Abstract
To evaluate clinical and molecular predictors of the risk of mortality in people with neurofibromatosis 2 (NF2), we analyzed the mortality experience of 368 patients from 261 families in the United Kingdom NF2 registry, using the Cox proportional-hazards model and the jackknife method. Age at diagnosis, intracranial meningiomas, and type of treatment center were informative predictors of the risk of mortality. In Cox models, the relative risk of mortality increased 1.13-fold per year decrease in age at diagnosis (95% confidence interval [CI] 1.08–1.18) and was 2.51-fold greater in people with meningiomas compared with those without meningiomas (95% CI 1.38–4.57). The relative risk of mortality in patients treated at specialty centers was 0.34 compared with those treated at nonspecialty centers (95% CI 0.12–0.98). In a separate model, the relative risk of mortality in people with constitutional NF2 missense mutations was very low compared with those with other types of mutations (nonsense or frameshift mutations, splice-site mutations, and large deletions), but the CI could not be well quantified because there was only one death among people with missense mutations. We conclude that age at diagnosis, the strongest single predictor of the risk of mortality, is a useful index for patient counseling and clinical management (as are intracranial meningiomas). To ensure optimal care, we recommend that people with NF2 be referred to specialty treatment centers.
Introduction
Neurofibromatosis 2 (NF2 [MIM 101000]) is an autosomal dominant disorder that is caused by inactivating mutations or loss of both alleles of the NF2 tumor-suppressor gene (Rouleau et al. 1993; Trofatter et al. 1993). Vestibular schwannomas (VSs), intracranial meningiomas, spinal tumors, peripheral nerve tumors, and presenile lens opacities are common in NF2 (Evans et al. 1992a; Parry et al. 1994; Mautner et al. 1996). VSs occur in ∼95% of adults with NF2 (bilateral VSs are pathognomonic for NF2), meningiomas in ∼50%, and presenile lens opacities in ∼60%–80%. Spinal tumors occur in ∼90% of people with NF2, although only 30% of these people have symptomatic spinal tumors (Mautner et al. 1995).
Cross-sectional studies of genotype-phenotype correlations in NF2 have found that, in general, constitutional nonsense and frameshift NF2 mutations are associated with severe disease; missense mutations, in-frame deletions, and large deletions with mild disease; and splice-site mutations are associated with variable disease severity (Mérel et al. 1995; Kluwe et al. 1996, 1998; Parry et al. 1996; Ruttledge et al. 1996; Evans et al. 1998a). There have been few longitudinal studies of NF2, because of the rarity of the disease (Evans et al. 1992b; Antinheimo et al. 2000). In two recent short-term longitudinal studies of the predictors of VS growth rates in NF2, VS growth rates tended to be higher in people with a younger age at onset or diagnosis of NF2, but they were highly variable, even among affected relatives of similar ages in a single family (Baser et al. 2002; Mautner et al. 2002). As yet, there have not been any longitudinal studies of other common tumors in NF2, such as intracranial meningiomas or spinal tumors, which also cause considerable morbidity (Evans et al. 2000).
NF2 is a chronic disease in which life expectancy, although often shortened, is lengthy. Short-term studies of the growth rates of NF2-associated tumors, especially studies of a single tumor type, do not reflect the total disease burden of NF2 or the efficacy of treatment as well as do long-term studies that utilize a more inclusive measure of health, such as mortality. Evans et al. (1992a) found that mean actuarial survival in people with NF2 was 62 years, and Parry et al. (1994) reported that broad categories of NF2 disease severity (mild, intermediate, and severe) were correlated with age at death. Neither of these studies evaluated specific clinical or molecular characteristics as potential predictors of the risk of mortality. In the present study, we evaluated clinical and molecular predictors of the risk of mortality in a large series of people with NF2.
Subjects and Methods
Patient Population
The United Kingdom NF2 registry is based in the Department of Medical Genetics, St. Mary's Hospital, Manchester. People are ascertained by contacting neurosurgeons, otolaryngologists, neurologists, pediatricians, dermatologists, and geneticists throughout the United Kingdom, and the collection is augmented in the North West Region by the Regional Cancer Registry. The present study was subject to continuing ethics committee evaluation, and participants gave informed consent.
