Abstract
Physician caseload may be a predictor of patient outcomes associated with various medical conditions and procedures, but the association between patient-physician ratio and mortality among patients undergoing hemodialysis has not been determined. We examined whether a higher patient-nephrologist ratio affects patient mortality risk using de-identified data from DaVita dialysis clinics and the U.S. Renal Data System. A total of 41 nephrologists with a caseload of 50–200 hemodialysis patients from an urban California region were retrospectively ranked according to their hemodialysis patient mortality rate during a 6-year period between 2001 and 2007. We calculated all-cause mortality hazard ratios for each nephrologist and compared patient- and provider-level characteristics between the 10 nephrologists with the highest patient mortality rates and the 10 nephrologists with the lowest patient mortality rates. Nephrologists with the lowest patient mortality rates had significantly lower patient caseloads than nephrologists with the highest mortality rates (median [interquartile range], 65 [55–76] versus 103 [78–144] patients per nephrologist, respectively; P<0.001). Additionally, patients treated by nephrologists with the lowest patient mortality rates received higher dialysis doses, had longer sessions, and received more kidney transplants. In demographic characteristic–adjusted analyses, each 50-patient increase in caseload was associated with a 2% increase in patient mortality risk (hazard ratio, 1.02; 95% confidence interval, 1.00 to 1.04; P<0.001). Hence, these results suggest that nephrologist caseload influences hemodialysis patient outcomes, and future research should focus on identifying the factors underlying this association.
Currently more than 400,000 patients are receiving long-term hemodialysis (HD) therapy for ESRD in the United States. Despite advancements in technology, patients with ESRD experience a seven-fold higher risk of death compared with the general population.1 To reduce the high burden of morbidity and mortality among dialysis patients,2,3 clinical practice guidelines, such as the National Kidney Foundation’s Kidney Disease Outcome Quality Initiative, have established targets for dialysis prescription and kidney disease complications (e.g., anemia, mineral bone disease, and malnutrition).4 In the past decade, several studies have sought to identify determinants of HD patient morbidity and mortality at the patient level (such as case mix and socioeconomic factors) and at the facility level (such as urban versus rural dialysis center and remote distance from dialysis center5–7).
In addition to patient and facility-level characteristics, there has been increasing examination of provider-level factors, such as physician caseload (patient-to-physician ratio), as predictors of mortality among various conditions and procedures.8–19 Physician caseload has been inversely associated with risk of adverse outcomes for both surgical procedures12 and medical conditions (e.g., AIDS,13 sepsis,9 and myocardial infarction8). However, for some complex medical conditions (such as diabetes18 and pneumonia16), high physician caseload has been associated with reduced adherence to clinical practice guidelines and process measures, but not worse outcomes per se.
Patients with ESRD are a unique population with a high burden of complex comorbid conditions who are routinely subjected to specialized procedures. In this regard, there may be a differential association between provider caseload and outcomes in this population. In one study, high HD patient-to-nurse ratios were associated with an increased likelihood of patient dissatisfaction, reduced adherence to dialysis (i.e., skipped and/or shortened treatments), and increased intradialytic hypotension.20 However, to our knowledge no studies have examined the association between patient-to-physician ratio and HD patient mortality. To better inform the field, we examined the association between nephrologist caseload and HD patient all-cause mortality within a large for-profit dialysis provider in a densely populated urban region in California during a 6-year period. We hypothesized that patients receiving care from nephrologists with higher caseloads had incrementally greater mortality than those receiving care from lower-caseload nephrologists.
Results
Cohort Description
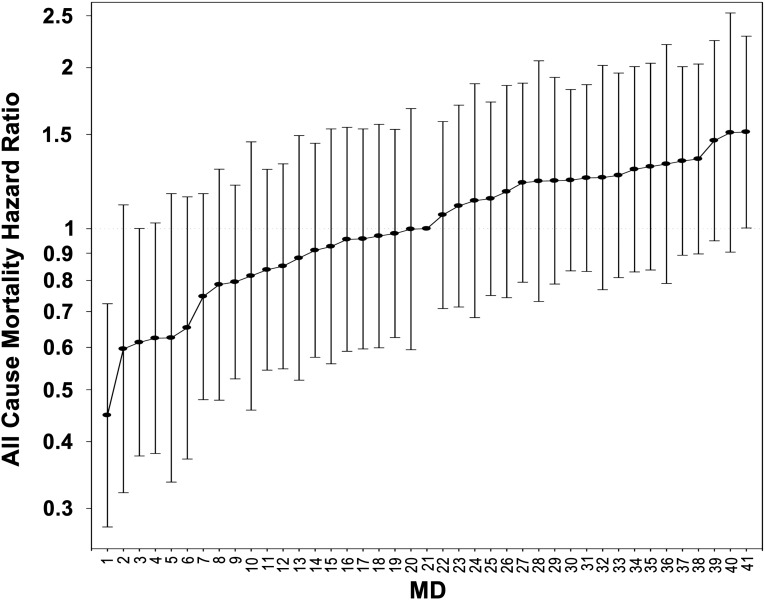
The national database of all DaVita dialysis patients included 164,789 patients during the 6-year period from July 1, 2001, through June 30, 2007. From this cohort, we excluded patients who did not have information in the base quarter, resided outside of Los Angeles County (as determined by a non-“90xxx” ZIP code), were <18 years of age, received treatment from a nephrologist with a discrepancy in patient assignment and outcomes, were receiving a non-HD modality (i.e., peritoneal dialysis), or had missing cohort time. Table 1 shows patient characteristics and HD treatment data for the Los Angeles County HD patient population irrespective of nephrologist caseload (n=7600) and broken down in caseload increments of 50. We then excluded patients who received care from nephrologists with caseload of <50 or >200 HD. The final cohort consisted of 4073 HD patients under the care of 41 Los Angeles County nephrologists. Table 2 presents further analysis of patient characteristics of the 10 nephrologists with the lowest hazard ratios (HRs) (n=820) and the highest HRs (n=1051). Figure 1 shows the death hazard ratios of the 41 nephrologists included in Table 2.
