Abstract
Background
The purpose of this study was to examine the biological environment of the esophageal hiatus through analysis of the collagen content within the gastrohepatic ligament (GHL), gastrophrenic ligament (GPL), and phrenoesophageal ligament (PEL) in patients with type I hiatal hernias (HH) and type III paraesophageal hernias (PEH).
Methods
A control group (N = 10) and patients with type I HH (N = 10) and type III PEH (N = 10) were included in the analysis. Specimens of the GHL, PEL, and GPL were collected intraoperatively. Slides stained with sirius red/fast green were created and ten photos at 400 × magnification were taken of each specimen. Axiovision 4.7 (Zeiss) photo analysis software was employed for quantification of collagen I (red) and III (green) by calculating color area (μm2). Statistical significance (p < 0.05) was determined using a one-way ANOVA and Fisher’s LSD post-test.
Results
Cross-polarization microscopy revealed that the collagen I content was similar in the three study groups for the GHL, greater in the type III PEH group and in the control group compared to the type I HH group for the PEL, and greater in the type III PEH group compared to control group for the GPL. Collagen III quantity was greater in the control group than in the type I HH group for each ligament, and greater in the GHL and PEL when compared to the type III PEH group. Type III PEH patients had greater collagen III quantity than did type I HH patients for each ligament. Collagen type I:III ratio of the GHL was greater in both hernia groups compared to the control group. Type III PEH patients contained a higher I:III ratio than both the control and type I HH groups with respect to the PEL. There was no difference in the ratio with evaluation of the GPL for the three groups.
Conclusion
Evaluation of the esophageal hiatus revealed that patients with PEH have a different biological environment with regard to collagen content compared to control patients. The collagen I:III ratio of the study groups was equal to or greater than the control group. Collagen deficiency in the GE junction supporting ligaments does not appear to be an etiology of PEH formation.
Keywords: Paraesophageal hernia, Collagen ratio, Collagen I, Collagen III
The integrity of the esophageal hiatus is maintained by several ligaments surrounding the gastroesophageal (GE) junction, including the phrenoesophageal ligament (PEL), gastrohepatic ligament (GHL), and the gastrophrenic ligament (GPL). There are four types of hiatal hernias (HH), defined by the content of the hernia sac and the location of the GE junction in relation to the diaphragm. Type I HH, also known as a sliding hiatal hernia, is the most common and is associated with laxity of the phrenoesophageal membrane. This enables the GE junction along with the cardia of the stomach to herniate above the diaphragm. Type II paraesophageal hernia (PEH) is unique in that the GE junction remains in the appropriate anatomic position, but a defect in the phrenoesophageal membrane allows the fundus of the stomach to herniate into the thorax. Type III PEH is a combination of type I and II with the GE junction and fundus located above the diaphragm. Finally, type IV PEH consists of a large defect in the phrenoesophageal membrane, permitting other abdominal organs to herniate into the thorax (i.e., colon, spleen, small bowel).
Hiatal hernia is a common condition encountered in surgical practice with prevalence between 15 and 60% [1]. There are several indications to repair a PEH depending on the hernia type and associated symptoms. Hiatal hernias are frequently associated with gastroesophageal reflux disease, and surgical repair is often required in these patients to treat their heartburn and/or regurgitation adequately [2]. Type II–IV PEHs are often associated with obstructive symptoms (chest pain, dysphagia, and early satiety) and are generally repaired upon diagnosis because of the inherent risk of serious complications related to organo-axial rotation, resulting in incarceration, necrosis, and perforation [3]. Surgical repair of a PEH via a transthoracic or minimally invasive abdominal approach is commonly divided into five components, including reduction of herniated organs into the abdominal cavity, excision of the hernia sac, closure of the hiatal defect, an antireflux procedure, and gastropexy [4].
A fundamental question is whether hernia formation is the sequelae of excessive mechanical stress or the result of an ongoing biological disorder of collagen deficiency or integrity. Recent investigations have shifted research from understanding the technical aspect of hernia development to exploring the biological processes involved in its development. The extracellular matrix (ECM), specifically collagen metabolism, has become the target of multiple investigations [5-9]. The observed correlation of several connective tissue diseases and hernia formation has spurred research in this direction [10, 11]. Previous studies evaluating the extracellular environment in patients with both primary and recurrent incisional hernias have revealed alterations in the composition of collagen types I and III [9, 12, 13]. In addition, a decrease in the collagen I:III ratio has been associated with recurrent hernia formation [6]. While these studies have focused on inguinal and incisional hernias, to date there is no study addressing the collagenous environment of the phrenoesophageal membrane related to PEH formation.
