Abstract
Reactive oxygen species (ROS) play an important role in normal cellular physiology. They regulate different biologic processes such as cell defense, hormone synthesis and signaling, activation of G protein-coupled receptors, and ion channels and kinases/phosphatases. ROS are also important regulators of transcription factors and gene expression. On the other hand, in pathologic conditions, a surplus of ROS in tissue results in oxidative stress with various injurious consequences such as inflammation and fibrosis. NADPH oxidases are one of the many sources of ROS in biologic systems, and there are seven isoforms (Nox1–5, Duox1, Duox2). Nox4 is the predominant form in the kidney, although Nox2 is also expressed. Nox4 has been implicated in the basal production of ROS in the kidney and in pathologic conditions such as diabetic nephropathy and CKD; upregulation of Nox4 may be important in renal oxidative stress and kidney injury. Although there is growing evidence indicating the involvement of NADPH oxidase in renal pathology, there is a paucity of information on the role of NADPH oxidase in the regulation of normal renal function. Here we provide an update on the role of NADPH oxidases and ROS in renal physiology and pathology.
Reactive oxygen species (ROS) produced by NADPH oxidases are implicated in many physiologic and pathophysiologic processes. NADPH oxidase catalyzes the transfer of electrons from NADPH to molecular oxygen, through the Nox catalytic subunit, to produce ROS. This mechanism distinguishes NADPH oxidases from other oxidases in which ROS production occurs either as a by-product of another oxidative reaction such as the mitochondrial electron transport chain or from a dysfunctional variant of the parent enzyme such as xanthine dehydrogenase → xanthine oxidase.1 The classic NADPH oxidase is gp91phox, also called Nox2, which is found primarily in phagocytic cells. Seven NADPH oxidase isoforms have been identified (Nox1–5, Duox1, Duox2). The isoforms differ in their use of the catalytic Nox subunit as well as in their Nox-binding proteins, tissue distribution, intracellular localization, ROS formation pattern, and regulation.2,3
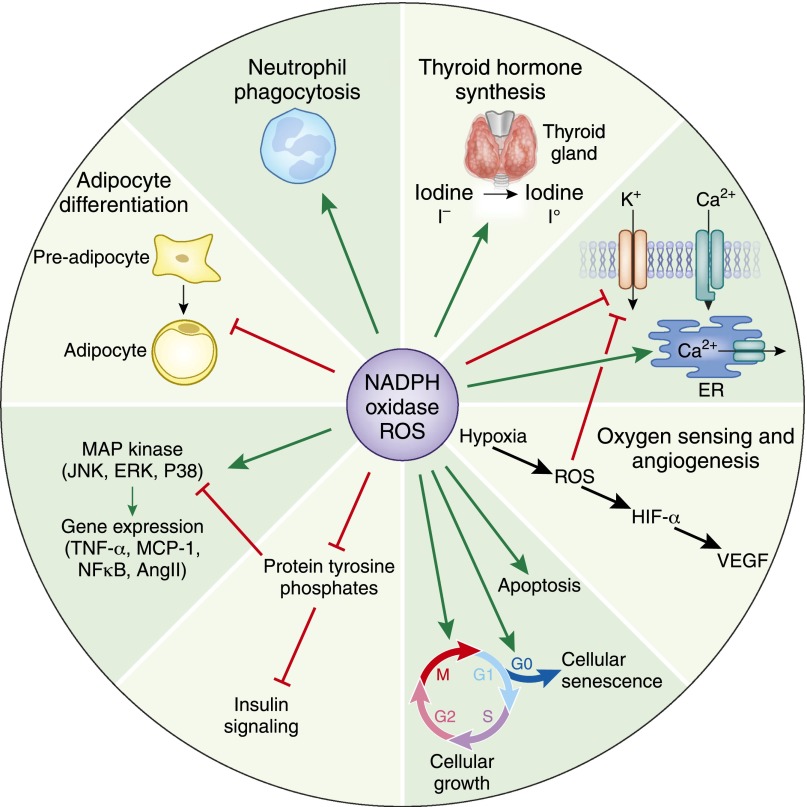
The family of NADPH oxidases consists of Nox1, Nox2 (gp91phox), Nox3, Nox4, Nox5, Duox1, and Duox2.4 The catalytic subunits of Nox1, Nox2, Nox3, and Nox4 isoforms depend on p22 phox, a common subunit required for the activity of the enzyme. On the other hand, Nox5, Duox1, and Duox2 are p22 phox-independent isoforms; they possess an additional peroxidase domain and are calcium dependent.5 NADPH oxidase-derived ROS play an important role in cell signaling as second messengers,6 mediating hormonal effects,7,8 regulation of ion channel activity, oxygen sensing,9 adipocyte differentiation,10 gene expression,6,11 reproduction,12 cell growth, senescence, and apoptosis (Figure 1).13,14
Figure 1.
