Abstract
The pathophysiology of contrast-induced AKI (CIAKI) is incompletely understood due to the lack of an appropriate in vivo model that demonstrates reduced kidney function before administration of radiocontrast media (RCM). Here, we examine the effects of CIAKI in vitro and introduce a murine ischemia/reperfusion injury (IRI)–based approach that allows induction of CIAKI by a single intravenous application of standard RCM after injury for in vivo studies. Whereas murine renal tubular cells and freshly isolated renal tubules rapidly absorbed RCM, plasma membrane integrity and cell viability remained preserved in vitro and ex vivo, indicating that RCM do not induce apoptosis or regulated necrosis of renal tubular cells. In vivo, the IRI-based CIAKI model exhibited typical features of clinical CIAKI, including RCM-induced osmotic nephrosis and increased serum levels of urea and creatinine that were not altered by inhibition of apoptosis. Direct evaluation of renal morphology by intravital microscopy revealed dilation of renal tubules and peritubular capillaries within 20 minutes of RCM application in uninjured mice and similar, but less dramatic, responses after IRI pretreatment. Necrostatin-1 (Nec-1), a specific inhibitor of the receptor-interacting protein 1 (RIP1) kinase domain, prevented osmotic nephrosis and CIAKI, whereas an inactive Nec-1 derivate (Nec-1i) or the pan-caspase inhibitor zVAD did not. In addition, Nec-1 prevented RCM-induced dilation of peritubular capillaries, suggesting a novel role unrelated to cell death for the RIP1 kinase domain in the regulation of microvascular hemodynamics and pathophysiology of CIAKI.
Contrast-induced AKI (CIAKI) is the consensus name for what was formally called contrast-induced nephropathy or radiocontrast-induced AKI.1–3 CIAKI is a common and potentially serious complication4 after the administration of contrast media,5–7 especially in patients who are at risk for AKI, and is the most common cause of iatrogenic, inpatient, drug-induced AKI,3,8,9 with outstanding implications for patients with diabetes.1 CIAKI was recognized as the third commonest cause of hospital-acquired renal failure accounting for 11% of the cases10 even before magnetic resonance imaging contrast media were found to be associated with nephrogenic systemic fibrosis. Preclinical research thus far has failed to unravel the underlying pathophysiology of CIAKI.
Programmed cell death (PCD) was used synonymously with apoptosis until regulated necrosis (RN) was discovered.11 Apoptosis has been proposed to contribute to CIAKI12–14 and asialoerythropoietin was recently demonstrated in this context to prevent CIAKI.15 Apoptosis is a process that is characterized by the activity of caspases that cleave hundreds of intracellular proteins to ultimately cause membrane blebbing, nuclear fragmentation, and regulated cellular shrinkage as a consequence of their proteolytic activity.16,17 Within this process, caspases are capable of cleaving NFs like poly(ADP-ribose)-polymerase (PARP)-family proteins.18 PARP-1 has also been demonstrated to elicit a necrotic phenotype in kidney cells and therefore exhibits a subroutine of the RN.19,20 It was suggested that tubular cell death by caspase-3–mediated apoptosis substantially contributes to the overall pathogenesis of CIAKI,14,15 and one report investigated the activation of the cell death molecules PARP, Bad, and BIM.14 On the basis of these findings, the currently proposed model ascribes apoptosis a major pathophysiologic function in CIAKI.12,13
Apart from PARP-mediated RN, necroptosis, another RN pathway, is mediated by activation of the “necrosome” consisting of receptor-interacting protein kinases 1 and 3 (RIP1 and RIP3).11,21–23 Necroptosis involves all necrotic cellular hallmarks such as early loss of membrane integrity as well as rupture of the plasma membrane after cellular swelling. We recently described the functional relevance of both apoptosis and necroptosis in AKI.24,25
Here, we demonstrate that necrostatin-1 (Nec-1), a highly specific inhibitor of the RIP1 kinase domain, prevents CIAKI in a new and easy-to-use preclinical model for the in vivo analysis of CIAKI. Our model reliably mimics “osmotic nephrosis,” a pathologic feature that is typical of CIAKI in humans. In vitro and in vivo, we found that apoptosis is of minor pathophysiologic importance. Mechanistically, the data implicate RIP1 in the functional renal failure in vivo and provide evidence for the prevention of CIAKI by the RIP1 kinase inhibitor Nec-1 that also prevented the functional changes in the peritubular vasculature after RCM injection as demonstrated by intravital microscopy. Because of the outstanding specificity of Nec-1 that has been subject to extensive investigation,26–29 we consider it justified to conclude that a novel non-cell death role of RIP1 might account for the functional kidney failure in CIAKI. In addition, we introduce Nec-1 as a potential inhibitor of CIAKI.