Table 1.
Baseline demographic, clinical, and biochemical characteristics of all HD patients within the study cohort
| Variables | All Patients (n=7600) and MDs (n=341) | Nephrologist Caseload | P Value | |||
|---|---|---|---|---|---|---|
| 0 to <50: Patients (n=1899) MDs (n=294) | 50 to <100: Patients (n=1443) MDs (n=22) | 100 to <150: Patients (n=1576) MDs (n=13) | ≥150: Patients (n=2682) MDs (n=12) | |||
| Mortality, n (%) | 3,900 (51) | 908 (48) | 716 (50) | 843 (53) | 1,433 (53) | <0.001 |
| Transplant, n (%) | 580 (8) | 148 (8) | 137 (9) | 102 (6) | 193 (7) | 0.11 |
| Mean dialysis session time (min) | 200±41 | 200±40 | 203±46 | 198±41 | 199±40 | 0.11 |
| Median dialysis session time (min) | 210 (180, 210) | 210 (180, 210) | 210 (180, 230) | 210 (180, 210) | 210 (180, 210) | 0.11 |
| Nephrologist caseload | 112 (49, 192) | 21 (8, 36) | 67 (60, 78) | 119 (111, 138) | 205 (192, 345) | n/a |
| Cohort time (d) | 902 (472, 1461) | 879 (484, 1350) | 983 (519, 1703) | 843 (456, 1422) | 911 (457, 1488) | 0.12 |
| Average patient cohort time per MD (d) | 996 (737, 1218) | 962 (720, 1242) | 1071 (1013, 1184) | 933 (869, 1018) | 999 (965, 1052) | 0.69 |
| Age | 60±16 | 61±16 | 60±16 | 60±15 | 60±15 | 0.03 |
| Women (%) | 47 | 46 | 47 | 48 | 49 | 0.08 |
| Diabetes mellitus (%) | 63 | 60 | 59 | 66 | 67 | <0.001 |
| Race (%) | ||||||
| White | 13 | 18 | 16 | 12 | 8 | <0.001 |
| Black | 25 | 25 | 22 | 29 | 23 | 0.78 |
| Hispanic | 48 | 37 | 43 | 47 | 58 | <0.001 |
| Asian | 7 | 11 | 8 | 5 | 5 | <0.001 |
| Other | 7 | 9 | 11 | 7 | 6 | <0.001 |
| Vintage (time on dialysis) (%) | ||||||
| 0–6 mo | 59 | 59 | 54 | 61 | 60 | 0.15 |
| 6–24 mo | 16 | 18 | 18 | 14 | 15 | <0.001 |
| 2–5 yr | 16 | 15 | 18 | 15 | 16 | 0.56 |
| >5 yr | 9 | 8 | 10 | 10 | 9 | 0.28 |
| Primary insurance (%) | ||||||
| Medicare | 55 | 58 | 58 | 52 | 54 | <0.001 |
| Medicaid | 21 | 18 | 24 | 20 | 24 | <0.001 |
| Private insurance | 12 | 7 | 11 | 15 | 13 | <0.001 |
| Other | 12 | 17 | 7 | 13 | 9 | <0.001 |
| Marital status (%) | ||||||
| Married | 45 | 43 | 44 | 46 | 47 | 0.03 |
| Divorced | 6 | 7 | 6 | 6 | 6 | 0.75 |
| Single | 35 | 36 | 36 | 32 | 34 | 0.14 |
| Widowed | 14 | 14 | 14 | 16 | 13 | 0.30 |
| Kt/V (dialysis dose) | 1.50±0.36 | 1.52±0.39 | 1.55±0.37 | 1.46±0.32 | 1.50±0.35 | <0.001 |
| Residual renal function (ml/min) | 0.31±1.15 | 0.40±1.31 | 0.46±1.35 | 0.24±1.05 | 0.20±0.95 | <0.001 |
| Access type (%) | ||||||
| Arteriovenous fistula | 26 | 25 | 29 | 23 | 27 | 0.68 |
| Graft | 29 | 28 | 28 | 30 | 30 | 0.14 |
| Catheter | 31 | 32 | 28 | 34 | 31 | 0.80 |
| Other | 1 | 1 | 1 | 1 | 1 | 0.59 |
| Unknown | 13 | 14 | 14 | 12 | 11 | 0.003 |
| Comorbid conditions (%) | ||||||
| AIDS | <1 | 3 | 6 | 6 | <1 | 0.30 |
| Cancer | 2 | 2 | 2 | 2 | 2 | 0.11 |
| Heart failure | 21 | 19 | 17 | 23 | 23 | <0.001 |
| Peripheral vascular disease | 5 | 5 | 4 | 4 | 7 | 0.001 |
| HIV-positive | <1 | 7 | 6 | <1 | <1 | 0.05 |
| Nonambulatory | 4 | 3 | 3 | 4 | 4 | 0.16 |
| Pulmonary disease | 2 | 2 | 2 | 2 | 2 | 0.84 |
| Current Smoker | 2 | 2 | 2 | 2 | 2 | 0.88 |
| Atherosclerosis | 10 | 9 | 9 | 11 | 10 | 0.14 |
| Cerebrovascular disease | 5 | 5 | 3 | 5 | 5 | 0.14 |
| Hypertension | 76 | 75 | 73 | 81 | 76 | 0.05 |
| Other cardiovascular | 3 | 3 | 3 | 3 | 3 | 0.21 |
| Serum levels | ||||||
| Creatinine (mg/dl) | 8.3±3.4 | 8.0±3.2 | 8.5±3.3 | 8.3±3.4 | 8.4±3.5 | 0.006 |
