Abstract
Cytomegalovirus (CMV) infection increases the risk of complications after renal transplantation, but the mechanisms controlling donor-derived infection are not adequately characterized. Here, we assessed the risk of clinically significant CMV disease in donor-seropositive, recipient-seropositive (D+R+) renal transplantation and examined recipients’ CMV antigen-specific cellular immune responses primed directly by donor cells. In a retrospective cohort of 569 patients administered standardized basiliximab-tacrolimus-mycophenolate-corticosteroid immunosuppressive therapy, CMV disease rates increased in D+R+ serostatus pairings compared with D−R+ pairings (hazard ratio [HR], 2.61; 95% confidence interval [CI], 1.36 to 5.01; P=0.004) and associated with increased donor-recipient HLA mismatch in the D+R+ group (HR [per class 1 mismatch], 1.43; 95% CI, 1.12 to 1.82]; P=0.02). D+R+ and D+R− transplants in which the donor and recipient differentially expressed at least one HLA class I allele were followed prospectively from the time of transplantation. During the first year after transplantation, four of eight seropositive recipients and one of three seronegative recipients displayed peripheral blood CD8+ T cell responses to CMV presented by recipient-specific HLA. Notably, no recipients mounted responses to CMV presented by donor-specific HLA, despite the detection of CMV antigen expression in all seropositive donor organs examined (n=10), suggesting that the allograft of Class I HLA-mismatched seropositive donors is inaccessible to CD8+ T cell responses. Finally, pretransplant assays of anti-CMV cellular immunity predicted post-transplant CMV replication less accurately in D+R+ pairings than in D−R+ pairings, possibly reflecting in vitro assay specificity for recipient, rather than donor, HLA. These findings are relevant to the clinical management and immunologic understanding of donor-transmitted viral infection.
Cytomegalovirus (CMV) infection remains an important complication of kidney transplantation, being associated with increased graft failure rates, morbidity, and mortality. Although the cellular immune response to CMV, especially the CD8+ T cell response, is of primary importance in controlling infection,1–3 the CMV-specific antibody serostatus of donor (D) and recipient (R) is a useful surrogate to aid in risk stratification because it identifies individuals with latent CMV infection that may subsequently reactivate. High disease rates in primary infection (D+R− transplantation) are well recognized, and requirement for antiviral prophylaxis in this context is largely uncontested. CMV disease is also seen in seropositive recipients (R+) and may occur irrespective of donor serostatus. Seropositive recipients are often grouped together as “intermediate risk,” irrespective of donor serostatus. However, historical evidence suggests that D+R+ transplantation is associated with increased disease rates compared with D−R+ transplantation.4 Also, two recent trials5,6 and a single-center study7 showed higher infection rates in D+R+ than in D−R+ transplantation. Because these latter studies focused on (often asymptomatic) CMV infection, rather than symptomatic disease, and because all used antiviral prophylaxis or pre-emptive therapy for CMV infection, it remains unclear whether disease rates differ between D+R+ and D−R+ transplantation, particularly under contemporary immunosuppression without antiviral prophylaxis.
After D+R+ transplantation, some CMV cases may result from donor-derived infection, although the mechanism behind this is incompletely understood.4,8 Although lack of protective immunity to newly infecting strains may be important,9 other mechanisms may also play a role. For example, HLA mismatch between donor and recipient may result in failure of “cognate” immunity to control viral infection in donor tissue. In this regard, clinical data are conflicting, with studies from the 1980s and 1990s suggesting increased CMV disease rates with increased HLA class II mismatch10,11 but others suggesting reduced rates in this setting.12,13 One of these studies also identified HLA class I mismatch as a risk factor for disease.11
The initial purpose of this study was to evaluate the effect of donor CMV serostatus on disease risk in seropositive recipients and whether HLA class I mismatch modifies the risk. We then used HLA-peptide tetramers to determine the specificity of cellular immunity to defined CMV peptides presented through donor or recipient HLA alleles and examined the development of T cell responses (or lack of them) against CMV primed “directly” on donor cells. These findings should be of value in the clinical management of CMV infection in this setting and are of considerable interest in further the understanding of the mechanism of viral infection in solid organ transplantation.
Results
The Incidence of CMV Disease Is Related to Donor and Recipient CMV Serostatus
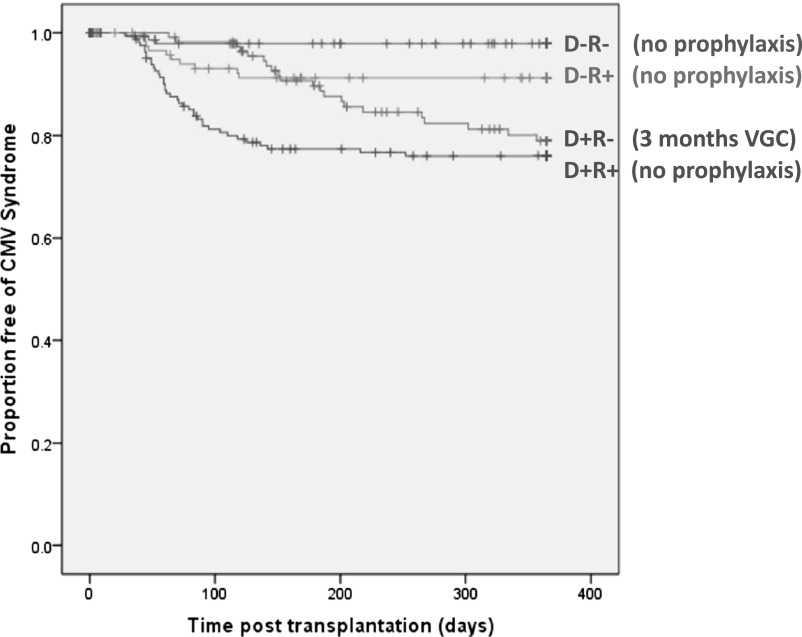
The demographic and clinical features of the 569 patients are described in Table 1. In these patients, 77 episodes of CMV disease occurred and were associated with donor and recipient serostatus (Figure 1) (P=0.003). D−R− transplantation was associated with the lowest risk of disease (3 of 151 [2%]). CMV disease was seen in 19.8% (23 of 117 patients) of the D+R− group. Of these, 3 patients experienced CMV disease during the 3-month period of antiviral prophylaxis, with suboptimal valganciclovir dosing (adjusted for allograft function) in all cases. Interestingly, risk was highest in seropositive recipients of seropositive donors (D+R+), where 39 of 179 patients (21.9%) experienced CMV disease. In contrast, 12 of 122 patients (9.9%) in the D−R+ group developed CMV disease.
Table 1.
