Abstract
Tenofovir disoproxil fumarate (TDF), the first nucleotidic inhibitor of HIV reverse transcription, became available in 2001. It has been extensively used worldwide and is now the most prescribed antiretroviral (ARV) drug. Its high antiviral activity and favorable metabolic profile are responsible for its success. Furthermore, TDF has been associated with other ARVs to form new combined antiretroviral treatments in only one tablet once-a-day, which increases treatment adherence. Fears of potential nephrotoxicity that tenofovir would have in common with two other drugs from the same family (adefovir, used to treat hepatitis B, and cidofovir, used to treat cytomegalovirus infections) were alleviated by the early clinical trials. Yet, in 2001, the first case of TDF-induced acute nephrotoxicity was published. Numerous cases have been published since then, and it is now established that TDF presents a tubular toxicity risk. Some facilitating factors have been identified, such as co-prescription of didanosine or boosted protease inhibitor, preexisting CKD, low body weight, and associated diabetes mellitus. Conversely, whether TDF is nephrotoxic in the long term is a highly debated question. Some studies suggest a decreased GFR when TDF is prescribed for a long period, while others indicate that TDF is safe for the kidneys even after many years of use. Here we review the differences in patient characteristics, study designs, and measured outcomes that can possibly explain these conflicting findings. We conclude with rational recommendation for appropriate TDF prescription.
Tenofovir disoproxil fumarate (TDF) is the only available nucleotidic reverse transcription inhibitor. It is the prodrug of tenofovir diphosphate, a structural analogue of deoxy-ATP. It halts DNA synthesis from the RNA-dependent DNA polymerase of HIV and is a weak inhibitor of host cell α and β DNA polymerases and of mitochondrial γ DNA polymerase.1,2
TDF was approved by the U.S. Food and Drug Administration (FDA) in October 2001 and has been widely used worldwide since then. Many countries have included it in their list of recommended first-line drugs for the treatment of HIV infection. It has been the most widely prescribed antiretroviral molecule since 2006 in the United States, and more than half of all treated patients living with HIV/AIDS are taking a tenofovir-containing regimen.3 In addition, tenofovir activity on human hepatitis B virus (HBV) is higher than that of adefovir.4 TDF is now indicated in the treatment of chronic HBV infection5 and is the drug of choice for HIV/HBV-coinfected patients.
The knowledge that has been accumulated on TDF is substantial (a recent publication refers to >450,000 person/years6), and it is a safe and highly effective drug. However, publications concerning its renal safety are still ambiguous. There may be a gap between what is observed in clinical trials and real life.7 Here we offer a basis to explain this seeming paradox.
TDF-Induced Kidney Injury
Tubular Toxicity
The first published case of acute tubular toxicity due to TDF consisted of both a proximal tubular injury with the combination of Fanconi syndrome and acute renal failure (ARF) and a distal tubular injury in the form of nephrogenic diabetes insipidus.8 The Fanconi syndrome was comprehensive and comprised metabolic acidosis with normal plasma ion gap, hypophosphatemia, hyperphosphaturia, hypokalemia, hypouricemia, urinary tubular protein waste, glycosuria with normal blood glucose, and aminoaciduria. Kidney biopsy showed extensive acute tubular necrosis with vacuoles in the proximal epithelial cells, brush cell effacement, and an unusual apical localization of the cell nuclei. All biologic measures returned to normal within a few months after TDF withdrawal.
This first case encapsulates all potential acute tubular toxicity with TDF. At least a dozen other case reports have been published since then, combining some or all of the abnormalities described in the original case.9–23 One hundred sixty-four complete or partial cases of TDF-induced Fanconi syndrome occurring between 2001 and 2006 have been retrospectively analyzed using the FDA reported adverse effects registry.24 Men were affected in 78% of the cases, at an average age of 46 years. Associated antiretroviral drugs (ARVs) played a prominent role, as 74% of the patients were also prescribed a ritonavir-boosted protease inhibitor (PI), mostly ritonavir-boosted lopinavir. Didanosine was also abundantly co-prescribed (43%). One third of the patients were treated with TDF, didanosine (DDI), and ritonavir/lopinavir. Dialysis was required transiently in 2% of the patients, and 2% died of a cause possibly related to their Fanconi syndrome.
ARF
In some instances of proximal tubulopathy, ARF from tubular necrosis ensues as the consequence of the tubular damage. Kidney function improves in the months following TDF withdrawal, but it does not always revert completely. In a study of 24 patients who stopped TDF treatment because of ARF, only 42% recovered their initial kidney function.25 Estimated GFR (eGFR) was 10 ml/min lower several months after TDF withdrawal than before treatment (P=0.03). The patients whose kidney function improved by >20 ml/min after stopping TDF had faster kidney decline at the time of ARF, were more frequently prescribed a PI, and had been treated with TDF for a shorter period than the patients whose function improved by <20 ml/min. Conversely, pretreatment kidney function, lowest kidney function during the ARF episode, the proportion of DDI-treated patients, and HIV viral load were similar between the two groups.
A meta-analysis including eight studies and 7496 patients showed that the risk for ARF was 0.7% higher (95% confidence interval [CI], 0.2 to 1.2) in TDF-treated patients than in patients receiving combined antiretroviral treatment (cART) without TDF.26
Chronic Renal Failure
Whether long-term use of TDF is detrimental to kidney function is also highly debated. On the one hand, TDF certainly represents a risk for incompletely reversible acute tubular damage. On the other, numerous studies show that long-term use of TDF is safe for the kidneys.6,27–34 However, these latter studies usually have an optimistic interpretation of results. In some instances, it was concluded that TDF is safe despite a slight but significant decline in kidney function as early as mid-term use. In others, kidney injury was said to be a rare adverse effect even though it affected 1%–5% patients. Three studies concluded that long-term use of TDF results in declining kidney function (Table 1).35–37 The deterioration in kidney function can be as low as 13.3 ml/min per 1.73 m2 after 1 year of treatment in TDF-treated groups.35
Table 1.
