Abstract
The severe side effects of long-term corticosteroid or cyclosporin A (CsA) therapy complicate the treatment of children with frequently relapsing steroid-sensitive nephrotic syndrome (FR-SSNS). We conducted a randomized, multicenter, open-label, crossover study comparing the efficacy and safety of a 1-year treatment with mycophenolate mofetil (MMF; target plasma mycophenolic acid trough level of 1.5–2.5 µg/ml) or CsA (target trough level of 80–100 ng/ml) in 60 pediatric patients with FR-SSNS. We assessed the frequency of relapse as the primary endpoint and evaluated pharmacokinetic profiles (area under the curve [AUC]) after 3 and 6 months of treatment. More relapses per patient per year occurred with MMF than with CsA during the first year (P=0.03), but not during the second year (P=0.14). No relapses occurred in 85% of patients during CsA therapy and in 64% of patients during MMF therapy (P=0.06). However, the time without relapse was significantly longer with CsA than with MMF during the first year (P<0.05), but not during the second year (P=0.36). In post hoc analysis, patients with low mycophenolic acid exposure (AUC <50 µg⋅h/ml) experienced 1.4 relapses per year compared with 0.27 relapses per year in those with high exposure (AUC>50 µg⋅h/ml; P<0.05). There were no significant differences between groups with respect to BP, growth, lipid levels, or adverse events. However, cystatin clearance, estimated GFR, and hemoglobin levels increased significantly with MMF compared with CsA. These results indicate that MMF is inferior to CsA in preventing relapses in pediatric patients with FR-SSNS, but may be a less nephrotoxic treatment option.
Idiopathic nephrotic syndrome, the most common form of childhood nephrotic syndrome, is most often associated with renal biopsy findings of minimal glomerular and tubulointerstitial changes (minimal change disease). Most patients respond to therapy with corticosteroids,1 but about 70% experience a relapsing course.2 Approximately 30% develop frequently relapsing steroid-sensitive nephrotic syndrome (FR-SSNS), defined as ≥4 relapses per year.3
Although corticosteroids are the mainstay of therapy in pediatric patients with minimal change disease, their repeated use in FR-SSNS results in severe side effects, and other therapeutic options are needed to prevent steroid toxicity.4 A 2- to 3-month course of cyclophosphamide or chlorambucil has been shown to produce a longer remission period in many patients5; however, these drugs may have serious side effects and their long-term efficacy is limited.6,7 Levamisole has been shown to reduce the risk of relapse in several small studies, but information is limited regarding long-term efficacy and adverse effects, and the drug is currently not available in most countries. Treatment with cyclosporin A (CsA) is highly effective in maintaining remission in patients with FR-SSNS allowing withdrawal of corticosteroids, but most patients relapse after withdrawal of CsA.8 Importantly, prolonged CsA therapy is accompanied by time- and dose-dependent nephrotoxicity.9
Mycophenolate mofetil (MMF), the prodrug of mycophenolic acid (MPA), is a non-nephrotoxic immunosuppressive drug with inhibitory effects on T and B lymphocytes, cell-surface markers, and cytokine gene expression10 and has proven efficacy and tolerability in renal allograft recipients. Several small studies with limited statistical power have shown that MMF has steroid-sparing effects and reduces relapse rates in patients with FR-SSNS, albeit with varying efficacy.11–18
We studied efficacy and safety of MMF in patients with FR-SSNS in comparison with CsA in a prospective randomized multicenter open-label crossover trial.
Results
Patient Characteristics
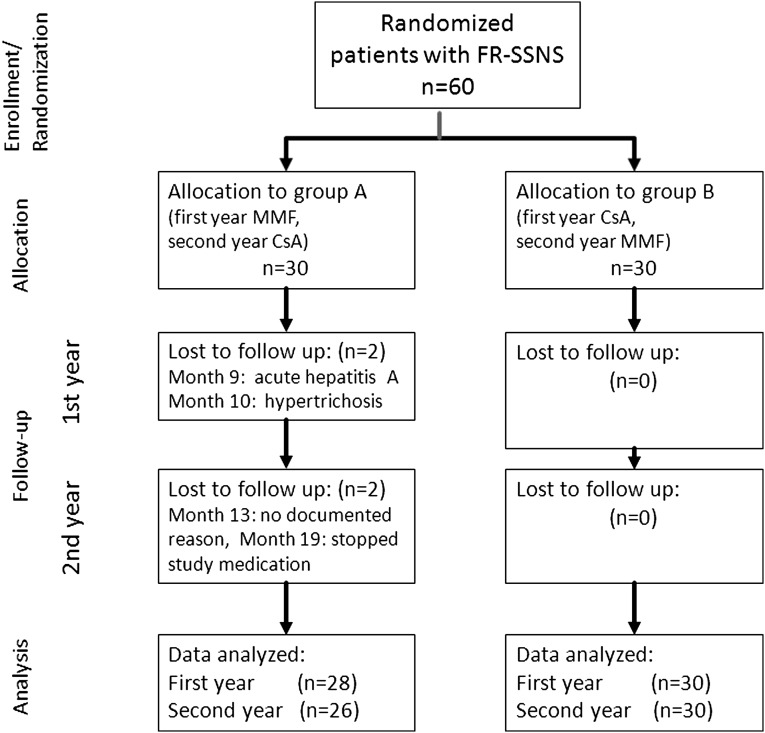
During December 2003 to April 2008, a total of 60 patients (48 boys, 12 girls) were recruited in 15 German centers for pediatric nephrology (Supplemental Figure 1). Patient characteristics at study entry are summarized in Table 1. Most of the patients (n=45 of 60; 75%) had received previous courses of immunosuppressive therapy (Supplemental Table 1). Premature termination of the study was recorded in four patients (7%; Figure 1). No relapses or other complications were recorded during the crossover period.