Results
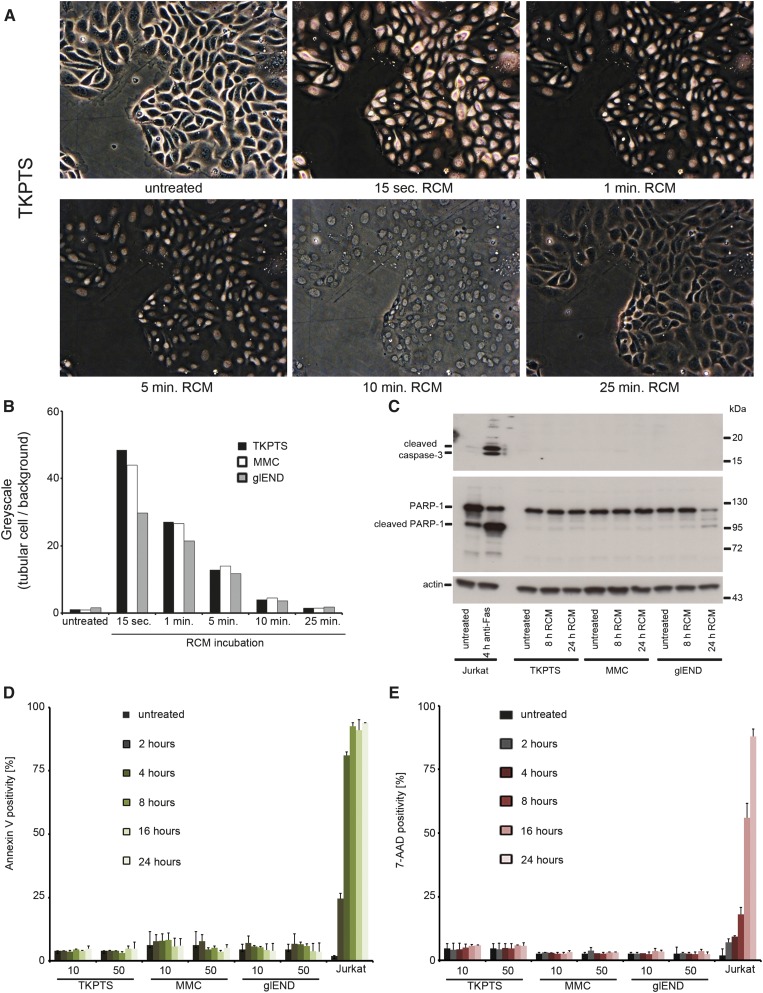
Rapid Nuclear RCM Uptake in Kidney Cell Lines Does Not Induce Cell Death In Vitro
Previous reports suggested that kidney cells undergo PCD after application of diverse RCM.12–15,30 We aimed to investigate the direct influence of clinically used standard RCM (Imeron) on renal cells and initially demonstrated the direct uptake of RCM into murine proximal tubular cells (TKPTS), mesangial cells (MMCs), and glomerular endothelial cells (glENDs) upon incubation with 50 µl RCM/ml (Figure 1A and Supplemental Figure 1). Greyscale analysis of contrast phase identified a peak RCM concentration within 15 seconds and a regular decline within 25 minutes (Figure 1B). We further investigated RCM-induced cell death by evaluation of annexin V positivity and persistence of membrane integrity (exclusion of 7-AAD) upon medium (10 µl/ml) and high (50 µl/ml) concentrations of RCM. No significant induction of cleaved caspase-3 or PARP-1 was detected in comparison with anti-Fas-treated Jurkat cells that serve as an apoptotic positive control, suggesting that classic caspase-mediated apoptosis is not initiated after RCM application (Figure 1C and Supplemental Figure 2). Minimal levels of PARP1-cleavage in glENDs upon long Western blot exposure (Supplemental Figure 3A) did not correlate with any detectable cell death (Supplemental Figure 3, B and C). Consistent with this, annexin V positivity in FACS analysis was minimal (Figure 1D). To assess other necrotic-type cell death modalities, we applied 7-AAD that was excluded over time in all renal cell lines (Figure 1E) at both 10 and 50 µl/ml RCM. However, in accordance with previously published data13–15 and Supplemental Figure 3A, very high concentrations of RCM (250 µl/ml) did result in some annexin V positivity after 24 hours (Supplemental Figure 4A). With 400 µl RCM/ml, we found an increase in PARP-1 cleavage that was unaffected by the addition of the caspase-8 inhibitor zIETD, the pan-caspase inhibitor zVAD, TAT-crmA (a previously published fusion protein that utilized the viral caspase-8 inhibitor31,32), or the cyclophilin D inhibitor cyclosporin A (Supplemental Figure 4B). We conclude that only doses that are high enough to induce artifacts lead to significant amounts of cell death even when applied in cell culture for 24 hours or longer.
Figure 1.
Regular doses of RCM do not induce cell death in TKPTS cells, MMCs, and glENDs. (A) TKPTS cells are left untreated or are treated with 50 µl/ml standard RCM (Imeron) for the indicated time points. Rapid nuclear RCM uptake is visualized by light microscopy followed over a period of 25 minutes. (B) Greyscale quantification of A and Supplemental Figure 1, A and B, after application of RCM. (C) TKPTS cells, MMCs, and glENDs are left untreated or treated with 50 µl/ml RCM for the indicated time periods. Jurkat cells serve as positive controls for induction of apoptosis after stimulation with 100 ng/ml anti-Fas mAb. The classic apoptosis markers cleaved caspase-3 (upper blot) and full-length PARP-1 versus cleaved PARP-1 (central blot) indicate the induction of apoptosis in Jurkat cells, but not in any of the remaining cell lines tested. GAPDH serves as a loading control. (D and E) Cells are stimulated as in C for the indicated time periods. Positivity for the apoptosis-marker annexin V (D) and the necrosis marker 7-AAD (E) are depicted. Again, Jurkat cells serve as a positive control that is known to undergo secondary necrosis 8 hours after induction of apoptosis. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
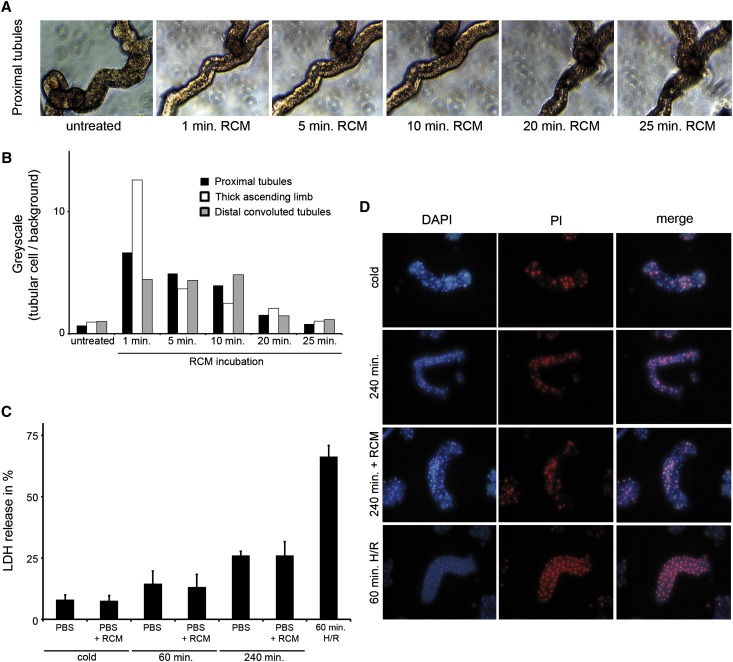
RCM Do Not Induce Cell Death in Freshly Isolated Renal Tubules
To transfer our in vitro data into an ex vivo setting, we treated freshly isolated proximal tubule segments with RCM. Comparable to the results in TKPTS cells, epithelial cell nuclei in the proximal tubule segments rapidly took up contrast media (Figure 2A). Similar results were obtained for thick ascending limb segments and segments from the distal convoluted tubules (Supplemental Figure 5). Greyscale analysis revealed similar RCM uptake kinetics in all tubular segments investigated (Figure 2B). Given the rapid direct RCM uptake, we investigated RCM-induced cell death as measured by lactate dehydrogenase (LDH) release (Figure 2C). Tubules treated for 60 minutes with hypoxia followed by 60 minutes of reoxygenation served as a positive control. As expected, LDH release increased over time in the isolated tubules, but no further increase in LDH release was detected when RCM was added, despite the use of high concentration (100 µl/ml). Accordingly, and in line with the finding from TKPTS cells in Figure 1E, positivity for propidium iodide in the tubules increased over time without further increases caused by RCM (Figure 2D). Similarly, no significant changes were detected by Western blotting regarding cleaved caspase-3 and PARP-1 (Supplemental Figure 6). From these data, we conclude that the minimal amount of RCM-induced cell death does not provide a convincing pathophysiologic concept to explain organ failure in CIAKI. To functionally address this question, we developed a new model for the analysis of CIAKI in vivo.
