Abstract
A1 adenosine receptor activation ameliorates ischemic AKI through the induction of renal proximal tubular sphingosine kinase-1. However, systemic adverse effects may limit A1 adenosine receptor–based therapy for ischemic AKI, indicating a need to identify alternative therapeutic targets within this pathway. Here, we evaluated the function of renal proximal tubular IL-11, a clinically approved hematopoietic cytokine, in A1 adenosine receptor–mediated induction of sphingosine kinase-1 and renal protection. Treatment of human proximal tubule epithelial (HK-2) cells with a selective A1 adenosine receptor agonist, chloro-N(6)-cyclopentyladenosine (CCPA), induced the expression of IL-11 mRNA and protein in an extracellular signal–regulated kinase–dependent manner, and administration of CCPA in mice induced renal synthesis of IL-11. Pretreatment with CCPA protected against renal ischemia-reperfusion injury in wild-type mice, but not in IL-11 receptor–deficient mice. Administration of an IL-11–neutralizing antibody abolished the renal protection provided by CCPA. Similarly, CCPA did not induce renal IL-11 expression or protect against renal ischemia-reperfusion injury in mice lacking the renal proximal tubular A1 adenosine receptor. Finally, treatment with CCPA induced sphingosine kinase-1 in HK-2 cells and wild-type mice, but not in IL-11 receptor–deficient or renal proximal tubule A1 adenosine receptor–deficient mice. Taken together, these results suggest that induction of renal proximal tubule IL-11 is a critical intermediary in A1 adenosine receptor–mediated renal protection that warrants investigation as a novel therapeutic target for the treatment of ischemic AKI.
AKI results in extremely high mortality and morbidity costing more than $10 billion per year in the United States.1 Furthermore, patients with AKI frequently die of multiorgan failure and sepsis, and those who survive have an increased incidence of CKD. Renal ischemia and reperfusion (IR) injury or ischemic AKI is a frequent and serious complication for patients subjected to major cardiac, liver, vascular, or kidney surgery.2 Attenuation of renal tubular cell necrosis, inflammation, and apoptosis ameliorates ischemic AKI in preclinical animal models.3 Unfortunately, there is no clinically effective therapy or drug to treat or prevent AKI.4
Adenosine is an endogenous paracrine molecule with potent physiologic and cytoprotective properties.5 Activation of cell surface adenosine receptors (ARs) powerfully attenuates all three components of cell death that contribute to ischemic AKI.6 In particular, we previously showed that activation of the A1 AR protects against ischemic AKI in mice and rats by reducing necrosis, inflammation, and apoptosis.7–9 However, A1AR-based therapy for ischemic AKI may be limited by the extrarenal systemic adverse effects of cardiac and central nervous system A1AR activation (bradycardia, hypotension, and sedation). In particular, A1AR agonist therapy may not be tolerated by critically ill patients at risk for developing AKI. One way to mitigate this problem is to target the distal signaling molecules synthesized after renal tubular A1AR activation.
IL-11 is a 20-kDa member of the IL-6–type cytokine family and is clinically approved to promote megakaryocyte maturation in patients receiving chemotherapy.10 In addition to its powerful hematopoietic properties, IL-11 protects against intestinal, cardiomyocyte, and endothelial cell death.11 We recently showed that recombinant human IL-11 treatment before or after renal ischemia attenuated ischemic AKI in mice.12 Specifically, IL-11 administration significantly attenuated all three components of renal cell death (necrosis, inflammation, and apoptosis) after ischemic AKI, closely mimicking the renal protective effects of A1AR activation. This IL-11–mediated protection against ischemic AKI requires the downstream induction of sphingosine kinase-1 (SK-1) as recombinant IL-11 induced SK-1 synthesis and SK-1–deficient mice were not protected against renal IR with IL-11 treatment. We were intrigued by this finding as we recently discovered that A1AR-mediated protection against ischemic AKI also requires SK-1 induction and activation.13
Collectively, our previous studies suggest that A1AR activation and IL-11 therapy induce the identical downstream cytoprotective enzyme SK-1. Therefore, in this study we tested the hypothesis that A1AR-mediated protection against ischemic AKI, as well as A1AR-mediated induction of SK-1, requires the induction of renal tubular IL-11. We first tested whether A1AR activation induces IL-11 synthesis in renal proximal tubule cells via extracellular signal–regulated kinase/mitogen-activated protein kinase (ERK MAPK) and/or protein kinase B (Akt) activation. We then tested whether A1AR-mediated protection against renal IR is attenuated or abolished in mice lacking IL-11 signaling. Finally, to investigate the direct role of proximal tubule A1AR in generating IL-11 and mediating renal protection in vivo, we generated mice with renal proximal tubule–specific deletion of A1AR by crossing A1AR floxed mice14 with mice expressing phosphoenolpyruvate carboxykinase promoter driven Cre recombinase.15
Results
A1AR Activation Induces IL-11 in HK-2 Cells via ERK MAPK
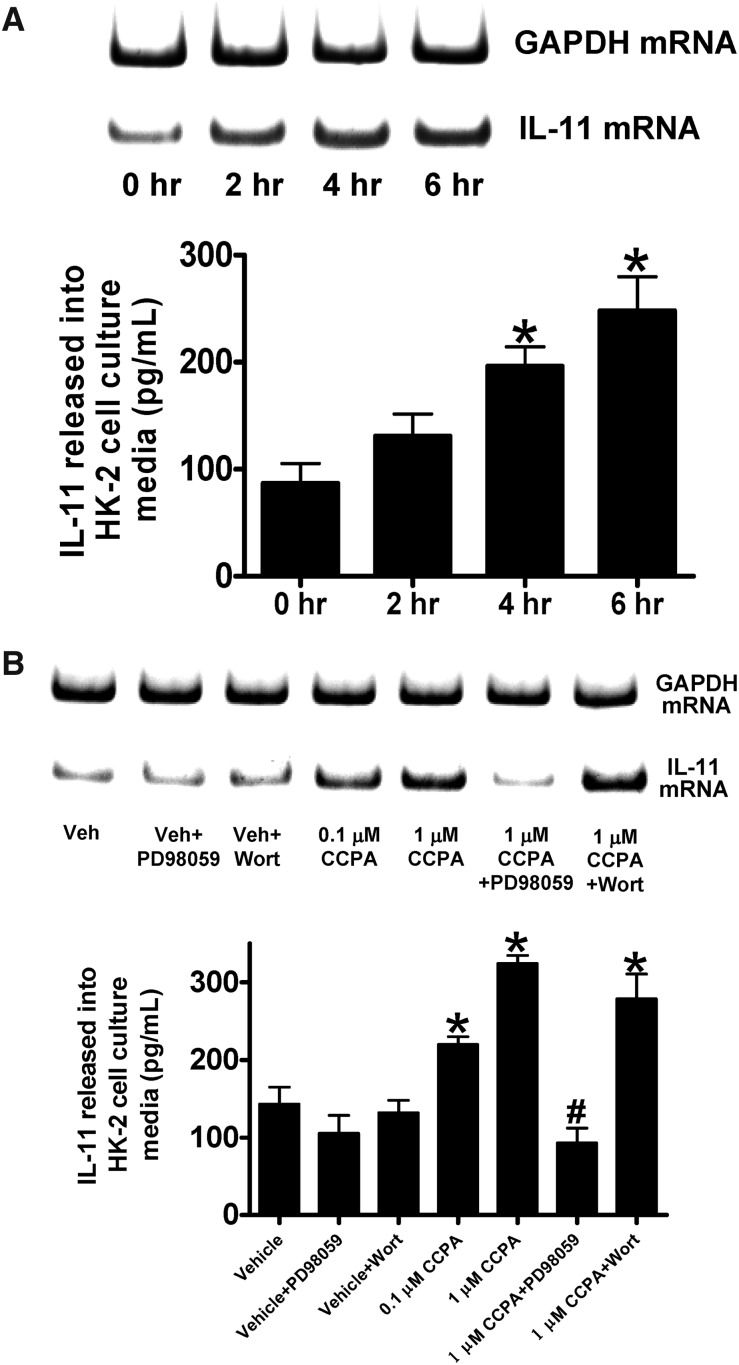
Figure 1 shows a time-dependent (0–6 hours, part A) and dose-dependent (0.1–1 μM, part B) induction of IL-11 mRNA (top) and IL-11 protein (released into cell culture media, bottom) in human proximal tubule (HK-2) cells with chloro-N(6)-cyclopentyladenosine (CCPA, a selective A1AR agonist) treatment. A1AR phosphorylates ERK MAPK as well as Akt in renal proximal tubule cells.16 We therefore tested the hypothesis that A1AR-mediated ERK and/or Akt phosphorylation directly induces IL-11 in HK-2 cells. We show that CCPA failed to induce IL-11 in HK-2 cells pretreated with a specific ERK MAPK inhibitor (PD98059; Figure 1B), whereas a selective Akt inhibitor (wortmannin) had no effect. Inhibitors alone had no significant effect on IL-11 levels in HK-2 cells.