Table 1.
Patient characteristics at start of study
| Characteristic | Group A | Group B |
|---|---|---|
| Patients (n) | 30 | 30 |
| Age (yr) | 10±3.3 | 9.5±4.0 |
| Height (cm) | 138.0±17.0 | 137.7±21.5 |
| eGFR (Schwartz) | 114.7±24.5 | 119.03±28.3 |
| Previous medicationsa | ||
| None | 8 | 6 |
| Levamisole | 5 | 3 |
| Cyclophosphamide | 13 | 22 |
| Chlorambucil | 1 | 1 |
| CsA | 14 | 12 |
| MMF | 2 | 1 |
| Duration of disease before study entry (yr) | 5.61±4.17 (0.4–14.6); median 4.7 | 5.52±4.09 (0.5–15.9); median 4.1 |
| Relapses before study entry (n) | 9.3±6.71 | 10.0±5.59 |
| Patients with steroid-dependent nephrotic syndrome (n) | 23 | 23 |
Some patients had received more than one previous course of immunosuppressive therapy before study (Supplemental Table 1).
Figure 1.
Diagram of patient flow through the clinical trial.
Primary Endpoint: Frequency of Relapses
There were significantly more relapses per patient per year with MMF compared with CsA therapy during the first year (mean 1.10 versus 0.24; P=0.03), but not during the second year (0.40 versus 0.20; P=0.14). During the first year, there was a single patient with nine relapses; if this patient was considered an outlier, the difference in relapse rate was insignificant (P=1.00). A total of 38 patients (64%) showed no relapses with MMF therapy, whereas 50 patients (85%) showed no relapses with CsA (Table 2). Relapses occurred in 21 patients with MMF therapy and in 9 patients with CsA therapy (Table 3); however, the number of patients relapsing with MMF or CsA during both treatment periods was not significantly different (P=0.06, two-sided Fisher’s exact test).
Table 2.
Relapses during therapy with MMF or CsA
| MMF Therapy | CsA Therapy | ||||
|---|---|---|---|---|---|
| n | Frequency | Summation of Patients (%) | n | Frequency | Summation of Patients (%) |
| 0 | 38 | 38 (64) | 0 | 50 | 50 (85) |
| 1 | 13 | 51 | 1 | 5 | 55 |
| 2 | 2 | 53 | 2 | 4 | 59 |
| 3 | 3 | 56 | |||
| 4 | 1 | 57 | |||
| 5 | 1 | 58 | |||
| 9 | 1 | 59 | |||
Table 3.
Patients relapsing with CsA, MMF, with both therapies, or never
| CsA Therapy | MMF Therapy | |
|---|---|---|
| Relapse | ||
| No | Yes | |
| Relapse | ||
| No | 35 | 15 |
| Yes | 3 | 6 |
| Summation (Patients) | 38 | 21 |
The cross-tabulation (McNemar test) shows the number of patients relapsing with CsA, MMF, in both therapies, or never. Differences were not significant (P=0.06). The number of relapses is not considered.
The first relapse occurred after a median of 195 days in group A (MMF first) and after 543 days in group B (CsA first).
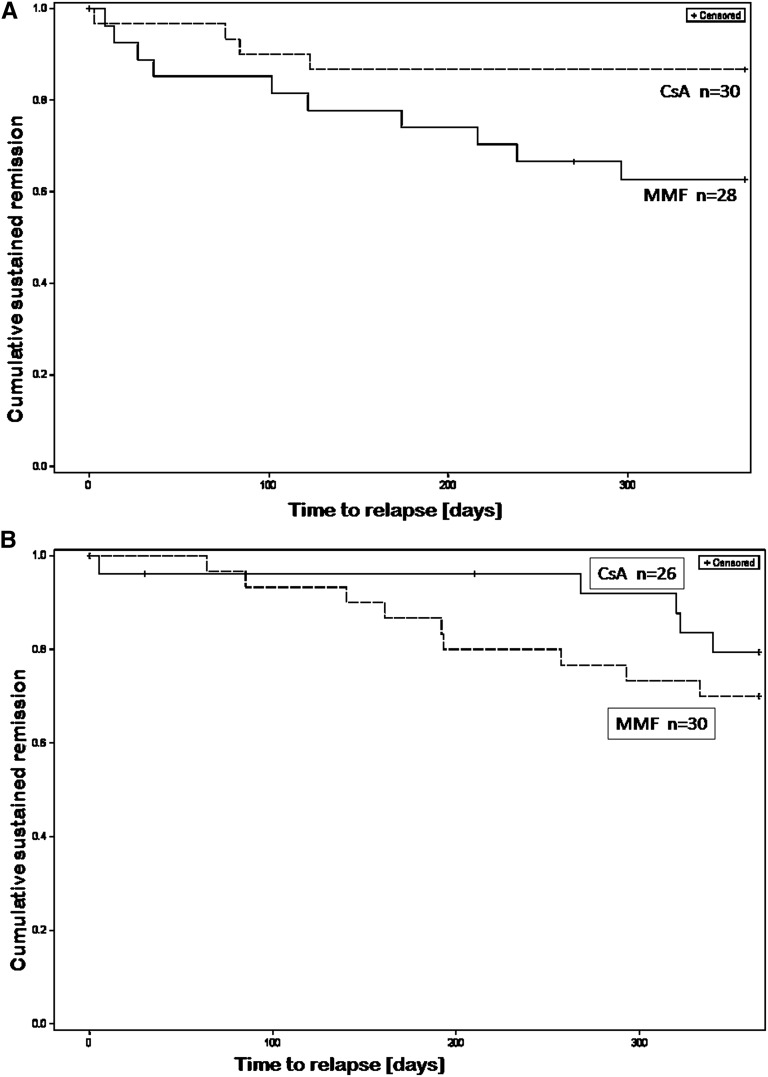
The time without relapse was significantly longer with CsA compared with MMF therapy during the first year of treatment (P<0.05), but not during the second year (P=0.367; Figure 2).