Physiologic role of NADPH oxidase-derived ROS. Green arrows indicate activation, and red lines with blunted ends indicate inhibition. NoxS, renal NADPH oxidases; VEGF, vascular endothelial growth factor; HIF, hypoxia inducible factor; ER, endoplasmic reticulum; MCP-1, monocyte chemotactic protein-1; MAP kinase, mitogen activated protein kinase; JNK, c-Jun N-terminal kinase 1; ERK, extracellular signal-regulated kinases; P38, p38 kinase.
NADPH Oxidases and the Kidney
In the kidney, NADPH oxidases are expressed in a regional and cell-specific manner. The localization of different renal Nox isoforms and their subunits as well as their distribution in nephron segments were previously extensively reviewed15,16 and are not further discussed here. Of the Nox isoforms, Nox4 is abundantly expressed in the kidney and is an important source of renal ROS. Other NADPH oxidases, such as Nox1 and Nox2, have also been identified in the kidney, but their functional significance remains unclear. Nox-derived ROS are implicated in physiologic processes of the kidney, including gluconeogenesis, glucose transport, tubuloglomerular feedback, hemodynamics, and electrolyte transport. This review discusses each of these topics, relative to NADPH oxidases in the kidney.
NADPH Oxidases, Renal Gluconeogenesis, and Glucose Transport
ROS generated by NADPH oxidases regulate renal glucose production and glucose handling. The kidney contributes to glucose homeostasis by producing glucose from glutamine (gluconeogenesis) in proximal tubular cells, and by reabsorbing filtered glucose into the blood. The amount of glucose released to the circulation through renal gluconeogenesis is almost equal to that produced by the liver (20%–25% versus 25%–30%).17,18 This amount is increased in patients with type II diabetes due to the lack of the inhibitory effect of insulin on gluconeogenic hormones.19 Winiarska et al.20 showed that pretreatment with apocynin (an NADPH oxidase inhibitor and ROS scavenger) attenuated proximal tubular cell gluconeogenesis in diabetic rabbits, suggesting that NADPH oxidases and ROS could be targeted to maintain glucose balance in the diabetic kidney.
NADPH oxidase also regulates glucose transport across the kidney tubules, including Na+/K+-ATPase–dependent transport of glucose (sodium/glucose cotransporters [SGLTs]) across the brush border of the proximal tubules.21 Although Gerich19 reported increased SGLT2 activity in patients with diabetes, Han et al.21 observed reduced activity of SGLTs in response to high glucose in rabbit proximal tubular cells that was blocked by pretreatment with apocynin, indicating a NADPH oxidase/redox-sensitive mechanism. This discrepancy may be due to an adaptive response of SGLTs in vivo to prolonged hyperglycemia. Taken together, these data indicate that NADPH oxidase is involved in the regulation of blood glucose levels, especially during diabetes.