Figure 1.
A1AR activation induces IL-11 mRNA and protein synthesis in human proximal tubule (HK-2) cells via ERK MAPK. HK-2 cells were treated with 1 μM CCPA for 0–6 hours (A, n=4) or with 0–1 μM CCPA for 6 hours (B, n=4). Some cells were pretreated with PD98059 (a specific MEK inhibitor, 50 mM) or with wortmannin (a specific phosphoinositide 3-kinases inhibitor, 100 nM) before CCPA treatment (B). GAPDH served as internal loading controls for RTPCR. Data are presented as mean ± SEM. *P<0.05 versus IL-11 protein expression at 0 hours or versus vehicle-treated control. #P<0.05 versus IL-11 protein expression with 1 μM CCPA treatment.
A1AR-Mediated Induction of Kidney IL-11 mRNA and Protein Synthesis In Vivo
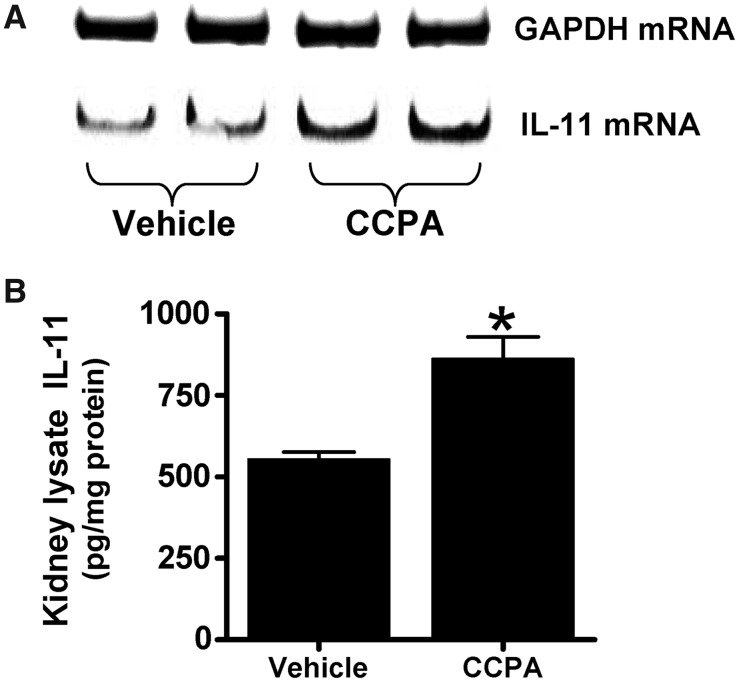
We previously showed that the dose of CCPA used (0.1 mg/kg intraperitoneally) produced a small decrease (approximately 9%) in systolic BP without any effect on renal blood flow.16 Figure 2 shows that CCPA (0.1 mg/kg) significantly induced IL-11 mRNA (top) and protein expression (bottom) measured in mouse kidneys compared with vehicle-treated group. However, CCPA did not change liver or small intestine IL-11 mRNA expression (data not shown). We also determined that a single dose of CCPA did not change leukocyte (vehicle-treated mice: 5087±466 cells/μl, n=3; CCPA-treated mice: 7233±1868 cells/μl, n=3; P=0.37) or platelet count (vehicle-treated mice: 894,667±50,584 cells/μl, n=3; CCPA-treated mice: 935,333±132,942 cells/μl, n=3; P=0.80).
Figure 2.
A1AR activation increases IL-11 mRNA and protein synthesis in mouse kidney. Representative bands for IL-11 mRNA (RT-PCR, A) and protein (ELISA, B) expression in mouse kidney (n=4 for each group) are shown. Mice were treated with vehicle (1% DMSO in saline) or with CCPA (0.1 mg/kg intraperitoneally); 6 hours later, kidneys (cortex and corticomedullary junction) were collected for analyses. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as internal loading controls for RT-PCR. *P<0.05 versus vehicle-treated group.
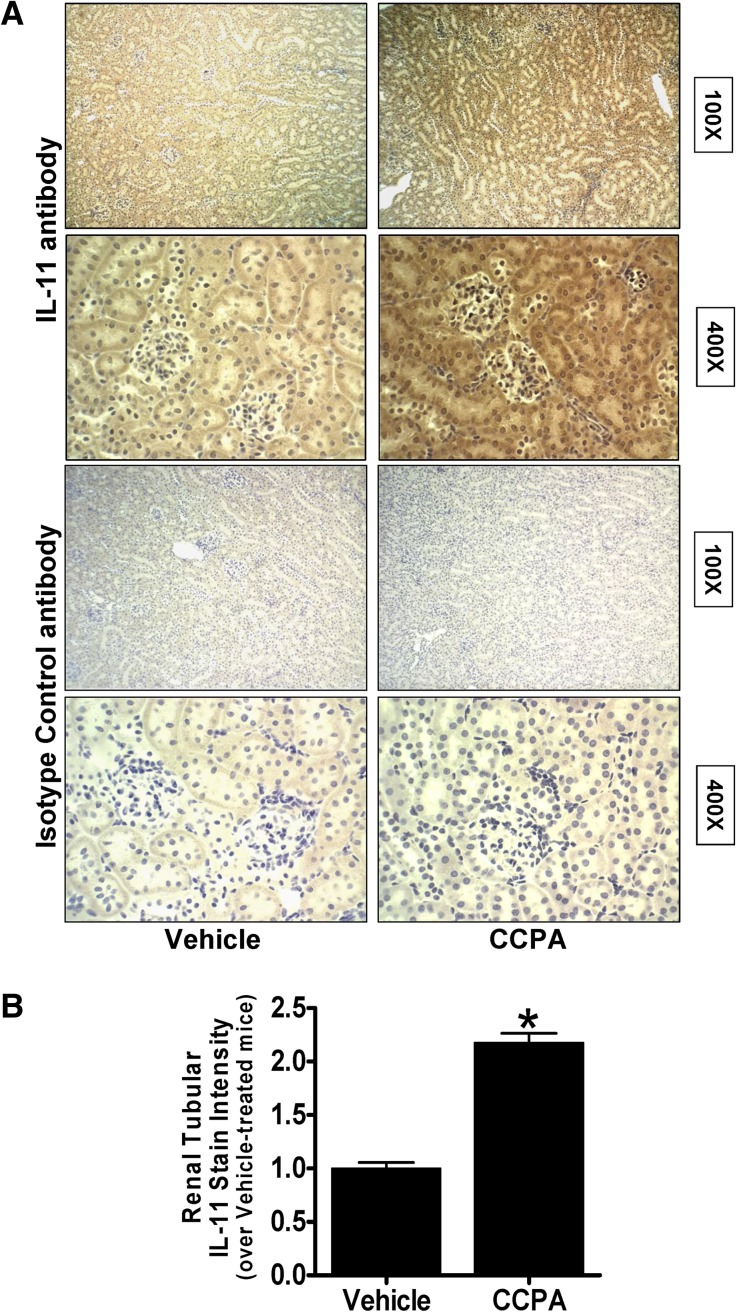
Immunohistochemistry (Figure 3A) shows diffuse renal tubular IL-11 staining in mice anesthetized with pentobarbital and increased IL-11 staining in the kidneys of mice treated with 0.1 mg/kg CCPA 6 hours earlier. Interestingly, this increase in IL-11 staining was more intense in the cortex compared with medulla. IL-11 staining was not visible in the kidneys stained with negative isotype control antibody. Quantification of immunohistochemical staining (400×) confirmed a significant increase in renal tubular IL-11 immunoreactivity in kidneys from CCPA-treated mice (Figure 3B). Therefore, from these experiments we can conclude that A1AR activation induces IL-11 mRNA and protein expression in vivo as well as in vitro.
Figure 3.
A1AR activation increases IL-11 immunoreactivity in mouse kidney. (A) IL-11 immunohistochemistry (original magnification, ×100 and ×400 shown) in IL-11 receptor WT mouse kidneys treated with vehicle or 0.l mg/kg CCPA 6 hours earlier (representative of four experiments). (B) Quantifications of renal tubular IL-11 staining in mice treated with vehicle or with CCPA 6 hours earlier (n=4). *P<0.05 versus vehicle-treated mice. Error bars represent 1 SEM.