Figure 2.
Regular doses of RCM do not induce cell death in freshly isolated primary renal tubules. (A) Primary murine proximal renal tubules are left untreated or are treated with 50 µl/ml RCM for the indicated time points. RCM uptake into the tubular compartment is visualized by light microscopy and followed over a period of 25 minutes. (B) Greyscale quantification of A and Supplemental Figure 3, A and B, after application of RCM. (C) Primary isolated murine renal tubules are left untreated (cold) or treated with 100 ml/ml RCM for the indicated time periods. Tubules incubated in hypoxia for 60 minutes followed by 60 minutes of reoxygenation serve as positive controls. LDH release is measured and is shown as the percentage of overall LDH present in the whole tubules. (D) Primary renal tubules treated as in C are stained for positivity of propidium iodide. No statistically significant difference is observed in untreated versus RCM-treated tubules after 240 minutes of incubation. DAPI, 4',6-diamidino-2-phenylindole; PI, propidium iodide.
An Ischemia/Reperfusion Injury–Based Model System Allows Investigation of CIAKI
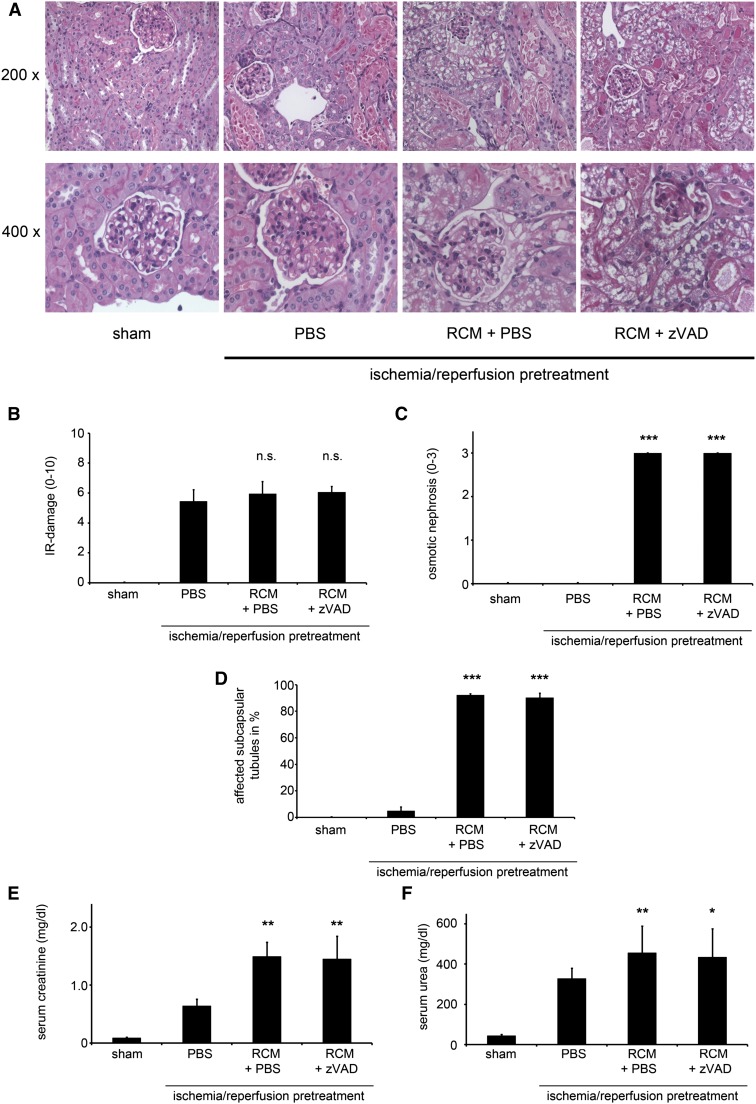
In humans, several studies have reported osmotic nephrosis as a typical morphologic feature that accompanies renal failure in CIAKI.33–35 With regard to serum parameters and histologically, however, doses as high as 250 µl RCM injected intravenously did not result in any detectable alterations 24 hours after application (Supplemental Figure 7). In previously published reports,15,30 CIAKI only develops if the GFR is reduced before RCM application. This previous work utilized water deprivation, unilateral nephrectomy, indomethacin, and N-nitro-l-arginine methyl ester.30,36 We therefore treated unilaterally nephrectomized mice with high doses of indomethacin (up to 100 µg/kg) in addition to high doses of N-nitro-l-arginine methyl ester using concentrations up to 100 µg/kg, respectively, after 16 hours of water deprivation followed by intravenous application of 250 µl RCM. In our hands, this did not induce detectable AKI as measured by unchanged serum urea or creatinine concentrations 24 or 48 hours after injection of RCM (Supplemental Figure 8). Our subsequent approach was based on achieving the required loss of renal function through an easy-to-reproduce ischemia/reperfusion injury (IRI)–based setting. After 24 hours of reperfusion, serum urea and creatinine levels and histologic analysis of the renal damage were used to demonstrate low SDs in this system. At 24 hours after reperfusion, we analyzed various concentrations of RCM to induce CIAKI and found that 250 µl of RCM that was rapidly injected via the tail vein confers an ideal setting (Supplemental Figure 9). We subsequently used this dose to characterize the time course of CIAKI in this model for within the first 96 hours after RCM injection (Supplemental Figure 10). According to these data, we performed the following experiments with 250 µl of RCM applied via the tail vein (RCM group) and read out serum markers and histology 24 hours later (48 hours after reperfusion). These were compared with the IRI-treated mice that received 250 µl of PBS instead of contrast media (PBS group) (Figure 3, A–G). We will further refer to the difference between the RCM group and the PBS group as our model for CIAKI. In addition, we assessed the effect of volume and N-acetylcysteine application in this model and found no protective effects for these regimes (Supplemental Figure 11). As quantified in Figure 3C and to the best of our knowledge, this is the first in vivo model that reliably and closely mimics the phenotype of tubular cell osmotic nephrosis in quantifiable resolution (Supplemental Figure 12). As expected from the in vitro data, blockade of apoptosis did not influence this CIAKI model in all parameters tested (Figure 3), but also did not worsen the outcome, as would be anticipated in a purely necroptotic cell death.32
Figure 3.