NADPH Oxidases, Tubuloglomerular Feedback Mechanisms, and Renal Hemodynamics
Tubuloglomerular feedback is an important mechanism by which the kidney regulates its hemodynamics in response to changes in renal tubular salt load. Superoxide anion (· O2−) enhances tubuloglomerular feedback responses both directly by constricting the afferent arteriole and indirectly by scavenging nitric oxide in the macula densa.22 Single-cell RT-PCR demonstrated that Nox2 and Nox4, but not Nox1, are expressed in the rat macula densa. Silencing Nox2 but not Nox4 in a mouse macula densa cell line (MMDD1) inhibited high salt induced superoxide production. Moreover, the addition of apocynin reduced superoxide production in response to high salt, suggesting a role for NADPH oxidase, but not xanthine oxidase or cyclooxygenase-2, as a source of ROS in macula densa cells.23 Liu et al.24,25 observed that high salt induces sodium/hydrogen exchangers (NHEs) in the macula densa, resulting in increased intracellular pH and depolarization of macula densa cells and, consequently, in activation of Nox-induced superoxide production. This effect was blocked by Tempol, a SOD inhibitor, and by apocynin, suggesting that NADPH oxidases and ROS are involved in the regulation of macula densa pH in response to high salt. These data highlight the potential importance of NADPH oxidases in the regulation of tubuloglomerular feedback and macula densa responses to high salt.
In addition to influencing tubuloglomerular feedback, NADPH oxidases also affect afferent arteriolar autoregulatory responses. For instance, Nox-derived ROS impaired juxtamedullary afferent arteriolar autoregulation in response to TGF-β stimulation. Apocynin restored the afferent arteriolar autoregulatory response.26 Both Nox2 and Nox4, but not Nox1, are expressed in renal resistance arteries; in addition, approximately 60% of superoxide formed is due to NADPH oxidase activation, which impairs endothelium-dependent vasodilation in elderly individuals.27 Similarly, Carlström et al.28 reported the role of Nox2-derived ROS in the regulation of afferent arteriolar tone and impaired endothelial dependent vasodilation. These observations emphasize the role played by NADPH oxidase-derived ROS in the regulation of renal hemodynamics and, consequently, in systemic BP.
NADPH Oxidases and Electrolyte Transport
NADPH oxidase regulates ion transport in different segments along the nephron, including the proximal tubule, thick ascending limb (TAL), and collecting duct. In the proximal tubule, Panico et al.29 showed that ROS blocked NHE activity; pretreatment with apocynin abrogated the effect of ROS on NHE in nephrons from spontaneously hypertensive rats (SHRs). These data suggest that increased NADPH oxidase activity in SHRs may be an adaptive mechanism to reduce salt and water reabsorption in an already hypertensive animal. These findings also suggest that NADPH oxidases regulate fluid uptake in the proximal convoluted tubules through inhibition of NHEs.
Another important regulator of renal fluid reabsorption is renal proximal tubular Na+/K+-ATPase. Expressed on the basolateral membranes, these channels are the driving force for Na+ and fluid reabsorption along the nephron. Silva and Soares-da-Silva30 examined the effect of aging on Na+/K+-ATPase in opossum kidney cells. They observed increased expression and activity of Na+/K+-ATPase in aged, highly passaged cells. This was associated with increased Nox1, SOD1, SOD2, SOD3, and accumulation of H2O2. Preincubation with apocynin resulted in reduced H2O2 levels and reduced Na+/K+-ATPase activity. Similarly, angiotensin II (AngII)–stimulated Na+/K+-ATPase activity in SHRs was attenuated by NADPH oxidase inhibition, partly mediated by a reduction in nitric oxide availability.31 These data support a role for NADPH oxidase–derived ROS in proximal tubular Na+/K+-ATPase regulation, which may affect BP regulation, especially in relation to high salt, AngII, and aging.
