, Cai H: WNK4 enhances the degradation of NCC through a sortilin-mediated lysosomal pathway. J Am Soc Nephrol 21: 82–92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newell-Litwa K, Seong E, Burmeister M, Faundez V: Neuronal and non-neuronal functions of the AP-3 sorting machinery. J Cell Sci 120: 531–541, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Nakatsu F, Ohno H: Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct Funct 28: 419–429, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Ronzaud C, Loffing-Cueni D, Hausel P, Debonneville A, Malsure SR, Fowler-Jaeger N, Boase NA, Perrier R, Maillard M, Yang B, Stokes JB, Koesters R, Kumar S, Hummler E, Loffing J, Staub O: Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J Clin Invest 123: 657–665, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellison DH: Adaptation in Gitelman syndrome: “We just want to pump you up”. Clin J Am Soc Nephrol 7: 379–382, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Favre GA, Nau V, Kolb I, Vargas-Poussou R, Hannedouche T, Moulin B: Localization of tubular adaptation to renal sodium loss in Gitelman syndrome. Clin J Am Soc Nephrol 7: 472–478, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng CJ, Shiang JC, Hsu YJ, Yang SS, Lin SH: Hypocalciuria in patients with Gitelman syndrome: Role of blood volume. Am J Kidney Dis 49: 693–700, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Lin SH, Yu IS, Jiang ST, Lin SW, Chu P, Chen A, Sytwu HK, Sohara E, Uchida S, Sasaki S, Yang SS: Impaired phosphorylation of Na(+)-K(+)-2Cl(-) cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and Bartter-like syndrome. Proc Natl Acad Sci U S A 108: 17538–17543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang SS, Hsu YJ, Chiga M, Rai T, Sasaki S, Uchida S, Lin SH: Mechanisms for hypercalciuria in pseudohypoaldosteronism type II-causing WNK4 knock-in mice. Endocrinology 151: 1829–1836, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Promeneur D, Kwon TH, Frøkiaer J, Knepper MA, Nielsen S: Vasopressin V(2)-receptor-dependent regulation of AQP2 expression in Brattleboro rats. Am J Physiol Renal Physiol 279: F370–F382, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Yang SS, Yamauchi K, Rai T, Hiyama A, Sohara E, Suzuki T, Itoh T, Suda S, Sasaki S, Uchida S: Regulation of apical localization of the thiazide-sensitive NaCl cotransporter by WNK4 in polarized epithelial cells. Biochem Biophys Res Commun 330: 410–414, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Sokolova O, Bozko PM, Naumann M: Helicobacter pylori suppresses glycogen synthase kinase 3β to promote β-catenin activity. J Biol Chem 283: 29367–29374, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.