Patient demographic characteristics
| Characteristic | Value |
|---|---|
| Recipient age (yr) | 47±13 |
| Male recipients, % (n/n) | 42.4 (241/569) |
| Recipient ethnicity, n (%) | |
| White | 431 (75.7) |
| Indo-Asian | 88 (15.5) |
| African-Caribbean | 42 (7.4) |
| Other | 8 (1.4) |
| Cause of renal failure, n (%) | |
| Glomerular | 185 (32.5) |
| Hereditary/cystic | 170 (29.9) |
| Diabetes | 31 (5.4) |
| Vascular | 29 (5.1) |
| Interstitial | 15 (2.6) |
| Other | 139 (24.4) |
| Donor age (yr) | 47±15 |
| Transplant source, n (%) | |
| Deceased donor | 313 (55.0) |
| DBD | 247 (43.4) |
| DCD | 66 (11.6) |
| Live donor | 256 (45.0) |
| Donor-recipient HLA mismatch | |
| HLA-A | 1.0±0.7 Ag |
| HLA-B | 1.0±0.6 Ag |
| HLA-DR | 0.7±0.6 Ag |
| Donor-recipient CMV serostatus | |
| D−R− | 151 (26.5) |
| D−R+ | 122 (21.5) |
| D+R+ | 179 (31.4) |
| D+R− | 117 (20.6) |
Values expressed with a plus/minus sign are the mean ± SD. DBD, donation after brain death; DCD, donation after cardiac death; Ag, antigen.
Figure 1.
Development of CMV disease by donor-recipient risk based on serostatus combination. Data shown for first 12 months in light of few episodes of CMV disease beyond this point. VGC, valganciclovir.
Median peak viral loads (copies/ml) during infections were also associated with serostatus: D−R−, 2.2×107 (range, 1.6×106–3.1×107); D+R−, 3.1×105 (range, 571–7.7×106); D+R+, 1.3×104 (range, 504–1.7×108); D−R+, 1.1×104 (range, 1025–1.9×105) (P<0.001). Histologic evidence of CMV infection was gastrointestinal in all cases but was uncommonly sought and proven in only five patients.
Multivariate Analysis Confirms That Donor Serostatus Influences CMV Disease Rates in Seropositive Recipients
Table 2 shows the univariate relationships between CMV serostatus (and other demographic variables) and time to CMV disease. Compared with D−R+ transplantation (selected as the reference group), D−R− transplantation was associated with reduced risk, and D+R− with increased risk for CMV disease (Table 2). In addition, increased risk of CMV was seen in comparing D+R+ and D−R+ serostatus groups (HR, 2.61 [95% confidence interval (CI), 1.36 to 5.01]; P=0.004 for this specific comparison).
Table 2.
Risk factors for CMV disease
| Risk Factor per Category | Hazard Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| CMV serostatus | ||
| D−R+ | 1 | <0.001 |
| D−R− | 0.20 (0.05 to 0.70) | |
| D+R+ | 2.61 (1.36 to 5.01) | |
| D+R− | 2.03 (1.10 to 4.08) | |
| Recipient age per 10 year increase | 1.18 (0.98 to 1.41) | 0.08 |
| Recipient age | ||
| Recipient sex: male | 1.10 (0.70 to 0.74) | 0.67 |
| Recipient ethnicity | ||
| White | 1 | 0.01 |
| Indo-Asian | 2.22 (1.31 to 3.76) | |
| African-Caribbean | 1.50 (0.69 to 3.22) | |
| Other | 1.32 (0.39 to 4.47) | |
| Cause of ESRD | ||
| Glomerular | 1 | 0.07 |
| Hereditary/cystic | 1.15 (0.62 to 2.15) | |
| Diabetes | 3.22 (1.46 to 7.08) | |
| Vascular | 2.10 (0.84 to 5.26) | |
| Interstitial | 1.93 (0.57 to 6.52) | |
| Other | 1.43 (0.76 to 2.67) | |
| Source | ||
| DBD | 1 | 0.62 |
| DCD | 0.92 (0.46 to 1.85) | |
| Live donor | 0.73 (0.38 to 1.43) | |
| Donor age per 10-yr increase | 1.19 (1.01 to 1.41) | 0.04 |
| Donor sex: male | 0.95 (0.61 to 1.48) | 0.82 |
| Class I mismatch per Ag | 1.16 (0.96 to 1.40) | 0.12 |
| Class II mismatch per Ag | 0.97 (0.69 to 1.35) | 0.84 |
DBD, donation after brain death; DCD, donation after cardiac death; Ag, antigen.
In the multivariate model, the only independent factor associated with time to CMV disease was donor-recipient serostatus, and therefore the effect identical to that in the univariate analysis (Table 2). Adjusting the model for post-transplant delayed graft function and acute rejection resulted in no material difference in the relationship (HR [D+R+ versus D−R+], 2.42 [95% CI, 1.31 to 4.47]; P=0.007).
Total HLA Class I Mismatch between Donor and Recipient Increases CMV Disease Risk in D+R+ Transplantation
No association was evident between HLA mismatch at either class I (combined HLA-A and HLA-B) or class II (HLA-DR) loci and time to CMV disease (Table 2). However, a significant statistical interaction was seen between serostatus group and total HLA class I mismatch (P=0.02).
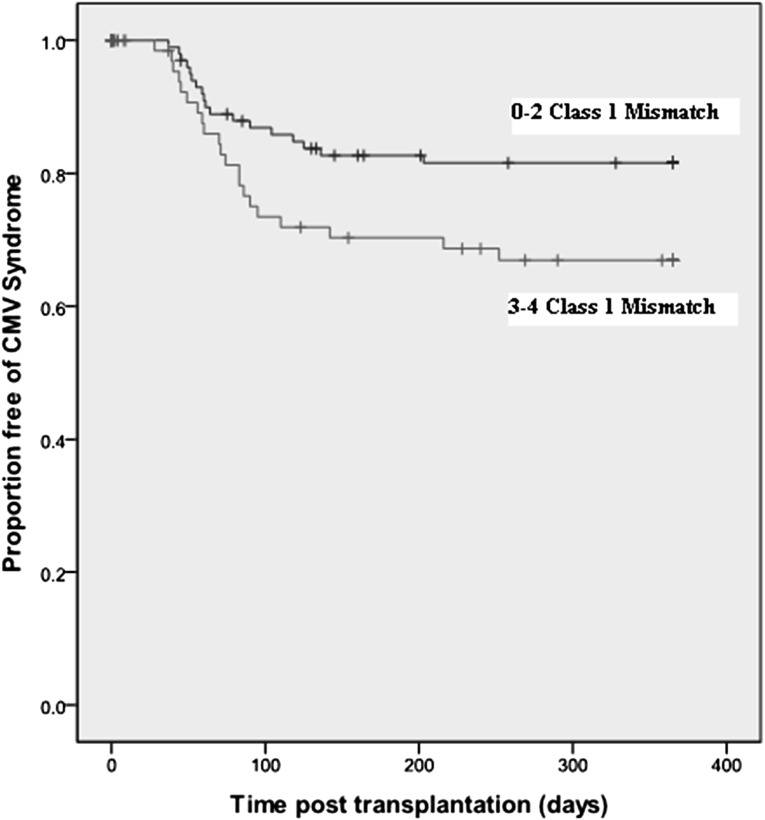
To illustrate this, the association between total HLA class I mismatch and time to CMV was evaluated for each serostatus risk group. In D+R+ transplantation (n=179), a significant relationship was evident between HLA class I mismatch and time to CMV disease, and this remained significant in the multivariate model (HR [per mismatch], 1.43 [95% CI, 1.12 to 1.82]; P=0.02). Kaplan-Meier estimates of CMV-free survival in the D+R+ cohort are shown in Figure 2 (P=0.03).