Blockade of apoptosis does not protect from RCM-induced osmotic nephrosis or AKI (CIAKI). (A) Eight-week-old male C57Bl/6 mice undergo sham surgery or bilateral renal pedicle clamping 24 hours before intraperitoneal injection of either PBS or the pan-caspase inhibitor zVAD followed by intravenous injection of RCM. Renal sections stained with periodic acid–Schiff are shown at magnifications of 200-fold and 400-fold. Ischemia-reperfusion damage is not significantly altered in any of the groups (B), whereas quantification of osmotic nephrosis appears exclusively in the RCM-treated mice (C). Subcapsular tubules that are affected by osmotic nephrosis are quantified in D. CIAKI is evaluated 48 hours after reperfusion (24 hours after application of RCM) by determination of serum creatinine (E) and serum urea (F) concentrations. We will further refer to the difference between the PBS-treated and the RCM + PBS-treated group as our murine model of CIAKI. n=8 per group. *P<0.05; **P<0.01; ***P<0.001 compared with the IRI-pretreated PBS group. n.s., not statistically significant .
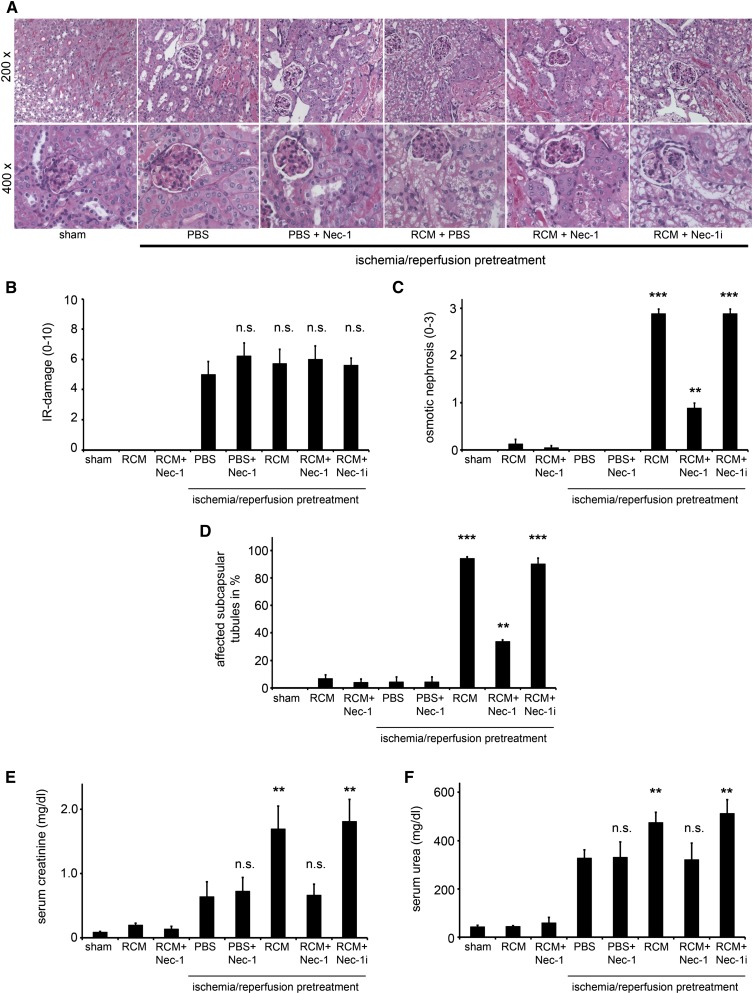
CIAKI Is Attenuated by Necrostatin-1, an Inhibitor of the Kinase Domain of Receptor-Interacting Protein Kinase-1
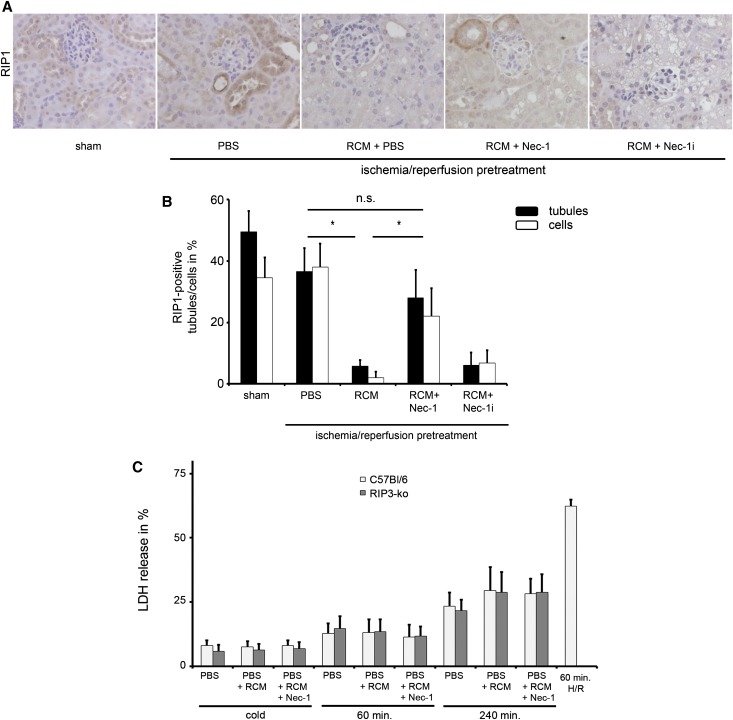
Because other forms of PCD have recently emerged,20 and renal cells preferentially appear to undergo programmed necrosis rather than apoptosis,19,25,37,38 we investigated the influence of the RIP1 inhibitor necrostatin-1 (Nec-1) in the in vivo CIAKI model. Importantly for the design, and in line with previously published data,25 application of Nec-1 24 hours after reperfusion without RCM application did not alter the amount of IRI damage (Figure 4, A and B) and had no influence on serum concentrations of urea and creatinine measured 48 hours after reperfusion; thus, effects of Nec-1 at the time 24 hours after ischemia/reperfusion (the time of RCM-administration) can be attributed to its effects on CIAKI. We found an almost complete prevention of RCM-induced osmotic nephrosis in the Nec-1–treated mice, but not in mice treated with an inactive derivate of Nec-132 called Nec-1i (Figure 4C). CIAKI-induced affection of subcapsular tubules was attenuated but not completely prevented by Nec-1 (Figure 4, D and E). Furthermore, CIAKI was markedly attenuated by Nec-1 as demonstrated by the prevention of the RCM-induced increase in serum concentrations of creatinine and urea (Figure 4, E and F). We conclude that the RIP1 kinase blocker, Nec-1, protects from CIAKI in our model.