The purpose of this study was to examine the biological environment of the esophageal hiatus through analysis of the collagen content within the gastrohepatic ligament (GHL), gastrophrenic ligament (GPL), and phrenoesophageal ligament (PEL) in patients with type I hiatal hernias (HH) and type III paraesophageal hernias (PEH).
Methods
Patient selection and tissue collection
Patients with the preoperative diagnosis of HH and PEH were enrolled in a prospective trial. The control group included patients with achalasia or morbid obesity without a hiatal hernia who were to undergo laparoscopic esophageal myotomy or laparoscopic gastric bypass, respectively. Participants consented to tissue sampling preoperatively and intraoperative specimens were harvested from ten patients in each group. From each patient a 1-cm2 section of tissue was collected from each of the supporting ligaments: GPL, GHL, and PEL. The samples were immediately placed in 10% neutral buffered formalin for storage. This study protocol (No. 07-0066) was approved by the Human Research Protection Office (HRPO) of Washington University School of Medicine.
Histological methods
Tissue samples were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin for evaluation of tissue architecture. The slides were washed with tap water and stained a with 0.1% fast green FCF for 10 min, followed by washing with acetic acid and staining with picrosirius red F3BA for 1 h. The slides were then washed with acidified water, dehydrated, cleared, and mounted in resinous medium. Sirius red/fast green (SR/FG) stain was chosen for its ability to differentiate type I from type III collagen. Sirius red is a strong anionic dye that stains collagen by reacting, via its sulfonic acid groups, with basic groups present in the collagen molecule. The dye molecules attach to the collagen fibers in such a way that their long axes are parallel. This configuration between the dye and the collagen results in an enhanced birefringency [14]. When viewed under cross-polarized light, the collagen fibers appear distinctly different from one another, with the type I collagen fibers appearing bright yellow-red/orange, while the type III collagen fibers appear green-blue. Slides were examined using cross-polarized light microscopy with an Axioskop 40 microscope (Carl Zeiss, Thornwood, NY) equipped with a Zeiss Axiocam at 400× magnification. A total of ten high-resolution images were captured of each slide, taking care not to overlap the images, ensuring ample representation of the field. The images were stored as multidimensional ZVI files for analysis.
Image analysis
The high-resolution images were analyzed using the automated measurement feature of Carl Zeiss’ Axiovision software. The area (μm2) stained red (type I collagen) and the area stained green (type III collagen) were quantified for each slide. The scaling was adjusted to correlate to the magnification of the digital image, with 0.1679 μm/pixel. With ten photos of each slide and ten patients in each study group, there were 100 images available for analysis for each ligament in a given study group. After the quantity of type I and type III collagens was recorded from each slide, the average quantity for each ligament was calculated and recorded. In addition, the collagen I:III ratio was calculated for each ligament by dividing the area stained red by the area stained green (μm2/μm2).
Statistical analysis
Data are expressed as mean ± standard error of the mean unless otherwise indicated, and statistical significance was defined as p < 0.05. Each set of SR/FG data was analyzed using a one-way ANOVA with “patient type” (i.e., control, HH, or PEH) as the independent variable. Variables with p < 0.05 were subsequently subjected to Fisher’s LSD post-test.
Results
Demographics
A total of 30 patients (9 males) with a mean age of 56.2 ± 2.35 years were enrolled in the study (Table 1). The control group included eight patients with achalasia who underwent laparoscopic esophageal myotomy and two patients with morbid obesity who underwent laparoscopic Roux-en-Y gastric bypass. The hernia groups all underwent laparoscopic hiatal hernia repair and fundoplication. Tissue samples of the GPL, GHL, and PEL were successfully collected from all patients and were included in the analysis.
Table 1.