Figure 2.
Efficacy of CsA and MMF in preventing relapses in FR-SSNS patients. Kaplan–Meier survival analysis: Time without relapse (cumulative sustained remission) during treatment with CsA or MMF. (A) In the first treatment year (P<0.05, long-rank test). (B) In the second treatment year (P=0.36, long-rank test). Group A, MMF (dashed line); group B, CsA (straight line).
The frequency of relapses was modulated by previous therapy with cytotoxic drugs. During CsA treatment, 83% of patients were relapse free if previously treated and 91% if previously untreated with cyclophosphamide, respectively. During MMF treatment, 64% of patients were relapse free if previously treated and 74% if previously untreated with cyclophosphamide, respectively; however, these differences were not significant (P=0.80, chi-squared test).There was no significant association of relapse frequency with age, both during the MMF and the CsA periods.
Effect of MPA Exposure on the Relapse Rate
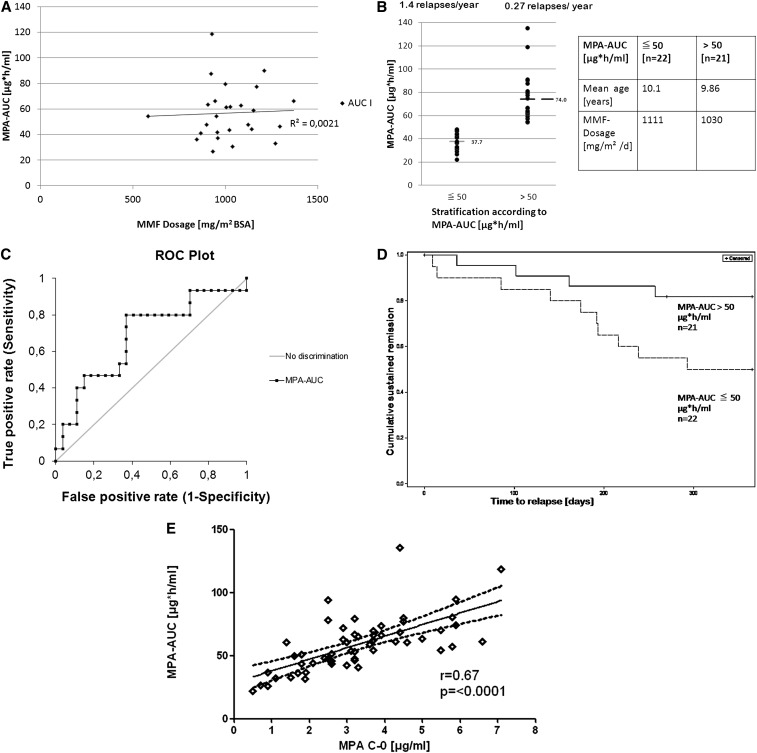
All pharmacokinetic profiles were performed during remission of FR-SSNS; 43 and 36 patients had complete measurements at 3 and 6 months, respectively. There was no correlation of the body surface area (BSA)–adjusted MMF dosage with the corresponding MPA area under the curve (AUC) (Figure 3A). To analyze intraindividual exposure over time, we analyzed the MPA-AUC after 3 and 6 months in 26 children with an unchanged MMF dosage. The mean AUC at 6 months was significantly lower in comparison with the 3-month AUC (P<0.05; Supplemental Figure 2). The decrease in AUC after 6 months was observed in both groups of patients and not related to the rate of relapses, use of corticosteroids, or change in estimated GFR (eGFR). The lowest MPA-AUC was observed in the patient who had nine relapses during MMF therapy.
Figure 3.