As of February 15, 2002, the registry had 425 people from 282 families. For the present study, we excluded three groups of people: (1) We excluded known somatic mosaics (N=17; shown to be mosaic at the molecular level). Almost all reported NF2 somatic mosaics have mild disease, despite having nonsense or frameshift mutations that typically cause severe disease in classical NF2 (Evans et al. 1998b; Kluwe and Mautner 1998). (2) We excluded people who were born before 1930 (N=34). All such individuals in the United Kingdom NF2 registry were identified through younger affected relatives. The pre-1930 group was excluded because it did not meet the proportional-hazards assumption for the Cox analysis. Specifically, type of treatment center and meningiomas did not predict the risk of mortality in people who were born before 1930, in contrast to those born after 1930. (3) We excluded people with missing information on either age at diagnosis (N=2) or presence of intracranial meningiomas (N=4), two covariates that are necessary for analysis of the risk of mortality.
The resultant study group had 368 people from 261 families, all of whom met the Manchester clinical diagnostic criteria for NF2 (Evans et al. 1992a) or had identified constitutional NF2 mutations. The distribution of the number of affected family members was 206 families with one affected member, 27 families with two affected members, 15 families with three affected members, 7 families with four affected members, 3 families with five affected members, 1 family with six affected members, and 2 families with seven affected members. There were 43 two-generation families and 9 three-generation families. There were 223 people with new mutations (individuals with sporadic disease and founders) and 145 people with inherited mutations.
NF2 Mutation Analysis
Genomic DNA samples prepared from peripheral leukocytes were amplified with primers for all 17 exons of the NF2 gene, and screening was performed for constitutional NF2 mutations, using SSCP analysis, as described elsewhere (Evans et al. 1998a).
Statistical Analysis
Potential predictors of the risk of mortality were first assessed using univariate Kaplan-Meier survival curves. The covariates examined were age at onset of symptoms, age at diagnosis, sex, type of constitutional NF2 mutation, inheritance (new mutation or inherited case), generation (in the 52 multigeneration families), presence and number of each type of NF2 nervous system tumor (VSs, intracranial meningiomas, spinal tumors, and peripheral nerve tumors), lens opacities, number of surgical operations, calendar year of diagnosis, and type of treatment center (specialty or nonspecialty). The specialty treatment centers were hospitals with NF2 specialist management teams (Manchester Royal Infirmary, Addenbrooke's Hospital [Cambridge], and Royal London Hospital).
The number of tumors at diagnosis was used because data from serial examinations were not routinely available. A potential concern about this choice is that imaging done prior to the late 1980s (i.e., with computerized tomography) may be of poorer quality and may tend to underestimate the number of tumors, in comparison with imaging done more recently (i.e., with magnetic resonance imaging). Several lines of evidence indicate that this potential bias is negligible in people with meningiomas or spinal tumors. The number of meningiomas did not vary significantly by calendar year of diagnosis (P=.95), after adjustment, using linear regression, for age at diagnosis of the first intracranial meningioma. Some people present with VSs but later develop intracranial meningiomas or have meningiomas that may not have been detected at presentation because of suboptimal imaging. Of the 136 people with both VSs and meningiomas, 25% were diagnosed with meningiomas after their VSs were diagnosed, and the median time between detection of VSs and meningiomas in these people was only four years. Some people may not have spinal imaging early in the course of their disease, but the median time between initial assessment and diagnosis of spinal tumors was only one year.
The presentation of NF2 is different in adults than in children, whose initial sign or symptom is often unrelated to VSs (Mautner et al. 1993; MacCollin and Mautner 1998). For this reason, interactions between age at diagnosis and number of each type of nervous system tumor were evaluated in the Cox models; none of these interactions were statistically significant. Age at onset of symptoms and age at diagnosis were highly correlated (r2=.63, P<.001); age at diagnosis was used in the analysis because tumor burden was usually first evaluated at this time. Age at diagnosis and calendar year of diagnosis were not highly correlated (r2=.01, P=.15). Initial data analyses included some separate analyses for people with new mutations and those with inherited disease, but the two groups were similar in survival rates (as shown by Kaplan-Meier curves), so people with new mutations and those with inherited disease were combined in further analyses. Proband/nonproband status and new mutation/inherited mutation status were highly correlated, so proband status was not considered further as a covariate.