Figure 2.
Influence of donor-recipient HLA mismatch on time to CMV disease in the D+R+ serostatus pairing. Data are shown for first 12 months in light of few episodes of CMV disease beyond this point.
The effect of HLA class I mismatch was not evident in the other three serostatus groups (P>0.3 for all). In addition, no statistical interaction was seen between serostatus group and class II mismatch (HLA-DR only; P=0.13).
Transplant Patients Fail to Mount a CD8+ T Cell Response to Immunodominant CMV Epitopes Selectively Presented by Donor MHC
We next investigated the CD8+ T cell response to CMV peptides presented exclusively by donor or recipient HLA alleles. We prospectively identified 11 instances of transplantation from CMV-seropositive donors where both the donor and recipient expressed at least one unique HLA class I allele and where appropriate HLA-peptide tetramers were available to study T cell immune responses against immunodominant CMV peptides presented through these two alleles. In eight cases the recipient was also CMV seropositive; in three cases the recipient was seronegative.
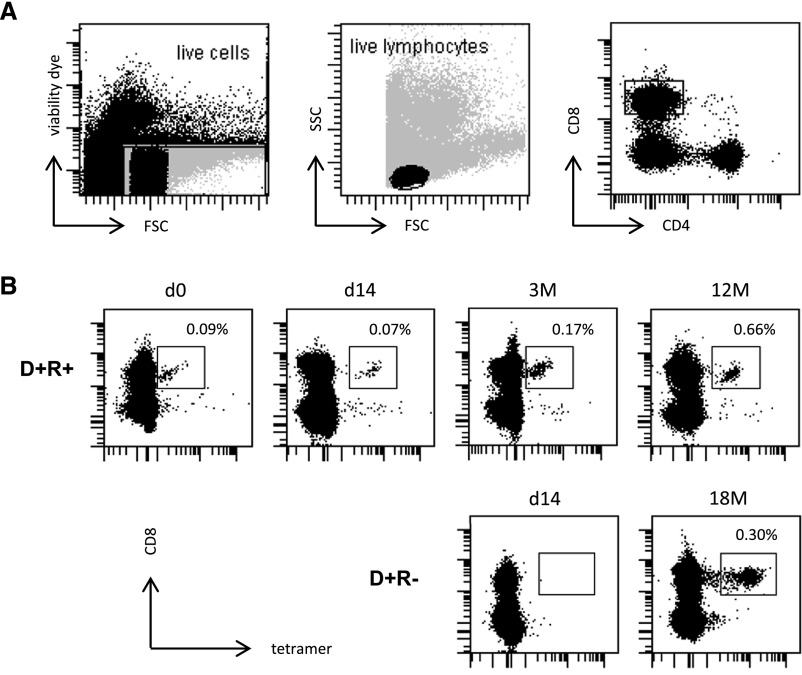
After transplantation, four of the eight recipients in the D+R+ group displayed CD8+ T cell responses to peptides presented by recipient-specific HLA alleles. Representative results for one individual (and gating strategy) are shown in Figure 3A and the upper row of Figure 3B, demonstrating the HLA A1-restricted CD8+ T cell response to the VTEHDTLLY peptide (recipient type: HLA-A1, A8; donor: HLA-A2, A3). In the remaining four patients, no such CD8+ T cell response was detected. However, and of particular interest, no immune response restricted through a donor-specific HLA allele was detected in any of the eight recipients at any point during the first year after transplantation (P=0.02 comparing recipient and donor-restricted responses). Similarly, none of the three patients in the D+R− group mounted a CD8+ T cell response to CMV peptides restricted by a donor-specific HLA allele. However, one recipient did develop a response to a peptide restricted by a recipient-specific allele; this was not evident early after transplantation but was then evident at 6 months and persisted to 18 months after transplantation (HLA-A1 restricted YSE peptide; Figure 3B, lower row).
Figure 3.
Assessment of recipient CD8+ cellular responses to HLA-specific CMV tetramers. CD8+ T cell responses against recipient-HLA-presented CMV-derived epitopes can be detected in both CMV-seropositive recipients of seropositive donor organs (D+R+) and CMV-negative recipients of seropositive organs (D+R−). (A) Gating strategy . (B) The upper row shows an example of a serial evaluation of the CD8+ T cell response to the HLA-A1 restricted epitope VTE (pp50) in the D+R+ setting at time of transplantation (d0), day 14, 3 months and 12 months after transplantation (top row). The lower row of B shows the T cell response to the HLA-A1 restricted epitope YSE (pp65) in a CMV-seronegative recipient of a CMV-seropositive donor organ, which was not evident early after transplantation, but then emerged and persisted through to 18 months after transplantation.
Taken together across 11 transplant procedures, five recipients developed a CD8+ T cell response to CMV peptides restricted by recipient-specific HLA alleles, whereas none demonstrated a response to epitopes restricted by donor-specific alleles (P=0.01).
CMV within Transplanted Tissue from Seropositive Kidney Transplant Donors Is Common
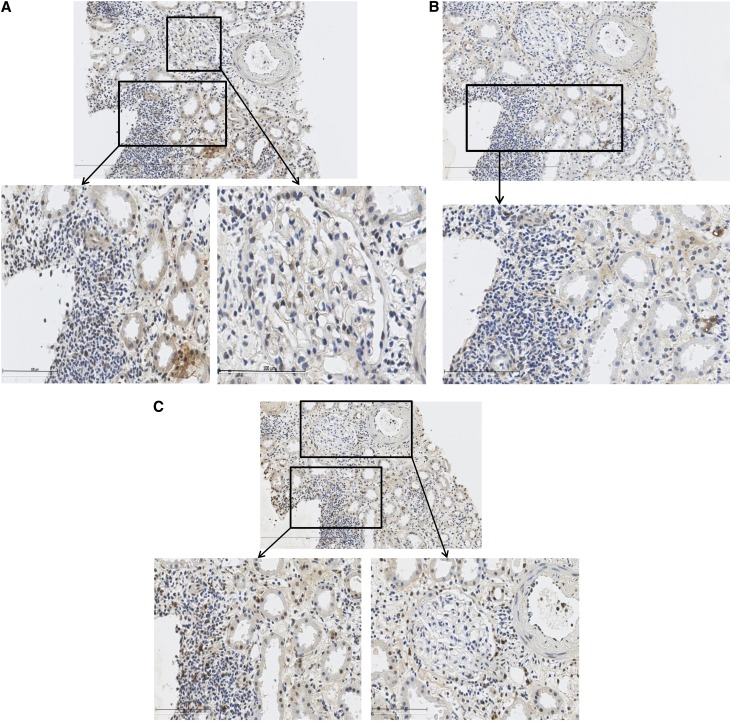
Histologic examination of implantation biopsy samples from 10 seropositive kidney transplant donors revealed evidence of CMV protein expression in all cases (HCMV-IE, HCMV-LA, and HCMV-pp65; Figure 4). Staining was absent in seronegative controls.