Pharmacokinetics of MPA and efficacy. (A) MPA exposure (MPA-AUC) estimated by three-point measuring21 demonstrating lack of correlation between drug dosage and drug concentration in serum. (B) Rate of relapses depending on MPA-exposition: The patients are divided in two groups according to an MPA-AUC <50 µg⋅h/ml or >50 µg⋅h/ml. The number of relapses (P<0.05) and the mean MPA-AUC are significantly different between groups (P=0.0001). (C) ROC curves computed for 3-month MPA-AUC values (n=43). Diagnostic sensitivities (true positives) are calculated for each individual AUC value as the fraction of patients with a recurrence to have lower values. The corresponding diagnostic specificities (false negatives) are calculated as the fraction of patients with no recurrence to have higher values. At a cutoff of 57.1 µg⋅h/ml, the MPA-AUC has a diagnostic sensitivity of 80.0% and a diagnostic specificity of 63.0% to discriminate relapsing from nonrelapsing patients (ROC-AUC=0.68, P=0.02). (D) Kaplan–Meier survival analysis comparing the time without relapse (cumulative sustained remission) in patients with low (dashed line) and high (straight line) MPA exposure (AUC) (P=0.03, long-rank test). (E) Linear regression and confidence intervals (dotted lines) of MPA drug exposure (MPA-AUC) and MPA predose concentration (MPA-C0).
To analyze the effect of MPA exposure on relapse frequency, we compared patients with a low and high MPA exposure. Using the median 3-month MPA-AUC of 50.4 µg⋅h/ml as a cutoff, patients were divided in two groups with a 3-month MPA-AUC <50 µg⋅h/ml or >50 µg⋅h/ml. Although both groups were comparable with respect to age and dosage of MMF, the group with lower MPA exposure (mean MPA-AUC 37.6 µg⋅h/ml) experienced significantly (P<0.05) more relapses (mean 1.4 per year) than those with a higher MPA exposure (mean MPA-AUC 74.0 µg⋅h/ml) (Figure 3B). The relapse rate of the latter group was similar (mean 0.27 per year) to the average frequency of all patients during the CsA treatment period (mean 0.23 relapses per year). To establish whether MPA exposure was a predictor of relapse, receiver operating characteristic (ROC) curves were computed for 3-month MPA-AUC values (Figure 3C). An MPA-AUC of 57.1 µg⋅h/ml had a diagnostic sensitivity of 80.0% and a diagnostic specificity of 63.0% to discriminate relapsing from nonrelapsing patients (ROC-AUC=0.68, P=0.02).
Similarly, the time without relapse was related to the degree of MPA exposure. As shown in Figure 3D, patients with a high MPA exposure (AUC >50 µg⋅h/ml) had a significantly longer time without relapse (P=0.03) than those with a lower exposure. In fact, time without relapse in the subgroup of patients with a high MPA exposure was not significantly different with MMF or CsA treatment during both the first and second treatment year (Supplemental Figure 3, A and B).
To analyze the predictive value of the predose concentrations (trough levels) of MPA (C0), we analyzed the association of C0 with MPA-AUC and with the relapse rate. C0 showed a linear correlation with the MPA-AUC (r2=0.45; Figure 3E). ROC analysis (Supplemental Figure 4) showed that C0 concentrations had no significant predictive value for relapses (ROC-MPA-C0-AUC=0.50; P=0.45). However, predose levels >3.5 µg/ml were 100% predictive of an MPA-AUC >50 µg⋅h/ml.
The mean CsA-AUC (n=36) was 3372±869 ng⋅h/ml (range, 974–4893). Due to the small number of relapses observed during CsA treatment (n=13; 0–2 in a single individual), it was not possible to calculate the effect of CsA exposure on relapse frequency.
Secondary Endpoints
Renal Function
When compared in both treatment groups during the second treatment year (after patients had a defined drug exposure with either MMF or CsA for 12 months), the cystatin C clearance estimated in 34 patients decreased from 146±26 ml/min per 1.73 m2 after 12 months to 120±25 ml/min per 1.73 m2 after 24 months (P=0.004) in group A (receiving MMF first). Group B (receiving CsA first) had an increase from 115±24 ml/min per 1.73 m2 after 12 months to 144±20 ml/min per 1.73 m2 after 24 months (P=0.0001). The results for eGFR were similar (Supplemental Figure 5).
Corticosteroid Use
The mean cumulative prednisone dose in group A was 1.825 g/m2 with MMF and 0.99 g/m2 with CsA therapy (P=0.02). In group B, the mean cumulative prednisone dose was 0.261 g/m2 with CsA and 0.359 g/m2 with MMF (P=0.74). The mean cumulative dose was similar in the CsA period (0.22 g/m2) and in the MMF period (0.28 g/m2) in patients with a high MMF exposure (MPA-AUC >50 µg⋅h/ml).
BP
The 24-hour ambulatory BP monitoring (ABPM) after 12 and 24 months (n=30) showed no differences between both treatment periods: Mean BP was 116/67 mmHg during MMF therapy and 117/69 mmHg during CsA therapy. Four patients required antihypertensive medication during MMF therapy, and six patients required antihypertensive medication during the CsA period (Supplemental Table 2). There was no association of the requirement for antihypertensive therapy with the number of relapses or the cumulative use of corticosteroids.
Growth
Growth was not significantly different during both treatment periods (mean 6.04 cm per year versus 6.05 cm per year with MMF versus CsA, respectively).
Lipid and Hemoglobin Levels
Cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides were not significantly different during treatment periods (Supplemental Table 3). Hemoglobin levels were stable in the first year of treatment in group A and tended to decrease (P=0.27) during the second year of treatment with CsA. In group B, hemoglobin levels were significantly lower during the first year with CsA therapy compared with the second year with MMF (P=0.003).
Adverse Events
Numerous mostly minor adverse effects were reported in 30 of 60 patients (Table 4 and Supplemental Table 4). Adverse events were not related to higher drug exposure with either CsA or MMF.