In addition to regulating tubular Na+/K+-ATPase, ROS affect sodium transport by Na+/K+/2Cl− cotransporters in the TAL of the loop of Henle. Silva and Garvin32 showed that ROS is increased by loop fluid NaCl, and this effect was inhibited by Na+/K+/2Cl− cotransporter inhibitors and by apocynin. These data allude to a possible interaction between Na+/K+/2Cl− channels and NADPH oxidases in the regulation of electrolyte transport at the TAL of the loop of Henle. Superoxide production was also stimulated by an increased urine flow rate in the TAL, and was similarly inhibited by apocynin or p47phox (NADPH oxidase subunit) deficiency in mice. Stimulation of protein kinase C (PKC) mimicked the effect of an increased flow rate on superoxide production and was inhibited by apocynin. These findings suggest that an increased flow rate in the TAL stimulates PKC, which in turn induces activation of p47phox-dependent NADPH oxidase, resulting in increased superoxide production and sodium reabsorption in the thick limb.33
The role of NADPH oxidase in electrolyte transport in collecting ducts was also investigated. Application of losartan (an AngII type 1 receptor blocker), diphenyleneiodonium chloride (a nonspecific inhibitor of NADPH oxidase), or a PKC inhibitor in rat cortical collecting ducts led to inhibition of the AngII-stimulated epithelial sodium channel (ENaC). In contrast, PKC activators and superoxide donors (xanthine and xanthine oxidase) increased ENaC activity, suggesting that the effect of AngII on the ENaC is mediated in part by ROS produced by PKC-dependent NADPH oxidase.34 Mamenko et al.35 also showed that the effect of AngII on ENaC channel activation was mediated by activation of NADPH oxidase in the mouse split-open distal nephron. Both studies confirmed a regulatory role of NADPH oxidase-derived ROS on ENaC activities and highlight the putative role of NADPH oxidase activation in mediating hypertension in response to increased intrarenal renin-angiotensin system activation.
NADPH oxidase also regulates potassium transport in the collecting duct. For instance, low potassium intake increased superoxide production in the renal cortex, which was associated with a reduction in renal outer medullary potassium channel (ROMK) activity and consequently decreased K+ excretion in the rat cortical collecting duct. The administration of Tempol to rats on a low K+ diet resulted in reduced cortical superoxide levels and increased ROMK activity.36 In addition, low potassium intake resulted in decreased superoxide production, increased ROMK staining in renal cortex, and increased urinary potassium levels in gp91phox null mice. The effect of gp91phox deletion was mimicked by the administration of Tempol to wild-type mice on a low potassium diet.37 These results are supported by the findings of Wei et al.,38 who observed that NAPDH oxidase mediated the effect of AngII on the reduction of ROMK channel activity in rats treated with a low potassium diet. Potassium restriction also inhibited protein phosphatase 2B (PP2B) in rat and mouse kidney; suppression of PP2B decreased ROMK channel activity in the cortical collecting duct. Deletion of gp91phox prevented low K+ intake–mediated reduction of PP2B.39 Although it seems that increased ROS production in response to a low potassium diet may be an adaptive mechanism to decrease potassium loss, none of the aforementioned studies investigated the effect of potassium restriction on BP, which is a critical component of these experiments. However, together these findings highlight the important role of NADPH oxidase in potassium homeostasis.
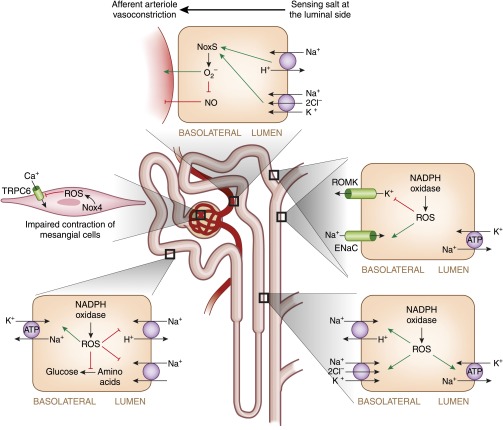
A summary of the putative role of NADPH oxidase in different segments of the nephron is illustrated in Figure 2.
Figure 2.