The covariates that were most highly associated with the risk of mortality in single-predictor Cox proportional hazards models were added sequentially to a multiple-predictor Cox model until there was only a minor decrease in the log partial likelihood. The log partial likelihood, an output-summary value of Cox regression analysis, using S-Plus (Venables and Ripley 1997, is considered to be an indicator of how well a set of potential predictors explain the variation in survival times; because of the dependence within families, it is not the actual partial likelihood. We constructed two Cox models, one without and one with information on the type of constitutional NF2 mutation. The resulting two models are called the “clinical model” and the “molecular model.” The mutation type covariate is categorical and is coded as several binary variables. These variables were indicators of splice-site mutations, missense mutations, large deletions, and unidentified mutations, each compared with nonsense or frameshift mutations. The category of unidentified mutations can include different types of mutations but was treated as a single category for the purpose of this analysis. The molecular model excluded 46 people who had not been screened for constitutional NF2 mutations, 2 people with in-frame deletions, and 2 people with chromosomal translocations.
The Cox model assumes independence of families and independence of members within families. The latter part of this assumption is violated when there is correlation between family members. When the data are positively correlated, the SEs of parameters estimated under the assumption of independence will tend to be too small, which could lead to an erroneous conclusion that an effect is important. To surmount this problem for the Cox models, the delete-one jackknife method, with family as the unit, was used to determine the adjusted parameter estimates and their SEs in the Cox model. The jackknife is a standard statistical method that is commonly used to correct for bias in parameter estimates and to provide SEs in nonstandard sampling situations (Mosteller and Tukey 1977).
In addition to fitting Cox models, we fitted log-normal regression models (regressing log survival on sets of predictor variables), using the S-Plus function called “survreg.” The results are qualitatively the same as those of the Cox model. To assess the amount of intrafamilial correlation, ρ, the simplest approach is to add familial dependence to the log-normal regression model and use a multivariate log-normal distribution for the vector of log survival for each family. The simplest dependence structure is exchangeable dependence; this assumes a common correlation for each pair of family members. This dependence obtains from a random-effects model with a common family effect plus individual effects. In notation, the model is logYij=β0+β′xij+Fi+Eij, where i is the index for families, j is the index for members within a family, Y is the survival time, xij is a vector of covariates, Fis are independent family effects that have a normal distribution with mean 0 and variance σ2F, Eijs are independent individual effects that have a normal distribution with mean 0 and variance σ2E. The ratio of variances between and within families is θ=σ2F/σ2E, and the intrafamilial correlation is ρ=θ/(1+θ). For a family with left-censored Yij, the likelihood contribution involves a multivariate normal-rectangle probability; for the random-effects model given above, the rectangle probability can be computed as a one-dimensional numerical integral. The log-likelihood can be numerically maximized with a quasi-Newton routine, to obtain maximum-likelihood estimates of the parameters.
Results
The characteristics of the study population are presented in table 1. The mean ± SE age at onset of symptoms was 22 ± 1 years, and the mean age at diagnosis was 27 ± 1 years. The median length of follow-up from initial clinical evaluation was 7 years (range 0–37 years). Ninety-eight percent of people were diagnosed after 1970. Ninety-four percent of people had VSs, and 45% had intracranial meningiomas. Age at diagnosis did not vary significantly by meningioma status (presence or absence of intracranial meningiomas) (mean ± SE 28 ± 1 years with meningiomas absent, 27 ± 1 years with meningiomas present). Constitutional NF2 mutations were identified in 120 (56%) of the 216 families that were screened for mutations. Seventy-four (20%) of the 368 people died during follow-up: 51 of tumor burden, 14 of complications in the immediate postoperative period, 3 of malignancies arising from an NF2-associated tumor, 2 each of traffic accidents and suicide, and 1 each of a fall due to NF2-associated imbalance and a myocardial infarction.
Table 1.
Characteristics of 368 People with NF2 in the Study Population
| Characteristic | No. | % |
| Vital status:a | ||
| Alive | 295 | 80.2 |
| Dead | 73 | 19.8 |
| Sex: | ||
| Female | 191 | 51.9 |
| Male | 177 | 48.1 |
| Inheritance: | ||
| New mutation | 223 | 60.6 |
| Inherited mutation | 145 | 39.4 |
| Age at onset of symptoms (years): | ||
| 1–19 | 153 | 41.8 |
| 20–39 | 155 | 42.3 |
| ⩾40 | 34 | 9.3 |
| Asymptomatic | 24 | 6.6 |
| Age at diagnosis (years): | ||
| 1–19 | 116 | 31.5 |
| 20–39 | 186 | 50.6 |
| ⩾40 | 66 | 17.9 |
| VS: | ||
| None | 23 | 6.3 |
| Unilateral | 33 | 9.0 |
| Bilateral | 310 | 84.7 |
| Intracranial meningiomas: | ||
| Absent | 203 | 55.2 |
| Present | 165 | 44.8 |
| Type of constitutional NF2 mutation:b | ||
| Nonsense | 43 | 13.5 |
| Frameshift deletion | 27 | 8.5 |
| Frameshift insertion | 10 | 3.1 |
| Splice donor site | 19 | 6.0 |
| Splice acceptor site | 36 | 11.3 |
| Missense | 22 | 6.9 |
| Large deletion | 47 | 14.8 |
| Not identified | 114 | 35.8 |
| Type of treatment center: | ||
| Nonspecialty | 259 | 70.4 |
| Specialty | 109 | 29.6 |
As of February 15, 2002.