Table 4.
Reports of AEs and SAEs
| Adverse Event | MMF Therapy (52 reports, 20 patients) | CsA Therapy (59 reports, 21 patients) |
|---|---|---|
| Infections | ||
| Acute respiratory infection, bronchitis, otitis media | 20 (1 SAE) | 19 (3 SAE) |
| Streptococcus infection | 2 | 2 |
| Herpes simplex | 3 (1 SAE) | 1 |
| Stomatitis | 1 | |
| Enteritis | 7 | 6 (1 SAE) |
| Acute hepatitis | 1 (SAE) | |
| Urinary tract infection | 2 | |
| Abdominal pain/vomiting | ||
| Hyperemesis, no appetite | 4 | 2 |
| Skin/hair/mucous membrane affections | ||
| Hypertrichosis | 8 | |
| Hyperplasia of the gingiva | 4 | |
| Alopecia | 1 | 1 |
| Verrucae | 2 | |
| Eczema/dry skin | 3 | 1 |
| Muscle/articulation | ||
| Muscle pain/tremor | 1 | 1 |
| Joint pain | 2 | 2 |
| Psychiatry/neurology | ||
| Sleep disorder | 1 | |
| Mood changes | 1 | 1 |
| Other | ||
| Anemia | 1 | 1 |
| Leukopenia | 1 | |
| Low magnesium level | 1 | |
| Miscellaneousa | 5 | 3 |
AE, adverse events; SAE, serious adverse events.
A detailed account of miscellaneous side effects is given in the Supplemental Material.
Discussion
In this randomized prospective crossover trial, 85% of patients remained without relapses with CsA compared with 64% with MMF. There were significantly more relapses per patient per year with MMF compared with CsA therapy during the first year, but not during the second year. The time without relapse was significantly shorter in the first year of treatment with MMF compared with CsA, but not in the second year. Thus, MMF seemed more effective in the second year than during the first year, which was most likely due to carry-over effects from the first treatment period (with CsA). In addition, the median time until a first relapse was almost three times longer (543 days) in group B (receiving CsA first) than in group A (receiving MMF first; 195 days). The higher efficacy of CsA was also evident when considering the total number of relapses per patient (up to nine during MMF therapy, up to two with CsA), and the number of patients relapsing with each therapy: Only 3 patients with no relapse during the MMF period had a relapse when treated with CsA, whereas 15 patients without a relapse during CsA relapsed with MMF treatment. Thus, considering the primary end point of the study (i.e., frequency of relapses), CsA treatment was significantly more effective than MMF. Moreover, prior CsA treatment was associated with higher efficacy of MMF in the second treatment year. In the only other randomized controlled trial in children with FR-SSNS published to date, 24 patients were treated for one year with a fixed dose of either MMF or CsA; relapse rates were higher (0.83 per year) in the MMF group than in the CsA group (0.08 per year).15
Our study shows a high interindividual variability of MPA exposure at a given dose of MMF as previously reported.19 At a dose of 800–1200 mg/m2, the achieved MPA-AUC varied from 24 to 120 µg⋅h/ml. Lack of correlation was not due to variations in albumin levels, which are known to strongly influence MPA plasma levels, because all patients were in remission at the time of measurement.
A similar MPA-AUC range was observed in previous studies estimating population pharmacokinetics of MPA in children with nephrotic syndrome.20 We also observed considerable intraindividual variability, as suggested by a significant decrease in achieved MPA-AUCs (with an unchanged MMF dose) after 3 and 6 months of treatment. This is in contrast to the increase in MPA-AUC observed after renal transplantation.19 Taken together, our data as well as recent pharmacokinetic studies confirm the value of therapeutic drug monitoring accompanying MMF therapy in patients with the nephrotic syndrome.13,21
To estimate the effect of MPA exposure, we compared relapse rates in patients with low and high MPA exposure in a post hoc analysis. MPA exposure was strongly associated with therapeutic efficacy, as confirmed by ROC curve analysis. Patients achieving an MPA-AUC >50 µg⋅h/ml not only had significantly fewer relapses than patients with an MPA-AUC <50 µg⋅h/ml (1.4 versus 0.27 per year), but also a similar number of relapses as observed in the whole study population during the CsA treatment period (0.23 per year) and a similar relapse-free observation time as CsA-treated patients during both treatment periods. This post hoc analysis in a subpopulation of study patients suggests that a high MPA exposure, corresponding to a MPA-AUC of >50 µg⋅h/ml, might have similar therapeutic efficacy as treatment with CsA in patients with FR-SSNS. However, these data need confirmation by future prospective studies comparing prespecified MPA-AUCs.
Several previous studies have suggested that low MPA predose levels (i.e., <2.013 or <2.5 µg/ml15,17) in children with the nephrotic syndrome treated with MMF are associated with an increased relapse rate. In our study, predose levels were significantly correlated with drug exposure, but explained <50% of the variability of the MPA-AUC. Overall, predose concentrations (C0) were of no significant value in predicting relapses. However, predose levels of >3.5 µg/ml reliably predicted an AUC >50 µg⋅h/ml. These data indicate a limited value of MPA predose levels in clinical practice, but suggest a beneficial effect of a higher MPA exposure in patients with FR-SSNS.