Summary of the main characteristics and findings of studies describing long-term kidney function loss in TDF-treated patients.
| Variable | United States | Australia | Germany |
|---|---|---|---|
| Study type | Longitudinal cohort | Retrospective cohort | Retrospective cohort |
| Treated patients (n) | 344 | 290 | 82 |
| Controls (n) | 314 | 618 | 92 |
| Age (yr)a | |||
| Cases | 38 (34–43) | 46 (23 to 38) | 42.6±8.1 |
| Controls | 32 (32–45); NS | 45 (21 to 75); NS | 42.3±8.4; NS |
| CD4 count (cells/mm3)a | |||
| Cases | 220 (77–433) | 460 (425 to 496) | 501±267 |
| Controls | 210 (94–380); NS | 523 (499 to 547); NS | 571±266; NS |
| Average treatment duration (mo)b | 9.9 (5.5, 12.0) | 20.4 (18.0 to 21.6) | 9±7.3 months |
| Change in kidney function | −13.3 ml/min (cases) versus −7.5 ml/min (controls); P=0.005 | −5.6 ml/min (cases) versus 1.3 ml/min (controls); P=0.002 | 97±49 ml/min (cases) versus 107±39 ml/min (controls); P<0.05 |
| Kidney function assessment | Cockcroft-Gault | Cockcroft-Gault indexed to ideal body weight | Measured creatinine clearance |
| Reference | 35 | 36 | 37 |
NS, not significant.
Values for United States are median (first–third quartile); values for Australia are mean (95% confidence interval); values for Germany are mean ± SD.
Values for United States are mean (minimum, maximum); values for Australia are mean (95% confidence interval); values for Germany are mean ± SD.
A Brazilian transversal study of 213 consecutively recruited patients over a 6-month period showed that prevalence of CKD was 8% in TDF-treated patients who had a 2.25 times higher risk of developing CKD than non–TDF-treated patients (95% CI, 1.02 to 4.95).38
A meta-analysis using 11 studies to estimate chronic nephrotoxicity associated with TDF therapy26 included 5767 patients treated for a mean of 48 weeks (range, 24–144 weeks). TDF-treated patients experienced a decrease in estimated creatinine clearance (Cockcroft-Gault formula) of 3.92 (95% CI, 2.13 to 5.70) ml/min per 1.72 m2 compared with non–TDF-treated patients.26 The difference in kidney function between TDF-treated and nontreated patients was qualified as moderate by the authors.
One has to keep in mind the relatively short treatment period in the included studies (<1 year on the average). Patients can receive TDF for several decades. On the contrary, a loss of kidney function of about 4 ml/min per 1.72 m2 per year appears quite significant. This is similar to what is observed in polycystic kidney disease or Fabry disease.39 In comparison, normal kidney function declines because of aging at 0.4 ml/min per year.40 However, whether decay of kidney function after a year of TDF treatment continues with the same rate, continues with a slower rate, or stops and remains stable after an initial decline is largely unknown.
Two recent studies have addressed this question. A retrospective cohort compared >6500 TDF-exposed patients with 4000 nonexposed patients between 1997 and 2007 (38,132 person-years of follow-up; median follow-up >3.9 years).41 The hazard ratios for proteinuria (two consecutive dipsticks showing proteinuria >30 mg/dl), rapid decline in kidney function (eGFR decline >3 ml/min per year using Modification of Diet in Renal Disease equation), or CKD (eGFR <60 ml/min), were 1.30, 1.17, and 1.44 per year of TDF exposure, respectively (95% CI, 1.22 to 1.37, 1.11 to 1.24, and 1.30 to 1.60, respectively). Patients ever exposed to TDF had more than twice the risk of CKD (eGFR < 60 ml/min; 95% CI, 1.76 to 2.54 ml/min). The risk of renal events did not decrease after TDF withdrawal during the study period. In a very similar study, the risk of progression from CKD stage 0–1 to stage 2 or 3 was higher in naive patients exposed to TDF than in TDF-free patients (48.8% versus 23.7%, P<0.001 for CKD stage 2; 5.8% versus 0.0%, P=0.03 for CKD stage 3).42 Tenofovir treatment was the only independent factor associated with progression to CKD stage 2 (hazard ratio, 2.12; 95% CI, 1.41 to 3.18) and to CKD stage 3 (hazard ratio, 4.91; 95% CI, 1.02 to 23.7).
The meta-analysis found substantial statistical heterogeneity between studies (I2=66%), and only 6 of the 11 studies in the meta-analysis found declining kidney function during TDF treatment.26 Heterogeneity reflected differences in study design; kidney function decline was lower in the intention-to-treat studies, in the studies that systematically reported adverse effects, and in randomized clinical trials (RCTs). This last observation is extremely important and probably explains most of the discrepancies observed in literature. Most of the studies concluding that TDF is nephrotoxic are case-control studies or retrospective cohort studies. In contrast, prospective RCTs usually conclude that TDF is safe in the long run. In the meta-analysis, loss of kidney function in TDF-treated patients was 1.5 ml/min per 1.72 m2 in clinical trials, a barely significant difference, with a CI of 0.05 to 2.96 ml/min per 1.72 m2, compared with 5.45 ml/min per 1.72 m2 for cohort studies (95% CI, 3.89 to 7.02 ml/min per 1.72 m2). This corresponds to a mean difference in kidney function loss of 4.32 ml/min between RCTs and cohort studies (95% CI, 2.15 to 6.49 ml/min).
Some of the heterogeneity in the meta-analysis was also due to pre-TDF treatment. Kidney function loss was inferior in treatment-naive patients for whom TDF was part of the initial cART (2.5 ml/min per 1.72 m2) than in treatment-experienced patients (5.15 ml/min per 1.72 m2). There was also a nonsignificant trend toward a smaller decline in kidney function in pharmaceutical company–funded studies than in independent studies.
Finally, this meta-analysis found that the risk for CKD and ESRD did not significantly differ between TDF-treated and nontreated patients. This suggests that declining kidney function was still within the boundaries of normal function.
Other Consequences of TDF-Induced Kidney Injury
Vitamin D deficiency is very common among HIV-infected patients.43,44 TDF-induced impairment of tubular function leads to decreased 1-α hydroxylation of vitamin D and to lower tubular reabsorption of vitamin D–binding protein.2 TDF-induced secondary hyperparathyroidism has been described in vitamin D–deficient patients.44–46
Hypophosphatemia, osteomalacia, bone pain, decreased bone mineralization, and bone fractures have also been described in patients prescribed TDF.16,47–49 Because bone mineral diseases are quite common among HIV-infected patients and have many possible causes, it is hard to conclude whether these complications are a direct consequence of TDF. In a meta-analysis regarding bone consequences of TDF treatment, the risk of fracture, the bone mineral density, and the incidence of hypophosphoremia could be analyzed in three studies that included 1111, 1224, or 1402 patients, respectively.26 These three criteria did not differ between TDF-treated and nontreated patients.
Finally, five cases of lactic acidosis, four of which were lethal, have been described in TDF-treated patients.50–55 In each case, a drug with a favoring role was co-prescribed: abacavir, DDI, or metformin.