Frequency of mutations in 318 people from 216 families that were screened for constitutional NF2 mutations (molecular model).
In single-predictor Cox models, five covariates were at least moderately associated with the risk of mortality: age at diagnosis, intracranial meningioma status, type of constitutional NF2 mutation, type of treatment center, and calendar year of diagnosis. Kaplan-Meier survival curves are presented in figures 1–4. These covariates were included sequentially in multiple-predictor Cox models.
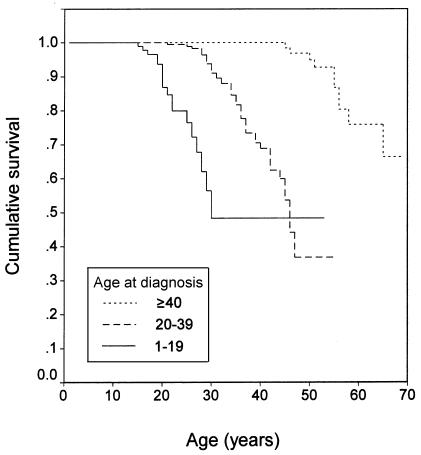
Figure 1.
Kaplan-Meier survival curve (log-rank test): age at diagnosis (P<.0001).
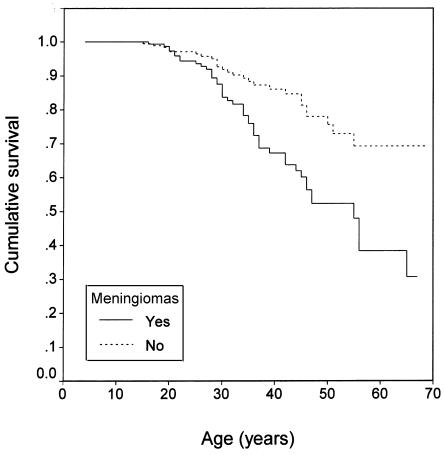
Figure 2.
Kaplan-Meier survival curve (log-rank test): intracranial meningiomas (P=.0003).
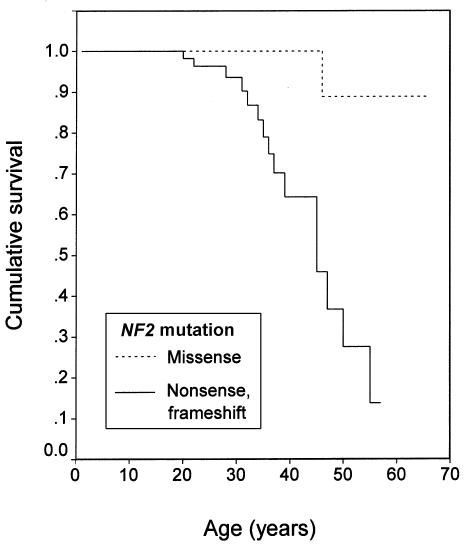
Figure 3.
Kaplan-Meier survival curve (log-rank test): type of constitutional NF2 mutation (P=.0020).
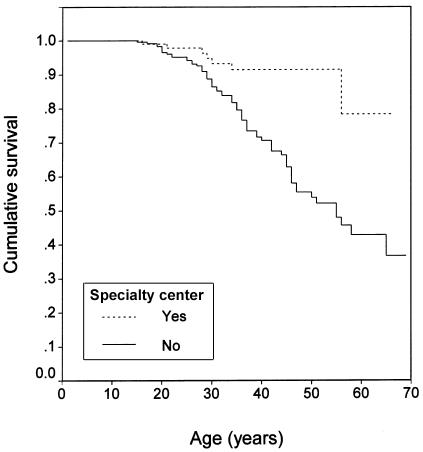
Figure 4.
Kaplan-Meier survival curve (log-rank test): type of treatment center (P=.0001).