| Albumin (g/dl) | 3.67±0.48 | 3.64±0.49 | 3.75±0.45 | 3.66±0.49 | 3.65±0.48 | 0.19 |
| Blood hemoglobin (g/dl) | 11.9±1.3 | 11.9±1.2 | 11.9±1.2 | 11.9±1.2 | 11.9±1.3 | 0.88 |
| White blood cell count (×103/μl) | 7.5±2.4 | 7.5±2.6 | 7.3±2.3 | 7.6±2.4 | 7.5±2.3 | 0.61 |
| Ferritin (ng/ml) | 415 (185, 780) | 411 (188, 736) | 451 (205, 838) | 409 (187, 767) | 398 (173, 785) | 0.96 |
| TIBC (mg/dl) | 207±46 | 206±48 | 207±45 | 205±45 | 207±45 | 0.75 |
| Lymphocyte (% of total white blood cells) | 21±8 | 22±8 | 22±7 | 21±8 | 21±7 | 0.36 |
| Calcium (mg/dl) | 9.1±0.7 | 9.1±0.7 | 9.2±0.7 | 9.2±0.7 | 9.1±0.7 | 0.54 |
| Parathyroid hormone (pg/ml) | 251 (143, 440) | 255 (145, 454) | 252 (143, 438) | 255 (160, 463) | 240 (135, 415) | 0.34 |
| Alkaline phosphatase (U/L) | 100 (78, 134) | 99 (77, 131) | 100 (76, 134) | 101 (77, 135) | 100 (79, 134) | 0.38 |
| Phosphorus (mg/dl) | 5.5±1.5 | 5.5±1.5 | 5.5±1.4 | 5.5±1.5 | 5.6±1.5 | 0.05 |
| Bicarbonate (mg/dl) | 22.8±3.0 | 22.9±3.0 | 23.3±2.9 | 22.6±2.9 | 22.5±2.9 | <0.001 |
| Body mass index (kg/m2) | 26.0±6.5 | 25.9±7.8 | 25.7±5.8 | 26.1±6.2 | 26.1±6.1 | 0.21 |
| Protein catabolic rate (g/kg per day) | 0.98±0.26 | 0.99±0.27 | 1.01±0.26 | 0.97±0.25 | 0.97±0.26 | <0.001 |
Data presented as percentages, means ± SDs, and medians (25th percentile, 75th percentile). Significant differences between lowest versus highest mortality MD groups estimated using chi-square test, t test, and Wilcoxon rank-sum test according to data type. MD, nephrologist; TIBC, total iron-binding capacity.
Table 2.
Baseline demographic, clinical, and biochemical characteristics of all HD patients within the study cohort and those receiving care from the 10 0nephrologists with the lowest mortality hazard ratios and the 10 nephrologists with the highest mortality hazard ratios
| Variables | All Patients (n=4073) and MDs (n=41) | Lowest Mortality: Patients (n=820) MDs (n=10) | Highest Mortality: Patients (n=1051) MDs (n=10) | P Value |
|---|---|---|---|---|
| Mortality, n (%) | 2102 (52) | 344 (42) | 594 (57) | <0.0001 |
| Transplant, n (%) | 297 (7) | 75 (9) | 68 (6) | 0.031 |
| Mean dialysis session time (min) | 205±26 | 210±24 | 201±22 | <0.0001 |
| Median dialysis session time (min) | 210 (180, 212) | 210 (188, 230) | 210 (180, 210) | |
| Nephrologist caseload | 86 (62, 123) | 65 (55, 76) | 103 (78, 144) | <0.0001 |
| Cohort time (d) | 893 (467, 1523) | 997 (542, 1705) | 816 (440, 1461) | <0.0001 |
| Average patient cohort time per MD (d) | 1018 (924, 1088) | 1145 (1013,1289) | 987 (869, 1058) | <0.0001 |
| Age | 60±16 | 60±16 | 60±15 | 0.90 |
| Women (%) | 48 | 47 | 47 | 0.86 |
| Diabetes mellitus (%) | 63 | 59 | 65 | 0.006 |
| Race (%) | ||||
| White | 13 | 16 | 8 | <0.0001 |
| Black | 27 | 28 | 24 | 0.05 |
| Hispanic | 47 | 44 | 57 | <0.0001 |
| Asian | 6 | 4 | 4 | 0.44 |
| Other | 7 | 8 | 7 | 0.38 |
| Vintage (time on dialysis) (%) | ||||
| 0–6 mo | 12 | 12 | 11 | 0.72 |
| 6–24 mo | 30 | 31 | 29 | 0.42 |
| 2–5 yr | 33 | 33 | 32 | 0.57 |
| >5 yr | 25 | 25 | 28 | 0.09 |
| Primary insurance (%) | ||||
| Medicare | 53 | 53 | 54 | 0.51 |
| Medicaid | 22 | 26 | 21 | 0.007 |
| Private insurance | 14 | 12 | 15 | 0.04 |
| Other | 11 | 9 | 10 | 0.76 |
| Marital status (%) | ||||
| Married | 45 | 47 | 48 | 0.63 |
| Divorced | 6 | 7 | 6 | 0.22 |
| Single | 35 | 35 | 31 | 0.13 |
| Widowed | 14 | 11 | 15 | 0.03 |
| Kt/V (dialysis dose) | 1.51±0.35 | 1.53±0.34 | 1.49±0.36 | 0.01 |