The role of NADPH oxidase along the nephron. Green arrows indicate activation, red lines with blunted ends indicate inhibition, and black lines indicate the direction of reactions. TRPC6, transient receptor potentials calcium-permeable cation channels; Na+, sodium ions, K+, potassium ions; H+, hydrogen ions; Cl−, chloride ions; Ca2+, calcium ions.
NADPH Oxidases and Kidney Disease—Focus on Nox2 and Nox4
Of the seven Nox isoforms, Nox4 has been best characterized in renal pathology; however, there is some evidence suggesting that Nox2 may also play a role. The renal expression of Nox2 is altered in various mouse models of diabetes. Leptin receptor–deficient db/db mice (type II diabetes) have reduced Nox2 gene expression in the kidney cortex compared with their heterozygous littermates.11 However, Fukuda et al.40 demonstrated upregulation of Nox2 in the kidney cortex of C57BL/KsJ diabetic mice compared with heterozygous nondiabetic mice. They showed an additive effect of candesartan and pioglitazone, a peroxisome proliferator–activated receptor γ agonist, on Nox2 inhibition in the heart, aorta, and kidney. Downregulation of Nox2 was accompanied by increased SOD expression, decreased oxidative stress, improved glucose tolerance, and reduced renal and cardiac fibrosis. These data suggest a possible new strategy in combining peroxisome proliferator–activated receptor γ agonists with AngII receptor blockers for the treatment of diabetic complications40 through ROS inhibition. In further support of a role for Nox2 and AngII in diabetic nephropathy, type I diabetic Akita(Ins2WT/C96Y) mice showed higher Nox2 gene expression in the kidney cortex.41 The elevated level of renal Nox2 was downregulated in mice pretreated with human recombinant angiotensin converting enzyme 2. This response was also associated with reduced renal oxidative stress, urinary albumin, and renal fibrosis, as well as decreased systolic BP. In streptozotocin-treated mice, the Nox2 gene is upregulated in the kidneys.42–44 Taken together, these data indicate that Nox2 may be involved in the development of renal oxidative stress during diabetes and, consequently, in the development of diabetic nephropathy.
Renal Nox4 has been implicated in the pathophysiology of diabetic nephropathy. Findings from our laboratory and others showed increased Nox4 expression in mouse proximal tubular cells exposed to high glucose as well as increased fibronectin and TGF-β expression.11 Nox4 siRNA or treatment with a Nox1/4 inhibitor (GKT136901) was able to block the high glucose effect on renal fibronectin deposition.11 In diabetic mice treated with the Nox1/4 inhibitor, albuminuria was attenuated despite persistent hyperglycemia, suggesting that the Nox1/4 inhibitor improves renal function in experimental diabetes independently of glucose control.45
Graham et al.46 showed that ROS produced by Nox4 in response to high glucose decreased the expression of TRPC6 receptors in rat mesangial cells. TRPC6s are canonical transient receptor potentials Ca2+-permeable cation channels that play a pivotal role in regulating Ca2+ signaling in a variety of cell types, including mesangial cells, suggesting that increased Nox4-mediated ROS plays a role in impaired mesangial cell contractility and results in impaired glomerular hemodynamics during diabetes. To further address the role of Nox4 in mediating the damaging effect of hyperglycemia during diabetes, Fujii et al.47,48 showed a reduction in the signs of diabetic nephropathy in mice treated with bilirubin, biliverdin (antioxidants), or pitavastatin (statin) through downregulation of the Nox4 protein and Nox4 gene in db/db mice.
The role of Nox4 upregulation in the induction of renal damage was also described in C57BL/6J mice treated with cisplatin. These mice exhibit upregulation of the renal Nox4 gene, renal fibrosis, and functional deterioration, associated with increased oxidative stress. The Nox4 gene expression induced by cisplatin was attenuated by both deletion of cannabinoid receptor type 1 (CB1) or by pretreatment with a CB1 antagonist, suggesting that cisplatin induces its nephrotoxic effect through the CB1 receptor linked to Nox4.49 Such findings shed light on possible involvement of Nox4 in obesity-induced nephropathy because increased circulating endocannabinoids may play an important role in inducing CB1 receptors and Nox4 expression and, consequently, in increasing renal oxidative stress and damage.