In the clinical model, the best multiple-predictor Cox model had three covariates, which were, in order of importance, age at diagnosis, type of treatment center, and meningioma status. The relative risk (RR) of mortality increased 1.13-fold per year decrease in age at diagnosis (95% CI 1.08–1.18) and was 2.51-fold greater in people with meningiomas compared with those without meningiomas (95% CI 1.38–4.57). The RR of mortality in people treated at specialty centers was 0.34 compared with those treated at nonspecialty centers (95% CI 0.12–0.98). The coefficients for these covariates were stable across Cox models with different sets of predictors. See table 2 for the summary of this Cox model; SEs were based on the jackknife method with the family as the sampling unit. Calendar year of diagnosis had little additional predictive value after age at diagnosis was included. In a separate model that included the covariates of age at diagnosis and type of treatment center but substituted number of meningiomas for presence of meningiomas, the RR of mortality did not significantly increase with increasing number of meningiomas; however, each group had relatively few people (61 people with one meningioma, 63 with two or three meningiomas, and 35 with more than three meningiomas).
Table 2.
Parameter Estimates and SEs for the Clinical Model (261 Families with NF2)
| Variable | β | SEβ | RR | 95% CIRR |
| Age at diagnosis (per year decrease) | .12 | .02 | 1.13 | 1.08–1.18 |
| Intracranial meningiomas (present vs. absent) | .92 | .31 | 2.51 | 1.38–4.57 |
| Type of treatment center (specialty vs. nonspecialty) | −1.07 | .54 | .34 | .12–.98 |
In the molecular model, the best multiple-predictor Cox model had three covariates, which were, in order of importance, age at diagnosis, type of treatment center, and mutation type. Meningioma status had little additional predictive value after these three variables were in the model. See table 3 for a summary of this Cox model; the βs for the four mutation types are relative to nonsense or frameshift mutations. The main conclusion is that people with missense mutations have a much lower risk of mortality than do people with any of the other types of mutations. This can be seen from the number of deaths among people with the five types of mutations: 15 of 80 for nonsense or frameshift mutations, 15 of 55 for splice-site mutations, 9 of 47 for large deletions, 19 of 114 for unidentified mutations, and 1 of 22 for missense mutations. Because there is only one death in the missense mutation group, the jackknife does not provide a reliable SE estimate for β for the missense mutation group; the data in table 3 for this variable are based on the omission of two jackknife outliers.
Table 3.
Parameter Estimates and SEs for the Molecular Model (216 Families with NF2)
| Variable | β | SEβ | RR | 95% CIRR |
| Age at diagnosis (per year decrease) | .14 | .03 | 1.15 | 1.09–1.21 |
| Type of treatment center (specialty vs. nonspecialty) | −1.21 | .63 | .30 | .08–1.05 |
| Type of constitutional NF2 mutation (vs. nonsense or frameshift): | ||||
| Splice-site | .22 | .67 | 1.24 | .33–4.70 |
| Missense | −2.49 | .51 | .08 | .03–.23 |
| Large deletion | −.29 | .49 | .75 | .28–2.00 |
| Unidentified | −.31 | .51 | .73 | .26–2.00 |
For the random-effects model for log survival time, the covariates of age at diagnosis, type of treatment center, and presence of meningiomas were fitted. The summary of the parameter estimates is given in table 4. The estimated intrafamilial correlation is 0.56=1.25/(1+1.25), with a wide 95% CI of 0.0–0.73. The data set is small and has a high censoring rate, so the intrafamilial correlation cannot be estimated precisely. However, the random-effects model appears to adapt well to censored data.
Table 4.
Parameter Estimates and SEs for the Random-Effects Model for Log Survival (261 Families with NF2)
| Variable | Estimate | SE |
| σE | .18 | .03 |
| θ | 1.25 | .73 |
| β0 | 3.15 | .06 |
| Age at diagnosis (per year increase) | −.23 | .02 |
| Intracranial meningiomas (present vs. absent) | −.07 | .04 |
| Type of treatment center (specialty vs. nonspecialty) | .21 | .06 |
Discussion
In the present study, we found that four covariates were informative predictors of the risk of mortality in people with NF2. The risk of mortality increased with decreasing age at diagnosis and was greater in people with intracranial meningiomas compared with those without meningiomas. The risk of mortality was lower in people with constitutional NF2 missense mutations than in those with other types of mutations and in people who were treated at specialty centers compared with those who were treated at nonspecialty centers. The simplicity of age at diagnosis, by far the strongest single predictor of risk of mortality in NF2, makes it a useful index for patient counseling and clinical management; intracranial meningiomas are also useful in this regard. A possible reason for the higher risk of mortality in people with NF2 who are diagnosed at younger ages is that tumor growth is generally more rapid in these patients (Baser et al. 2002; Mautner et al. 2002), perhaps because of a higher rate of somatic cell growth or a larger proportion of growing cells in young people. Age at onset (which is highly correlated with age at diagnosis) and number of non-VS intracranial tumors are key indices of NF2 disease severity (Parry et al. 1994). Other covariates, such as sex, inheritance, and year of diagnosis, were not as useful predictors of the risk of mortality. However, as more information is collected on people with NF2 in the future, the assessment of the risk of mortality should be repeated with these covariates, since it is possible that the present data are insufficient to detect some associations.