Clinical and Histologic Description of TDF-Induced Tubular Damage
In a retrospective study of 13 cases of TDF-induced tubulopathy, no ethnic or sex predominance was noted.56 The mean patient age was 51 years, and the patients had been treated from 1 month to 8 years when AKI occurred. This highlights the importance of unidentified triggering factors. AKI was noted in 66% of the cases, and 15% of the patients presented with anuria. Dialysis treatment was transiently necessary in one third of the patients. Proteinuria was noted in all the cases, ranging from 1 to 2 g/24 hours. Conversely, hematuria was unusual (15% of the cases).
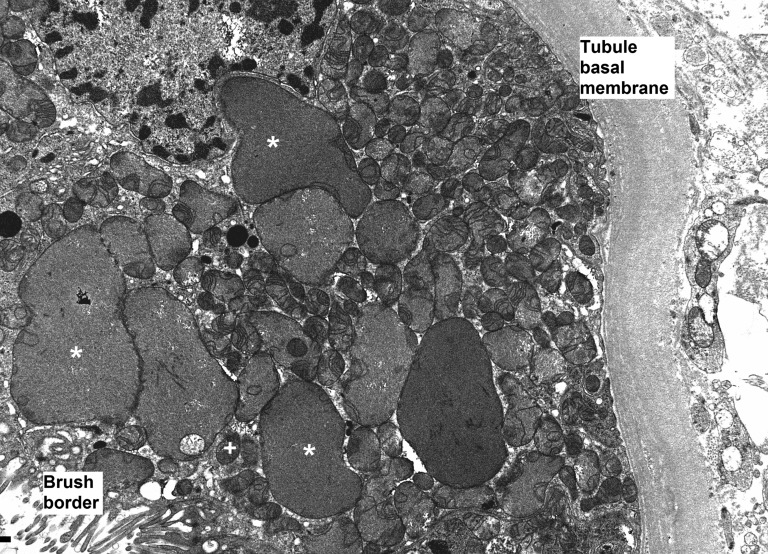
Histologic examination mainly revealed acute tubular necrosis, primarily affecting proximal tubules. Tubular ectasia, cytoplasmic simplification, prominent nucleoli, and loss of brush border indicated a toxic origin. The only sign of TDF-specific toxicity was giant mitochondria visible as prominent eosinophilic inclusions in the cytoplasm of proximal tubular epithelial cells.
Electron microscopy revealed abnormal mitochondria in almost all cases (Figure 1). Some were greatly increased in size, while others appeared shrunken. They were usually grouped within the cell. Some epithelial cells showed marked mitochondrial depletion. Mitochondrial cristae could be absent or less abundant than normal, sometimes grouped at one pole of the mitochondria.
Figure 1.
TDF-induced epithelial cell dysfunction is due to mitochondrial damage. Enlarged mitochondria (*) are visible adjacent to normal-size mitochondria (+). Large mitochondria appear devoid of cristae, while other mitochondria show normal cristae content. When cristae are visible in dysmorphic mitochondria, they are usually grouped at a pole. (Original magnification ×8000.) Photograph kindly provided by Leal C. Herlitz, MD, Department of Pathology, Columbia University Medical Center in New York, New York.
Pathophysiology of TDF-Induced Damage
TDF can be toxic to mitochondria. This is supported by ultrastructural mitochondrial abnormalities found in TDF-induced tubulopathy.56 Kidney mitochondrial DNA (mtDNA) content was also depleted, and dysfunction in the oxidative respiratory chain has been noted in animal models.57
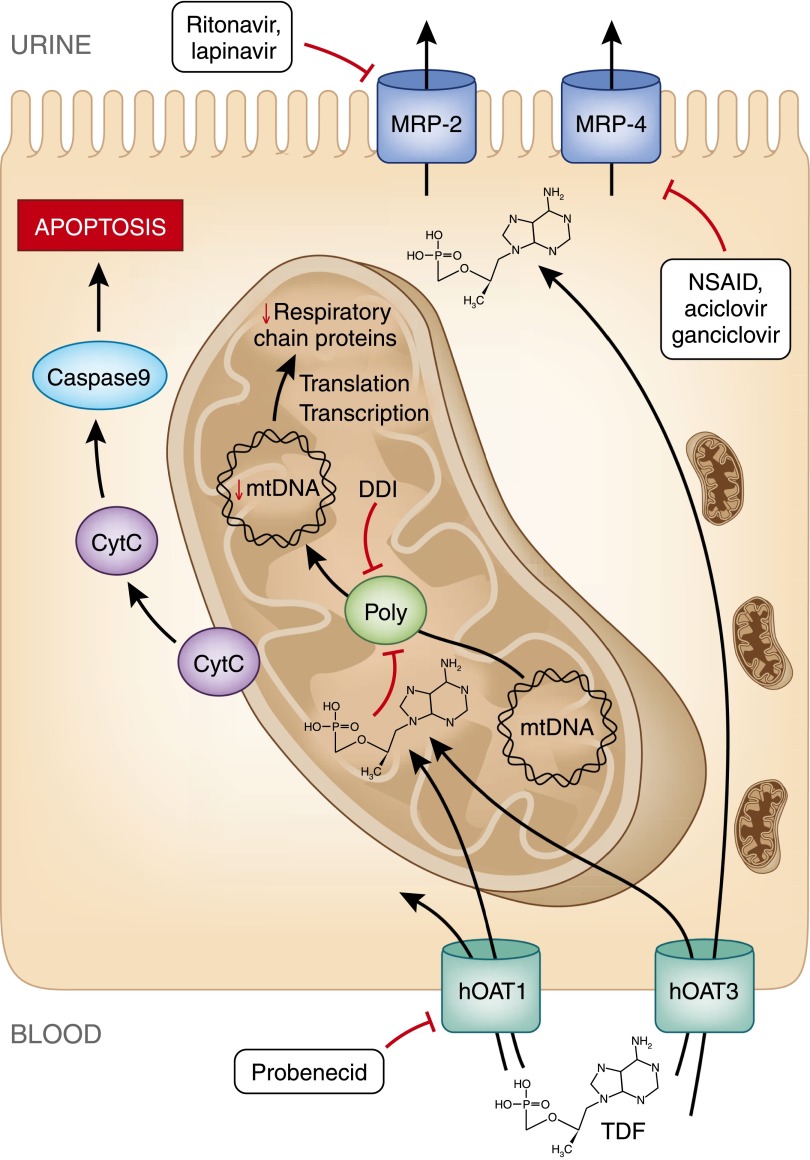
The following scheme is currently used to explain TDF-induced kidney damages (Figure 2). Unmodified TDF is excreted in the urine both by glomerular filtration and tubular secretion.58,59 To be secreted, TDF enters the epithelial cell at its basolateral pole using human organic anion transporter (hOAT) 160 and to a lesser extend hOAT3.61 It is then secreted in the tubular lumen through multidrug resistance protein (MRP) 2,62 MRP4,60 and possibly MRP3.63 When plasma concentration increases, or when apical secretion is inhibited, intracellular concentration of TDF increases.60 This results in partial inhibition of mtDNA polymerase γ,64 depletion in mtDNA,60,65,66 and oxidative respiratory chain dysfunction.66 Mitochondrial structural changes are induced in proximal tubular epithelial cells.56,66 Because of a shortage of ATP production, tubular cells cannot properly ensure reabsorption of ions and small molecules, such as potassium, glucose, phosphate, uric acid, amino acids, and β2-microglobulin. Therefore, these molecules are secreted in abnormal quantities in the urine, defining the Fanconi syndrome.