Figure 4.
The kinase domain of RIP1 mediates osmotic nephrosis and CIAKI. (A) Eight-week-old male C57Bl/6 mice undergo sham surgery or bilateral renal pedicle clamping 24 hours before intraperitoneal injection of either PBS, the highly specific RIP1 kinase inhibitor Nec-1, or the inactive derivate of necrostatin-1 (Nec-1i) in the presence or absence of RCM. (B) Histologic quantification of renal IRI is not significantly changed in any of the groups. (C) Osmotic nephrosis in RCM-treated mice was reduced in Nec-1–treated mice, but not in those that received Nec-1i. Osmotic nephrosis in subcapsular tubules is quantified in D. Concentrations of serum creatinine (E) and serum urea (F) are evaluated 48 hours after reperfusion (24 hours after application of RCM). n=8 per group. **P<0.05; ***P<0.001 compared with the IRI-pretreated PBS group. Sham versus IRI-treated mice: P<0.001 (not indicated in E and F). n.s., not significant.
CIAKI-Induced Loss of RIP1 Positivity Is Attenuated by Nec-1
To further investigate RIP1 biology in CIAKI, we performed immunohistochemistry in sections from sham-treated and CIAKI-treated mice. RIP1 expression did not significantly change upon renal IRI. In CIAKI, however, positivity for RIP1 disappeared, and this effect was prevented by Nec-1, but not by Nec-1i (Figure 5, A and B). Because RIP1 has been well described to activate RIP3 to form the so-called necroptosome and mediate necroptosis11,20,39–41 but our in vitro results suggested that RCM do not induce cell death, we hypothesized that in CIAKI, the kinase domain of RIP1 might exert novel non-cell death function. To exclude the involvement of RIP3 in this context, we investigated RIP3-deficient freshly isolated tubules and found no significant alterations in LDH release (Figure 5C), pointing to an effect that is RIP3 independent. To gain further information about the functional pathophysiology of CIAKI in vivo, we utilized intravital microscopy (IVM).
Figure 5.
Nec-1 prevents osmotic nephrosis and CIAKI. (A and B) Eight-week-old mice undergo sham surgery or CIAKI treatment as described for Figures 3 and 4, and kidney sections are stained for immunohistochemistry of RIP1 at 400-fold magnification in the presence or absence of PBS, Nec-1, or Nec-1i as indicated (A) and RIP1-positive tubules/tubular cells are counted and quantified for each group (B) (n=6 per group). (C) LDH release of primary freshly isolated renal tubules from wild-type mice (open bars) compared with tubules from RIP3-deficient mice (gray bars) after cold preparation or 60 minutes and 240 minutes of incubation at 37°C in PBS in the presence and absence of RCM and Nec-1 as indicated. Wild-type tubules that are incubated 60 minutes in hypoxia followed by 60 minutes of reoxygenation serve as positive controls. n=8 per group. *P<0.05 compared with the IRI-pretreated PBS group. n.s., not statistically significant.
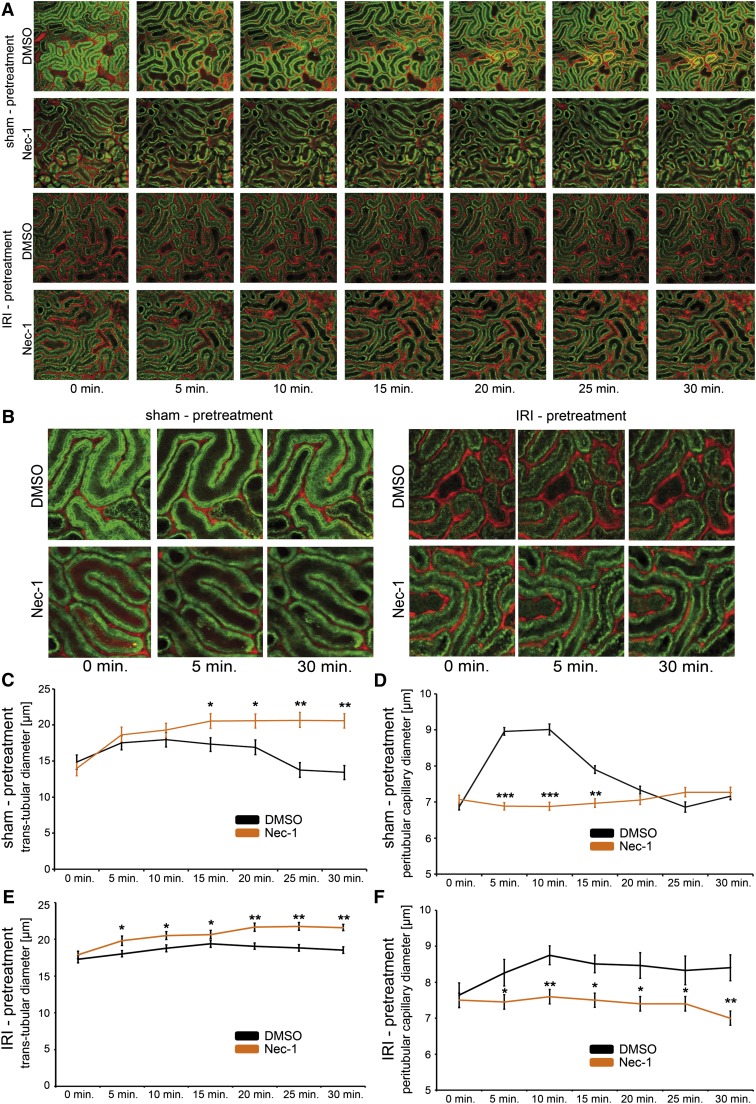
Nec-1 Induces Tubular Dilation and Affects the Kinetics of the Dilation of Peritubular Capillaries after RCM Application
The applicability of IVM to investigate AKI has recently been established by others.42,43 We utilized IVM to detect the initial events in tubules and peritubular capillaries upon RCM application in mice with an in vivo detection of renal tubules to a depth of 150 µm below the kidney capsule. Transtubular dilation after intravenous application of 250 µl RCM injection appeared throughout the first 20 minutes before diameters returned to baseline levels. Upon a single intraperitoneal application of a single dose of Nec-1 15 minutes before RCM, the return to baseline levels was prevented within the observation period. In line with this finding, we detected significantly wider tubules in the Nec-1 group after IRI pretreatment (Figure 6, A, B, C, and E). In addition, and in line with a previous report,44 RCM resulted in a significant increase in the diameter of the peritubular capillaries in the first 10 minutes (6.9 µm versus 7.9 µm, vasodilative phase) followed by a graduate decline between 10 and 25 minutes (7.9 µm versus 5.8 µm, vasoconstrictive phase). As expected upon IRI pretreatment, peritubular capillaries remained in a dilated state, but diameters remained stable and significantly different to the wild-type mice in the Nec-1 group (Figure 6, A, B, D, and E). The latter effect was also recently demonstrated for the vasa recta.45 According to the laminar flow equation of Hagen-Poiseuille, the increased blood flow might reflect the functional decrease in kidney function in CIAKI, without causing cell death, an effect that is not observed upon Nec-1 treatment. In addition, the peritubular dilation was completely abolished by intraperitoneal application of Nec-1 15 minutes before application of RCM, suggesting a functional role for RIP1 in this setting, even after IRI pretreatment. In combination with our in vitro results, these data suggested that RCM might not kill tubular cells, but rather lead to a RIP1-associated functional renal failure that involves vasodilation of the peritubular vessels, the mechanism of which remains unclear.