In the clinical model, the empirical importance of the type of treatment center is comparable to that of meningioma status. The effect of treatment in specialty centers is not due to marked differences in the characteristics of people who were seen at specialty and nonspecialty treatment centers. New mutations in these two groups were similar with respect to age at onset of symptoms, age at diagnosis, and prevalence of meningiomas. Similar proportions of people with inherited mutations were treated at specialty and nonspecialty centers.
In all likelihood, a major cause of the lower risk of mortality in people with NF2 who are treated in specialty centers is the increasing rate of favorable operative outcomes and the decreasing rate of postoperative complications with increasing surgical experience. When new surgical teams are being trained for VS surgery, there are clear trends, as the number of surgeries increases, toward improved postoperative preservation of facial nerve function, complete resection rate, and hearing preservation, as well as a lowered incidence of serious complications, such as cerebrospinal fluid leaks (Buchman et al. 1996; Welling et al. 1999). In Denmark, decentralized VS neurosurgery was associated with very high rates of perioperative mortality (8.5%) and serious surgical complications (35.6%) (Charabi et al. 1992). People with benign meningioma who are treated in academic hospitals have significantly lower mortality, after adjustment for other risk factors, than do those who are treated in community hospitals (McCarthy et al. 1998). In addition to more extensive surgical experience, specialty centers have coordinated expertise in the multiple clinical specialties that are needed to properly diagnose NF2 and to treat affected individuals and their at-risk family members (Evans et al. 1993; Jackler 1998). To ensure optimal care, we recommend that people with NF2 be referred to specialty centers.
Almost all of the people in this study were diagnosed after 1970. During the last three decades, there have been considerable improvements in neuroimaging techniques that have permitted earlier detection of small tumors. In combination with improvements in neurosurgical treatments, this has led to better clinical management and a much greater incentive to diagnose VSs and NF2. The noninclusion of the more recent years of diagnosis in the multiple-predictor Cox models does not suggest that improvements in clinical care lack benefit. In all likelihood, insufficient time has elapsed for such benefit to be reflected in decreased mortality. In addition, the benefits from incremental improvements in clinical care throughout the post-1970 era may be more subtle, with respect to risk of mortality, than those that occurred with the advent of computerized tomography scanning in the early 1970s.
Of the people with NF2 who have access to treatment, relatively few die of their VSs. In the modern era of improved microsurgical techniques, operative mortality in specialized neuro-otology treatment centers is ⩽1%, and recurrence rates are nil when the entire VS and vestibular nerves are excised. Intracranial meningiomas and spinal tumors are common in NF2, and these recurrent tumors often require repeated surgeries. There is considerable pre- and postoperative morbidity due to seizures, paralysis, wasting, pneumonia, and accidents associated with meningiomas and spinal tumors (Evans et al. 2000).
In the molecular model, the risk of mortality appeared to be much lower in people with missense mutations than in those with other types of mutations. Experimental studies have suggested possible mechanisms through which constitutional NF2 missense mutations cause milder disease than do nonsense or frameshift NF2 mutations. Missense mutations produce an NF2 protein (termed “merlin” or “schwannomin”) that is stable but defective in negative growth regulation, whereas nonsense mutations do not produce stable protein (Gutmann et al. 1998). Naturally occurring missense mutations have reduced, but not absent, activity of the merlin-binding protein βII-spectrin (Scoles et al. 1998).