| Residual renal function (ml/min) | 0.32±1.19 | 0.37±1.15 | 0.43±1.39 | 0.35 |
| Access type (%) | ||||
| Arteriovenous fistula | 25 | 31 | 24 | 0.001 |
| Graft | 29 | 30 | 31 | 0.6 |
| Catheter | 31 | 29 | 30 | 0.62 |
| Other | 1 | 1 | 1 | 0.83 |
| Unknown | 13 | 9 | 14 | 0.001 |
| Comorbid conditions (%) | ||||
| AIDS | <1 | <1 | <1 | 0.37 |
| Cancer | 1 | 2 | 2 | 0.54 |
| Heart failure | 20 | 18 | 23 | 0.02 |
| Peripheral vascular disease | 4 | 4 | 4 | 0.75 |
| HIV-positive | 2 | 0 | 0 | NA |
| Nonambulatory | 3 | 3 | 3 | 0.55 |
| Pulmonary disease | 2 | 2 | 2 | 0.58 |
| Current Smoker | 2 | 1 | 2 | 0.68 |
| Atherosclerosis | 10 | 11 | 11 | 0.75 |
| Cerebrovascular disease | 4 | 3 | 5 | 0.14 |
| Hypertension | 76 | 72 | 79 | <0.001 |
| Other cardiovascular | 3 | 2 | 2 | 0.66 |
| Serum levels | ||||
| Creatinine (mg/dl) | 8.4±3.4 | 8.3±3.3 | 8.6±3.5 | 0.05 |
| Albumin (g/dl) | 3.69±0.48 | 3.72±0.46 | 3.69±0.47 | 0.13 |
| Blood hemoglobin (g/dl) | 11.9±1.2 | 11.9±1.2 | 12.0±1.3 | 0.64 |
| White blood cell count (×103/μl) | 7.5±2.4 | 7.2±2.2 | 7.6±2.3 | <0.001 |
| Ferritin (ng/ml) | 567±581 | 567±611 | 567±565 | 0.99 |
| TIBC (mg/dl) | 206±45 | 208±44 | 205±47 | 0.15 |
| Lymphocyte (% of total white blood cells) | 21±8 | 22±7 | 21±7 | <0.001 |
| Calcium (mg/dl) | 9.2±0.7 | 9.1±0.7 | 9.2±0.7 | 0.19 |
| Parathyroid hormone (pg/ml) | 369±375 | 375±350 | 368±391 | 0.73 |
| Alkaline phosphatase (U/L) | 120±86 | 119±86 | 122±91 | 0.43 |
| Phosphorus (mg/dl) | 5.5±1.5 | 5.5±1.4 | 5.5±1.4 | 0.37 |
| Bicarbonate (mg/dl) | 22.9±2.9 | 23.1±2.9 | 22.7±3.0 | 0.007 |
| Body mass index (kg/m2) | 25.9±6.1 | 25.6±5.9 | 25.9±5.6 | 0.31 |
| Protein catabolic rate (g/kg per day) | 0.98±0.26 | 1.01±0.26 | 0.98±0.26 | 0.02 |
Data presented as percentages, means ± SDs, and medians (25th percentile, 75th percentile). Significant differences between lowest versus highest mortality MD groups estimated using chi-square test, t test, and Wilcoxon rank-sum test according to data type. MD, nephrologist; NA, not available; TIBC, total iron-binding capacity.
Figure 1.
All cause mortality hazard ratios for patients of 41 Los Angeles County Nephrologists. Analyses adjusted for entry calendar quarter, age, sex, diabetes, race/ethnicity, vintage, primary insurance, marital status, dialysis dose, residual renal function, 10 comorbid conditions, and vascular access type.
During the follow-up period, a greater proportion of deaths and a lower proportion of kidney transplantations occurred among patients receiving treatment from nephrologists associated with the highest mortality risk than those with the lowest mortality risk. Compared with patients cared for by nephrologists with the highest mortality risk, those cared for by nephrologists with the lowest mortality risk tended to have a lower prevalence of Hispanic race/ethnicity, diabetes, heart failure, hypertension, and private insurance as their primary insurer and a higher prevalence of African-American and white race/ethnicity, arteriovenous fistulas, and Medicaid as their primary insurer. Additionally, patients cared for by nephrologists with the lowest mortality risk had, on average, longer dialysis session lengths, higher delivered dialysis doses, and some but not all laboratory measures consistent with less inflammation (lower white blood cell count) and greater protein intake (increased normalized protein catabolic rate).