Figure 6.
Functional influence of RCM on peritubular capillaries is affected by Nec-1 in vivo. Eight-week-old male C57BL/6N mice receive 250 µl RCM via the tail vein 15 minutes after intraperitoneal injection of DMSO or Nec-1 with and without IRI pretreatment, as indicated. IVM is performed to detect changes in the diameters of peritubular capillaries and renal tubules. Representative images are depicted in A, and in higher magnification in B. Nec-1 prevents the return to baseline transtubular diameters after 20–25 minutes (C) and affects the dilation phase (injection to 10 minutes) in peritubular capillaries (D). Upon IRI pretreatment, application of Nec-1 leads to significantly increased transtubular diameters (E) and prevents the increase in peritubular capillary diameters that is seen in the control mice (F). Note the artificial yellow appearance of the heat-exposed area in the intravital microscope. This area is not included in the quantification of tubular diameters (n>200 per value) and peritubular capillaries (n>200 per value). t tests reveal statistical significance as depicted by the following: *P<0.05; **P<0.01; ***P<0.001.
Discussion
We previously reported that application of Nec-1 protects from renal IRI when it is applied before reperfusion.25 Mechanistically, the effect of Nec-1 on CIAKI presented in this article is completely different, and must carefully be separated from interference with necroptosis. Most importantly, addition of Nec-1 24 hours after reperfusion does not influence the course of IRI, which is a prerequisite for the present investigation in which we address three considerations that might be of interest in the context of CIAKI. First, we question the functional relevance of apoptosis, and PCD in general, in the pathogenesis of CIAKI. Second, we introduce a new and easy-to-reproduce CIAKI mouse model. Third, we provide evidence for a non-cell death function for the kinase domain of RIP1 in regulation of vascular tone and CIAKI and its inhibitor Nec-1.
Apoptosis in tubular cells has been reported after various iodide RCM in vitro such as 200 mg/ml of Iodixanol,14 320 mg/ml of Iodixanol,13 and 320 mg/ml of Ioversol.15 These agents have several limitations. First, these concentrations to our understanding are artificially high and do not mimic the clinical situation. Second, unlike RCM used in this study, those RCM are not in everyday clinical use. Third, the detection of apoptosis was performed using nonspecific terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling assays.39,46,47 Some reports also investigated caspase-3 activity, a very good marker for apoptosis, but the activity increased only by 1.4-fold.15 In typical apoptotic settings, caspase-3 activity increases by ≥10-fold.31,48,49 Here we demonstrate that apoptosis only occurs in vitro using artificially high RCM concentrations (Supplemental Figure 4), which is generally in line with some of the previously mentioned reports.13,15 In vivo, we cannot find any protection from CIAKI that is based on blockade of caspases (Figure 3) and do not find any significant amount of cell death that might provide a concept to explain AKI.
A preclinical model for CIAKI should meet three major criteria. First, it must not be a lethal model to be comparable to clinically relevant CIAKI. Second, intravenous application of commonly used contrast media should lead to a measurable increase in serum urea and creatinine concentrations. Third, typical histologic changes that are regularly seen in renal biopsies, like osmotic nephrosis, should be easily detected by standard histology. We undertook several approaches that had been suggested in the literature, all of which did not meet the above-mentioned three criteria in our hands using mice (Supplemental Figure 8). From several ischemia/reperfusion studies, it is well known that kidney function was comparable in medium-sized groups 24 and 48 hours after reperfusion with low SD in mice.25,50–52 Therefore, we chose the time point at 24 hours after reperfusion for the induction of CIAKI. In this regard, our model is restricted to the investigation of those compounds and knockout models that do not influence IRI when applied 24 hours after reperfusion. For any agent that is investigated in this model, it needs to be assured that IRI by 48 hours is not significantly different from the PBS control at 48 hours. For instance, blockade of Fas ligand by the mAb MFL3 did exert protection from IRI when applied 24 hours after reperfusion (data not shown) with the consequence that our model does not allow evaluation of the effectiveness of the blockade of this death receptor in CIAKI and RIP3-deficient mice could not be investigated due to the protection from IRI compared with wild-type mice (data not shown). Nec-1, however, did not influence IRI when applied later than 30 minutes after the onset of reperfusion and certainly not when applied 24 hours from the onset of reperfusion.25
Mechanistically, our study suggests a functional role of the RIP1 kinase domain that is not associated with cell death in CIAKI based on three lines of evidence. First, Nec-1 blocks CIAKI in our model. Second, the inactive Nec-1 derivate Nec-1i25,28 does not affect this model, ruling out off-target effects of this compound. Third, the RIP1 expression pattern, which is unaffected by IRI (as reported earlier25), substantially changes after applications of RCM (Figure 5, A and B). RIP1 was described to be involved in both apoptosis and necroptosis, but also in activating the NF-κB pathway after TNFR1 ligation by TNFα.53 We are only beginning to understand non-cell death functions of RIP1 that can be prevented by Nec-1.54 It is of interest in this regard that the histologic pattern of osmotic nephrosis is not restricted to CIAKI, but has also been described in severe sepsis.55–57 RIP1 is also critically involved in sepsis as recently demonstrated in a model of TNFα-induced shock,58 although in this model, we could not detect osmotic nephrosis due to the early death of the mice within 24 hours after TNFα application.32 Currently, we cannot exclude a common underlying role for RIP1 in the pathogenesis of osmotic nephrosis in both CIAKI and sepsis because RIP1-deficient mice die perinatally.59 Organ-specific floxed RIP1-deficient mice will be required to further investigate the role of RIP-1 in both models. Unlike in the sepsis model, Nec-1 prevented all signs of CIAKI almost completely. Besides hydration and application of N-acetylcysteine, both of which did not influence our model (Supplemental Figure 11), and the recently suggested application of atorvastatin,60 blocking the RIP1 kinase activity may be considered a new strategy to prevent CIAKI.