Critical Role of IL-11 in A1AR-Mediated Renal Protection In Vivo
IL-11R wild-type (WT) mice treated with vehicle or with 0.1 mg/kg CCPA and subjected to sham operation had similar plasma creatinine (Figure 4A). Similarly, IL-11R knockout (KO) mice or IL-11R WT mice pretreated with IL-11 neutralizing antibody had similar creatinine values after sham operation. Plasma creatinine significantly increased in vehicle-treated IL-11R WT mice subjected to 30 minutes of renal ischemia and 24-hour reperfusion compared with sham-operated mice (Figure 4A). However, IL-11R WT mice pretreated with 0.1 mg/kg CCPA before renal ischemia had significantly decreased plasma creatinine 24 hours after injury compared with vehicle-treated mice subjected to renal IR. Supporting a critical role for IL-11 in A1AR-mediated renal protection against IR, IL-11R KO mice as well as IL-11R WT mice treated with IL-11–neutralizing antibody before renal ischemia were not protected against renal injury with CCPA treatment (Figure 4A). Next we tested whether endogenous IL-11 provides resistance against renal IR injury. Figure 4B shows that IL-11R KO mice had significantly worse renal injury after 20 minutes of renal ischemia and 24-hour reperfusion.
Figure 4.
IL-11 is critical for A1AR-mediated renal protection against ischemic AKI. (A) Plasma creatinine values obtained from mice subjected to sham operation or to 30 minutes of renal ischemia and 24 hours of reperfusion (RIR). IL-11 WT or IL-11R–deficient (IL-11R KO) mice were treated with vehicle or with a selective A1AR agonist (CCPA, 0.1 mg/kg) 15 minutes before sham operation to renal IR. Some IL-11R WT mice were pretreated with an IL-11–neutralizing antibody (1 mg/kg intravenously) 30 minutes before CCPA treatment and then subjected to sham operation or to renal IR injury. *P<0.05 versus respective sham-operated mice. #P<0.05 versus vehicle treated mice subjected to renal IR. n=5–6 for each group. Data presented as mean ± SEM. (B) Endogenous IL-11 provides resistance against renal IR injury. IL-11R WT and deficient (KO) mice were subjected to 20 minutes of renal ischemia and 24 hours of reperfusion (n=5). *P<0.05 versus IL-11R WT mice subjected to 20 minutes of renal IR.
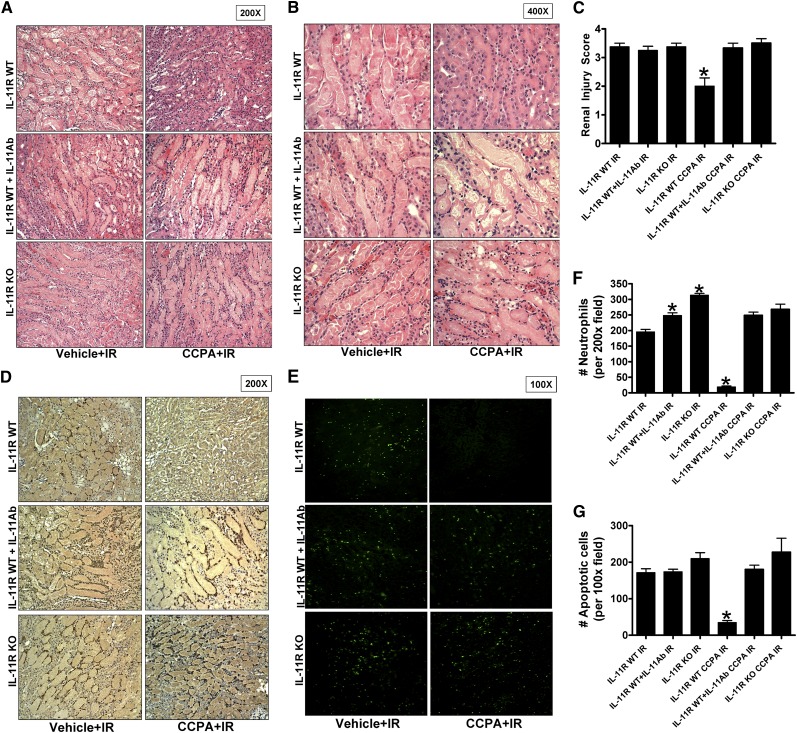
Kidney histologic assessment (Figure 5A [original magnification, ×200] and Figure 5B [original magnification, ×400]) demonstrated severe necrotic renal injury in vehicle-treated IL-11R WT or IL-11R KO mice subjected to renal IR. Compared with sham-operated vehicle-treated mice (not shown), the kidneys of IL-11R WT mice subjected to renal IR showed significant tubular necrosis, cast formation, and increased congestion. In contrast, consistent with the plasma creatinine data, CCPA-pretreated IL-11R WT mice had reduced renal tubular necrosis 24 hours after renal IR. Supporting a critical role of IL-11 in A1AR-mediated renal protection against IR, CCPA pretreatment failed to reduce renal tubular necrosis in IL-11R WT mice pretreated with a neutralizing IL-11 antibody or in IL-11R KO mice. The Jablonski scale17 renal injury score was used to grade renal tubular necrosis in the corticomedullary junction 24 hours after renal IR (Figure 5C). Thirty minutes of renal ischemia and 24 hours of reperfusion resulted in severe acute tubular necrosis in vehicle-treated IL-11R WT and IL-1R KO mice. A1AR activation significantly reduced the renal injury score in IL-11R WT mice but not in IL-11R KO mice or in IL11R WT mice pretreated with IL-11 neutralizing antibody.
Figure 5.
IL-11 induction is critical for A1AR-mediated reduction in renal tubular necrosis, renal neutrophil infiltration, and renal tubular apoptosis after IR. (A and B) Representative photomicrographs (A: original magnification, ×200; B: original magnification, ×400) hematoxylin and eosin staining of kidney sections of mice subjected to 30 minutes of renal ischemia followed by 24 hours of reperfusion after treatment with a selective A1AR agonist (CCPA, 0.1 mg/kg, intraperitoneally 15 minutes before renal ischemia) or with vehicle. Photographs are representative of 4–5 independent experiments. (C) Summary of Jablonski scale renal injury scores (scale 0–4) for mice subjected to sham operation or renal IR. (D and E) Representative photomicrographs of 4–5 experiments for immunohistochemistry (brown staining) for neutrophil infiltration (D: original magnification, ×200) and TUNEL staining (E, representing apoptotic nuclei: original magnification, ×100) from kidneys of mice subjected to 30 minutes of renal ischemia and 24 hours of reperfusion (IR) after treatment with a selective A1AR agonist (CCPA, 0.1 mg/kg, intraperitoneally 15 minutes before renal ischemia) or with vehicle. Photographs are representative of four independent experiments. (F and G) Quantifications of infiltrated neutrophils per ×200 field (F) and apoptotic cells per ×100 field (G) in the kidneys of mice after renal IR. *P<0.05 versus vehicle-treated mice subjected to renal IR. Error bars represent 1 SEM.
Critical Role of IL-11 in A1AR-Mediated Reduction in Kidney Neutrophil Infiltration and Apoptosis
Figure 5D shows representative images (from four experiments) of immunohistochemistry of neutrophils infiltrating the kidneys of mice subjected to 30 minutes of renal ischemia and 24-hour reperfusion (original magnification, ×200). In sham-operated mice, we were unable to detect any neutrophils in the kidney (data not shown). There was heavy neutrophil infiltration (dark brown) in the kidneys (corticomedullary junction) of vehicle-treated IL-11R WT and IL-11R KO mice subjected to renal IR. In contrast, IL-11R WT mice pretreated with a selective A1AR agonist CCPA showed significantly reduced neutrophil infiltration in the kidney 24 hours after IR (Figure 5E). In contrast, CCPA treatment failed to reduce kidney neutrophil infiltration in IL-11R WT mice treated with IL-11–neutralizing antibody and in IL-11R KO mice.
TUNEL staining detected apoptotic renal tubular cells in kidneys of mice. In sham-operated mice, very few TUNEL-positive cells were detected in the kidney (data not shown). Vehicle-treated IL-11R WT and IL-11R KO mice subjected to renal IR demonstrated numerous renal tubule cell apoptosis detected with TUNEL staining (Figure 5F [original magnification, ×100] and Figure 5G). However, IL-11R WT mice pretreated with CCPA before renal ischemia had a significantly reduced number of apoptotic TUNEL-positive cells in the kidney 24 hours after IR. Again, CCPA treatment failed to reduce renal cell apoptosis in IL-11R WT mice treated with IL-11–neutralizing antibody and in IL-11R KO mice supporting a critical role for IL-11 in CCPA-mediated reduction of kidney apoptosis.