Figure 2.
Cytoplasmic accumulation of TDF is responsible for mDNA depletion and oxidative respiratory chain dysfunction resulting in epithelial cell apoptosis. TDF enters tubular epithelial cells through hOAT1 and hOAT3 receptor at the basolateral pole. It is excreted in tubular lumen through receptors MRP2 and MRP4. TDF intracellular concentrations can be modified by drugs that specifically inhibit these receptors. Once inside a mitochondrion, TDF inhibits DNA polymerase γ, which results in a progressive depletion of mitochondrial DNA, a decreased synthesis of respiratory chain proteins and morphologic abnormalities of mitochondria (enlargement, loss of cristae). Some respiratory chain protein are released in the cytoplasm which can be detected by the caspase pathway and induce apoptosis of the cell. Polγ, DNA polymerase γ; CytC, cytochrome C; NSAID, nonsteroid anti-inflammatory drug.
It is possible that mitochondrial abnormalities induce apoptosis of epithelial cells through the caspase pathway, as it is the case with cidofovir, a very closely related drug.67 This would explain the acute tubular necrosis.
Hypophosphatemia could have a dual origin: decreased proximal reabsorption of phosphate and decreased vitamin D activation. Interestingly, fibroblast growth factor-23 concentrations seem to be normal in TDF-induced hypophosphatemic osteomalacia.68
TDF treatment decreases aquaporin-2 expression in epithelial cells along the medullary collecting ducts.58,69 The resulting deficit of water reabsorption could explain the nephrogenic diabetes insipidus that is sometime associated with TDF-induced Fanconi syndrome.
Incidence and Risk Factors for TDF Nephrotoxicity
Incidence of AKI after initiation of TDF treatment greatly varies: from 1.6 per 100 person-years28,33 to 1.5 per 1000 person-years,6 again illustrating inconsistency in the literature on the subject.
Risk factors for AKI include older age (odds ratio, 1.05 per year; P=0.02), CD4 cell count (odds ratio, 0.46 for each additional 50 CD4 cells/mm3; P<0.001), weight (odds ratio, 0.96 for each additional kilogram; P<0.001), prescription of a nephrotoxic drug at the same time as TDF (odds ratio, 2.40; P=0.03),6 male sex, hepatitis C virus co-infection, and advanced HIV disease.33
Two studies indicate that kidney damage risk (acute tubular dysfunction70 or chronic decline of GFR71) increases with TDF trough levels. The links between trough levels and nephrotoxicity need to be more thoroughly studied because they might help to generate management recommendations in case of TDF nephrotoxicity.
A Japanese retrospective study of 493 patients treated with TDF reported an unusually high incidence of declining chronic kidney function: 10.5 per 100 patient-years.72 The main risk factor identified in this study was a lower body weight.
The possible role of DDI and lopinavir in TDF nephrotoxicity is unclear. These two ARTs are often associated with TDF in cases of Fanconi syndrome.24,73 Kidney function decline was more frequent (odds ratio, 3.1)74 and more pronounced (14.7 versus 4.7 ml/min per 1.72 m2 per year)75 in TDF-treated patients also receiving DDI or a boosted PI, respectively.
TDF excretion in the urinary lumen through MRP4 is partly inhibited by ritonavir and lopinavir.76 This may result in a decreased clearance of TDF77 and its cytoplasmic accumulation in epithelial tubular cells. DDI also induces mitochondrial toxicity, which could exacerbate TDF-induced mitochondrial damage.66 Yet, these observations are challenged by clinical78,79 and pharmacologic76,80 studies.
Genetic background is also probably involved in certain cases of TDF tubular toxicity. Two studies showed that polymorphisms in the MRP2 gene were associated with a five to six times higher risk of tubular toxicity.62,81 MRP4 polymorphisms could also regulate TDF intracellular concentration.82
TDF Nephrotoxicity: How to Reconcile Clinical Trials and Observed Cases
The difference between the abundance of tubular injuries described in the literature and good tolerance found in clinical trials can be explained by several observations. First, Fanconi syndrome can occur without any increase in serum creatinine. Tubulopathy is a diagnosis that requires very specific analyses, which are not always performed in RCTs. TDF-induced kidney failure is therefore probably less frequent than TDF-induced tubulopathy. Second, creatinine is a late marker of kidney dysfunction and usually does not increase before GFR decreases to 60 ml/min per 1.72 m2. Finally, it is important to distinguish CKD and chronic decline in kidney function. Patients whose GFR changes from 100 ml/min per 1.72 m2 before TDF treatment to 90 ml/min per 1.72 m2 after treatment have experienced a significant loss of kidney function but are not classified as having CKD.
DDI and boosted PIs might also play a role in TDF nephrotoxicity. If these drugs were very commonly associated in cohorts reporting numerous cases of nephrotoxicity, this high-risk combination was usually not prescribed in RCTs, where TDF is prescribed with efavirenz (a non-nucleoside reverse transcriptase inhibitor).
Patients with possible risk factors, such as CKD or very low body weight, are commonly excluded from RCTs in favor of highly selected, homogenous patients.
The most important issue is that two very distinct categories of kidney disease risk in HIV-infected patients need to be recognized (Table 2). There are patients whose kidney risk stems from HIV itself; their kidney function is altered directly or indirectly by HIV. For these patients, a cART including TDF will most often be beneficial to the kidneys because of its high antiviral activity. This is the case when TDF is prescribed as part of a first-line ART. These patients are preferentially selected to participate in RCTs. The other category consists of HIV-infected patients whose kidney risk does not depend on HIV. These patients have controlled HIV disease thanks to a long period of previous ART. Their kidney risk is the consequence of many causes, such as age, diabetes, previous CKD, history of nephrotoxic exposure, and high cardiovascular risk due to chronic HIV infection and its long-term treatment. These patients are probably more exposed to TDF-induced decline in chronic kidney function. They are probably over-represented in cohort studies and absent from RCTs. It may very well be because of differences in patient recruitment that cohort studies and RCTs come to opposite conclusions.
Table 2.