In summary, we conclude that living renal cells are put into a nonfunctioning state by RCM and that this functional inhibition, rather than cell death, is prevented by Nec-1, suggesting a role for the kinase domain of RIP1 in this process. With respect to the fact that probably no other kinase inhibitor was examined in this much detail for its selectivity,26–28 we consider it justified to conclude that the RIP1 kinase domain essentially mediates the mentioned effects without the need for its default partner RIP3. These results should focus our efforts on the inhibition of the RIP1 kinase domain in this scenario rather than on the prevention of cell death. Finally, the possibility that Nec-1 and presumably other RIP1 kinase inhibitors can strongly alter peritubular perfusion might be of relevance beyond CIAKI.
Concise Methods
Complete methods are available in the Supplemental Material.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Vishva Dixit for the RIP3-deficient mice, Elsa Bello-Reuss for the TKPTS cells, and Harald Schöcklmann for the MMC and glEND cells. We also thank Silvia Iversen, Mareike Newsky, Alina Pape, and Katja Bruch (Kiel) and Nancy F. Roeser (Ann Arbor) for excellent technical support.
This work was funded by grants from the German Society for Nephrology (to A.L.); Novartis Pharma GmbH, Germany (to A.L. and S.K.); Fresenius Medical Care, Germany (to S.K. and U.K.); Else Kröner-Fresenius Stiftung (to U.K.); INTERREG 4A, South Denmark-Schlewig- K.E.R.N. program no.: 62-1.2-10, J-no.: 10/13123 (to U.K.); and the National Institutes of Health (NIH-DK34275 to J.M.W.). A.J.S. was funded by a grant from TAMOP (4.2.2/B-10/1-2010-0013). This work is part of the thesis of J.O.H.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012121169/-/DCSupplemental.
References
- 1.Calvin AD, Misra S, Pflueger A: Contrast-induced acute kidney injury and diabetic nephropathy. Nat Rev Nephrol 6: 679–688, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCullough PA: Radiocontrast-induced acute kidney injury. Nephron, Physiol 109: 61–72, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Weisbord SD, Mor MK, Resnick AL, Hartwig KC, Sonel AF, Fine MJ, Palevsky PM: Prevention, incidence, and outcomes of contrast-induced acute kidney injury. Arch Intern Med 168: 1325–1332, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Maioli M, Toso A, Leoncini M, Gallopin M, Musilli N, Bellandi F: Persistent renal damage after contrast-induced acute kidney injury: Incidence, evolution, risk factors, and prognosis. Circulation 125: 3099–3107, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Budano C, Levis M, D’Amico M, Usmiani T, Fava A, Sbarra P, Burdese M, Segoloni GP, Colombo A, Marra S: Impact of contrast-induced acute kidney injury definition on clinical outcomes. Am Heart J 161: 963–971, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Solomon R: Contrast-induced acute kidney injury (CIAKI). Radiol Clin North Am 47: 783–788, v, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Weisbord SD, Palevsky PM: Contrast-induced acute kidney injury: Short- and long-term implications. Semin Nephrol 31: 300–309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudnick M, Feldman H: Contrast-induced nephropathy: What are the true clinical consequences? Clin J Am Soc Nephrol 3: 263–272, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Rudnick MR, Berns JS, Cohen RM, Goldfarb S: Contrast media-associated nephrotoxicity. Curr Opin Nephrol Hypertens 5: 127–133, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Nash K, Hafeez A, Hou S: Hospital-acquired renal insufficiency. Am J Kidney Dis 39: 930–936, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G: Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat Rev Mol Cell Biol 11: 700–714, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Tao Z, Xu Z, Tao Z, Chen B, Wang L, Li C, Chen L, Jia Q, Jia E, Zhu T, Yang Z: Toxic effects of a high dose of non-ionic iodinated contrast media on renal glomerular and aortic endothelial cells in aged rats in vivo. Toxicol Lett 202: 253–260, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Quintavalle C, Brenca M, De Micco F, Fiore D, Romano S, Romano MF, Apone F, Bianco A, Zabatta MA, Troncone G, Briguori C, Condorelli G: In vivo and in vitro assessment of pathways involved in contrast media-induced renal cells apoptosis. Cell Death Dis 2: e155, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romano G, Briguori C, Quintavalle C, Zanca C, Rivera NV, Colombo A, Condorelli G: Contrast agents and renal cell apoptosis. Eur Heart J 29: 2569–2576, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Yokomaku Y, Sugimoto T, Kume S, Araki S, Isshiki K, Chin-Kanasaki M, Sakaguchi M, Nitta N, Haneda M, Koya D, Uzu T, Kashiwagi A: Asialoerythropoietin prevents contrast-induced nephropathy. J Am Soc Nephrol 19: 321–328, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boatright KM, Salvesen GS: Mechanisms of caspase activation. Curr Opin Cell Biol 15: 725–731, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Pop C, Salvesen GS: Human caspases: Activation, specificity, and regulation. J Biol Chem 284: 21777–21781, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroemer G, Galluzzi L, Brenner C: Mitochondrial membrane permeabilization in cell death. Physiol Rev 87: 99–163, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Devalaraja-Narashimha K, Padanilam BJ: PARP-1 inhibits glycolysis in ischemic kidneys. J Am Soc Nephrol 20: 95–103, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linkermann A, De Zen F, Weinberg J, Kunzendorf U, Krautwald S: Programmed necrosis in acute kidney injury. Nephrol Dial Transplant 27: 3412–3419, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Challa S, Chan FK: Going up in flames: Necrotic cell injury and inflammatory diseases. Cell Mol Life Sci 67: 3241–3253, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christofferson DE, Yuan J: Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol 22: 263–268, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Declercq W, Vanden Berghe T, Vandenabeele P: RIP kinases at the crossroads of cell death and survival. Cell 138: 229–232, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Linkermann A, Himmerkus N, Rölver L, Keyser KA, Steen P, Bräsen JH, Bleich M, Kunzendorf U, Krautwald S: Renal tubular Fas ligand mediates fratricide in cisplatin-induced acute kidney failure. Kidney Int 79: 169–178, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Linkermann A, Bräsen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S: Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 81: 751–761, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Biton S, Ashkenazi A: NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-α feedforward signaling. Cell 145: 92–103, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J: Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1: 112–119, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J: Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4: 313–321, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES: RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471: 368–372, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Billings FT, 4th, Chen SW, Kim M, Park SW, Song JH, Wang S, Herman J, D’Agati V, Lee HT: alpha2-Adrenergic agonists protect against radiocontrast-induced nephropathy in mice. Am J Physiol Renal Physiol 295: F741–F748, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Krautwald S, Ziegler E, Rölver L, Linkermann A, Keyser KA, Steen P, Wollert KC, Korf-Klingebiel M, Kunzendorf U: Effective blockage of both the extrinsic and intrinsic pathways of apoptosis in mice by TAT-crmA. J Biol Chem 285: 19997–20005, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linkermann A, Bräsen JH, De Zen F, Weinlich R, Schwendener RA, Green DR, Kunzendorf U, Krautwald S: Dichotomy between RIP1- and RIP3-mediated necroptosis in tumor necrosis factor-α-induced shock. Mol Med 18: 577–586, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinrich MC: Osmotic nephrosis and contrast media. Am J Kidney Dis 52: 629–630, author reply 629–630, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Dickenmann M, Oettl T, Mihatsch MJ: Osmotic nephrosis: Acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis 51: 491–503, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Moreau JF, Droz D, Sabto J, Jungers P, Kleinknecht D, Hinglais N, Michel JR: Osmotic nephrosis induced by water-soluble triiodinated contrast media in man. A retrospective study of 47 cases. Radiology 115: 329–336, 1975 [DOI] [PubMed] [Google Scholar]
- 36.Lee HT, Jan M, Bae SC, Joo JD, Goubaeva FR, Yang J, Kim M: A1 adenosine receptor knockout mice are protected against acute radiocontrast nephropathy in vivo. Am J Physiol Renal Physiol 290: F1367–F1375, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Devalaraja-Narashimha K, Diener AM, Padanilam BJ: Cyclophilin D gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol 297: F749–F759, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Sanz AB, Sanchez-Niño MD, Ortiz A: TWEAK, a multifunctional cytokine in kidney injury. Kidney Int 80: 708–718, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nuñez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G: Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19: 107–120, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moquin D, Chan FK: The molecular regulation of programmed necrotic cell injury. Trends Biochem Sci 35: 434–441, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oberst A, Green DR: It cuts both ways: Reconciling the dual roles of caspase 8 in cell death and survival. Nat Rev Mol Cell Biol 12: 757–763, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA: Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol 283: C905–C916, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Peti-Peterdi J, Burford JL, Hackl MJ: The first decade of using multiphoton microscopy for high-power kidney imaging. Am J Physiol Renal Physiol 302: F227–F233, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porter GA: Experimental contrast-associated nephropathy and its clinical implications. Am J Cardiol 66: 18F–22F, 1990 [DOI] [PubMed] [Google Scholar]
- 45.Sendeski MM, Persson AB, Liu ZZ, Busch JF, Weikert S, Persson PB, Hippenstiel S, Patzak A: Iodinated contrast media cause endothelial damage leading to vasoconstriction of human and rat vasa recta. Am J Physiol Renal Physiol 303: F1592–F1598, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Galluzzi L, Aaronson SA, Abrams J, Alnemri ES, Andrews DW, Baehrecke EH, Bazan NG, Blagosklonny MV, Blomgren K, Borner C, Bredesen DE, Brenner C, Castedo M, Cidlowski JA, Ciechanover A, Cohen GM, De Laurenzi V, De Maria R, Deshmukh M, Dynlacht BD, El-Deiry WS, Flavell RA, Fulda S, Garrido C, Golstein P, Gougeon ML, Green DR, Gronemeyer H, Hajnóczky G, Hardwick JM, Hengartner MO, Ichijo H, Jäättelä M, Kepp O, Kimchi A, Klionsky DJ, Knight RA, Kornbluth S, Kumar S, Levine B, Lipton SA, Lugli E, Madeo F, Malomi W, Marine JC, Martin SJ, Medema JP, Mehlen P, Melino G, Moll UM, Morselli E, Nagata S, Nicholson DW, Nicotera P, Nuñez G, Oren M, Penninger J, Pervaiz S, Peter ME, Piacentini M, Prehn JH, Puthalakath H, Rabinovich GA, Rizzuto R, Rodrigues CM, Rubinsztein DC, Rudel T, Scorrano L, Simon HU, Steller H, Tschopp J, Tsujimoto Y, Vandenabeele P, Vitale I, Vousden KH, Youle RJ, Yuan J, Zhivotovsky B, Kroemer G: Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ 16: 1093–1107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G, Nomenclature Committee on Cell Death 2009 : Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16: 3–11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS: Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467: 863–867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McStay GP, Salvesen GS, Green DR: Overlapping cleavage motif selectivity of caspases: Implications for analysis of apoptotic pathways. Cell Death Differ 15: 322–331, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD: Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20: 1744–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ko GJ, Jang HR, Huang Y, Womer KL, Liu M, Higbee E, Xiao Z, Yagita H, Racusen L, Hamad AR, Rabb H: Blocking Fas ligand on leukocytes attenuates kidney ischemia-reperfusion injury. J Am Soc Nephrol 22: 732–742, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L, Huang L, Ye H, Song SP, Bajwa A, Lee SJ, Moser EK, Jaworska K, Kinsey GR, Day YJ, Linden J, Lobo PI, Rosin DL, Okusa MD: Dendritic cells tolerized with adenosine A2AR agonist attenuate acute kidney injury. J Clin Invest 122: 3931–3942, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P: RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ 14: 400–410, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D: Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 38: 27–40, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Doi K, Leelahavanichkul A, Yuen PS, Star RA: Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest 119: 2868–2878, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebcioglu Z, Cohen DJ, Crew RJ, Hardy MA, Ratner LE, D’Agati VD, Markowitz GS: Osmotic nephrosis in a renal transplant recipient. Kidney Int 70: 1873–1876, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Venkatachalam MA, Weinberg JM: The tubule pathology of septic acute kidney injury: A neglected area of research comes of age. Kidney Int 81: 338–340, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, Declercq W, Libert C, Cauwels A, Vandenabeele P: RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 35: 908–918, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J: Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 471: 373–376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quintavalle C, Fiore D, De Micco F, Visconti G, Focaccio A, Golia B, Ricciardelli B, Donnarumma E, Bianco A, Zabatta MA, Troncone G, Colombo A, Briguori C, Condorelli G: Impact of a high loading dose of atorvastatin on contrast-induced acute kidney injury. Circulation 126: 3008–3016, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.