Demographic information of study groups
| Type I PEH | Type III PEH | Control | |
|---|---|---|---|
| Sex (M:F) | 3:7 | 1:9 | 5:5 |
| Age | 54.80 ± 3.86 | 58.20 ± 1.79 | 55.60 ± 5.86 |
Quantitative analysis
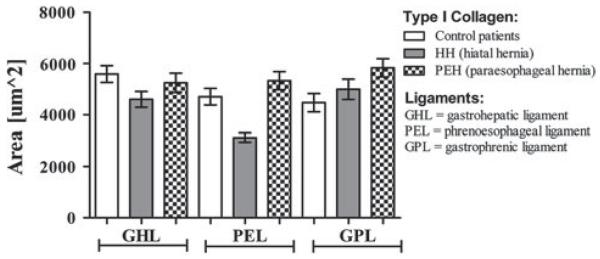
Type I collagen dominated in all three ligaments compared to type III collagen for each of the study groups. When comparing the type I collagen content among the study groups, no differences were detected for the GHL between any of the patient groups (p = 0.110) (Fig. 1). For the PEL, both the PEH group and the control group contained more type I collagen than the HH group (5331 ± 355.2 PEH and 4705 ± 324.6 control vs. 3126 ± 180.1 HH; p = 0.0001 for both cases). Analysis of the GPL data demonstrated that PEH patients contained more type I collagen than the control patients (5832 ± 356.9 PEH vs. 4474 ± 358.2 control; p = 0.010).
Fig. 1.
Type I collagen quantification in the supporting ligaments of the gastroesophageal junction
Analysis of the type III collagen content demonstrated that the control group had more type III collagen than did HH patients for all three ligaments (GHL, PEL, and GPL; p = 0.0001 for all cases) (Fig. 2). In addition, the control group had a greater quantity of type III collagen in the GHL and PEL than did the PEH group (GHL 5146 ± 255.9 vs. 3115 ± 192.5; p = 0.0001 and PEL 4317 ± 235.8 vs. 3378 ± 186.0; p = 0.001, respectively). When the hernia groups were compared to one another, PEH patients contained more type III collagen than did HH patients for all three ligaments (GHL; p = 0.006, PEL; p = 0.011, and GPL; p = 0.010, respectively).
Fig. 2.
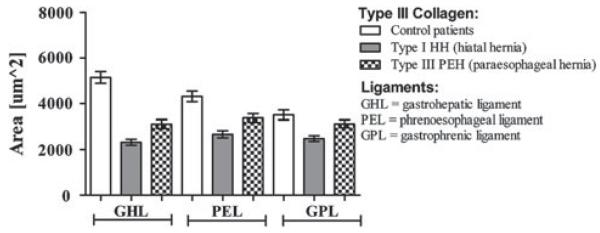
Type III collagen quantification in the supporting ligaments of the gastroesophageal junction
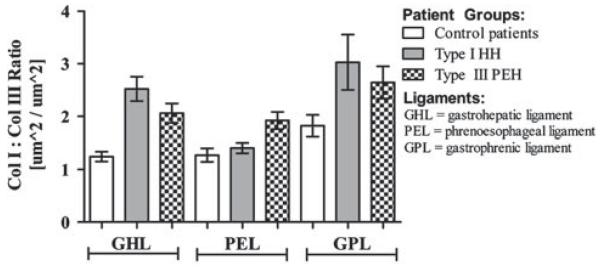
The collagen I:III ratio was calculated for each patient group (Fig. 3). With respect to the GHL, both hernia groups had a greater collagen I:III ratio than the control group (HH: 2.52 ± 0.2, p = 0.0001; PEH: 2.06 ± 0.2, p = 0.001; control: 1.25 ± 0.1). For the PEL, PEH patients had a higher ratio than did HH and control patients (PEH: 1.92 ± 0.2, HH: 1.41 ± 0.1, p = 0.001; control: 1.27 ± 0.1, p = 0.006). For GPL, no statistically significant difference was detected among the patient groups.
Fig. 3.
Collagen I:III ratio of supporting ligaments
Discussion
In the present study, the collagen content of the primary esophageal supporting ligaments was compared in control patients and those with type I and III hiatal hernias. The principal findings are that collagen content of the phrenoesophageal membrane of the hernia groups was different from that of the control group. Collagen I:III ratio was actually higher for both hiatal hernia groups compared to controls for the GHL, and higher in the PEH group compared to controls for the PEL. Collagen I:III ratios were the same for the three study groups with respect to the GPL. This is evident by the higher quantity of type I collagen in the PEL and the GPL of the PEH group compared to that of controls. The quantity of type III collagen was greater in the control group than in the PEH group for the GHL and PEL and greater in all three supporting ligaments compared to the HH group.