Summary of factors that explain differences in conclusions between randomized control trials and cohort studies
| Variable | Clinical Trial | Cohort Study |
|---|---|---|
| Selected patients | Patients are often selected; no kidney dysfunction before treatment | Unselected patients who can have CKD before TDF initiation |
| Therapeutic history | Frequently naive patients | Frequently ARV-experienced patients |
| Associated ARVs | Frequently NNRTI | Frequently boosted PI |
| HIV disease | Often, recent diagnosis of HIV infection | Patients more susceptible to having advanced HIV disease |
| Studied kidney measure | Decrease of GFR <90 ml/min per 1.72 m2 or even <60 ml/min per 1.72 m2 | Modification of eGFR that can significantly decrease while remaining >90 ml/min per 1.72. m2 |
| Follow-up and duration of TDF exposure | Short follow-up (<1 yr) and duration of TDF treatment | Frequently, longer follow-up and exposure duration |
| Study finding | More commonly financed by pharmaceutical companies | More commonly independent studies |
| Resulting effect | Patients whose kidney function is very likely to benefit from the antiviral effect of TDF | Patients more at risk of cardiovascular and kidney complications of long-term HIV infection and ARV treatment |
NNRTI, non-nucleoside reverse transcription inhibitor.
Recommendations for a Wise Prescription of TDF
The Infectious Diseases Society of America has published official recommendations about tenofovir use.83 It advises screening for kidney abnormalities in all patients recently diagnosed with HIV infection. The screening should consist of measuring BP and serum creatinine and looking for proteinuria with the use of a dipstick. GFR should be estimated using serum creatinine. Furthermore, the Society recommends measuring serum creatinine, serum phosphate, proteinuria, and glycosuria twice a year in all patients who are prescribed TDF and who have an eGFR <90 ml/min per 1.72 m2 at the introduction of TDF treatment, are prescribed other potentially nephrotoxic drugs or a ritonavir-boosted PI, or have a high-risk profile for kidney disease, especially hypertensive and diabetic patients.
The European Association for the Study of the Liver has very recently published guidelines on TDF monitoring in patients infected with HBV.84 Before TDF initiation, it recommends measuring serum creatinine and estimating GFR. High-risk patients are those with decompensated cirrhosis, creatinine clearance <60 ml/min, high BP, proteinuria, diabetes mellitus, GN, organ transplant, and concomitant nephrotoxic drugs. The Association recommends testing for serum creatinine, estimating GFR, and measuring serum phosphate in all TDF-treated patients every 1–3 months during the first year and every 3–6 months thereafter depending on the patient’s kidney risk profile. It is probably safer to avoid prescribing DDI in patients receiving TDF.
The management of TDF-induced kidney injury is unclear. In case of acute kidney failure, because of the higher risk of long-term declining kidney function, it is probably safer to withdraw and contraindicate prescription of TDF, unless some favoring cause has been clearly identified and corrected, such as withdrawal of another prescribed nephrotoxic drug. The use of trough levels to prevent AKI or to adapt TDF dose in case of AKI has not been determined.
In case of hypophosphatemia, the first measure is to search for and correct potential vitamin D deficiency, a very common condition in HIV-infected patients.85 If hypophosphatemia persists even after vitamin D storage has been reconstituted, a search for Fanconi syndrome should be undertaken. In the case of Fanconi syndrome, TDF should be withdrawn. If hypophosphatemia is isolated and moderate, risks of TDF prescription should be balanced against its benefits. On the one hand, the risk of bone mineral disease should be assessed (bone pain, fracture risk, bone mineral density). On the other hand, therapeutic options other than TDF should be considered.
No specific recommendations have been published for TDF-treated patients whose kidney function progressively declines. General management applies, and usual factors for CKD progression should be corrected if possible, with a special search for co-prescription of other nephrotoxic drugs. Nonsteroidal anti-inflammatory drugs,86 vancomycin,87 and metformin55 have been described as potentially nephrotoxic when prescribed with TDF. When these conditions have been treated, if CKD continues to progress, the decision to decrease TDF dose or to withdraw TDF treatment will need to be considered. No available data indicate whether a TDF dose reduction can slow declining of kidney function while maintaining proper control over HIV replication.
Conclusion
TDF is already contained in Atripla and Complera, the first medications available as a single pill taken once a day for combined antiretroviral therapy that complies with international recommendations. Stribild, the only once-a-day pill that contains an integrase inhibitor, has recently been approved by the FDA. It is very likely that Stribild will be a highly successful therapy. TDF is recommended as part of first-choice therapy in many African countries, where HIV prevalence is quite high. It is also recommended as part of first-line therapy in the very recently published guidelines of the International Antiviral Society–USA panel.88 Therefore, TDF will probably be prescribed to numerous patients for a prolonged period. The question of its long-term nephrotoxicity is of utmost importance. It is possible to significantly reduce this risk by observing simple rules, such as measuring kidney function carefully and assessing kidney disease risk before prescription. Long- term kidney safety of TDF still needs to be assessed in real-life prospective cohorts.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Birkus G, Hitchcock MJ, Cihlar T: Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother 46: 716–723, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez-Fernandez B, Montoya-Ferrer A, Sanz AB, Sanchez-Niño MD, Izquierdo MC, Poveda J, Sainz-Prestel V, Ortiz-Martin N, Parra-Rodriguez A, Selgas R, Ruiz-Ortega M, Egido J, Ortiz A: Tenofovir nephrotoxicity: 2011 update. Aids Res Treat 2011: 354908, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilead Science 2006 annual report on marketed products. 2007. Available at: http://www.gilead.ca/AR2006/hiv_aids.php Accessed September 9, 2013
- 4.Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, Manns M, Kotzev I, Tchernev K, Buggisch P, Weilert F, Kurdas OO, Shiffman ML, Trinh H, Washington MK, Sorbel J, Anderson J, Snow-Lampart A, Mondou E, Quinn J, Rousseau F: Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 359: 2442–2455, 2008 [DOI] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration. Viread. 2008. Modified indication. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021356s025lbl.pdf Accessed September 9, 2013