The results of CsA-AUC measurements were well within the target range for treatment of children with FR-SSNS.22,23
Confirming our previous observation,24 eGFR significantly increased with MMF and decreased with CsA, respectively.
The use of corticosteroids was significantly different between treatment periods, reflecting the higher efficacy of CsA. Although the average dose was about twice as high in the MMF period compared with the CsA period, this was not accompanied by an increase in BP. The mean 24-hour ABPM was almost identical between both treatment periods without a higher use of antihypertensives.
We found significantly higher hemoglobin levels during the second treatment year after switching from CsA to MMF (group B). It is currently unknown how CsA treatment could affect hemoglobin levels, but this observation has also been reported by others.15 The study was continuously monitored and numerous minor adverse effects were reported, without clearly favoring either treatment. Of note, gastrointestinal side effects such as colicky pain and diarrhea, frequently observed in transplanted children, were not seen during the MMF treatment period.
The strength of this study lies in the crossover design, allowing comparison of treatment effects in the same individuals, thus limiting genetic and disease-associated heterogeneity in participating patients and allowing statistical comparison of treatment effects in spite of a small study population. A further strength is added by the pharmacokinetic data illustrating the importance of individualized drug dosing for efficacy of treatment with MMF.
The main limitation of the study is the recruitment of a prevalent population with a history of multiple previous treatment cycles. Although patients with FR-SSNS by definition cannot be untreated when recruited for a study, it is unknown to what extent previous treatment periods affected our results, especially during the first year. It is a limitation that pharmacokinetic data were analyzed in a post hoc analysis of a subpopulation of the study group; therefore, MPA-AUC and MPA-C0 thresholds of efficacy of MMF should be validated in prospective studies. Because all patients were of Caucasian origin, results may not be valid for other populations.
In conclusion, new therapeutic options are needed in patients with FR-SSNS because side effects of prolonged treatment with corticosteroids and CsA are unacceptable. In a randomized controlled crossover trial, we could demonstrate superiority of treatment with CsA compared with MMF. Equal efficacy was observed in a subpopulation achieving a high MPA exposure, which was not accompanied by a higher frequency of side effects. A high variability in MPA pharmacokinetics and higher relapse rates with a low MPA-AUC indicate the need for repeated therapeutic drug monitoring to avoid underexposure of MPA in children with FR-SSNS treated with MMF. Therapy with MMF was associated with better renal function and hemoglobin levels, and therapy with CsA with less use of corticosteroids. Numerous minor adverse events were reported in both treatment periods. Considering risks and benefits, MMF therapy was associated with a higher risk for relapses, but remains an attractive steroid-sparing therapeutic option due to lack of nephrotoxicity.
Concise Methods
Study Design
Steroid sensitivity, steroid dependence, remission, and relapse were defined according to International Study of Kidney Disease in Children criteria.3,25
Corticosteroid therapy of relapses was performed in a standardized manner in all participating centers according to the recommendation of the German Society for Pediatric Nephrology before study (further information is included in the Supplemental Material).5
Patients were randomly allocated to two treatment groups, with blocking for age groups. Group A first received MMF for 12 months followed by CsA for 12 months; group B first received CsA for 12 months followed by MMF for 12 months (Supplemental Figure 1). At crossover time after 12 months, the respective new medication was started, and the respective previous medication was reduced by 50% and discontinued within 2 weeks without additional prednisone therapy. During the study period of 2 years, monthly follow-up visits included a physical examination, assessment of side effects, proteinuria screening, and therapeutic drug monitoring. Laboratory analysis for serum and urine parameters was performed every 3 months. ABPM was performed after 12 and 24 months.
A power calculation was performed for the hypothesis that treatment with MMF is not inferior to CsA in achieving sustained remission in FR-SSNS. Using a crossover design and assuming a similar relapse frequency of one relapse per 12 months with CsA and MMF therapy, respectively,24 and a clinically significant difference of >2 relapses per 12 months, a minimum sample size of 45 patients (undergoing both treatments for 1 year) was calculated to detect a significant difference (5% α, 80% power). To compensate for expected dropouts, we aimed at enrolling at least 55 patients into the trial.
The primary endpoint was the frequency of relapses during both treatment periods. Secondary endpoints were the cumulative dose of corticosteroids, changes in eGFR, 24-hour ABPM, and the lipid profile, respectively, during each treatment period. Side effects of MMF and CsA therapy, including clinical signs and symptoms and pathologic laboratory values, were monitored at study visits.
Ethical Approval and Informed Consent
The study was approved by the Ethics Committee of the Charité University Hospital in 2002 (ref: 1656/Si 238) and the ethics committees of each participating center. External monitoring was provided by the Coordinating Center for Clinical Studies Berlin Charité. All parts of the study (http://www.controlled-trials.com/ISRCTN61976169) were carried out according to good clinical practice guidelines. Written and informed consent was obtained from patients and their parents or guardians.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: frequently relapsing SSNS with or without steroid dependance and with or without previous immunosuppressive therapy, minimal change glomerulopathy proven by biopsy, eGFR ≥90 ml/min per 1.73 m2, age 3–18 years, and in remission (at study entry). At the time of randomization, all patients had ongoing disease, and were in remission after a recent relapse (during 0–6 months before study entry).