Nephrologist Caseload and Mortality
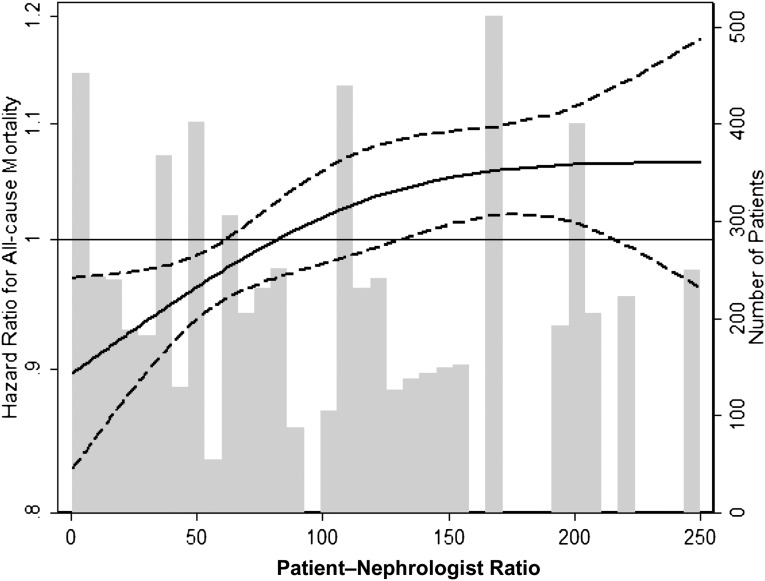
In survival analyses, nephrologists with the highest patient mortality HRs had a significantly larger caseload than nephrologists with the lowest patient mortality HRs (median [interquartile range], 103 [78, 144] versus 65 [55, 76] patients/nephrologists, respectively; P<0.001). In case mix–adjusted analyses, patients receiving care from higher-caseload nephrologists had greater mortality, such that for every increase in caseload of 50 patients/nephrologist, there was a 2% higher mortality risk (HR, 1.02; 95% confidence interval, 1.00 to 1.04; P<0.001). When nephrologist caseload was examined as a restricted cubic spline function, excluding two outlier physicians, each with >250 patients, there appeared to be a monotic increase in mortality risk from 50 patients up to a peak of 140 patients, after which risk plateaued (Figure 2).
Figure 2.
Cubic spline of increasing all cause mortality with increasing patient-nephrologist ratio. Analyses adjusted for entry calendar quarter, age, sex, diabetes, race/ethnicity, vintage, primary insurance, marital status, dialysis dose, residual renal function, 10 comorbid conditions, and vascular access type.
Discussion
To our knowledge, this is the first study to evaluate the relationship between individual nephrologists, nephrologist caseload, and HD patient mortality risk. In this study, we examined a contemporary cohort of 7600 patients undergoing maintenance HD treated in a large United States–based dialysis organization during a period of 6 years. We found that among Los Angeles County nephrologists caring for 50–200 patients, nephrologists with lower patient mortality HRs had a significantly smaller caseload than those with higher patient mortality HRs. Additionally, nephrologists with lower patient mortality HRs tended to have more favorable case-mix and delivered-dialysis characteristics (e.g., longer dialysis session lengths and greater dialysis doses). After accounting for differences in case mix, we found that patients receiving care from higher-caseload nephrologists had greater mortality than those receiving care from lower-caseload nephrologists and that this association was robust in both categorical and restricted cubic spline analyses.
The findings contrast with those of prior studies showing an inverse association between provider caseload and patient outcomes in the context of surgical12,21 and invasive cardiovascular11 procedures and medical conditions (e.g., acute myocardial infarction,8 sepsis,9 pulmonary embolus,14 and AIDS13). In a systematic review evaluating the effect of physician volume on outcomes, 69% of studies showed that high provider volume was a determinant of improved patient outcomes.10 There have been several explanations for this pattern of association. First, it has been hypothesized that provider caseload may be a proxy for a physician's experience, knowledge, skills, and/or quality of care with which to manage particular diseases.8,9,13 Second, there may be a selection factor in which (1) physicians who achieve favorable patient outcomes will receive more patient referrals or (2) patients may self-refer to physicians who care for patients with similar characteristics.9,10 Third, these observations may reflect not only physician practice patterns but also the system in which the provider works. For example, physicians with high patient caseloads may belong to high-volume facilities with greater access to resources (e.g., multidisciplinary care teams) that confer improved outcomes.15 In contrast, in studies of ambulatory diabetes and inpatient pneumonia cohorts, high patient volume was associated with reduced adherence to process measures (e.g., decreased hemoglobin A1c screening and vaccine screening/administration, respectively); however, this did not translate into worse outcomes (e.g., no differences in A1c levels or patient mortality, respectively).16,18
Several factors may explain why a differential association between physician caseload and mortality may exist among HD patients. First, the aforementioned studies of patients without ESRD examined acute procedures and conditions (e.g., pulmonary embolus14) or short-term outcomes of chronic conditions (i.e., in-hospital or 30-day mortality).8,9,11,13–15,17,19 In contrast, our study evaluated long-term outcomes in a largely ambulatory setting, which is more representative of the longitudinal care that nephrologists provide to maintenance HD patients. Second, HD patients have a disproportionate burden of comorbid conditions and kidney disease–related complications and routinely undergo specialized procedures that require complex decision-making from a multidisciplinary team of providers (i.e., nephrologist, dialysis nurse, vascular access surgeon, dietician, social worker). This population may thus require a substantial amount of individualized management and communication with multidisciplinary team members that may be more difficult to achieve among high-caseload nephrologists. Third, in caring for HD patients, there may be a caseload threshold above which the benefits of acquiring greater experience and knowledge is outweighed by insufficient time and attention provided to individual patients. In this study, we focused on patients receiving care from nephrologists with a caseload of 50–200, and it is possible that this threshold exists below this lower limit.
The association between nephrologist caseload and outcomes may have important policy implications given the high prevalence, costs, mortality rate, and health care burden of ESRD. First, identifying mechanisms by which increased caseload confers worse outcomes may provide an opportunity to improve the static morbidity and mortality rates of patients with ESRD. For example, when we compared nephrologists with the highest patient mortality HRs (with a higher median caseload) to those with the lowest patient HRs, patients from the former group had shorter median dialysis session lengths than those in the latter group. Given the association between shorter dialysis session lengths and greater mortality,22 this may be a process of care by which high caseload confers greater mortality. However, it should be noted that these values represent delivered as opposed to prescribed indicators, which may be influenced by patient-level (e.g., nonadherence) or facility-level (e.g., overcrowding) practices as opposed to physician-level factors. Second, there has been increasing concern about an impending nephrologist workforce crisis in the context of a declining interest in nephrology as a career among trainees.23 If these concerns are realized, a nephrologist shortage may exacerbate the associations between high caseload and higher mortality.