The stability of the ECM is directly related to the rate of collagen synthesis and degradation of collagen by matrix metalloproteinases (MMPs) [15]. Collagen comprises 80% of the dry weight of connective tissue. Type I and type III collagens are the predominant types of collagen found within the dermis, together accounting for 95% of the collagen present [16-18]. Type I collagen is mature, mechanically stable, and responsible for tensile strength [19]. Type III collagen, typically present during the early phase of wound healing, is thinner and generally regarded as immature and weak [20, 21].
Alterations in the ECM, specifically the quality of collagen formation, and the relationship to hernia development have become the focus of several investigations [6, 9, 13]. The quality of collagen has been characterized by the ratio of type I:III collagen [6]. Compared to control patients, a decrease in this ratio would indicate that the quantity of strong type I collagen was diminished or that the weaker type III collagen was more prevalent. In either case, the integrity of such a structure would be compromised. In normal skin, the natural ratio of type I:III collagen is 4:1 [22]. Klinge et al. [23] found that the collagen type I:III ratio was diminished in patients with both direct and indirect inguinal hernias when compared to a control group. Another study evaluating the collagen ratio in incisional hernias demonstrated a decrease in the collagen ratio of both incisional hernias and recurrent incisional hernias [7]. However, these investigations have focused on inguinal and incisional hernias which are distinctly different from PEH, and an alteration in the collagen ratio may not contribute to hernia formation in this anatomic location.
With the recent increased interest in the biological basis of hernia formation, various techniques have been employed to analyze collagen quantity and collagen I:III ratio. Immunohistochemical staining and polymerase chain reaction (PCR) techniques have been used to assess the expression of collagen and MMPs in relation to hernia development [13, 24]. The current study concentrated on the use of cross-polarization microscopy for evaluation of collagen quantity in the supporting ligaments of the GE junction. Although this is a well-established method for analysis [5, 25], recent data by our institution suggests that measuring the MMP concentrations within the supporting ligaments may yield additional insight into the pathophysiology of PEH formation [26].
Previous tissue analyses by our research group have demonstrated a decrease in the amount of elastin found in the supporting ligaments of the gastroesophageal junction in hiatal hernia patients [27]. It is possible that there is a relationship between “stiff” collagenous tissue and “elastin-rich” tissues that can recoil. In the formation of hiatal hernias, it is possible that the collagenous ligaments stretch but do not recoil. Another possibility is that hiatal hernia formation is independent of the gastroesophageal junction ligaments. Perhaps increased intra-abdominal forces due to weight, pulmonary disease, gastrointestinal (chronic constipation), genitourinary (benign prostatic hypertrophy), or genetic deficiencies are more significant than the structure of the gastroesophageal ligaments themselves [28].
This study had some limitations. The control group included patients with achalasia and morbid obesity because the operations performed to treat these conditions are localized to the foregut, providing easy access to the supporting ligaments. However, morbid obesity is associated with increased intra-abdominal pressure and possibly increased stress at the GE junction, which could influence the results of this study.
Conclusion
The collagen I:III ratio of patients with a hiatal hernia was equal to or greater than the control patients. Collagen deficiency in the supporting ligaments of the GE junction does not appear to be an etiology of hiatal hernia formation and the pathophysiology of hiatal hernia formation remains elusive. As research transitions from the technical aspect of hernia formation to a more biological basis, further investigation is needed to elucidate the origin of hiatal hernia formation.
Acknowledgment
This work was funded by the Musculoskeletal Transplantation Foundation.
Footnotes
Presented at the 12th World Congress of Endoscopic Surgery, National Harbor, MD, April 14-17, 2010.
Disclosures Dr. Brown, Dr. Melman, Dr. Jenkins, and Mrs. Frisella have no conflicts of interest or financial ties to disclose. Dr. Deeken has received consulting fees and an honorarium from Davol, Incorporated. Dr. Eagon is a consultant for Ethicon Endosurgical. Dr. Brunt has received educational grants and research support from Ethicon Endosurgical, Karl Storz Endoscopy, Stryker endoscopy, and Lifecell, and an honorarium for speaking from Ethicon EndoSurgery. Dr. Matthews has received consulting fees from Atrium Medical, Ethicon EndoSurgery, and Muskuloskeletal Transplant Foundation, and an honorarium for speaking from W.L. Gore.