Exclusion criteria were steroid-resistant or familial nephrotic syndrome, eGFR <90 ml/min per 1.73 m2, other histologic findings, or severe comorbidities (cardiac, liver, hematologic or gastrointestinal disease, cancer, infections, or pregnancy).
Study Medication
MMF (CellCept) was given as capsules (250 mg) or suspension (in small children). The starting dose was 1000–1200 mg/m2 BSA per day in two divided doses. The MMF dose was adjusted to blood MPA predose concentrations of 1.5–2.5 µg/ml.
CsA microemulsion (Sandimmun Optoral) was given as capsules or suspension at a dose of 150 mg/m2 BSA per day in two divided doses adjusted to blood trough concentrations of 80–100 ng/ml.
Pharmacokinetic Studies
A four-point abbreviated pharmacokinetic profile for CsA was measured 4 weeks after start of CsA therapy by the CEDIA-Cyclosporine Plus Combi Kit (Thermo Fisher Microgenics Corporation) and the CsA-AUC was calculated as described.26
A three-point abbreviated pharmacokinetic profile for MPA was performed during MMF therapy after 3 and 6 months based on MPA plasma concentrations before oral intake of MMF (C0) and 30 min (C0.5) and 2 hours (C2) thereafter (further information is included in the Supplemental Material).27 All pharmacokinetic profiles were analyzed in a central laboratory (Department of Clinical Pharmacology, Charité Berlin). MPA was measured by a CEDIA-MPA Immunoassay (Thermo Fisher Microgenics Corporation).
Renal Function
Renal function (eGFR) was calculated according to the method of Schwartz et al.28 and by cystatin C clearance according to the method of Hoek et al.29 Cystatin C was measured by Dimension Vista assay (Siemens AG, Erlangen, Germany).
Statistical Analyses
Sample size calculation was performed with NCSS Trial and PASS 2000 software.
Data were collected by secuTrial software (http://www.secutrial.com; InterActive Systems, Berlin, Germany) and analyzed by SAS software (http://www.sas.com/; version 9.2 TS Level 1M0; SAS Institute Inc., Cary, NC).
Results are expressed as median (range) or mean ± SD, and percentages. Correlations of variables were calculated by Pearson’s correlation coefficient. In an intention-to-treat analysis, the number of relapses in each treatment group was compared by the Mann–Whitney test and differences in cumulative sustained remission by Kaplan–Meier survival analysis. Differences in medication and eGFR before and after MMF treatment were estimated by the Wilcoxon signed-rank test. A P value <0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The help of the following persons at the Coordinating Center for Clinical Studies (KKS Charité) is gratefully acknowledged: Cornelia Schäfer (study assistant and monitoring), Maria Wiese and The-Hoang Do (data management), and Gerald Splettstößer and Alexander Krannich (biostatistics). We thank Dr. Jan Bartel (Limbach Laboratories, Heidelberg, Germany) for providing the cystatin C measurements and Gudrun Cimander (ClinSupport, Germany) for assistance with the study protocol.
The following individuals are local investigators of the GPN Nephrotic Syndrome Study Group: J. Gellermann and U. Querfeld (Berlin), I. Franke (Bonn), E.M. Rüth and W. Rascher (Erlangen), A.K. Arbeiter and P. Hoyer (Essen), M. Pohl (Freiburg), L. Pape (Hannover), R. Feneberg and B. Tönshoff (Heidelberg), J. Misselwitz (Jena), S. Wygoda (Leipzig), H. Fehrenbach (Memmingen), M. Benz and L. Weber (Munich), O. Schofer (Neunkirchen), M. Wigger (Rostock), A. Rudolph (Schwerin), and O. Beringer (Tübingen).
This study was supported in part by grants from Roche Pharma AG (Grenzach, Germany), Novartis Pharma (Germany), and Teva Pharma AG (Basel, Switzerland) and by funds dedicated to clinical research by the Charité Universitätsmedizin Berlin. All funding sources had no involvement in data analysis or interpretation.
Results of this study were presented at the Late Breaking Clinical Trials Session of the American Society of Nephrology Kidney Week 2011, November 8–13, 2011, in Philadelphia, Pennsylvania.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012121200/-/DCSupplemental.