Critical Role of Renal Proximal Tubule A1AR for Renal Protection and IL-11 Induction
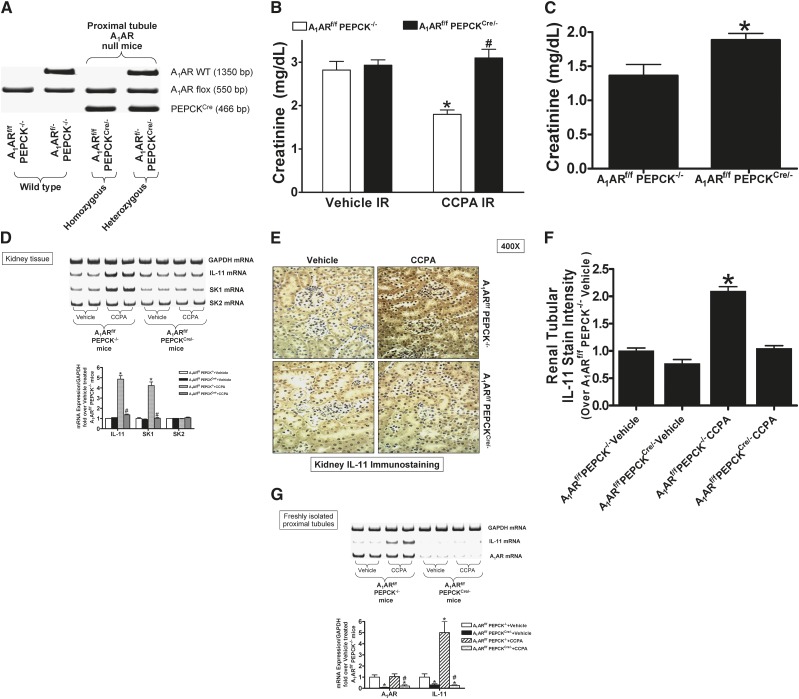
Mating mice carrying the floxed A1AR gene (A1ARf/f mice14) with phosphoenolpyruvate carboxykinase (PEPCK)Cre recombinase mice15 resulted in mice carrying floxed A1AR with (null) or without (WT) PEPCK Cre recombinase. Tail PCR confirmed the genotypes of proximal tubule A1AR null mice (A1ARf/f PEPCKCre/−) and the WT control transgenic mice (A1ARf/fPEPCK−/−) generated (Figure 6A).
Figure 6.
Critical role of renal proximal tubule A1AR in IL-11 generation and A1AR-mediated protection against ischemic AKI. (A) Tail PCR confirming the genotypes of proximal tubule specific A1AR null mice (A1ARf/f PEPCKCre/- or A1ARf/- PEPCKCre/−) and the control transgenic mice (A1ARf/f PEPCK−/− or A1ARf/- PEPCK−/−). (B) Plasma creatinine from WT (A1ARf/f PEPCK−/−) or from proximal tubule A1AR null mice (A1ARf/f PEPCKCre/−) subjected to 30 minutes of renal ischemia and 24 hours of reperfusion (n=6). Treatment with a selective A1AR agonist (CCPA, 0.1 mg/kg intraperitoneally 15 minutes before renal ischemia) or with vehicle. *P<0.05 versus vehicle-treated A1ARf/f PEPCK−/− mice. #P<0.05 versus CCPA-treated A1ARf/f PEPCK−/− mice. (C) Proximal tubule A1ARs provide endogenous resistance against ischemic AKI. WT (A1ARf/f PEPCK−/−) mice and proximal tubule A1AR null (A1ARf/f PEPCKCre/−) mice were subjected to 20 minutes of renal ischemia and 24 hours of reperfusion. *P<0.05 versus A1ARf/f PEPCK−/− mice subjected to renal IR. (D) Lack of kidney IL-11 and SK-1 mRNA induction in proximal tubule A1AR null mice treated with CCPA (n=4). Representative images (top) and band intensity quantifications (bottom) expressed as fold increases in kidney IL-11, SK-1, and SK-2 mRNA expression over vehicle-treated WT (A1ARf/f PEPCK−/−) mice (n=4). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was also quantified to normalize lane loading. *P<0.05 versus vehicle-treated WT (A1ARf/f PEPCK−/−) mice. #P<0.05 versus CCPA-treated WT (A1ARf/f PEPCK−/−) mice. (E) Kidney IL-11 immunohistochemistry (original magnification, ×400) in proximal tubule A1AR null (A1ARf/f PEPCKCre/−) mice and WT (A1ARf/f PEPCK−/−) mice treated with vehicle or 0.l mg/kg CCPA 6 hours earlier (n=4). (F) Quantifications of renal tubular IL-11 staining in proximal tubule A1AR null (A1ARf/f PEPCKCre/-) mice and WT (A1ARf/f PEPCK−/−) mice treated with vehicle or with CCPA 6 hours earlier. *P<0.05 versus vehicle-treated WT (A1ARf/f PEPCK−/−) mice. Error bars represent 1 SEM. (G) Representative images (top) and band intensity quantifications (bottom) for A1AR and IL-11 mRNA expression in freshly isolated renal proximal tubules from proximal tubule A1AR null (A1ARf/f PEPCKCre/-) mice and WT (A1ARf/f PEPCK−/−) mice (n=4). *P<0.05 versus vehicle-treated WT (A1ARf/f PEPCK−/−) mice. #P<0.05 versus CCPA-treated WT (A1ARf/f PEPCK−/−) mice.
Figure 6B shows plasma creatinine from WT (A1ARf/f PEPCK−/−) or from proximal tubule A1AR null mice (A1ARf/f PEPCKCre/-) subjected to 30 minutes of renal ischemia and 24 hours of reperfusion. Treatment with a selective A1AR agonist (CCPA, 0.1 mg/kg intraperitoneally 15 minutes before renal ischemia) protected against ischemic AKI in WT mice. However, CCPA failed to protect proximal tubule A1AR null mice against ischemic AKI. We also performed experiments to test whether proximal tubule A1ARs provide endogenous resistance against a shorter period of renal IR injury. We show that proximal tubule A1AR null mice had significantly worse renal injury after 20 minutes of renal ischemia and 24 hours of reperfusion.
Figure 6D shows that CCPA treatment (0.1 mg/kg) 6 hours earlier induced kidney IL-11 in WT mice. However, CCPA failed to induce kidney IL-11 in proximal tubule A1AR null mice. We previously showed that A1AR activation induced SK-1 in mouse renal proximal tubules.13 Consistent with this, we demonstrate here that SK-1 induction was also selectively abolished in proximal tubule A1AR null mice (Figure 6D). Kidney immunohistochemistry from proximal tubule A1AR null (A1ARf/f PEPCKCre/−) and WT (A1ARf/f PEPCK−/−) mice (Figure 6E) shows that IL-11 staining was darker in kidneys of WT mice treated with CCPA. IL-11 staining did not increase in proximal tubule A1AR null mice. Quantification of immunohistochemical staining confirmed that renal tubular IL-11 immunoreactivity significantly increased in CCPA-treated WT mice but not in proximal tubule A1AR null mice (Figure 6F). These experiments suggest that renal proximal tubule A1AR activation is required for induction of IL-11 mRNA and protein expression in vivo.
We also determined whether A1AR-mediated IL-11 induction is attenuated in proximal tubules from proximal tubule A1AR null mice. We first noted significantly reduced A1AR mRNA in proximal tubule cells from proximal tubule A1AR null mice compared with WT mice (Figure 6G). In addition, baseline proximal tubule IL-11 mRNA expression was lower in proximal tubule A1AR null mice compared with WT mice. Furthermore, Figure 6G also shows that CCPA treatment (0.1 mg/kg) 6 hours earlier induced IL-11 mRNA in proximal tubules isolated from WT mice but not in proximal tubules from proximal tubule A1AR null mice. These experiments further support the hypothesis that renal proximal tubule A1AR activation is required for induction of IL-11 mRNA expression in vivo.