References
- 1.Berstad A, Weberg R, Froyshov Larsen I, Hoel B, Hauer-Jensen M. Relationship of hiatus hernia to reflux oesophagitis. A prospective study of coincidence, using endoscopy. Scand J Gastroenterol. 1986;21(1):55–58. doi: 10.3109/00365528609034622. [DOI] [PubMed] [Google Scholar]
- 2.Soper NJ. Laparoscopic management of hiatal hernia and gastroesophageal reflux. Curr Probl Surg. 1999;36(10):765–838. doi: 10.1016/s0011-3840(99)80301-3. [DOI] [PubMed] [Google Scholar]
- 3.Hashemi M, Sillin LF, Peters JH. Current concepts in the management of paraesophageal hiatal hernia. J Clin Gastroenterol. 1999;29(1):8–13. doi: 10.1097/00004836-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 4.El Sherif A, Yano F, Mittal S, Filipi CJ. Collagen metabolism and recurrent hiatal hernia: cause and effect? Hernia. 2006;10(6):511–520. doi: 10.1007/s10029-006-0152-9. [DOI] [PubMed] [Google Scholar]
- 5.Junge K, Klinge U, Rosch R, Lynen P, Binnebosel M, Conze J, Mertens PR, Schwab R, Schumpelick V. Improved collagen type I/III ratio at the interface of gentamicin-supplemented polyvinylidenfluoride mesh materials. Langenbecks Arch Surg. 2007;392(4):465–471. doi: 10.1007/s00423-006-0138-1. [DOI] [PubMed] [Google Scholar]
- 6.Junge K, Klinge U, Rosch R, Mertens PR, Kirch J, Klosterhalfen B, Lynen P, Schumpelick V. Decreased collagen type I/III ratio in patients with recurring hernia after implantation of alloplastic prostheses. Langenbecks Arch Surg. 2004;389(1):17–22. doi: 10.1007/s00423-003-0429-8. [DOI] [PubMed] [Google Scholar]
- 7.Klinge U, Si ZY, Zheng H, Schumpelick V, Bhardwaj RS, Klosterhalfen B. Abnormal collagen I to III distribution in the skin of patients with incisional hernia. Eur Surg Res. 2000;32(1):43–48. doi: 10.1159/000008740. [DOI] [PubMed] [Google Scholar]
- 8.Klinge U, Binnebosel M, Mertens PR. Are collagens the culprits in the development of incisional and inguinal hernia disease? Hernia. 2006;10(6):472–477. doi: 10.1007/s10029-006-0145-8. [DOI] [PubMed] [Google Scholar]
- 9.Klinge U, Si ZY, Zheng H, Schumpelick V, Bhardwaj RS, Klosterhalfen B. Collagen I/III and matrix metalloproteinases (MMP) 1 and 13 in the fascia of patients with incisional hernias. J Invest Surg. 2001;14(1):47–54. doi: 10.1080/089419301750072202. [DOI] [PubMed] [Google Scholar]
- 10.Girotto JA, Malaisrie SC, Bulkely G, Manson PN. Recurrent ventral herniation in Ehlers-Danlos syndrome. Plast Reconstr Surg. 2000;106(7):1520–1526. doi: 10.1097/00006534-200012000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Uden A, Lindhagen T. Inguinal hernia in patients with congenital dislocation of the hip. A sign of general connective tissue disorder. Acta Orthop Scand. 1988;59(6):667–668. doi: 10.3109/17453678809149421. [DOI] [PubMed] [Google Scholar]
- 12.Si Z, Bhardwaj R, Rosch R, Mertens PR, Klosterhalfen B, Klinge U. Impaired balance of type I and type III procollagen mRNA in cultured fibroblasts of patients with incisional hernia. Surgery. 2002;131(3):324–331. doi: 10.1067/msy.2002.121376. [DOI] [PubMed] [Google Scholar]
- 13.Rosch R, Klinge U, Si Z, Junge K, Klosterhalfen B, Schumpelick V. A role for the collagen I/III and MMP-1/-13 genes in primary inguinal hernia? BMC Med Genet. 2002;3:2. doi: 10.1186/1471-2350-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11(4):447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 15.Basson MD. Invited research review: cell-matrix interactions in the gut epithelium. Surgery. 2003;133(3):263–267. doi: 10.1067/msy.2003.24. [DOI] [PubMed] [Google Scholar]