Cuevas et al.44 investigated the mechanism whereby stimulation of dopamine type 2 receptors ameliorates the development of hypertension in mice. Their results showed that the BP-lowering effect of dopamine type 2 receptors was mediated by downregulation of renal Nox4 expression and activity as well as reduced ROS production. These results support a role for Nox4 activation in the development of renal complications and hypertension.
Although advances have been made in the characterization of renal Nox isoforms and mechanisms of ROS generation, the exact roles of NADPH oxidase–derived ROS and specific Nox isoforms in the kidney still remain unclear. It is becoming increasingly apparent that Nox and ROS are involved in the regulation of renal hemodynamics and renal ion transport. ROS also mediate the effects of numerous pathologic stimuli, such as hyperglycemia and AngII, on renal structure and function. Through actions on sodium reabsorption and on renal hemodynamics, ROS could be a key regulator of BP in different pathologic conditions associated with increased NADPH oxidase activity in the kidney. Growing evidence indicates a role for Nox4 in renal fibrosis and development of diabetic nephropathy, at least in experimental models. Accordingly, there has been much interest in advancing strategies to selectively inhibit Nox4 as a potential target in the treatment of kidney disease in diabetes. Despite the developments in renal Nox biology, there is still a paucity of information on the exact physiologic and pathologic roles of NADPH oxidases in the kidney and the clinical significance of these systems in humans remains unclear. Improved research tools and new experimental mouse models should advance the field.
Disclosures
None.
Acknowledgments
The studies performed by the authors were supported by grants from the Juvenile Diabetes Research Foundation, the Canadian Institutes for Health Research, and the Kidney Foundation of Canada/Pfizer. M.S. was supported by a fellowship through the KRESCENT program of the Kidney Foundation of Canada.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Opitz N, Drummond GR, Selemidis S, Meurer S, Schmidt HH: The ‘A’s and ‘O’s of NADPH oxidase regulation: A commentary on “Subcellular localization and function of alternatively spliced Noxo1 isoforms”. Free Radic Biol Med 42: 175–179, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD: Homologs of gp91phox: Cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD: Cell transformation by the superoxide-generating oxidase Mox1. Nature 401: 79–82, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Kawahara T, Quinn MT, Lambeth JD: Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol 7: 109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Touyz RM, Briones AM, Sedeek M, Burger D, Montezano AC: NOX isoforms and reactive oxygen species in vascular health. Mol Interv 11: 27–35, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Ha H, Lee HB: Reactive oxygen species as glucose signaling molecules in mesangial cells cultured under high glucose. Kidney Int Suppl 77: S19–S25, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Ohye H, Sugawara M: Dual oxidase, hydrogen peroxide and thyroid diseases. Exp Biol Med (Maywood) 235: 424–433, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Goldstein BJ, Mahadev K, Wu X, Zhu L, Motoshima H: Role of insulin-induced reactive oxygen species in the insulin signaling pathway. Antioxid Redox Signal 7: 1021–1031, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaelin WG, Jr: ROS: Really involved in oxygen sensing. Cell Metab 1: 357–358, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Mouche S, Mkaddem SB, Wang W, Katic M, Tseng YH, Carnesecchi S, Steger K, Foti M, Meier CA, Muzzin P, Kahn CR, Ogier-Denis E, Szanto I: Reduced expression of the NADPH oxidase NOX4 is a hallmark of adipocyte differentiation. Biochim Biophys Acta 1773: 1015–1027, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, Hébert RL: Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: Implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299: F1348–F1358, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Bedard K, Krause KH: The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Buetler TM, Krauskopf A, Ruegg UT: Role of superoxide as a signaling molecule. News Physiol Sci 19: 120–123, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Shen HM, Pervaiz S: Reactive oxygen species in cell fate decisions biomedical and life sciences. In: Essentials of Apoptosis, New York, Humana Press, 2009, pp 199–221