References
- 1.International Study of Kidney Disease in Children : The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr 98: 561–564, 1981 [DOI] [PubMed] [Google Scholar]
- 2.Hodson EM, Willis NS, Craig JC: Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev (4): CD001533, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Tarshish P, Tobin JN, Bernstein J, Edelmann CM, Jr: Prognostic significance of the early course of minimal change nephrotic syndrome: Report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 8: 769–776, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Hodson EM, Willis NS, Craig JC: Non-corticosteroid treatment for nephrotic syndrome in children. Cochrane Database Syst Rev (1): CD002290, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Cyclophosphamide treatment of steroid dependent nephrotic syndrome: Comparison of eight week with 12 week course. Report of Arbeitsgemeinschaft für Pädiatrische Nephrologie. Arch Dis Child 62: 1102–1106, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vester U, Kranz B, Zimmermann S, Hoyer PF: Cyclophosphamide in steroid-sensitive nephrotic syndrome: Outcome and outlook. Pediatr Nephrol 18: 661–664, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Cammas B, Harambat J, Bertholet-Thomas A, Bouissou F, Morin D, Guigonis V, Bendeddouche S, Afroukh-Hacini N, Cochat P, Llanas B, Decramer S, Ranchin B: Long-term effects of cyclophosphamide therapy in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. Nephrol Dial Transplant 26: 178–184, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Ishikura K, Ikeda M, Hattori S, Yoshikawa N, Sasaki S, Iijima K, Nakanishi K, Yata N, Honda M: Effective and safe treatment with cyclosporine in nephrotic children: A prospective, randomized multicenter trial. Kidney Int 73: 1167–1173, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Kengne-Wafo S, Massella L, Diomedi-Camassei F, Gianviti A, Vivarelli M, Greco M, Stringini GR, Emma F: Risk factors for cyclosporin A nephrotoxicity in children with steroid-dependant nephrotic syndrome. Clin J Am Soc Nephrol 4: 1409–1416, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moudgil A, Bagga A, Jordan SC: Mycophenolate mofetil therapy in frequently relapsing steroid-dependent and steroid-resistant nephrotic syndrome of childhood: Current status and future directions. Pediatr Nephrol 20: 1376–1381, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Bagga A, Hari P, Moudgil A, Jordan SC: Mycophenolate mofetil and prednisolone therapy in children with steroid-dependent nephrotic syndrome. Am J Kidney Dis 42: 1114–1120, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Novak I, Frank R, Vento S, Vergara M, Gauthier B, Trachtman H: Efficacy of mycophenolate mofetil in pediatric patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol 20: 1265–1268, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Fujinaga S, Ohtomo Y, Umino D, Takemoto M, Shimizu T, Yamashiro Y, Kaneko K: A prospective study on the use of mycophenolate mofetil in children with cyclosporine-dependent nephrotic syndrome. Pediatr Nephrol 22: 71–76, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Afzal K, Bagga A, Menon S, Hari P, Jordan SC: Treatment with mycophenolate mofetil and prednisolone for steroid-dependent nephrotic syndrome. Pediatr Nephrol 22: 2059–2065, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Dorresteijn EM, Kist-van Holthe JE, Levtchenko EN, Nauta J, Hop WC, van der Heijden AJ: Mycophenolate mofetil versus cyclosporine for remission maintenance in nephrotic syndrome. Pediatr Nephrol 23: 2013–2020, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baudouin V, Alberti C, Lapeyraque AL, Bensman A, André JL, Broux F, Cailliez M, Decramer S, Niaudet P, Deschênes G, Jacqz-Aigrain E, Loirat C: Mycophenolate mofetil for steroid-dependent nephrotic syndrome: A phase II Bayesian trial. Pediatr Nephrol 27: 389–396, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Mendizábal S, Zamora I, Berbel O, Sanahuja MJ, Fuentes J, Simon J: Mycophenolate mofetil in steroid/cyclosporine-dependent/resistant nephrotic syndrome. Pediatr Nephrol 20: 914–919, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Hogg RJ, Fitzgibbons L, Bruick J, Bunke M, Ault B, Baqi N, Trachtman H, Swinford R: Mycophenolate mofetil in children with frequently relapsing nephrotic syndrome: A report from the Southwest Pediatric Nephrology Study Group. Clin J Am Soc Nephrol 1: 1173–1178, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Weber LT, Shipkova M, Armstrong VW, Wagner N, Schütz E, Mehls O, Zimmerhackl LB, Oellerich M, Tönshoff B: The pharmacokinetic-pharmacodynamic relationship for total and free mycophenolic acid in pediatric renal transplant recipients: A report of the german study group on mycophenolate mofetil therapy. J Am Soc Nephrol 13: 759–768, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Saint-Marcoux F, Guigonis V, Decramer S, Gandia P, Ranchin B, Parant F, Bessenay L, Libert F, Harambat J, Bouchet S, Broux F, Compagnon P, Marquet P: Development of a Bayesian estimator for the therapeutic drug monitoring of mycophenolate mofetil in children with idiopathic nephrotic syndrome. Pharmacol Res 63: 423–431, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Kuypers DR, Le Meur Y, Cantarovich M, Tredger MJ, Tett SE, Cattaneo D, Tönshoff B, Holt DW, Chapman J, Gelder T, Transplantation Society (TTS) Consensus Group on TDM of MPA : Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol 5: 341–358, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Niaudet P, Reigneau O, Humbert H: A pharmacokinetic study of Neoral in childhood steroid-dependent nephrotic syndrome. Pediatr Nephrol 16: 154–155, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Filler G: How should microemulsified cyclosporine A (Neoral) therapy in patients with nephrotic syndrome be monitored? Nephrol Dial Transplant 20: 1032–1034, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Gellermann J, Querfeld U: Frequently relapsing nephrotic syndrome: Treatment with mycophenolate mofetil. Pediatr Nephrol 19: 101–104, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Kidney Disease Improving Global Outcomes: KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2: 139–274, 2012 [Google Scholar]
- 26.Filler G, Feber J, Lepage N, Weiler G, Mai I: Universal approach to pharmacokinetic monitoring of immunosuppressive agents in children. Pediatr Transplant 6: 411–418, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Pawinski T, Kunicki PK, Sobieszczanska-Malek M, Gralak B, Szlaska I: A limited sampling strategy for estimating mycophenolic acid area under the curve in adult heart transplant patients treated with concomitant cyclosporine. J Clin Pharm Ther 34: 89–101, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoek FJ, Kemperman FA, Krediet RT: A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant 18: 2024–2031, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.