- 16.Friedman DW, Boyd CD, Norton P, Greco RS, Boyarsky AH, Mackenzie JW, Deak SB. Increases in type III collagen gene expression and protein synthesis in patients with inguinal hernias. Ann Surg. 1993;218(6):754–760. doi: 10.1097/00000658-199312000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krane SM, Byrne MH, Lemaitre V, Henriet P, Jeffrey JJ, Witter JP, Liu X, Wu H, Jaenisch R, Eeckhout Y. Different collagenase gene products have different roles in degradation of type I collagen. J Biol Chem. 1996;271(45):28509–28515. doi: 10.1074/jbc.271.45.28509. [DOI] [PubMed] [Google Scholar]
- 18.Arakawa M, Hatamochi A, Mori Y, Mori K, Ueki H, Moriguchi T. Reduced collagenase gene expression in fibroblasts from hypertrophic scar tissue. Br J Dermatol. 1996;134(5):863–868. [PubMed] [Google Scholar]
- 19.Schaffer M, Becker HD. Immune regulation of wound healing. Chirurg. 1999;70(8):897–908. doi: 10.1007/s001040050740. [DOI] [PubMed] [Google Scholar]
- 20.Hurme T, Kalimo H, Sandberg M, Lehto M, Vuorio E. Localization of type I and III collagen and fibronectin production in injured gastrocnemius muscle. Lab Invest. 1991;64(1):76–84. [PubMed] [Google Scholar]
- 21.Lehto M, Sims TJ, Bailey AJ. Skeletal muscle injury— molecular changes in the collagen during healing. Res Exp Med (Berl) 1985;185(2):95–106. doi: 10.1007/BF01854894. [DOI] [PubMed] [Google Scholar]
- 22.Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176(2A Suppl):26S–38S. doi: 10.1016/s0002-9610(98)00183-4. [DOI] [PubMed] [Google Scholar]
- 23.Klinge U, Zheng H, Si Z, Schumpelick V, Bhardwaj RS, Muys L, Klosterhalfen B. Expression of the extracellular matrix proteins collagen I, collagen III and fibronectin and matrix metalloproteinase-1 and -13 in the skin of patients with inguinal hernia. Eur Surg Res. 1999;31(6):480–490. doi: 10.1159/000008728. [DOI] [PubMed] [Google Scholar]
- 24.Rosch R, Lynen-Jansen P, Junge K, Knops M, Klosterhalfen B, Klinge U, Mertens PR, Schumpelick V. Biomaterial-dependent MMP-2 expression in fibroblasts from patients with recurrent incisional hernias. Hernia. 2006;10(2):125–130. doi: 10.1007/s10029-005-0060-4. [DOI] [PubMed] [Google Scholar]
- 25.Rosch R, Junge K, Knops M, Lynen P, Klinge U, Schumpelick V. Analysis of collagen-interacting proteins in patients with incisional hernias. Langenbecks Arch Surg. 2003;387(11-12):427–432. doi: 10.1007/s00423-002-0345-3. [DOI] [PubMed] [Google Scholar]
- 26.Melman L, Chisholm PR, Curci JA, Arif B, Pierce R, Jenkins ED, Brunt LM, Eagon C, Frisella M, Miller K, Matthews BD. Differential regulation of MMP-2 in the gastrohepatic ligament of the gastroesophageal junction. Surg Endosc. 2010;24(7):1562–1565. doi: 10.1007/s00464-009-0811-x. [DOI] [PubMed] [Google Scholar]
- 27.Curci JA, Melman LM, Thompson RW, Soper NJ, Matthews BD. Elastic fiber depletion in the supporting ligaments of the gastroesophageal junction: a structural basis for the development of hiatal hernia. J Am Coll Surg. 2008;207(2):191–196. doi: 10.1016/j.jamcollsurg.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 28.Asling B, Jirholt J, Hammond P, Knutsson M, Walentinsson A, Davidson G, Agreus L, Lehmann A, Lagerström-Fermer M. Collagen type III alpha I is a gastro-oesophageal reflux disease susceptibility gene and a male risk factor for hiatus hernia. Gut. 2009;58(8):1063–1069. doi: 10.1136/gut.2008.167353. [DOI] [PMC free article] [PubMed] [Google Scholar]