Critical Role of IL-11 in A1AR-Mediated SK-1 Induction in HK-2 Cells and in Mouse Kidney
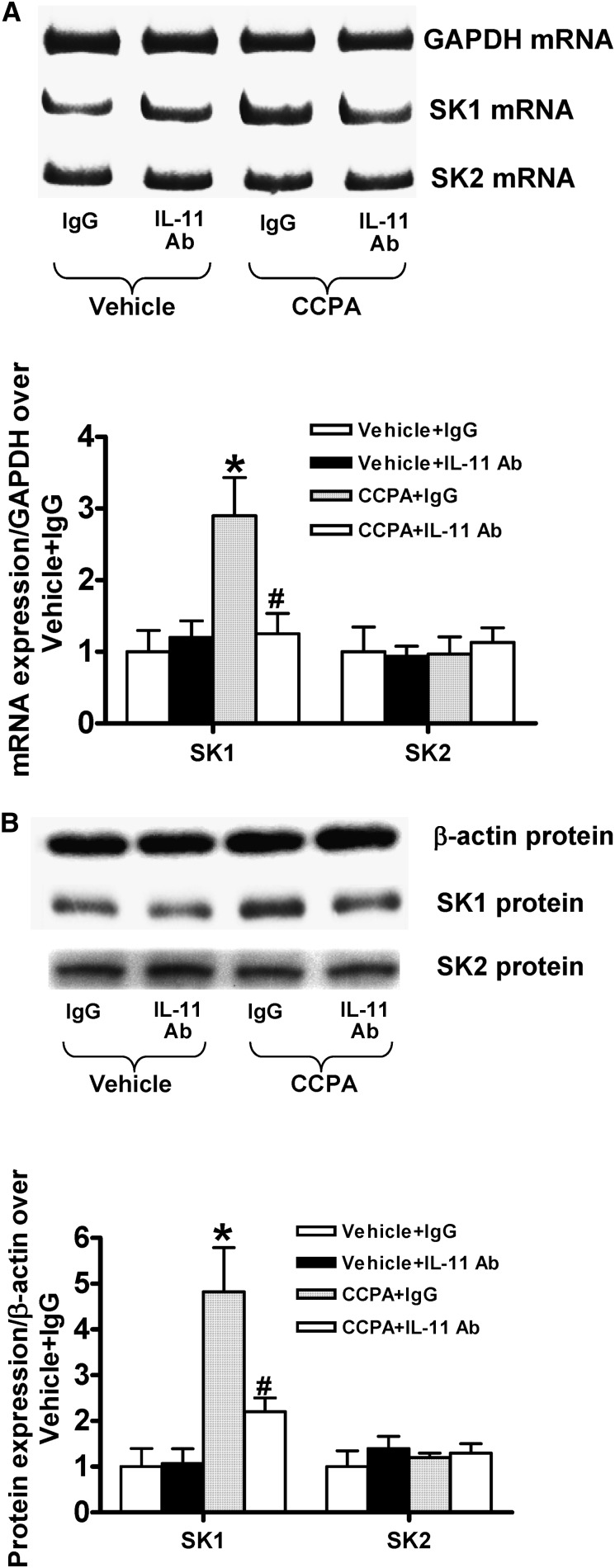
Figure 7 shows that CCPA induced SK-1 mRNA (Figure 7A) and protein (Figure 7B) in control isotype antibody–treated HK-2 cells. In contrast, IL-11–neutralizing antibody treatment significantly attenuated CCPA-mediated induction of SK-1 mRNA and protein in HK-2 cells. CCPA had no effects on SK2 expression. Consistent with these in vitro data, we also demonstrate that CCPA (0.1 mg/kg intraperitoneally) significantly induced SK-1 mRNA expression in the kidneys of IL-11R WT mice compared with kidneys from vehicle-treated mice. In contrast, CCPA failed to induce SK-1 mRNA expression in the kidneys from IL-11R KO mice (Figure 8). Again, CCPA had no effects on SK2 expression. Collectively, these in vivo and in vitro data suggest that IL-11 signaling is critical for A1AR-mediated induction of cytoprotective SK-1.
Figure 7.
IL-11 is critical for A1AR-mediated induction of SK-1 in HK-2 cells. SK-1 and SK2 mRNA (RT-PCR) (A) and protein (immunoblotting) (B) expression in HK-2 cells treated with vehicle or with 1 μM CCPA for 6 hours (n=4–5). Representative images (top) and band intensity quantifications (bottom) expressed as fold increases in SK-1 or SK2 expression over vehicle-treated cells. HK-2 cells were pretreated with control isotype antibody (IgG) or with IL-11–neutralizing antibody (10 μg/ml) 30 minutes before vehicle or CCPA treatment. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA and β-actin protein expression were also quantified to normalize lane loading. *P<0.05 versus vehicle-treatment cells pretreated with IgG isotype antibody. #P<0.05 versus CCPA-treated cells pretreated with IgG isotype antibody. Error bars represent 1 SEM.
Figure 8.
IL-11 is required for A1AR-mediated induction of SK-1 mRNA in mouse kidney. Representative images (top) and band intensity quantifications (bottom) expressed as fold increases in SK-1 or SK-2 expression over vehicle-treated mice. IL-11 WT or IL-11R–deficient (IL-11R KO) mice were treated with vehicle or with a selective A1AR agonist (CCPA, 0.1 mg/kg) 6 hours earlier. *P<0.05 versus IL-11R WT mice treated with vehicle. #P<0.05 versus IL-11R WT mice treated with CCPA. n=4. Data presented as mean ± SEM.
Discussion
AKI is a disastrous clinical complication with high mortality, morbidity, and health care costs.4,18 Surgical renal ischemia (e.g., kidney transplantation and partial nephrectomy) and renal hypoperfusion (e.g., due to cardiogenic shock or aortic surgery) are the leading causes of perioperative AKI.19 It is becoming clear that reduction in renal tubular necrosis, apoptosis, and inflammation during and after renal IR is required to attenuate the pathogenesis of ischemic AKI.3 Therapies aimed at attenuating these renal cell death pathways have great clinical significance because there is no clinically efficacious therapy for AKI.
Adenosine regulates the physiology and function of many organs and cell types. Adenosine regulates the function of the heart (heart rate, contractility), brain (sedation), and kidney (GFR and urine output), as well as vascular tone (vasomotor control), via four subtypes of G-protein–coupled purinergic receptors.20,21 In addition to its physiologic effects, we previously demonstrated that renal A1AR activation reduced kidney IR injury in mice and rats.7,9,16,22 This A1AR-mediated protection against ischemic AKI was characterized by significantly lower plasma creatinine and reductions in three major components of renal tubular cell death: necrosis, apoptosis, and inflammation.22
Although the A1AR agonist–mediated protection against ischemic AKI has significant therapeutic potential, the extrarenal effects of A1AR activation, especially at high doses (e.g., bradycardia, reduction in GFR, hypotension), may produce undesirable systemic adverse effects limiting its clinical applications.23 For example, A1AR agonist therapy may not be suitable for hypotensive patients in the intensive care unit at risk for developing AKI. Instead, exogenous application of the downstream signaling molecule generated with A1AR activation (such as IL-11) devoid of systemic hemodynamic and central nervous system effects would increase the clinical applicability to treat ischemic AKI. We also show in this study that IL-11–deficient mice had increased injury after mild renal IR (Figure 4B). In addition, we determined that proximal tubule A1AR null mice had significantly worse renal injury after 20 minutes of ischemic AKI (Figure 6C). These findings suggest that basal IL-11 production, as well as endogenous activation of proximal tubule A1ARs, provides endogenous resistance against ischemic AKI.
Recombinant human IL-11 (oprelvekin) is clinically approved to treat chemotherapy-induced thrombocytopenia. We show in this study that the A1AR activation induces IL-11 mRNA and protein synthesis in renal proximal tubule cells in vitro and mouse kidney in vivo. IL-11 is a member of the IL-6–type cytokine family isolated from bone marrow–derived stromal cells.24 IL-11 regulates hematopoiesis by promoting megakaryocyte maturation through activation of cell surface IL-11 receptors.25 In addition to its effect on platelets, recent studies suggest a cytoprotective role for IL-11, and exogenous IL-11 administration attenuates necrotic, inflammatory, and apoptotic cell death.12,26–28
We recently demonstrated that exogenous human recombinant IL-11 attenuated ischemic AKI in mice and reduced necrosis and apoptosis in HK-2 proximal tubule cells.12 In mice, IL-11 treatment attenuated renal tubular necrosis and apoptosis as well as the influx of proinflammatory neutrophils after renal IR. Not only did A1AR activation induced IL-11 in the kidney proximal tubule cells, but the antinecrotic, antiapoptotic, and anti-inflammatory effects of exogenous IL-11 treatment closely mimicked the renal protective effects of A1AR activation in mice.9,22 Furthermore, the A1AR selective agonist CCPA failed to attenuate renal histologic injury, apoptosis, and influx of proinflammatory neutrophils in IL-11R–deficient mouse kidneys after ischemic AKI. Taken together, we propose that IL-11 signaling is critical for A1AR-mediated protection against ischemic AKI. To our knowledge, the A1AR-mediated induction of IL-11 synthesis has not been described previously, and it represents a new paradigm in understanding of the cytoprotective mechanisms of A1AR activation.