We also demonstrate significantly more transplants among lower-mortality-risk (lower-caseload) doctors. Although racial differences between our high- and low-mortality cohorts may limit the significance of this finding, it may nonetheless prompt high-volume providers to examine whether ongoing patient education and transplant referrals are equally available to patients in a higher-volume setting. Further studies are needed to confirm these findings and to explore processes by which high caseload influences patient outcomes.
The strengths of our study include the following: examination of a contemporary dialysis cohort (2001–2007); uniform laboratory measurements, with all laboratory data analyzed within a single laboratory facility; large sample size; long period of follow-up; and adjustment for differences in comorbid conditions, sociodemographics, and laboratory value characteristics.
Several limitations of this study also exist. First, although we adjusted for differences in case mix in analyses examining the caseload-mortality association, we cannot exclude the possibility of clustering of patient characteristics among providers. There did appear to be a less favorable distribution of case-mix and delivered-dialysis characteristics among nephrologists with the highest patient mortality HRs (higher prevalence of diabetes,24 hypertension,25,26 and heart failure; lower prevalence of African Americans;27 and shorter dialysis sessions28,29) than those with the lowest mortality HRs. Second, given the observational and retrospective nature of our study, we cannot exclude the possibility of residual confounding due to limited severity of illness information. Third, we did not examine cause-specific mortality (i.e., cardiovascular, infectious), which may have provided greater insight into mechanisms. Fourth, our study did not account for peritoneal or home HD patients, which may have accounted for differences in in-center HD patient caseload among some nephrologists. We were also unable to take into account care provided by the examined nephrologists to HD patients outside our study cohort during the study period. Fifth, our data were restricted to patients who receive treatment in for-profit dialysis units in an urban Californian region30,31 and thus may not be generalizable to patients receiving care in not-for-profit dialysis units or those residing in other United States regions. Finally, our available study data and methods did not allow us to control for provider vintage, number of provider encounters per month, presence of ancillary providers (such as nurse practitioners or physician assistants), or association with academic centers where fellows may participate in dialysis patient care. We also had no direct information about socioeconomic status of the patients, although we used insurance status as a surrogate to this end. Given the ever-changing nature of healthcare practice models in the United States, future studies should explore the effect of these potential variables in hemodialysis patient care.
In conclusion, we believe our study is the first of its kind to examine the association between nephrologist caseload and mortality risk in a large urban United States setting. We have found that patients receiving treatment from higher-caseload nephrologists have greater mortality than those receiving care from lower-caseload nephrologists. Further studies are needed to confirm findings, to explore mechanisms by which caseload influences outcomes, and to determine the caseload threshold above which the benefits of increased experience are outweighed by a deterioration in quality of care and patient outcomes.
Concise Methods
Patient Cohort
Administrative data from all individuals with ESRD who underwent HD treatment from July 1, 2001, to June 30, 2006, for 20 consecutive calendar quarters in one of the United States outpatient dialysis facilities of DaVita, Inc., were included and were followed for 1 additional year (until June 30, 2007). The creation and analyses of this 6-year, nonconcurrent, dynamic cohort of HD patients have been described previously.32–37 The study was approved by the institutional review committees of the Los Angeles Biomedical Research Institute at Harbor-UCLA and DaVita Clinical Research. The requirement for a written consent form was exempted because of the large number and anonymity of the patients studied and the nonintrusive nature of the research.
Nephrologist Cohort
De-identified information regarding nephrologists and their respective patients was examined. Using random code identifiers to preserve anonymity, we restricted the nephrologist cohort to those who provided care to patients in Los Angeles County. At start of the data analyses, no members of the investigative team had the ability to link this de-identified code to the identity of any specific nephrologist. However, later in the study, one of the study co-investigators who was within the nephrologist cohort agreed to be identified for the sake of comparison once all analyses were completed.
Clinical and Demographic Measures
To minimize measurement variability and the effect of short-term variation in dietary and fluid intake on weight or laboratory measurements, we averaged all repeated measures for each patient during any given calendar quarter (i.e., over 13 consecutive weeks or 3 months).
Dialysis vintage was defined as the duration of time between the first day of dialysis treatment and the first day that the patient entered the cohort. In addition to quarterly laboratory values, postdialysis dry weight (to calculate averaged body mass index [BMI]) was also calculated. The first (baseline) study quarter for each patient was the calendar quarter in which patient’s vintage was >45 days during at least half of the time of that quarter. The administered dialysis dose was measured by single-pool Kt/V using urea kinetic modeling equations that are described elsewhere.34 HD treatment time was estimated in minutes based on the dialysis machine log of the facility.
The presence or absence of diabetes at baseline was obtained from DaVita data. History of tobacco smoking and preexisting comorbid conditions were obtained by linking the DaVita database to the Medical Evidence Form 2728 of the United States Renal Data System, and the latter were categorized into 10 comorbid conditions: ischemic heart disease, congestive heart failure, history of cerebrovascular events, history of myocardial infarction, pericarditis, cardiac dysrhythmia, peripheral vascular disease, chronic obstructive pulmonary disease, current tobacco smoking, and cancer.34
Laboratory Values
Most blood samples were collected before dialysis, with the exception of the postdialysis serum urea nitrogen, which was obtained to calculate urea kinetics. Blood samples were drawn using uniform techniques in all dialysis clinics and were transported within 24 hours to a single laboratory center (DaVita Laboratory in Deland, Florida), where the laboratory values were measured by automated and standardized methods.