Figure 4.
HCMV immunostaining of implantation biopsy specimens from seropositive donor. (A) HCMV IE immunostaining. (B) HCMV LA immunostaining. (C) HCMV pp65 immunostaining.
Predictive Utility of Assessment of the Pretransplant CMV-Specific T Cell Immune Response Differs between D+R+ and D−R+ Transplantation
Finally, the prognostic value of CMV-specific cellular immunity immediately before transplantation was evaluated in CMV-seropositive recipients. In D−R+ transplantation (n=19), a median 0.13% of total CD8+ T cells responded to an IE-1 peptide mix as measured by IFN-γ production (range, 0%–8.18%), and 0.06% (range, 0%–4.35%) of total CD4+ T cells responded to pp65-derived peptides. Six patients (31%) in this group developed detectable CMV replication during the first year. In D+R+ transplantation (n=19), a median 0.13% CD8+ T cells responded to IE-1 epitopes (range, 0%–23.05%), and 0.05% CD4+ T cells (range, 0%–1.43%) responded to pp65 epitopes, with nine patients (47%) displaying CMV replication.
Receiver-operating characteristic curve analysis defined the optimal prognostic cutoff, and then sensitivity, specificity, and positive and negative predictive values at that cutoff were calculated. Results are shown in Table 3. In D−R+ transplantation, a CD8+ T cell response of 0.16% (i.e., when 0.16% of total CD8+ cells produced IFN-γ after IE-1 peptide stimulation) was associated with moderate to good prediction (c-statistic, 0.76) for CMV replication. From a clinical perspective, in 83% of patients experiencing CMV replication, ≤0.16% total CD8+ T cells responded to IE-1 peptide stimulation before transplantation; 86% of patients with >0.16% total CD8+ T cells responding to IE-1 did not develop CMV replication within 12 months after transplantation. The CD8+ T cell response to pp65 demonstrated identical prognostic performance to the IE-1 CD8+ T cell response, although the optimal cutoff value was 0.08% total CD8+ T cells.
Table 3.
Prognostic performance of pretransplant assays of cellular immunity to CMV
| Variable | c-Statistic | Cut-off (Percentage IFN-γ–Releasing Cells) | Sensitivity | NPV | Specificity | PPV |
|---|---|---|---|---|---|---|
| D−R+ transplantation | ||||||
| IE-1 (CD8) | 0.76 | 0.16 | 0.83 | 0.86 | 0.50 | 0.45 |
| pp65 (CD4) | 0.54 | 0.40 | 1.00 | 1.00 | 0.08 | 0.35 |
| D+R+ transplantation | ||||||
| IE-1 (CD8) | 0.56 | 0.82 | 0.67 | 0.50 | 0.38 | 0.55 |
| pp65 (CD4) | 0.52 | 0.05 | 0.56 | 0.50 | 0.50 | 0.56 |
Endpoint of CMV infection during first 12 months after transplantation. NPV, negative predictive value; PPV, positive predictive value.
Conversely, the pretransplant CD8+ T cell response did not demonstrate prognostic utility in D+R+ transplantation (Table 3). In fact, of nine recipients in this subgroup displaying an IE-1 response ≥0.16% total CD8+ T cells, five developed CMV replication and four did not. Interestingly, and despite the small numbers studied, there was some suggestion that these five patients developing post-transplant CMV replication displayed increased HLA class I mismatch in comparison with the four patients in whom this percentage of CMV-responding CD8+ T cells was “protective” (class I mismatch: 4,3,3,2,2 versus 3,2,1,1 respectively; P=0.1).
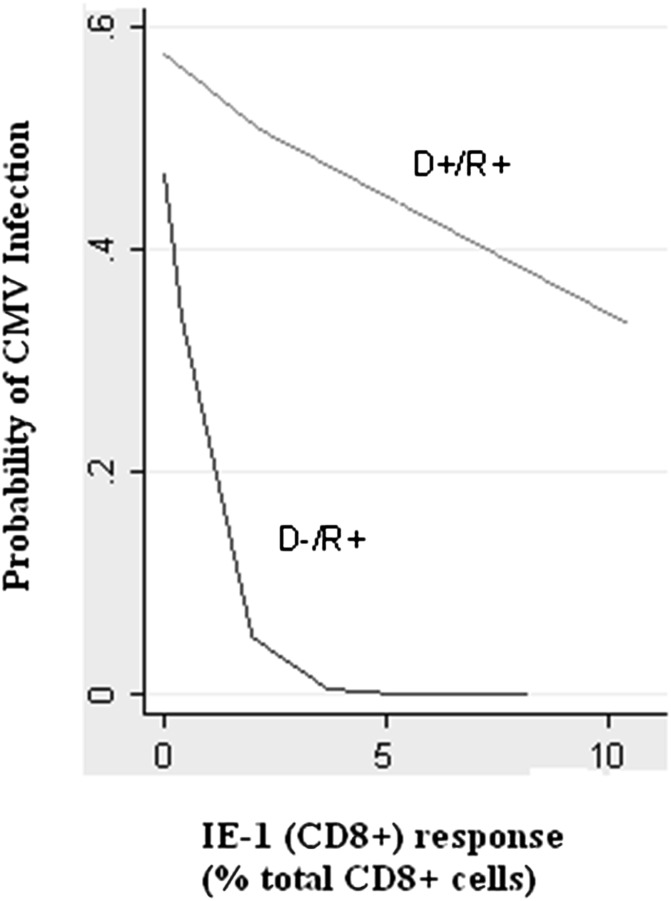
The relationship between the IE-1 CD8+ T cell response and risk of CMV replication in D−R+ and D+R+ transplantation is shown in Figure 5.
Figure 5.
Cellular immunity and risk of CMV infection. The relationship between the IE-1 CD8+ response and the probability of CMV infection over the first post-transplant year, comparing D−R+ and D+R+ serostatus pairings.