The A1AR-mediated induction of IL-11 appears to be specific for kidneys because CCPA failed to induce IL-11 mRNA expression in the liver or small intestine. Moreover, CCPA failed to induce kidney IL-11 in proximal tubule A1AR null mice (Figure 6D). Consistent with this, we demonstrated significantly reduced proximal tubule IL-11 mRNA expression in proximal tubules in proximal tubule A1AR null mice (Figure 6G). Finally, renal tubular IL-11 immunoreactivity did not increase in CCPA-treated proximal tubule A1AR null mice (Figure 6E). Taken together, these findings suggest that proximal tubular induction of IL-11 represents a major component of A1AR-mediated kidney IL-11 induction. In addition, we proposed that proximal tubular induction of IL-11 plays a major role in A1AR-mediated renal protection against ischemic AKI. However, to conclusively define the role for tubular IL-11 in A1AR-mediated protection against ischemic AKI, future studies with conditional deletion of proximal tubule IL-11 or IL-11 receptors will need to be performed.
We show in this study that A1AR-mediated induction of IL-11 requires ERK MAPK signaling because a selective inhibitor of ERK PD98059 prevented CCPA-mediated induction of IL-11. Our findings are consistent with those of previous studies implicating a critical role of ERK MAPK in induction of IL-11 in human bronchial epithelial cells and intestinal myofibroblasts.29,30 Furthermore, previous studies suggest that IL-11 protects against oxidant-induced necrosis and apoptosis via mechanisms involving ERK MAPK and induction of heat shock protein 27 in vascular endothelial and intestinal epithelial cells.28,31 Interestingly, we previously showed that the cytoprotective effects of renal A1AR activation also involve ERK MAPK and heat shock protein 27 pathways.16,32 These data suggest that the A1AR and IL-11–mediated renal protection may share common signaling mechanisms in renal proximal tubule cells.
We recently showed that the A1AR-mediated protection against renal tubular necrosis, apoptosis, and inflammation requires an induction of another cytoprotective enzyme sphingosine kinase-1 (SK-1) in renal proximal tubule cells.13 Interestingly, we show in this study that a selective A1AR agonist CCPA fails to induce SK-1 in the kidneys of IL-11 receptor–deficient mice, suggesting that IL-11 signaling is required for A1AR-mediated induction of SK-1. Furthermore, we recently showed that exogenous IL-11 produces renal protection by direct induction of SK-1 via nuclear translocation of HIF-1α.12 These previous and current data strongly suggest that A1AR-mediated induction of proximal tubule SK-1 requires an intermediate signaling molecule mediated by IL-11 and IL-11 receptor activation.
SK-1 phosphorylates sphingosine to make intracellular sphingosine 1-phosphate (S1P).33 S1P is a potent lipid signaling molecule that activates five S1P receptors (S1PRs) to regulate diverse biological effects, including cell growth, survival, and modulation of inflammation.34–36 S1P1R activation in particular has been shown to reduce ischemic AKI via direct renal tubular protection against necrosis, as well as via reducing T lymphocyte–mediated renal inflammation.37,38 Activation of the S1P1Rs on endothelial cells also protects the integrity of the vascular endothelial cell barrier function.39 Collectively, the previous and current findings suggest that renal tubular A1AR activation produces two sequential renal protective signaling molecules, including IL-11, followed by induction of SK-1 and S1P.
Renal proximal tubules in the corticomedullary junction (mainly the S3 segments) are extremely susceptible to IR injury,40 and our current and previous studies confirm severe proximal tubule necrosis and apoptosis in mice subjected to renal IR. The A1AR- and IL-11–mediated renal protection are associated with significantly preserved corticomedullary junction architecture and reduction in proximal tubule necrosis and apoptosis. Therefore, we tested the hypothesis that proximal tubular A1ARs are critical for A1AR-mediated IL-11 induction as well as renal protection. We show in this study that mice deficient in proximal tubule A1ARs cannot be protected against ischemic AKI with CCPA treatment. These findings suggest that proximal tubule A1AR is critical for CCPA-mediated renal protection against ischemic AKI. In addition, our findings suggest that other cell types, including endothelial cells and leukocytes, are dispensable for A1AR-mediated renal protection. Taken together, our findings indicate that renal proximal tubule A1ARs are critical for A1AR-mediated protection against ischemic AKI, as well as induction of additional cytoprotective signaling proteins, including IL-11 and SK-1.
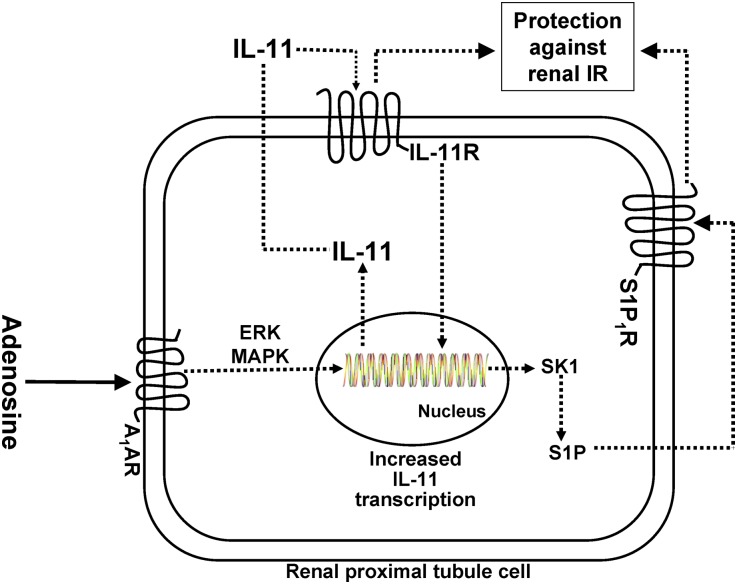
In summary, we demonstrated here that a selective A1AR agonist protects against renal tubular necrosis, apoptosis, and inflammation by inducing cytoprotective IL-11 generation. Figure 9 summarizes the potential mechanisms of A1AR-mediated renal protection involving IL-11 and SK-1 synthesis. We propose that IL-11 synthesized by A1AR activation binds to the IL-11 receptor, which then induces cytoprotective SK-1. S1P generated by SK-1 then produces additional renal tubular protection via activation of S1P1R. Our studies may lead to new therapeutic approaches (IL-11, S1P) with a drug that can reduce all three pathways of renal cell death (necrosis, apoptosis, and inflammation) to lessen the clinical perils from AKI and have implications in organ protection strategies beyond the kidney. Furthermore, IL-11–based renal protection would avoid some of the systemic adverse effects (e.g., bradycardia, hypotension, hypothermia, sedation) of A1AR agonist therapy in critically ill patients.
Figure 9.
The cytoprotective effects of A1AR activation against ischemic AKI are dependent on IL-11 mediated SK1 induction and activation. Collectively, our data suggest that A1AR activation results in increased IL-11 mRNA and protein synthesis via ERK MAPK–mediated transcription factor. We propose that IL-11 synthesized then subsequently activates IL-11R to induce additional cytoprotective signaling including SK-1 synthesis and increased S1P generation.
Concise Methods
Cell Culture and A1AR Activation
Immortalized HK-2 cells (American Type Culture Collection, Manassas, VA) were grown and passaged as described.41 To determine whether A1AR activation induces IL-11, HK-2 cells were treated with 0.1–1 μM CCPA, a selective A1AR agonist (Tocris Bioscience, Minneapolis, MN),42,43 or with vehicle (1% DMSO) for 0–6 hours. CCPA has approximately 10,000-fold selectivity and subnanomolar affinity for A1ARs. To elucidate whether MAPK or Akt signaling pathways are involved in A1AR-mediated IL-11 induction, some HK-2 cells were treated with 50 μM 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one (PD98059, a selective inhibitor of MAPK (Tocris Bioscience) or with 100 nM wortmannin (a specific, covalent inhibitor of phosphoinositide 3-kinases (Sigma, St. Louis, MO) 30 minutes before CCPA treatment. The doses of inhibitors were determined in preliminary experiments to selectively block MEK1 or PI3K to prevent ERK or Akt phosphorylation (data not shown). To determine whether A1AR-mediated IL-11 induction subsequently leads to the synthesis of new SK-1, some HK-2 cells were preincubated with 10 μg/ml of anti–IL-11 (MAB218, R&D Systems, Minneapolis, MN) 30 minutes before CCPA treatment.