Statistical Analyses
Patients were assigned a nephrologist caseload value according to their respective nephrologist’s total cumulative caseload (number of HD patients cared for over the study period).
Baseline characteristics within four groups of nephrologist caseload (<50, 50 to <100, 100 to <150, and ≥150 patients per nephrologist) were analyzed as proportions, means ± SDs, or medians (with interquartile ranges) as dictated by data type, with P value for test-for-trend to evaluate whether there was an increasing or decreasing trend linearly across groups.
For the 41 nephrologists who had 50–200 patients, we evaluated individual nephrologists’ HD patient all-cause mortality HRs in case mix–adjusted Cox proportional hazards models using the robust sandwich covariance estimates to take into account interdependency among patients from the same clinic. This limitation was set in order to focus our analysis on typical urban physician practice size while eliminating effects of very small or large caseload outliers. The case mix–adjusted models included each nephrologist, entry calendar quarter (Q1–Q20), age, sex, diabetes mellitus, race/ethnicity (non-Hispanic white, non-Hispanic black, referred to as white and African-American; also Hispanic, Asian, and other), dialysis vintage (<6 months, 6 months to <2 years, 2 to <5 years, ≥5 years), primary insurance (Medicare, Medicaid, private, and other), marital status (married, single, divorced, or widowed), dialysis dose as indicated by single-pool Kt/V, residual renal function during the entry quarter (i.e., urinary urea clearance), 10 comorbid conditions (ischemic heart disease, congestive heart failure, history of cerebrovascular events, history of myocardial infarction, pericarditis, cardiac dysrhythmia, peripheral vascular disease, chronic obstructive pulmonary disease, current tobacco smoking, and cancer), and vascular access type (arteriovenous fistula, graft, catheter, other, or unknown). We then compared the baseline characteristics of patients receiving treatment from the 10 nephrologists with highest all-cause mortality HRs and the 10 nephrologists with the lowest HRs using chi-squared, Wilcoxon rank sum, and two-sample t tests according to data type.
Using multivariable Cox models with robust sandwich covariance estimators adjusted for the case-mix covariates (except for individual nephrologist), we examined the association between nephrologist caseload and all-cause mortality in which caseload was examined as a continuous variable with caseload increments of 50 and as a continuous variable illustrated with restricted cubic splines with 2 degrees of freedom to examine the nonlinear associations with mortality.
Patients who underwent transplantation or left DaVita clinics were censored at the time of the event. Patients were followed for mortality outcomes through June 30, 2007, and records of death were obtained from the U.S. Renal Data Service. Missing covariate data (<1% for most laboratory and demographic variables) were imputed by means or medians of recorded values. For all analyses, two-sided P values are reported, and results were considered statistically significant if P<0.05. Analyses were carried out with SAS software, version 9.1 (SAS Institute, Inc., Cary, NC).
Disclosures
K.K.Z. was medical director of a DaVita dialysis clinic in California from 2008 to 2012.
Acknowledgments
We thank DaVita Clinical Research for providing access to database.
K.K.Z. is supported in part by National Institutes of Health grants K24-DK091419 and R01-DK078106. C.M.R. is supported by a National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Disease grant (F32 DK093201).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Bologa RM, Levine DM, Parker TS, Cheigh JS, Serur D, Stenzel KH, Rubin AL: Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis 32: 107–114, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD: Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: The 58th annual fall conference and scientific sessions. Hypertension 45: 811–817, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, Fonarow GC: Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 119: 671–679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemodialysis Adequacy 2006 Work Group : Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis 48[Suppl 1]: S2–S90, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Thompson S, Gill J, Wang X, Padwal R, Pelletier R, Bello A, Klarenbach S, Tonelli M: Higher mortality among remote compared to rural or urban dwelling hemodialysis patients in the United States. Kidney Int 82: 352–359, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Tonelli M, Manns B, Culleton B, Klarenbach S, Hemmelgarn B, Wiebe N, Gill JS; Alberta Kidney Disease Network: Association between proximity to the attending nephrologist and mortality among patients receiving hemodialysis. CMAJ 177: 1039–1044, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rucker D, Hemmelgarn BR, Lin M, Manns BJ, Klarenbach SW, Ayyalasomayajula B, James MT, Bello A, Gordon D, Jindal KK, Tonelli M: Quality of care and mortality are worse in chronic kidney disease patients living in remote areas. Kidney Int 79: 210–217, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Casale PN, Jones JL, Wolf FE, Pei Y, Eby LM: Patients treated by cardiologists have a lower in-hospital mortality for acute myocardial infarction. J Am Coll Cardiol 32: 885–889, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Chen CH, Chen YH, Lin HC: Association between physician caseload and patient outcome for sepsis treatment. Infect Control Hosp Epidemiol 30:556–562, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Halm EA, Lee C, Chassin MR: Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med 137: 511–520, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Hannan EL, Racz M, Ryan TJ, McCallister BD, Johnson LW, Arani DT, Guerci AD, Sosa J, Topol EJ: Coronary angioplasty volume-outcome relationships for hospitals and cardiologists. JAMA 277: 892–898, 1997 [PubMed] [Google Scholar]