The CD4+ response (pp65) did not demonstrate prognostic utility in D−R+ or D+R+ transplantation (Table 3).
Discussion
This study demonstrates important characteristics of CMV infection in kidney transplant recipients. First, CMV-seropositive recipients receiving contemporary immunosuppression are at increased risk of clinical CMV disease when receiving kidneys from CMV-seropositive compared with CMV-seronegative donors. This observation represents a real-world scenario, dealing specifically with patients experiencing clinically manifest CMV disease, thereby extending data from previous clinical trials addressing asymptomatic CMV infection/replication.5,7 Although “strain-specific” mismatch is cited as an underlying mechanism for this phenomenon,9 substantial data exist that question this hypothesis (discussed further below). We therefore investigated whether donor-recipient HLA mismatch impaired development of cognate cellular immunity to CMV within transplanted donor tissue. Indeed, further analysis demonstrated increased risk of CMV disease with greater HLA class I mismatch. To investigate the underlying mechanism, we used HLA-peptide tetramers to interrogate the development of CD8+ T cell immunity against peptides presented through HLA alleles specific for recipient or donor. Notably, we observed that although cellular responses to CMV peptides presented through recipient-specific HLA alleles were frequently detected, CD8+ T cell responses to peptides presented solely by donor-specific HLA alleles were not generated within 12 months after transplantation.
The latter observation is relevant to current theories of the relative roles of different antigen presentation pathways in viral infection. These include direct presentation by infected donor antigen-presenting cells (APCs), presentation by recipient APCs “cross-dressed” with donor-derived MHC:viral peptide complexes transferred from donor immune or parenchymal cells, and cross-presentation by recipient APCs that have engulfed and processed circulating virions.14,15 Recipients’ failure to develop CD8+ immune responses through donor-specific HLA alleles suggests that priming and expansion of immune responses to donor-transmitted CMV do not result from direct presentation or cross-dressing. This novel finding with regard to transplantation-associated viral transmission resonates with data from models of murine CMV, suggesting cross-presentation as the primary pathway to antiviral immunity,16–18 with direct priming inhibited due to CMV-mediated downregulation of activating co-stimulatory molecules (such as CD86) and upregulation of inhibitory ligands (such as PD-L1) on APCs,19–21 along with inhibition of dendritic cell maturation and antigen presentation.22,23
Absence of directly primed CD8+ T cell immune responses raises questions regarding how infection is ultimately controlled within donor tissue. Several possibilities include CD4+ T cell immunity, natural killer cells, and humoral immunity. However, sensitive DNA-based techniques commonly detect CMV persistence after transplantation in kidneys from seropositive donors,24 suggesting that donor tissue may act as a “reservoir” for infection, which may later become clinically manifest.25 Certainly the preimplantation histologic data from the current study suggest peri-transplantation CMV transmission is very common. After CMV reactivation, HLA class I upregulation occurs in infected and surrounding cells,26–29 with subsequent presentation of CMV antigens to CD8+ T cells.30,31 In D+R+ transplantation, pre-existing cellular immunity may control spread of donor-derived virus, but donor-recipient mismatching at class I loci may impair the efficacy of this response, and so increase the likelihood of clinical disease, as seen in the current study. HLA class I mismatch may be less important in the setting of D+R− or D−R+ transplantation because control of CMV infection in the former is primarily contingent on a de novo immune response (rather than a pre-existing one), and the latter depends on responses to recipient HLA-presented virus.
This study highlights differences in risk of CMV when seropositive recipients receive kidneys from donors with or without latent CMV infection (i.e., between D+R+ and D−R+ transplantation. This finding opposes the view that these groups be considered together as an “intermediate risk” category and supports policies of antiviral prophylaxis or heightened surveillance in D+R+ transplantation, particularly in instances of increased donor-recipient HLA class I mismatch. The introduction of a novel CMV strain has been implicated as enhancing the risk of CMV disease in D+R+ transplantation.32 However, it is noteworthy that the study showing this effect incorporated “high level antigenemia” without symptoms within the definition of CMV disease and did not show increased CMV infection rates with strain-specific donor-recipient serologic mismatch.32 Also, it is known that human immunity to CMV is broadly targeted,33 suggesting “redundancy” in the cellular repertoire. Furthermore mismatching for immunodominant CD8+ epitopes in murine models of CMV has minimal effect on disease severity.34 A primate model also shows that hepatitis C re-infection rates are similar irrespective of whether the re-infecting strain was homologous or heterologous to the original infection.35 Therefore “strain mismatch” may not necessarily fully explain increased CMV rates in D+R+ transplantation. Indeed, combinations of viral variation and HLA mismatch may be important, and future study is required to clarify their relative contributions. In light of the extreme difficulty in isolating latent viral DNA from transplant donors, we were unable to directly address this issue of genotype mismatch in this study.
Assays of cellular immunity to CMV are emerging research tools. However, this study suggests that such in vitro assessments, whereby CMV peptides are presented by recipient HLA (using recipient PBMCs) may be misleading, considering that in vivo the virus may be harbored within donor tissue, and therefore presented upon reactivation by donor HLA. This may account for the inferior prognostic utility of these assays in D+R+ (compared with D−R+) transplantation. There was also some suggestion that HLA mismatch played a role in this context, and future evaluation of this phenomenon in larger cohorts may be worthwhile. To our knowledge this is only the second study to evaluate the prognostic utility of pretransplant cellular immunity to CMV. The first study, published recently,36 also identified an association between pretransplant immunity and post-transplant viral replication in 55 kidney or lung transplant recipients undergoing antiviral prophylaxis or pre-emptive therapy, but did not compare D+R+ and D−R+ groups specifically or present metrics of prognostic utility.
It can be speculated that these findings may be relevant to other viruses that are transmitted in allograft tissue, such as Epstein-Barr virus or polyomavirus. They may also be relevant in considering targets for adoptive cellular transfer therapy in the treatment of viral infection of donor origin. The requirement for a cellular response against donor-presented epitopes may also have implications for vaccine development and efficacy in the context of viruses transmitted by transplantation, as identified in the context of antitumor vaccination.37
In conclusion, clinically relevant CMV disease is more common in nonprophylaxed seropositive recipients of kidneys from seropositive (compared with seronegative) donors; donor-recipient HLA class I mismatch modifies this risk. We suggest that CD8+ T cell immune responses against CMV transmitted within grafted tissue fail to develop, although cannot yet claim this as definitive pending further study in other cohorts. Nevertheless, these preliminary findings have considerable implications for the clinical management and immunologic understanding of viral infection in transplantation.