Modulation of Renal IR Injury in Mice with A1AR Activation and IL-11 Deletion/Neutralization
After Columbia University Institutional Animal Care and Use Committee approval, we subjected adult male C57BL/6 (Harlan, Indianapolis, IN) and IL-11 receptor–deficient mice (IL-11R KO mice) to 20 minutes (mild) or 30 minutes (severe) of renal IR or sham operation (laparotomy, right nephrectomy without renal ischemia) as described.44,45 Heterozygous breeding pairs of IL-11R KO mice on a C57BL/6 background (B6.129S1-Il11ra1tm1Wehi/J) were obtained from Jackson Labs (Bar Harbor, ME). IL-11R WT or IL-11R KO mice were pretreated with 0.1 mg/kg CCPA (a selective A1AR agonist) or vehicle (1% DMSO) intraperitoneally 15 minutes before renal ischemia. To neutralize IL-11 in vivo, some IL-11 WT mice were pretreated with 1 mg/kg intravenous monoclonal anti–IL-11 (MAB418, R&D Systems) 30 minutes before CCPA treatment. We collected kidney (cortex and corticomedullary junction) and plasma 6 or 24 hours after IR injury to examine the severity of renal dysfunction (plasma creatinine, renal histology, apoptosis, and neutrophil infiltration) and IL-11 mRNA and protein detection. Plasma creatinine was measured as described with an enzymatic creatinine reagent kit according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA).46 We also collected kidney, liver, and intestine samples 6 hours after treatment with vehicle or with 0.1 mg/kg CCPA. Because IL-11 is a regulator of hematopoiesis and affects megakaryocyte maturation,25 we performed complete blood counts in some mice to determine whether a single injection of A1AR agonist modifies systemic platelet and leukocyte count.
Histologic Detection of Kidney Necrosis, Apoptosis, and Neutrophil Infiltration
We examined and quantified kidney morphology (hematoxylin and eosin staining), neutrophil infiltration, and apoptosis, as described previously in our studies,9,22 based on the grading system developed by Jablonski et al.17
Generation and Genotyping of Mice with Proximal Tubule–Specific Deletion of A1AR
We crossed mice carrying the floxed A1AR gene (A1ARff mice14) with PEPCK-Cre recombinase mice (provided by Dr. Volker Haase, Vanderbilt University15). A1AR-floxed mice carrying the PEPCK-Cre gene (A1ARf/f PEPCKCre/-) and control mice (A1ARf/f PEPCK−/−) were genotyped by tail biopsy PCR using Cre transgene and A1ARloxP specific primers (Table 1). PEPCK-Cre primers generated a 466-bp fragment. Flox primers amplified a 550-bp fragment for the A1AR floxed allele and a 1350-bp fragment for the A1AR WT allele. Some A1ARf/f PEPCKCre/- and A1ARf/f PEPCK−/− (controls) mice were pretreated with 0.1 mg/kg CCPA or vehicle intraperitoneally 15 minutes before renal ischemia.
Table 1.
Primers used to amplify cDNAs based on published GenBank sequences for mice.
| Primers | Accession Number | Sequence (Sense/Antisense) | Product Size (bp) | Cycle Number | Annealing Temp (°C) |
|---|---|---|---|---|---|
| Mouse IL-11 | NM_008350.4 | 5′-AACTGTGTTTGTCGCCTGGT-3′ | 267 | 30 | 68 |
| 5′-AAGCTGCAAAGATCCCAATG-3′ | |||||
| Mouse SK1 | NM_011451 | 5′-GATGCATGAGGTGGTGAATG-3′ | 337 | 22 | 64 |
| 5′-GCCCACTGTGAAACGAATC-3′ | |||||
| Mouse SK2 | NM_020011.5 | 5′-ACTGCTCGCTTCTTCTCTGC-3′ | 437 | 23 | 68 |
| 5′-ACCATTGAGGGACAGGTCAG-3′ | |||||
| Human IL-11 | NM_000641.3 | 5′-CTGAAGACTCGGCTGTGACC-3′ | 300 | 22 | 66 |
| 5′-CAGGGCAGAAGTCTGTGGAC-3′ | |||||
| Human SK1 | NM_021972 | 5′-ATCTCCTTCACGCTGATGC-3′ | 330 | 26 | 66 |
| 5′-GTGCAGAGACAGCAGGTTCA-3′ | |||||
| Human SK2 | NM_020126 | 5′-GGAGGAAGCTGTGAAGATGC-3′ | 482 | 22 | 66 |
| 5′-GCAGGTCAGACACAGAACGA-3′ | |||||
| GAPDH | M32599 | 5′-ACCACAGTCCATGCCATCAC-3′ | 450 | 15 | 65 |
| 5′-CACCACCCTGTTGCTGTAGCC-3′ | |||||
| A1ARloxP | 5′-GCCTTCCGAATCCACAGTT-3′ | Lox: 450 | 35 | 62 | |
| 5′-AGCCTCTGTTCCACATACAC-3′ | WT: 1350 | ||||
| Cre transgene | 5′-TGGGCGGCATGGTGCAAGTT-3′ | 466 | 30 | 61 | |
| 5′-CGGTGCTAACCAGCGTTTTC-3′ |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
RT-PCR
With RT-PCR, we measured mRNAs encoding human (HK-2 cells) or mouse IL-11, SK-1, and SK-2 after vehicle or CCPA treatment, as described elsewhere,47 using primers listed in Table 1. We also performed IL-11 RT-PCR in mouse liver and small intestine samples 6 hours after treatment with vehicle or with 0.1 mg/kg CCPA.
IL-11 ELISA and SK Immunoblotting
HK-2 cell supernatant or mouse kidney cortex lysate IL-11 protein expression was measured using human or mouse-specific sandwich IL-11 ELISA kit (R&D Systems), respectively. HK-2 cell lysates were collected for immunoblotting analyses of SK-1, SK2, and β-actin (internal protein loading control) as described previously.48,49
IL-11 Immunohistochemistry
Immunohistochemistry detected mouse kidney IL-11 protein expression and localization 6 hours after vehicle or CCPA (0.1 mg/kg intraperitoneally) treatment with rat anti–IL-11 antibody (MAB418; 1:50 dilution; R&D Systems) and biotin-conjugated antirat IgG (1:100 dilution; Vector Laboratories, Burlingame, CA). Normal rat IgG2a (Vector Laboratories) was used at the same concentration as the primary antibody as a negative isotype control. Renal tubular IL-11 expression was quantified as described by Kristina et al., with some modifications.50
Isolation of Mouse Kidney Proximal Tubules
Mouse kidney proximal tubules were isolated using the methods described by Vinay et al. (collagenase digestion followed by Percoll density gradient separation) as we showed previously.51,52
Statistical Analyses
The data were analyzed with t test when comparing means between two groups or one-way ANOVA plus Tukey post hoc multiple comparison test when comparing multiple groups. The ordinal values of the renal injury scores were analyzed by the Mann-Whitney nonparametric test. In all cases, P<0.05 was taken to indicate significance. All data are expressed throughout the text as means ± SEM. Power analysis was performed with G.power 3.1 software.53
A complete description of the methods is available in the Supplemental Material.
Disclosures
None.
Supplementary Material
Acknowledgments
We appreciate the surgical, analytical, and technical assistance provided by Sang Won Park. We thank Dr. Volker Haase of Vanderbilt University for providing us with breeding pairs of PEPCK-Cre recombinase mice.
This work was supported in part by Department of Anesthesiology, Columbia University, R01 DK-058547 and R01 GM-067081 (to H.T.L.) and by Department of Veterans Affairs and R01NS075545 (to R.W.G.).