- 12.Hertzer NR: Outcome assessment in vascular surgery—results mean everything. J Vasc Surg 21: 6–15, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Kitahata MM, Koepsell TD, Deyo RA, Maxwell CL, Dodge WT, Wagner EH: Physicians’ experience with the acquired immunodeficiency syndrome as a factor in patients’ survival. N Engl J Med 334: 701–706, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Lin HC, Lee HC: Caseload volume-outcome relation for pulmonary embolism treatment: Association between physician and hospital caseload volume and 30-day mortality. J Thromb Haemost 6: 1707–1712, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Lin HC, Xirasagar S, Chen CH, Hwang YT: Physician’s case volume of intensive care unit pneumonia admissions and in-hospital mortality. Am J Respir Crit Care Med 177: 989–994, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Lindenauer PK, Behal R, Murray CK, Nsa W, Houck PM, Bratzler DW: Volume, quality of care, and outcome in pneumonia. Ann Intern Med 144: 262–269, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Tu JV, Austin PC, Chan BT: Relationship between annual volume of patients treated by admitting physician and mortality after acute myocardial infarction. JAMA 285: 3116–3122, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Turchin A, Shubina M, Pendergrass ML: Relationship of physician volume with process measures and outcomes in diabetes. Diabetes Care 30: 1442–1447, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Ward MM: Association between physician volume and in-hospital mortality in patients with systemic lupus erythematosus. Arthritis Rheum 52: 1646–1654, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Thomas-Hawkins C, Flynn L, Clarke SP. Relationships between registered nurse staffing, processes of nursing care, and nurse-reported patient outcomes in chronic hemodialysis units. Nephrol Nurs J 35:123–130, 145, 2008 [PMC free article] [PubMed] [Google Scholar]
- 21.Lavernia CJ, Guzman JF: Relationship of surgical volume to short-term mortality, morbidity, and hospital charges in arthroplasty. J Arthroplasty 10: 133–140, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, Kimata N, Gillespie BW, Combe C, Bommer J, Akiba T, Mapes DL, Young EW, Port FK: Longer treatment time and slower ultrafiltration in hemodialysis: Associations with reduced mortality in the DOPPS. Kidney Int 69: 1222–1228, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Parker MG, Ibrahim T, Shaffer R, Rosner MH, Molitoris BA: The future nephrology workforce: Will there be one? Clin J Am Soc Nephrol 6: 1501–1506, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Collins AJ, Foley RN, Herzog C, Chavers BM, Gilbertson D, Ishani A, Kasiske BL, Liu J, Mau LW, McBean M, Murray A, St. Peter W, Guo H, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers PW, Agodoa L: Excerpts from USRDS 2009 Annual Data Report. Am J Kidney Dis 55(Suppl 1):S1–A7, 2010 [Google Scholar]
- 25.Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG: Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med 115: 291–297, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Stidley CA, Hunt WC, Tentori F, Schmidt D, Rohrscheib M, Paine S, Bedrick EJ, Meyer KB, Johnson HK, Zager PG, Medical Directors of Dialysis Clinic Inc : Changing relationship of blood pressure with mortality over time among hemodialysis patients. J Am Soc Nephrol 17: 513–520, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Kucirka LM, Grams ME, Lessler J, Hall EC, James N, Massie AB, Montgomery RA, Segev DL: Association of race and age with survival among patients undergoing dialysis. JAMA 306: 620–626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker TF, 3rd, Husni L, Huang W, Lew N, Lowrie EG: Survival of hemodialysis patients in the United States is improved with a greater quantity of dialysis. Am J Kidney Dis 23: 670–680, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Held PJ, Levin NW, Bovbjerg RR, Pauly MV, Diamond LH: Mortality and duration of hemodialysis treatment. JAMA 265: 871–875, 1991 [PubMed] [Google Scholar]
- 30.Garg PP, Frick KD, Diener-West M, Powe NR: Effect of the ownership of dialysis facilities on patients’ survival and referral for transplantation. N Engl J Med 341: 1653–1660, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Devereaux PJ, Schünemann HJ, Ravindran N, Bhandari M, Garg AX, Choi PT, Grant BJ, Haines T, Lacchetti C, Weaver B, Lavis JN, Cook DJ, Haslam DR, Sullivan T, Guyatt GH: Comparison of mortality between private for-profit and private not-for-profit hemodialysis centers: A systematic review and meta-analysis. JAMA 288: 2449–2457, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, Nissenson AR, Krishnan M, Kopple JD, Mehrotra R, Anker SD: Obesity paradox and mortality-predictability of surrogates of body size and muscle mass in hemodialysis patients. Mayo Clin Proc 85: 991–1001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalantar-Zadeh K, Miller JE, Kovesdy CP, Mehrotra R, Lukowsky LR, Streja E, Ricks J, Jing J, Nissenson AR, Greenland S, Norris KC: Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res 25: 2724–2734, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller JE, Kovesdy CP, Nissenson AR, Mehrotra R, Streja E, Van Wyck D, Greenland S, Kalantar-Zadeh K: Association of hemodialysis treatment time and dose with mortality and the role of race and sex. Am J Kidney Dis 55: 100–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller JE, Kovesdy CP, Norris KC, Mehrotra R, Nissenson AR, Kopple JD, Kalantar-Zadeh K: Association of cumulatively low or high serum calcium levels with mortality in long-term hemodialysis patients. Am J Nephrol 32: 403–413, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Streja E, Kovesdy CP, Molnar MZ, Norris KC, Greenland S, Nissenson AR, Kopple JD, Kalantar-Zadeh K: Role of nutritional status and inflammation in higher survival of African American and Hispanic hemodialysis patients Am J Kid Dis 57: 883–893, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molnar MZ, Lukowsky LR, Streja E, Dukkipati R, Jing J, Nissenson AR, Kovesdy CP, Kalantar-Zadeh K: Blood pressure and survival in long-term hemodialysis patients with and without polycystic kidney disease. J Hypertens 28: 2475–2484, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]