- 6.Nelson MR, Katlama C, Montaner JS, Cooper DA, Gazzard B, Clotet B, Lazzarin A, Schewe K, Lange J, Wyatt C, Curtis S, Chen SS, Smith S, Bischofberger N, Rooney JF: The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: The first 4 years. AIDS 21: 1273–1281, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Atta MG, Fine DM: Editorial comment: tenofovir nephrotoxicity—the disconnect between clinical trials and real-world practice. AIDS Read 19: 118–119, 2009 [PubMed] [Google Scholar]
- 8.Verhelst D, Monge M, Meynard JL, Fouqueray B, Mougenot B, Girard PM, Ronco P, Rossert J: Fanconi syndrome and renal failure induced by tenofovir: A first case report. Am J Kidney Dis 40: 1331–1333, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Créput C, Gonzalez-Canali G, Hill G, Piketty C, Kazatchkine M, Nochy D: Renal lesions in HIV-1-positive patient treated with tenofovir. AIDS 17: 935–937, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Karras A, Lafaurie M, Furco A, Bourgarit A, Droz D, Sereni D, Legendre C, Martinez F, Molina JM: Tenofovir-related nephrotoxicity in human immunodeficiency virus-infected patients: Three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin Infect Dis 36: 1070–1073, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Rollot F, Nazal EM, Chauvelot-Moachon L, Kélaïdi C, Daniel N, Saba M, Abad S, Blanche P: Tenofovir-related Fanconi syndrome with nephrogenic diabetes insipidus in a patient with acquired immunodeficiency syndrome: The role of lopinavir-ritonavir-didanosine. Clin Infect Dis 37: e174–e176, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Breton G, Alexandre M, Duval X, Prie D, Peytavin G, Leport C, Vildei JL: Tubulopathy consecutive to tenofovir-containing antiretroviral therapy in two patients infected with human immunodeficiency virus-1. Scand J Infect Dis 36: 527–528, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Callens S, De Roo A, Colebunders R: Fanconi-like syndrome and rhabdomyolysis in a person with HIV infection on highly active antiretroviral treatment including tenofovir. J Infect 47: 262–263, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Earle KE, Seneviratne T, Shaker J, Shoback D: Fanconi’s syndrome in HIV+ adults: Report of three cases and literature review. J Bone Miner Res 19: 714–721, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Gaspar G, Monereo A, García-Reyne A, de Guzmán M: Fanconi syndrome and acute renal failure in a patient treated with tenofovir: A call for caution. AIDS 18: 351–352, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Haverkort ME, van der Spek BW, Lips P, Slieker WA, Ter Heine R, Huitema AD, Bronsveld W: Tenofovir-induced Fanconi syndrome and osteomalacia in two HIV-infected patients: Role of intracellular tenofovir diphosphate levels and review of the literature. Scand J Infect Dis [DOI] [PubMed]
- 17.Irizarry-Alvarado JM, Dwyer JP, Brumble LM, Alvarez S, Mendez JC: Proximal tubular dysfunction associated with tenofovir and didanosine causing Fanconi syndrome and diabetes insipidus: A report of 3 cases. AIDS Read 19: 114–121, 2009 [PubMed] [Google Scholar]
- 18.James CW, Steinhaus MC, Szabo S, Dressier RM: Tenofovir-related nephrotoxicity: Case report and review of the literature. Pharmacotherapy 24: 415–418, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Kapitsinou PP, Ansari N: Acute renal failure in an AIDS patient on tenofovir: A case report. J Med Case Reports 2: 94, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik A, Abraham P, Malik N: Acute renal failure and Fanconi syndrome in an AIDS patient on tenofovir treatment—case report and review of literature. J Infect 51: E61–E65, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Mathew G, Knaus SJ: Acquired Fanconi’s syndrome associated with tenofovir therapy. J Gen Intern Med 21: C3–C5, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quimby D, Brito MO: Fanconi syndrome associated with use of tenofovir in HIV-infected patients: A case report and review of the literature. AIDS Read 15: 357–364, 2005 [PubMed] [Google Scholar]
- 23.Rifkin BS, Perazella MA: Tenofovir-associated nephrotoxicity: Fanconi syndrome and renal failure. Am J Med 117: 282–284, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Gupta SK: Tenofovir-associated Fanconi syndrome: Review of the FDA adverse event reporting system. AIDS Patient Care STDS 22: 99–103, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Wever K, van Agtmael MA, Carr A: Incomplete reversibility of tenofovir-related renal toxicity in HIV-infected men. J Acquir Immune Defic Syndr 55: 78–81, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M: Systematic review and meta-analysis: Renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis 51: 496–505, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Izzedine H, Hulot JS, Vittecoq D, Gallant JE, Staszewski S, Launay-Vacher V, Cheng A, Deray G, Study 903 Team : Long-term renal safety of tenofovir disoproxil fumarate in antiretroviral-naive HIV-1-infected patients. Data from a double-blind randomized active-controlled multicentre study. Nephrol Dial Transplant 20: 743–746, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Antoniou T, Raboud J, Chirhin S, Yoong D, Govan V, Gough K, Rachlis A, Loutfy M: Incidence of and risk factors for tenofovir-induced nephrotoxicity: A retrospective cohort study. HIV Med 6: 284–290, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Gayet-Ageron A, Ananworanich J, Jupimai T, Chetchotisakd P, Prasithsirikul W, Ubolyam S, Le Braz M, Ruxrungtham K, Rooney JF, Hirschel B, Staccato Study Group : No change in calculated creatinine clearance after tenofovir initiation among Thai patients. J Antimicrob Chemother 59: 1034–1037, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Gérard L, Chazallon C, Taburet AM, Girard PM, Aboulker JP, Piketty C: Renal function in antiretroviral-experienced patients treated with tenofovir disoproxil fumarate associated with atazanavir/ritonavir. Antivir Ther 12: 31–39, 2007 [PubMed] [Google Scholar]