An abstract form of this work was presented at the 2012 American Society of Nephrology meeting in San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013010114/-/DCSupplemental.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Elapavaluru S, Kellum JA: Why do patients die of acute kidney injury? Acta Clin Belg Suppl 326–331, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Bonventre JV, Weinberg JM: Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Jones DR, Lee HT: Perioperative renal protection. Best Pract Res Clin Anaesthesiol 22: 193–208, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Haskó G, Linden J, Cronstein B, Pacher P: Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7: 759–770, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauerle JD, Grenz A, Kim JH, Lee HT, Eltzschig HK: Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol 22: 14–20, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Lee HT, Emala CW: Protective effects of renal ischemic preconditioning and adenosine pretreatment: Role of A(1) and A(3) receptors. Am J Physiol Renal Physiol 278: F380–F387, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Lee HT, Emala CW: Protein kinase C and G(i/o) proteins are involved in adenosine- and ischemic preconditioning-mediated renal protection. J Am Soc Nephrol 12: 233–240, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW: A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol 286: F298–F306, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Goldman SC, Bracho F, Davenport V, Slack R, Areman E, Shen V, Lenarsky C, Weinthal J, Hughes R, Cairo MS: Feasibility study of IL-11 and granulocyte colony-stimulating factor after myelosuppressive chemotherapy to mobilize peripheral blood stem cells from heavily pretreated patients. J Pediatr Hematol Oncol 23: 300–305, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Du X, Williams DA: Interleukin-11: Review of molecular, cell biology, and clinical use. Blood 89: 3897–3908, 1997 [PubMed] [Google Scholar]
- 12.Lee HT, Park SW, Kim M, Ham A, Anderson LJ, Brown KM, D’Agati VD, Cox GN: Interleukin-11 protects against renal ischemia and reperfusion injury. Am J Physiol Renal Physiol 303: F1216–F1224, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SW, Kim M, Kim JY, Brown KM, Haase VH, D’Agati VD, Lee HT: Proximal tubule sphingosine kinase-1 has a critical role in A1 adenosine receptor-mediated renal protection from ischemia. Kidney Int 82: 878–891, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scammell TE, Arrigoni E, Thompson MA, Ronan PJ, Saper CB, Greene RW: Focal deletion of the adenosine A1 receptor in adult mice using an adeno-associated viral vector. J Neurosci 23: 5762–5770, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rankin EB, Tomaszewski JE, Haase VH: Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joo JD, Kim M, Horst P, Kim J, D’Agati VD, Emala CW, Sr, Lee HT: Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol 293: F1847–F1857, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J: An experimental model for assessment of renal recovery from warm ischemia. Transplantation 35: 198–204, 1983 [DOI] [PubMed] [Google Scholar]
- 18.Faubel S: Acute kidney injury and multiple organ dysfunction syndrome. Minerva Urol Nefrol 61: 171–188, 2009 [PubMed] [Google Scholar]
- 19.Ikeda M, Prachasilchai W, Burne-Taney MJ, Rabb H, Yokota-Ikeda N: Ischemic acute tubular necrosis models and drug discovery: A focus on cellular inflammation. Drug Discov Today 11: 364–370, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Linden J: Molecular approach to adenosine receptors: Receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol 41: 775–787, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Vallon V,, Osswald H: Adenosine receptors and the kidney. Handb Exp Pharmacol 443–470, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HT, Gallos G, Nasr SH, Emala CW: A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 15: 102–111, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Baraldi PG, Iaconinoto MA, Moorman AR, Carrion MD, Cara CL, Preti D, López OC, Fruttarolo F, Tabrizi MA, Romagnoli R: Allosteric enhancers for A1 adenosine receptor. Mini Rev Med Chem 7: 559–569, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kaye JA: The clinical development of recombinant human interleukin 11 (NEUMEGA rhIL-11 growth factor). Stem Cells 14[Suppl 1]: 256–260, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Reynolds CH: Clinical efficacy of rhIL-11. Oncology (Williston Park) 14[Suppl 8]: 32–40, 2000 [PubMed] [Google Scholar]
- 26.Kimura R, Maeda M, Arita A, Oshima Y, Obana M, Ito T, Yamamoto Y, Mohri T, Kishimoto T, Kawase I, Fujio Y, Azuma J: Identification of cardiac myocytes as the target of interleukin 11, a cardioprotective cytokine. Cytokine 38: 107–115, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kuenzler KA, Pearson PY, Schwartz MZ: IL-11 pretreatment reduces cell death after intestinal ischemia-reperfusion. J Surg Res 108: 268–272, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Waxman AB, Mahboubi K, Knickelbein RG, Mantell LL, Manzo N, Pober JS, Elias JA: Interleukin-11 and interleukin-6 protect cultured human endothelial cells from H2O2-induced cell death. Am J Respir Cell Mol Biol 29: 513–522, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Bamba S, Andoh A, Yasui H, Makino J, Kim S, Fujiyama Y: Regulation of IL-11 expression in intestinal myofibroblasts: role of c-Jun AP-1- and MAPK-dependent pathways. Am J Physiol Gastrointest Liver Physiol 285: G529–G538, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Lecureur V, Arzel M, Ameziane S, Houlbert N, Le Vee M, Jouneau S, Fardel O: MAPK- and PKC/CREB-dependent induction of interleukin-11 by the environmental contaminant formaldehyde in human bronchial epithelial cells. Toxicology 292: 13–22, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Ropeleski MJ, Tang J, Walsh-Reitz MM, Musch MW, Chang EB: Interleukin-11-induced heat shock protein 25 confers intestinal epithelial-specific cytoprotection from oxidant stress. Gastroenterology 124: 1358–1368, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Lee HT, Kim M, Jan M, Penn RB, Emala CW: Renal tubule necrosis and apoptosis modulation by A1 adenosine receptor expression. Kidney Int 71: 1249–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Leclercq TM, Pitson SM: Cellular signalling by sphingosine kinase and sphingosine 1-phosphate. IUBMB Life 58: 467–472, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Allende ML, Dreier JL, Mandala S, Proia RL: Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem 279: 15396–15401, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Chae SS, Proia RL, Hla T: Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat 73: 141–150, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh NS, Khan F, Proia RL, Hla T: Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J 397: 461–471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jo SK, Bajwa A, Awad AS, Lynch KR, Okusa MD: Sphingosine-1-phosphate receptors: Biology and therapeutic potential in kidney disease. Kidney Int 73: 1220–1230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai LW, Yong KC, Igarashi S, Lien YH: A sphingosine-1-phosphate type 1 receptor agonist inhibits the early T-cell transient following renal ischemia-reperfusion injury. Kidney Int 71: 1223–1231, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Spiegel S: Sphingosine 1-phosphate: A ligand for the EDG-1 family of G-protein-coupled receptors. Ann N Y Acad Sci 905: 54–60, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Molitoris BA, Dahl R, Geerdes A: Cytoskeleton disruption and apical redistribution of proximal tubule Na(+)-K(+)-ATPase during ischemia. Am J Physiol 263: F488–F495, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Lee HT, Emala CW: Adenosine attenuates oxidant injury in human kidney proximal tubular cells via A(1) and A(2a) adenosine receptors. Am J Physiol Renal Physiol 282: F844–F852, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Klotz KN, Lohse MJ, Schwabe U, Cristalli G, Vittori S, Grifantini M: 2-Chloro-N6-[3H]cyclopentyladenosine ([3H]CCPA)—a high affinity agonist radioligand for A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol 340: 679–683, 1989 [DOI] [PubMed] [Google Scholar]
- 43.Lohse MJ, Klotz KN, Schwabe U, Cristalli G, Vittori S, Grifantini M: 2-Chloro-N6-cyclopentyladenosine: Aa highly selective agonist at A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol 337: 687–689, 1988 [DOI] [PubMed] [Google Scholar]
- 44.Kim M, Chen SW, Park SW, Kim M, D’Agati VD, Yang J, Lee HT: Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int 75: 809–823, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim M, Park SW, Kim M, Chen SW, Gerthoffer WT, D'Agati VD, Lee HT: Selective renal overexpression of human heat shock protein 27 reduces renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 299: F347–F358, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slot C: Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest 17: 381–387, 1965 [DOI] [PubMed] [Google Scholar]
- 47.Kim M, Kim M, Park SW, Pitson SM, Lee HT: Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol 31: 353–362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park SW, Kim M, Chen SW, Brown KM, D’Agati VD, Lee HT: Sphinganine-1-phosphate protects kidney and liver after hepatic ischemia and reperfusion in mice through S1P1 receptor activation. Lab Invest 90: 1209–1224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park SW, Kim M, Chen SW, D’Agati VD, Lee HT: Sphinganine-1-phosphate attenuates both hepatic and renal injury induced by hepatic ischemia and reperfusion in mice. Shock 33: 31–42, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matkowskyj KA, Schonfeld D, Benya RV: Quantitative immunohistochemistry by measuring cumulative signal strength using commercially available software photoshop and matlab. J Histochem Cytochem 48: 303–312, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Park SW, Kim M, Brown KM, D’Agati VD, Lee HT: Inhibition of sphingosine 1-phosphate receptor 2 protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 23: 266–280, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vinay P, Gougoux A, Lemieux G: Isolation of a pure suspension of rat proximal tubules. Am J Physiol 241: F403–F411, 1981 [DOI] [PubMed] [Google Scholar]
- 53.Faul F, Erdfelder E, Buchner A, Lang AG: Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods 41: 1149–1160, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.