Concise Methods
Risk of CMV Disease and Donor-Recipient Serostatus: Retrospective Cohort
A retrospective analysis of the prospectively maintained departmental database identified 569 adult patients (≥18 years) undergoing solitary kidney transplantation between May 2007 and June 2011 at Queen Elizabeth Hospital Birmingham, United Kingdom. This start date was chosen because at that time a standardized immunosuppression regimen was introduced. This consisted of basiliximab induction followed by tacrolimus (trough level 5–8 ng/ml initially, measured by liquid chromatography-tandem mass spectrometry), mycophenolate mofetil (2 g daily initially), and prednisolone (20 mg daily, reducing to 5 mg maintenance by 3 months after transplantation). Transplantation proceeded provided the cross-match between donor and recipient was negative by flow cytometry and cytotoxicity.
Baseline information was collected on the pretransplant CMV serostatus of the recipients and their donors, donor and recipient age and sex, cause of renal failure, HLA mismatch, source of transplant (living related, living unrelated, deceased donor following brain death, deceased donor following cardiac death). Postoperative events of delayed graft function (requirement for dialysis during the first postoperative week) and biopsy-proven acute rejection (any time; any histologic grade) were collected.
CMV prophylaxis with 100 days of valganciclovir was given to the D+R− group only, with dose adjustment for renal function. Testing for CMV was based on clinical suspicion of disease; no protocolized assessment of CMV infection was undertaken in this cohort. CMV disease was diagnosed according to international guidelines and was based on one or more of the following in association with the finding of CMV viremia: fever, new-onset severe malaise, leukopenia, thrombocytopenia, hepatitis (alanine aminotransferase or aspartate aminotransferase levels greater than twice the upper limit of normal), and tissue-invasive disease proven by histology. For our laboratory, a copy rate of >500 copies/ml of whole blood represents significant CMV viremia. For the current analysis, CMV disease of any severity was evaluated (i.e., no distinction was made between mild CMV syndrome and severe tissue-invasive disease).
Evaluation of HLA Restricted Cellular Response in CMV Reinfection (Seropositive Donors): Prospective Cohort
Between February 2008 and October 2010, 36 adult recipients of kidney transplants from CMV-seropositive donors were enrolled into a prospective study to evaluate the immune response to CMV (D+R−, 17; D+R+, 19). The immunosuppression and antiviral prophylaxis strategy was identical to the departmental protocol described above.
Class I CMV tetramers were used to examine HLA-specific cell-mediated immunity to CMV. We first identified donor-recipient pairs for which the donor expressed an HLA-A or HLA-B antigen not expressed by the recipient, and where the recipient expressed an HLA-A or HLA-B antigen not expressed by the donor. We then narrowed this selection down to donor-recipient pairs for which a class I CMV tetramer specific to the mismatched HLA for both the donor and recipient was available (see below for details of available tetramers). In this way, we were able to distinguish circulating CD8+ T cells that were specific for CMV epitopes presented by donor HLA (but not recipient HLA) on one hand and recipient HLA (but not donor HLA) on the other. Using these criteria, we identified 11 recipients of kidneys from seropositive donors (eight D+/R+ pairs and three D+/R− donor-recipient pairs) suitable for analysis. In these patients, serial blood samples were taken for analysis after transplantation (1, 3, 6, and 12 months).
All CMV peptides used were synthesized commercially by Alta Biosciences (Birmingham, United Kingdom). Peptides incorporated in the tetramers (and the protein from which they are derived) were as follows: HLA-A1 restricted epitopes YSEHPTFTSQY (pp65) and VTEHDTLLY (pp50), HLA-A2 restricted epitopes NLVPMVATV (pp65) and VLEETSVML (IE-1), HLA-B7 restricted epitopes TPRVTGGGAM and RPHERNGFTVL (both pp65) and HLA-B8 restricted epitopes ELKRKMIYM and QIKVRVDMV (both IE-1). Tetramerization was carried out using streptavidin-APC (Invitrogen, Paisley, United Kingdom). PBMCs were isolated from heparinized blood of the transplant recipient by density gradient centrifugation using RPMI-1640 medium (Sigma) and cryopreserved in FCS containing 10% DMSO. This was performed within 6 hours after venipuncture. To identify virus-specific CD8+ T cells by flow cytometry, 1×106 PBMCs were stained with tetramer at 37°C for 15 minutes, followed by staining of surface markers. Cells were then analyzed on a Becton-Dickinson LSRII flow cytometer and using DIVA software.
Predicting Post-transplant CMV Infection by Pretransplant Assessment of Cell-Mediated Immune Response to CMV: Prospective Cohort
The predictive utility of pretransplant cell-mediated immunity to CMV was evaluated in 38 adult seropositive transplant recipients receiving kidneys from seropositive or seronegative donors (19 D+R+; 19 D−R+) between February 2008 and October 2010, using the departmental immunosuppression protocol described above. No patient received CMV prophylaxis. The frequency of circulating CMV-reactive T cells was determined before transplantation by antigen stimulation and subsequent detection of cytokine production. Briefly, fresh PBMCs were stimulated with CMV-derived peptides for 16 hours. Peptide pools were derived from IE-1 (to assess the CD8 response) or pp65 (which can assess both CD4 and CD8 responses; see below) were used at a final concentration of 1 μg/ml per peptide (all Alta Biosciences, Birmingham, United Kingdom). Ten micrograms of BrefeldinA per milliliter (Sigma-Aldrich, Gillingham, United Kingdom) was added to block cytokine secretion after 1 hour of incubation. As a positive control, cells were stimulated with Staphylococcus enterotoxin B (0.2 μg/ml final concentration; Sigma-Aldrich), and unstimulated cells served as a negative control. Cells were then stained with anti-CD4 and anti-CD8 antibodies (BD Biosciences, Oxford, United Kingdom), fixed using 4% paraformaldehyde, and permeabilized with 0.5% saponin. Intracellular IFN-γ was detected with anti–IFN-γ FITC (BD Biosciences). Analysis was performed on a Becton-Dickinson LSRII flow cytometer with FlowJo software.
The endpoint for this investigation was the development of CMV replication (irrespective of the development of symptomatic disease) within the first 12 months after transplantation. Serial whole-blood samples were taken for CMV DNA PCR in these patients at day 0 (before transplantation) and then at weeks 1, 2, 3, 4, 6, 8, 10, 12, 16, 20, 24, 28, 34, 40, 46, and 52. This cohort of patients were contained within the larger “retrospective” cohort described above. However, the clinical team remained unaware of the results of the T cell responses or the PCR results, and no changes in clinical management resulted from this series of experiments.