- 31.Padilla S, Gutiérrez F, Masiá M, Cánovas V, Orozco C: Low frequency of renal function impairment during one-year of therapy with tenofovir-containing regimens in the real-world: A case-control study. AIDS Patient Care STDS 19: 421–424, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Scott JD, Wolfe PR, Bolan RK, Guyer B: Serious renal impairment occurs rarely with use of tenofovir DF. HIV Clin Trials 7: 55–58, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Madeddu G, Bonfanti P, De Socio GV, Carradori S, Grosso C, Marconi P, Penco G, Rosella E, Miccolis S, Melzi S, Mura MS, Landonio S, Ricci E, Quirino T, CISAI Group : Tenofovir renal safety in HIV-infected patients: Results from the SCOLTA Project. Biomed Pharmacother 62: 6–11, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Jones R, Stebbing J, Nelson M, Moyle G, Bower M, Mandalia S, Gazzard B: Renal dysfunction with tenofovir disoproxil fumarate-containing highly active antiretroviral therapy regimens is not observed more frequently: A cohort and case-control study. J Acquir Immune Defic Syndr 37: 1489–1495, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Gallant JE, Parish MA, Keruly JC, Moore RD: Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase inhibitor treatment. Clin Infect Dis 40: 1194–1198, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Winston A, Amin J, Mallon P, Marriott D, Carr A, Cooper DA, Emery S: Minor changes in calculated creatinine clearance and anion-gap are associated with tenofovir disoproxil fumarate-containing highly active antiretroviral therapy. HIV Med 7: 105–111, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Mauss S, Berger F, Schmutz G: Antiretroviral therapy with tenofovir is associated with mild renal dysfunction. AIDS 19: 93–95, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Menezes AM, Torelly J, Jr, Real L, Bay M, Poeta J, Sprinz E: Prevalence and risk factors associated to chronic kidney disease in HIV-infected patients on HAART and undetectable viral load in Brazil. PLoS ONE 6: e26042, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortiz A, Oliveira JP, Wanner C, Brenner BM, Waldek S, Warnock DG: Recommendations and guidelines for the diagnosis and treatment of Fabry nephropathy in adults. Nat Clin Pract Nephrol 4: 327–336, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M: Age- and gender-specific reference values of estimated GFR in Caucasians: The Nijmegen Biomedical Study. Kidney Int 72: 632–637, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Scherzer R, Estrella M, Li Y, Choi AI, Deeks SG, Grunfeld C, Shlipak MG: Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS 26: 867–875, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monteagudo-Chu MO, Chang MH, Fung HB, Bräu N: Renal toxicity of long-term therapy with tenofovir in HIV-infected patients. J Pharm Pract 25: 552–559, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Cotter AG, Powderly WG: Endocrine complications of human immunodeficiency virus infection: hypogonadism, bone disease and tenofovir-related toxicity. Best Pract Res Clin Endocrinol Metab 25: 501–515, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Rosenvinge MM, Gedela K, Copas AJ, Wilkinson A, Sheehy CA, Bano G, Hay PE, Pakianathan MR, Sadiq ST: Tenofovir-linked hyperparathyroidism is independently associated with the presence of vitamin D deficiency. J Acquir Immune Defic Syndr 54: 496–499, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Childs KE, Fishman SL, Constable C, Gutierrez JA, Wyatt CM, Dieterich DT, Mullen MP, Branch AD: Short communication: Inadequate vitamin D exacerbates parathyroid hormone elevations in tenofovir users. AIDS Res Hum Retroviruses 26: 855–859, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masia M, Padilla S, Robledano C, Lopez N, Ramos JM, Gutierrez F: Early changes in parathyroid hormone concentrations in HIV-infected patients initiating antiretroviral therapy with tenofovir. AIDS Res Hum Retroviruses 28:242–246, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Di Biagio A, Rosso R, Monteforte P, Russo R, Rovetta G, Viscoli C: Whole body bone scintigraphy in tenofovir-related osteomalacia: A case report. J Med Case Reports 3: 8136, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parsonage MJ, Wilkins EG, Snowden N, Issa BG, Savage MW: The development of hypophosphataemic osteomalacia with myopathy in two patients with HIV infection receiving tenofovir therapy. HIV Med 6: 341–346, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Perrot S, Aslangul E, Szwebel T, Caillat-Vigneron N, Le Jeunne C: Bone pain due to fractures revealing osteomalacia related to tenofovir-induced proximal renal tubular dysfunction in a human immunodeficiency virus-infected patient. J Clin Rheumatol 15: 72–74, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Giola M, Basilico C, Grossi P: Fatal lactic acidosis associated with tenofovir and abacavir. Int J Infect Dis 9: 228–229, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Guo Y, Fung HB: Fatal lactic acidosis associated with coadministration of didanosine and tenofovir disoproxil fumarate. Pharmacotherapy 24: 1089–1094, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Hansen AB, Mathiesen S, Gerstoft J: Severe metabolic acidosis and renal failure in an HIV-1 patient receiving tenofovir. Scand J Infect Dis 36: 389–392, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Rivas P, Polo J, de Górgolas M, Fernández-Guerrero ML: Drug points: Fatal lactic acidosis associated with tenofovir. BMJ 327: 711, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosso R, Di Biagio A, Ferrazin A, Bassetti M, Ciravegna BW, Bassetti D: Fatal lactic acidosis and mimicking Guillain-Barré syndrome in an adolescent with human immunodeficiency virus infection. Pediatr Infect Dis J 22: 668–670, 2003 [PubMed] [Google Scholar]
- 55.Aperis G, Paliouras C, Zervos A, Arvanitis A, Alivanis P: Lactic acidosis after concomitant treatment with metformin and tenofovir in a patient with HIV infection. J Ren Care 37: 25–29, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D’Agati VD, Markowitz GS: Tenofovir nephrotoxicity: Acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int 78: 1171–1177, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Lebrecht D, Venhoff AC, Kirschner J, Wiech T, Venhoff N, Walker UA: Mitochondrial tubulopathy in tenofovir disoproxil fumarate-treated rats. J Acquir Immune Defic Syndr 51: 258–263, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez-Novoa S, Labarga P, Soriano V: Pharmacogenetics of tenofovir treatment. Pharmacogenomics 10: 1675–1685, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Ray AS, Cihlar T, Robinson KL, Tong L, Vela JE, Fuller MD, Wieman LM, Eisenberg EJ, Rhodes GR: Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother 50: 3297–3304, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kohler JJ, Hosseini SH, Green E, Abuin A, Ludaway T, Russ R, Santoianni R, Lewis W: Tenofovir renal proximal tubular toxicity is regulated by OAT1 and MRP4 transporters. Lab Invest 91: 852–858, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uwai Y, Ida H, Tsuji Y, Katsura T, Inui K: Renal transport of adefovir, cidofovir, and tenofovir by SLC22A family members (hOAT1, hOAT3, and hOCT2). Pharm Res 24: 811–815, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Izzedine H, Hulot JS, Villard E, Goyenvalle C, Dominguez S, Ghosn J, Valantin MA, Lechat P, Deray AG: Association between ABCC2 gene