- 15.Gill PS, Wilcox CS: NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Nistala R, Whaley-Connell A, Sowers JR: Redox control of renal function and hypertension. Antioxid Redox Signal 10: 2047–2089, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC: Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest 98: 378–385, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerich JE: Physiology of glucose homeostasis. Diabetes Obes Metab 2: 345–350, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Gerich JE: Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: Therapeutic implications. Diabet Med 27: 136–142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winiarska K, Grabowski M, Rogacki MK: Inhibition of renal gluconeogenesis contributes to hypoglycaemic action of NADPH oxidase inhibitor, apocynin. Chem Biol Interact 189: 119–126, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Han HJ, Lee YJ, Park SH, Lee JH, Taub M: High glucose-induced oxidative stress inhibits Na+/glucose cotransporter activity in renal proximal tubule cells. Am J Physiol Renal Physiol 288: F988–F996, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Liu R, Ren Y, Garvin JL, Carretero OA: Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int 66: 268–274, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Zhang R, Harding P, Garvin JL, Juncos R, Peterson E, Juncos LA, Liu R: Isoforms and functions of NAD(P)H oxidase at the macula densa. Hypertension 53: 556–563, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu R, Garvin JL, Ren Y, Pagano PJ, Carretero OA: Depolarization of the macula densa induces superoxide production via NAD(P)H oxidase. Am J Physiol Renal Physiol 292: F1867–F1872, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Liu R, Carretero OA, Ren Y, Wang H, Garvin JL: Intracellular pH regulates superoxide production by the macula densa. Am J Physiol Renal Physiol 295: F851–F856, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma K, Cook A, Smith M, Valancius C, Inscho EW: TGF-β impairs renal autoregulation via generation of ROS. Am J Physiol Renal Physiol 288: F1069–F1077, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Schlüter T, Zimmermann U, Protzel C, Miehe B, Klebingat KJ, Rettig R, Grisk O: Intrarenal artery superoxide is mainly NADPH oxidase-derived and modulates endothelium-dependent dilation in elderly patients. Cardiovasc Res 85: 814–824, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Carlström M, Lai EY, Ma Z, Patzak A, Brown RD, Persson AE: Role of NOX2 in the regulation of afferent arteriole responsiveness. Am J Physiol Regul Integr Comp Physiol 296: R72–R79, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Panico C, Luo Z, Damiano S, Artigiano F, Gill P, Welch WJ: Renal proximal tubular reabsorption is reduced in adult spontaneously hypertensive rats: Roles of superoxide and Na+/H+ exchanger 3. Hypertension 54: 1291–1297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva E, Soares-da-Silva P: Reactive oxygen species and the regulation of renal Na+-K+-ATPase in opossum kidney cells. Am J Physiol Regul Integr Comp Physiol 293: R1764–R1770, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Javkhedkar AA, Lokhandwala MF, Banday AA: Defective nitric oxide production impairs angiotensin II-induced Na-K-ATPase regulation in spontaneously hypertensive rats. Am J Physiol Renal Physiol 302: F47–F51, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Silva GB, Garvin JL: Rac1 mediates NaCl-induced superoxide generation in the thick ascending limb. Am J Physiol Renal Physiol 298: F421–F425, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong NJ, Silva GB, Garvin JL: PKC-alpha mediates flow-stimulated superoxide production in thick ascending limbs. Am J Physiol Renal Physiol 298: F885–F891, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun P, Yue P, Wang WH: Angiotensin II stimulates epithelial sodium channels in the cortical collecting duct of the rat kidney. Am J Physiol Renal Physiol 302: F679–F687, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O: Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J Biol Chem 287: 660–671, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babilonia E, Wei Y, Sterling H, Kaminski P, Wolin M, Wang WH: Superoxide anions are involved in mediating the effect of low K intake on c-Src expression and renal K secretion in the cortical collecting duct. J Biol Chem 280: 10790–10796, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babilonia E, Lin D, Zhang Y, Wei Y, Yue P, Wang WH: Role of gp91phox -containing NADPH oxidase in mediating the effect of K restriction on ROMK channels and renal K excretion. J Am Soc Nephrol 18: 2037–2045, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei Y, Zavilowitz B, Satlin LM, Wang WH: Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J Biol Chem 282: 6455–6462, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Lin DH, Wang ZJ, Jin Y, Yang B, Wang WH: K restriction inhibits protein phosphatase 2B (PP2B) and suppression of PP2B decreases ROMK channel activity in the CCD. Am J Physiol Cell Physiol 294: C765–C773, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukuda M, Nakamura T, Kataoka K, Nako H, Tokutomi Y, Dong YF, Ogawa H, Kim-Mitsuyama S: Potentiation by candesartan of protective effects of pioglitazone against type 2 diabetic cardiovascular and renal complications in obese mice. J Hypertens 28: 340–352, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Oudit GY, Liu GC, Zhong J, Basu R, Chow FL, Zhou J, Loibner H, Janzek E, Schuster M, Penninger JM, Herzenberg AM, Kassiri Z, Scholey JW: Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes 59: 529–538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohshiro Y, Ma RC, Yasuda Y, Hiraoka-Yamamoto J, Clermont AC, Isshiki K, Yagi K, Arikawa E, Kern TS, King GL: Reduction of diabetes-induced oxidative stress, fibrotic cytokine expression, and renal dysfunction in protein kinase Cbeta-null mice. Diabetes 55: 3112–3120, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Chew P, Yuen DY, Stefanovic N, Pete J, Coughlan MT, Jandeleit-Dahm KA, Thomas MC, Rosenfeldt F, Cooper ME, de Haan JB: Antiatherosclerotic and renoprotective effects of ebselen in the diabetic apolipoprotein E/GPx1-double knockout mouse. Diabetes 59: 3198–3207, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuevas S, Zhang Y, Yang Y, Escano C, Asico L, Jones JE, Armando I, Jose PA: Role of renal DJ-1 in the pathogenesis of hypertension associated with increased reactive oxygen species production. Hypertension 59: 446–452, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sedeek M, Gutsol A, Montezano AC, Burger D, Nguyen Dinh Cat A, Kennedy CR, Burns KD, Cooper ME, Jandeleit-Dahm K, Page P, Szyndralewiez C, Heitz F, Hebert RL, Touyz RM: Renoprotective effects of a novel Nox1/4 inhibitor in a mouse model of type 2 diabetes. Clin Sci (Lond) 124: 191–202, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Graham S, Gorin Y, Abboud HE, Ding M, Lee DY, Shi H, Ding Y, Ma R: Abundance of TRPC6 protein in glomerular mesangial cells is decreased by ROS and PKC in diabetes. Am J Physiol Cell Physiol 301: C304–C315, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujii M, Inoguchi T, Maeda Y, Sasaki S, Sawada F, Saito R, Kobayashi K, Sumimoto H, Takayanagi R: Pitavastatin ameliorates albuminuria and renal mesangial expansion by downregulating NOX4 in db/db mice. Kidney Int 72: 473–480, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Fujii M, Inoguchi T, Sasaki S, Maeda Y, Zheng J, Kobayashi K, Takayanagi R: Bilirubin and biliverdin protect rodents against diabetic nephropathy by downregulating NAD(P)H oxidase. Kidney Int 78: 905–919, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Mukhopadhyay P, Pan H, Rajesh M, Bátkai S, Patel V, Harvey-White J, Mukhopadhyay B, Haskó G, Gao B, Mackie K, Pacher P: CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br J Pharmacol 160: 657–668, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]