Immunohistochemistry Analysis of Implantation Kidney Biopsy Tissue
Paraffin-embedded tissue sections obtained from five seropositive kidney transplant donors immediately before transplantation (implantation biopsy specimens) were examined for HCMV-IE (reacts with an immediate early nonstructural antigen of 68–72 kD, antibodies IgG2a, Chemicon International), HCMV-LA (reacts with a late protein of 47–55 kD, antibodies IgG2a, Chemicon International) and HCMV-pp65 (IgG1, Novacostra, CA). Antibodies against smooth muscle cells α-actin (IgG2a, Biogenex, San Ramon, CA), vwf (IgG1, DakoCytomation, Denmark), and tissue stained by exclusion of primary antibodies were used as controls. Comparison was made with biopsy samples from three seronegative individuals.
Briefly, the sections were deparaffinized in xylene (Sigma Aldrich), rehydrated in alcohol series, postfixed with 4% neutral buffered formalin (Apoteketpharmaci, Stockholm, Sweden), treated with pepsin (Biogenex, San Ramon, CA), and then incubated in citrate buffer (Biogenex). Endogenous peroxidase was blocked by treating sections with 3% H2O2 (Sigma-Aldrich), endogenous avidin/biotin was blocked using an avidin/biotin blocking kit (DakoCytomation, Glostrup, Denmark), and FC receptor blocker (Innovex Biosciences) was used to block FC receptors. Finally, the tissue sections were treated with background buster (Innovex Biosciences). All sections were incubated with primary antibodies as mentioned above or without primary antibodies (only diluents) overnight at 4°C. Antibodies were visualized using biotinylated secondary goat antibodies against primary mouse antibodies (Biogenex), streptavidin-conjugated horseradish peroxidase, and diaminobenzidine (Innovex Biosciences).
The study was approved by the North Staffordshire Research Ethics Committee and was conducted in accordance with the guidelines of the Declaration of Helsinki.
Statistical Analyses
Data are presented as mean ± SD unless otherwise described. Continuously distributed data were compared using a t test, and categorical data were compared using chi-squared testing.
Survival analysis was conducted using Kaplan-Meier methods, with the log-rank test used to assess significance. The development of CMV disease in the retrospective analysis was considered as a time-to-event outcome. This analysis factored both death and graft loss as competing risks, and so the analysis was performed using a competing-risks regression model using the methods described by Fine and Gray. For all analyses, initially the effect of each variable on the outcome was considered separately in a series of univariate analyses. Variables showing some evidence of effect (P<0.15) were included in a subsequently multivariate analysis. A stepwise backward selection procedure was performed to retain only the statistically significant variables in the final model.
In addition, there was specific interest with regard to the influence of donor-recipient HLA mismatch on the development of CMV disease. First, a statistical interaction between donor-recipient HLA mismatch and donor-recipient CMV serostatus with regard to time to CMV disease was evaluated. When such an interaction was found, subgroup analysis (by serostatus combination) was performed using the competing-risk model described above.
In the first instance, only pretransplant demographic characteristics were considered in the model, and episodes of delayed graft function and biopsy-proven acute rejection were not evaluated because these can be considered as intermediate end points that may confound the analysis. However, in a set of secondary analyses, these post-transplant events were evaluated as time-dependent covariates.
For all analyses, a type 1 error rate <5% (P<0.05) was considered to represent a statistically significant difference.
Assessment of the cell-mediated immunity assay in regard to predicting CMV replication in the first year was evaluated by the following parameters of predictive performance: sensitivity, specificity, and positive and negative predictive values. These measures were calculated following the determination of the optimal cutoff value from receiver-operating characteristic curve analysis. The area under the receiver-operating characteristic curve (c-statistic) was calculated as a global measure of prognostic performance.
Disclosures
None.
Acknowledgments
The research was carried out at the National Institute for Health Research (NIHR)/Wellcome Trust Birmingham Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health. We are especially grateful to Golaleh Didarzadeh and Theresa McCarthy for their invaluable assistance in the conduct of this study.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Moss P, Khan N: CD8(+) T-cell immunity to cytomegalovirus. Hum Immunol 65: 456–464, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Polić B, Hengel H, Krmpotić A, Trgovcich J, Pavić I, Luccaronin P, Jonjić S, Koszinowski UH: Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J Exp Med 188: 1047–1054, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pahl-Seibert MF, Juelch M, Podlech J, Thomas D, Deegen P, Reddehase MJ, Holtappels R: Highly protective in vivo function of cytomegalovirus IE1 epitope-specific memory CD8 T cells purified by T-cell receptor-based cell sorting. J Virol 79: 5400–5413, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundy JE, Lui SF, Super M, Berry NJ, Sweny P, Fernando ON, Moorhead J, Griffiths PD. Symptomatic cytomegalovirus infection in seropositive kidney recipients: Reinfection with donor virus rather than reactivation of recipient virus. Lancet 1988. 16;2(8603):132-5. [DOI] [PubMed]
- 5.Kliem V, Fricke L, Wollbrink T, Burg M, Radermacher J, Rohde F: Improvement in long-term renal graft survival due to CMV prophylaxis with oral ganciclovir: Results of a randomized clinical trial. Am J Transplant 8: 975–983, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Witzke O, Hauser IA, Bartels M, Wolf G, Wolters H, Nitschke M, VIPP Study Group : Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: 1-year results of a randomized clinical trial. Transplantation 93: 61–68, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Atabani SF, Smith C, Atkinson C, Aldridge RW, Rodriguez-Perálvarez M, Rolando N, Harber M, Jones G, O’Riordan A, Burroughs AK, Thorburn D, O’Beirne J, Milne RS, Emery VC, Griffiths PD: Cytomegalovirus replication kinetics in solid organ transplant recipients managed by preemptive therapy. Am J Transplant 12: 2457–2464, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manuel O, Asberg A, Pang X, Rollag H, Emery VC, Preiksaitis JK, Kumar D, Pescovitz MD, Bignamini AA, Hartmann A, Jardine AG, Humar A: Impact of genetic polymorphisms in cytomegalovirus glycoprotein B on outcomes in solid-organ transplant recipients with cytomegalovirus disease. Clin Infect Dis 49: 1160–1166, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Ishibashi K, Tokumoto T, Shirakawa H, Hashimoto K, Ikuta K, Kushida N, Yanagida T, Shishido K, Aikawa K, Toma H, Inoue N, Yamaguchi O, Tanabe K, Suzutani T: Lack of antibodies against the antigen domain 2 epitope of cytomegalovirus (CMV) glycoprotein B is associated with CMV disease after renal transplantation in recipients having the same glycoprotein H serotypes as their donors. Transpl Infect Dis 13: 318–323, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Schnitzler MA, Lowell JA, Hmiel SP, Hardinger KL, Liapis H, Ceriotti CS, Brennan DC: Cytomegalovirus disease after prophylaxis with oral ganciclovir in renal transplantation: The importance of HLA-DR matching. J Am Soc Nephrol 14: 780–785, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Pouteil-Noble C, Bétuel H, Raffaele P, Megri K, Louvier C, Lefrançois N, Bosshard S, Dubernard JM, Aymard M, Touraine JL: [Influence of HLA compatibility on cytomegalovirus infection in kidney transplantation]. Presse Med 20: 2022–2024, 1991 [PubMed] [Google Scholar]