Since NF2 mutation type is strongly associated with age at diagnosis in cross-sectional studies, a logical question is why age at diagnosis is more strongly associated than the type of NF2 mutation with the risk of mortality. Age at diagnosis may be more strongly associated because both age at diagnosis and age at death reflect a composite of disease-influencing factors, whereas mutation type is only one of these factors. Other factors are the stochastic loss of the second NF2 allele (Baser et al. 1996) and putative modifying genes (Bruder et al. 1999a, 1999b).
Constitutional NF2 mutations that are not found by SSCP could be mutations in the 3′ or 5′ UTRs, the promoter region, or untranscribed transcriptional control elements; intronic mutations that are not covered by SSCP primers; large deletions, insertions, or other rearrangements; or mutations in patients who are somatic mosaics (Zucman-Rossi et al. 1998). Other epigenetic events (i.e., methylation) could result in loss of NF2 expression (Kino et al. 2001). There is no evidence for locus heterogeneity in NF2 (Narod et al. 1992). In 60 United Kingdom families that have people with NF2 in two or more generations, all families have linkage to NF2, and NF2 mutations have been identified in all but six families (D.G.R.E., unpublished data).
Approximately 20% of NF2 new mutations are thought to be somatic mosaics, and almost all have mild disease, despite having constitutional nonsense or frameshift NF2 mutations (Evans et al. 1998b; Kluwe and Mautner 1998). We excluded known somatic mosaics from the analysis, and, although some somatic mosaics probably were not detected, the resultant bias is likely to be minor. Among the 187 people with new mutations who underwent molecular screening, pathogenic NF2 mutations were not identified in 88. Sixty-eight of these people with new mutations were ⩽20 years of age at the onset of symptoms, or they had two or more meningiomas or four or more spinal tumors. These people are unlikely to be mosaic, because they have severe disease. The remaining 20 people with new mutations had mild disease. If 20% of them were mosaic, then we failed to identify only four mosaic individuals in the screened group.
In summary, the strongest single predictor of the risk of mortality in NF2 is age at diagnosis, which is a useful index for patient counseling and clinical management, as is meningioma status. People with constitutional NF2 missense mutations appear to have a much lower risk of mortality than do those with other types of mutations. Our finding that people with NF2 who are treated at specialty centers have a lower risk of mortality is consistent with studies of unilateral sporadic VS, in which surgical outcomes were improved and operative complications were reduced in proportion to increasing surgical experience. To ensure optimal care, we recommend that people with NF2 be referred to specialty treatment centers.
Acknowledgments
This work was supported in part by the FBT Foundation and U.S. Army grant U.S.A.R.M.C. NF990038.
Footnotes
Presented in part at the 49th Annual Meeting of the American Society of Human Genetics, in San Francisco, on October 19–23, 1999.
Electronic-Database Information
Accession number and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for NF2 [MIM 101000]) [PubMed]
References
- Antinheimo J, Sankila R, Carpén O, Pukkala E, Sainio M, Jääkeläinen J (2000) Population-based analysis of sporadic and type 2 neurofibromatosis-associated meningiomas and schwannomas. Neurology 54:71–76 [DOI] [PubMed] [Google Scholar]
- Baser ME, Friedman JM, Evans DGR (1999) Predictors of survival in neurofibromatosis. Am J Hum Genet Suppl 65:A61 [Google Scholar]
- Baser ME, Makariou EV, Parry DM (2002) Predictors of vestibular Schwannoma growth in patients with neurofibromatosis type 2. J Neurosurg 96:217–222 [DOI] [PubMed] [Google Scholar]
- Baser ME, Ragge NK, Riccardi VM, Janus T, Gantz B, Pulst S (1996) Phenotypic variability in monozygotic twins with neurofibromatosis 2. Am J Med Genet 64:563–567 [DOI] [PubMed] [Google Scholar]