haplotypes and tenofovir-induced proximal tubulopathy. J Infect Dis 194: 1481–1491, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Izzedine H, Launay-Vacher V, Deray G: Antiviral drug-induced nephrotoxicity. Am J Kidney Dis 45: 804–817, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Lewis W, Day BJ, Copeland WC: Mitochondrial toxicity of NRTI antiviral drugs: An integrated cellular perspective. Nat Rev Drug Discov 2: 812–822, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Tanji N, Tanji K, Kambham N, Markowitz GS, Bell A, D’Agati VD: Adefovir nephrotoxicity: Possible role of mitochondrial DNA depletion. Hum Pathol 32: 734–740, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Côté HC, Magil AB, Harris M, Scarth BJ, Gadawski I, Wang N, Yu E, Yip B, Zalunardo N, Werb R, Hogg R, Harrigan PR, Montaner JS: Exploring mitochondrial nephrotoxicity as a potential mechanism of kidney dysfunction among HIV-infected patients on highly active antiretroviral therapy. Antivir Ther 11: 79–86, 2006 [PubMed] [Google Scholar]
- 67.Ortiz A, Justo P, Sanz A, Melero R, Caramelo C, Guerrero MF, Strutz F, Müller G, Barat A, Egido J: Tubular cell apoptosis and cidofovir-induced acute renal failure. Antivir Ther 10: 185–190, 2005 [PubMed] [Google Scholar]
- 68.Saidenberg-Kermanac’h N, Souabni L, Prendki V, Prie D, Boissier MC: Normal plasma FGF23 levels kinetic in tenofovir-related hypophosphatemic osteomalacia in an HIV-infected patient with von Recklinghausen disease. Joint Bone Spine 78: 306–308, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Libório AB, Andrade L, Pereira LV, Sanches TR, Shimizu MH, Seguro AC: Rosiglitazone reverses tenofovir-induced nephrotoxicity. Kidney Int 74: 910–918, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Rodríguez-Nóvoa S, Labarga P, D’avolio A, Barreiro P, Albalate M, Vispo E, Solera C, Siccardi M, Bonora S, Di Perri G, Soriano V: Impairment in kidney tubular function in patients receiving tenofovir is associated with higher tenofovir plasma concentrations. AIDS 24: 1064–1066, 2010 [DOI] [PubMed] [Google Scholar]
- 71.Poizot-Martin I, Solas C, Allemand JO-RBrégigeon S, Ménard A, Faucher O, Lacarelle B, Brégigeon S, Ménard A, Faucher O, Lacarelle B, editors. Renal impairment in patients receiving a tenofovir –cART regimen: impact of tenofovir concentration? Conference on Retroviruses and Opportunistic Infections (CROI); 2011; Boston. [Google Scholar]
- 72.Nishijima T, Komatsu H, Gatanaga H, Aoki T, Watanabe K, Kinai E, Honda H, Tanuma J, Yazaki H, Tsukada K, Honda M, Teruya K, Kikuchi Y, Oka S: Impact of small body weight on tenofovir-associated renal dysfunction in HIV-infected patients: A retrospective cohort study of Japanese patients. PLoS ONE 6: e22661, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zimmermann AE, Pizzoferrato T, Bedford J, Morris A, Hoffman R, Braden G: Tenofovir-associated acute and chronic kidney disease: A case of multiple drug interactions. Clin Infect Dis 42: 283–290, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Crane HM, Kestenbaum B, Harrington RD, Kitahata MM: Amprenavir and didanosine are associated with declining kidney function among patients receiving tenofovir. AIDS 21: 1431–1439, 2007 [DOI] [PubMed] [Google Scholar]
- 75.Goicoechea M, Liu S, Best B, Sun S, Jain S, Kemper C, Witt M, Diamond C, Haubrich R, Louie S, California Collaborative Treatment Group 578 Team : Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis 197: 102–108, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Cihlar T, Ray AS, Laflamme G, Vela JE, Tong L, Fuller MD, Roy A, Rhodes GR: Molecular assessment of the potential for renal drug interactions between tenofovir and HIV protease inhibitors. Antivir Ther 12: 267–272, 2007 [PubMed] [Google Scholar]
- 77.Kiser JJ, Carten ML, Aquilante CL, Anderson PL, Wolfe P, King TM, Delahunty T, Bushman LR, Fletcher CV: The effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clin Pharmacol Ther 83: 265–272, 2008 [DOI] [PubMed] [Google Scholar]
- 78.Ray AS, Wright MR, Rhodes GR: Lack of evidence for an effect of lopinavir/ritonavir on tenofovir renal clearance. Clin Pharmacol Ther 84: 660–, author reply 661., 2008 [DOI] [PubMed] [Google Scholar]
- 79.Buchacz K, Young B, Baker RK, Moorman A, Chmiel JS, Wood KC, Brooks JT: Renal function in patients receiving tenofovir with ritonavir/lopinavir or ritonavir/atazanavir in the HIV Outpatient Study (HOPS) cohort. J Acquir Immune Defic Syndr 43: 626–628, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Vidal F, Domingo JC, Guallar J, Saumoy M, Cordobilla B, Sánchez de la Rosa R, Giralt M, Alvarez ML, López-Dupla M, Torres F, Villarroya F, Cihlar T, Domingo P: In vitro cytotoxicity and mitochondrial toxicity of tenofovir alone and in combination with other antiretrovirals in human renal proximal tubule cells. Antimicrob Agents Chemother 50: 3824–3832, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodríguez-Nóvoa S, Labarga P, Soriano V, Egan D, Albalater M, Morello J, Cuenca L, González-Pardo G, Khoo S, Back D, Owen A: Predictors of kidney tubular dysfunction in HIV-infected patients treated with tenofovir: A pharmacogenetic study. Clin Infect Dis 48: e108–e116, 2009 [DOI] [PubMed] [Google Scholar]
- 82.Kiser JJ, Aquilante CL, Anderson PL, King TM, Carten ML, Fletcher CV: Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J Acquir Immune Defic Syndr 47: 298–303, 2008 [DOI] [PubMed] [Google Scholar]
- 83.Gupta SK, Eustace JA, Winston JA, Boydstun II, Ahuja TS, Rodriguez RA, Tashima KT, Roland M, Franceschini N, Palella FJ, Lennox JL, Klotman PE, Nachman SA, Hall SD, Szczech LA: Guidelines for the management of chronic kidney disease in HIV-infected patients: Recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 40: 1559–1585, 2005 [DOI] [PubMed] [Google Scholar]
- 84.European Association For The Study Of The Liver : EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 57: 167–185, 2012 [DOI] [PubMed] [Google Scholar]
- 85.Lake JE, Adams JS: Vitamin D in HIV-infected patients. Curr HIV/AIDS Rep 8:133–141, 2011 [DOI] [PMC free article] [PubMed]
- 86.Morelle J, Labriola L, Lambert M, Cosyns JP, Jouret F, Jadoul M: Tenofovir-related acute kidney injury and proximal tubule dysfunction precipitated by diclofenac: A case of drug-drug interaction. Clin Nephrol 71: 567–570, 2009 [DOI] [PubMed] [Google Scholar]
- 87.Psevdos G, Jr, Gonzalez E, Sharp V: Acute renal failure in patients with AIDS on tenofovir while receiving prolonged vancomycin course for osteomyelitis. AIDS Read 19: 245–248, 2009 [PubMed] [Google Scholar]
- 88.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Eron JJ, Günthard HF, Hammer SM, Reiss P, Richman DD, Rizzardini G, Thomas DL, Jacobsen DM, Volberding PA: Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA 308: 387–402, 2012 [DOI] [PubMed] [Google Scholar]