- 12.Blancho G, Josien R, Douillard D, Bignon JD, Cesbron A, Soulillou JP: The influence of HLA A-B-DR matching on cytomegalovirus disease after renal transplantation. Evidence that HLA-DR7-matched recipients are more susceptible to cytomegalovirus disease. Transplantation 54: 871–874, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Kraat YJ, Christiaans MH, Nieman FH, van den Berg-Loonen PM, van Hooff JP, Bruggeman CA: Increased frequency of CMV infection in HLA-DR7 matched renal allograft recipients. Lancet 341: 494–495, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Smyth LA, Hervouet C, Hayday T, Becker PD, Ellis R, Lechler RI, Lombardi G, Klavinskis LS: Acquisition of MHC:peptide complexes by dendritic cells contributes to the generation of antiviral CD8+ T cell immunity in vivo. J Immunol 189: 2274–2282, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Wakim LM, Bevan MJ: Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature 471: 629–632, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder CM, Allan JE, Bonnett EL, Doom CM, Hill AB: Cross-presentation of a spread-defective MCMV is sufficient to prime the majority of virus-specific CD8+ T cells. PLoS ONE 5: e9681, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munks MW, Pinto AK, Doom CM, Hill AB: Viral interference with antigen presentation does not alter acute or chronic CD8 T cell immunodominance in murine cytomegalovirus infection. J Immunol 178: 7235–7241, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Walton SM, Mandaric S, Torti N, Zimmermann A, Hengel H, Oxenius A: Absence of cross-presenting cells in the salivary gland and viral immune evasion confine cytomegalovirus immune control to effector CD4 T cells. PLoS Pathog 7: e1002214, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews DM, Andoniou CE, Granucci F, Ricciardi-Castagnoli P, Degli-Esposti MA: Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat Immunol 2: 1077–1084, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Loewendorf A, Krüger C, Borst EM, Wagner M, Just U, Messerle M: Identification of a mouse cytomegalovirus gene selectively targeting CD86 expression on antigen-presenting cells. J Virol 78: 13062–13071, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benedict CA, Loewendorf A, Garcia Z, Blazar BR, Janssen EM: Dendritic cell programming by cytomegalovirus stunts naive T cell responses via the PD-L1/PD-1 pathway. J Immunol 180: 4836–4847, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gredmark S, Söderberg-Nauclér C: Human cytomegalovirus inhibits differentiation of monocytes into dendritic cells with the consequence of depressed immunological functions. J Virol 77: 10943–10956, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins C, Garcia W, Godwin MJ, Spencer JV, Stern JL, Abendroth A, Slobedman B: Immunomodulatory properties of a viral homolog of human interleukin-10 expressed by human cytomegalovirus during the latent phase of infection. J Virol 82: 3736–3750, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liapis H, Storch GA, Hill DA, Rueda J, Brennan DC: CMV infection of the renal allograft is much more common than the pathology indicates: A retrospective analysis of qualitative and quantitative buffy coat CMV-PCR, renal biopsy pathology and tissue CMV-PCR. Nephrol Dial Transplant 18: 397–402, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Boudreault AA, Xie H, Rakita RM, Scott JD, Davis CL, Boeckh M, Limaye AP: Risk factors for late-onset cytomegalovirus disease in donor seropositive/recipient seronegative kidney transplant recipients who receive antiviral prophylaxis. Transpl Infect Dis 13: 244–249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundy JE, Ayles HM, McKeating JA, Butcher RG, Griffiths PD, Poulter LW: Enhancement of class I HLA antigen expression by cytomegalovirus: Role in amplification of virus infection. J Med Virol 25: 483–495, 1988 [DOI] [PubMed] [Google Scholar]
- 27.Hosenpud JD, Chou SW, Wagner CR: Cytomegalovirus-induced regulation of major histocompatibility complex class I antigen expression in human aortic smooth muscle cells. Transplantation 52: 896–903, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Grundy JE, McKeating JA, Ward PJ, Sanderson AR, Griffiths PD: Beta 2 microglobulin enhances the infectivity of cytomegalovirus and when bound to the virus enables class I HLA molecules to be used as a virus receptor. J Gen Virol 68: 793–803, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Waldman WJ, Knight DA: Cytokine-mediated induction of endothelial adhesion molecule and histocompatibility leukocyte antigen expression by cytomegalovirus-activated T cells. Am J Pathol 148: 105–119, 1996 [PMC free article] [PubMed] [Google Scholar]
- 30.Simon CO, Holtappels R, Tervo HM, Böhm V, Däubner T, Oehrlein-Karpi SA, Kühnapfel B, Renzaho A, Strand D, Podlech J, Reddehase MJ, Grzimek NK: CD8 T cells control cytomegalovirus latency by epitope-specific sensing of transcriptional reactivation. J Virol 80: 10436–10456, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holtappels R, Gillert-Marien D, Thomas D, Podlech J, Deegen P, Herter S, Oehrlein-Karpi SA, Strand D, Wagner M, Reddehase MJ: Cytomegalovirus encodes a positive regulator of antigen presentation. J Virol 80: 7613–7624, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishibashi K, Tokumoto T, Tanabe K, Shirakawa H, Hashimoto K, Kushida N, Yanagida T, Inoue N, Yamaguchi O, Toma H, Suzutani T: Association of the outcome of renal transplantation with antibody response to cytomegalovirus strain-specific glycoprotein H epitopes. Clin Infect Dis 45: 60–67, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ: Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 202: 673–685, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holtappels R, Simon CO, Munks MW, Thomas D, Deegen P, Kühnapfel B, Däubner T, Emde SF, Podlech J, Grzimek NK, Oehrlein-Karpi SA, Hill AB, Reddehase MJ: Subdominant CD8 T-cell epitopes account for protection against cytomegalovirus independent of immunodomination. J Virol 82: 5781–5796, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, Melpolder JC, Strazzera A, Chien DY, Munoz SJ, Balestrieri A, Purcell RH, Alter HJ: The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 288: 339–344, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Cantisán S, Lara R, Montejo M, Redel J, Rodríguez-Benot A, Gutiérrez-Aroca J, González-Padilla M, Bueno L, Rivero A, Solana R, Torre-Cisneros J: Pretransplant interferon-γ secretion by CMV-specific CD8+ T cells informs the risk of CMV replication after transplantation. Am J Transplant 13: 738–745, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Kratky W, Reis e Sousa C, Oxenius A, Spörri R: Direct activation of antigen-presenting cells is required for CD8+ T-cell priming and tumor vaccination. Proc Natl Acad Sci U S A 108: 17414–17419, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]