- Bruder CEG, Ichimura K, Blenow E, Ikeuchi T, Yamaguchi T, Yuasa Y, Collins VP, Dumanski JP (1999a) Severe phenotype of the neurofibromatosis type 2 gene in patients with a 7.4 Mbp constitutional deletion on chromosome 22: possible localization of a neurofibromatosis type 2 modifier gene? Genes Chromosomes Cancer 25:184–190 [PubMed] [Google Scholar]
- Bruder CEG, Ichimura K, Tingby O, Hirakawa K, Komatsuzaki A, Tamura A, Yuasa Y, Collins VP, Dumanski JP (1999b) A group of schwannomas with interstitial deletions on 22q located outside the NF2 locus shows no detectable mutations in the NF2 gene. Hum Genet 104:418–424 [DOI] [PubMed] [Google Scholar]
- Buchman CA, Chen DA, Flannagan P, Wilberger JE, Maroon JC (1996) The learning curve for acoustic tumor surgery. Laryngoscope 106:1406–1411 [DOI] [PubMed] [Google Scholar]
- Charabi S, Tos M, Thomsen J, Borgesen SE (1992) Suboccipital acoustic neuroma surgery: results of decentralized neurosurgical tumor removal in Denmark. Acta Otolaryngol 112:810–815 [DOI] [PubMed] [Google Scholar]
- Evans DG, Sainio M, Baser ME (2000). Neurofibromatosis type 2. J Med Genet 37:897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DGR, Huson SM, Donnai D, Neary W, Blair V, Newton V, Harris R (1992a) A clinical study of type 2 neurofibromatosis. Q J Med 84:603–618 [PubMed] [Google Scholar]
- Evans DGR, Huson SM, Donnai D, Neary W, Blair V, Teare D, Newton V, Strachan T, Ramsden R, Harris R (1992b) A genetic study of type 2 neurofibromatosis in the United Kingdom. I. Prevalence, mutation rate, fitness, and confirmation of maternal transmission effect on severity. J Med Genet 29:841–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DGR, Ramsden R, Huson SM, Harris R, Lye R, King TT (1993) Type 2 neurofibromatosis: the need for supraregional care? J Laryngol Otol 107:401–406 [DOI] [PubMed] [Google Scholar]
- Evans DGR, Trueman L, Wallace A, Collins S, Strachan T (1998a) Genotype/phenotype correlations in type 2 neurofibromatosis (NF2): evidence for more severe disease associated with truncating mutations. J Med Genet 35:450–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DGR, Wallace AJ, Wu CL, Trueman L, Ramsden RT, Strachan T (1998b) Somatic mosaicism: a common cause of classic disease in tumor-prone syndromes? lessons from type 2 neurofibromatosis. Am J Hum Genet 63:727–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann DH, Geist RT, Xu H-m, Kim JS, Saporito-Irwin S (1998) Defects in neurofibromatosis 2 protein function can arise at multiple levels. Hum Mol Genet 7:335–345 [DOI] [PubMed] [Google Scholar]
- Jackler RK (1998) The perils of decentralized care in otology/neuro-otology. Am J Otol 19:691–692 [PubMed] [Google Scholar]
- Kino T, Takeshima H, Nakao M, Nishi T, Yamamoto K, Kimura T, Saito Y, Kochi M, Kuratsu J, Saya H, Ushio Y (2001) Identification of the cis-acting region in the NF2 gene promoter as a potential target for mutation and methylation-dependent silencing in schwannoma. Genes Cells 6:441–454 [DOI] [PubMed] [Google Scholar]
- Kluwe L, Beyer S, Baser ME, Hazim W, Haase W, Fünsterer C, Mautner VF (1996) Identification of NF2 germ-line mutations and comparison with NF2 phenotypes. Hum Genet 98:534–538 [DOI] [PubMed] [Google Scholar]
- Kluwe L, MacCollin M, Tatagiba M, Thomas S, Hazim W, Haase W, Mautner VF (1998) Phenotypic variability associated with 14 splice-site mutations in the NF2 gene. Am J Med Genet 77:228–233 [PubMed] [Google Scholar]
- Kluwe L, Mautner VF (1998) Mosaicism in sporadic neurofibromatosis 2 patients. Hum Mol Genet 7:2051–2055 [DOI] [PubMed] [Google Scholar]
- MacCollin M, Mautner VF (1998) The diagnosis and management of neurofibromatosis 2 in childhood. Semin Pediatr Neurol 5:243–252 [DOI] [PubMed] [Google Scholar]
- Mautner VF, Baser ME, Thakkar SD, Feigen UM, Friedman JM, Kluwe L (2002) Vestibular schwannoma growth in patients with neurofibromatosis type 2: a longitudinal study. J Neurosurg 96:223–228 [DOI] [PubMed] [Google Scholar]
- Mautner VF, Lindenau M, Baser ME, Hazim W, Tatagiba M, Haase W, Samii M, Wais R, Pulst SM (1996) The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery 38:880–885 [DOI] [PubMed] [Google Scholar]
- Mautner VF, Tatagiba M, Guthoff R, Samii M, Pulst SM (1993) Neurofibromatosis 2 in the pediatric age group. Neurosurgery 33:92–96 [DOI] [PubMed] [Google Scholar]
- Mautner VF, Tatagiba M, Lindenau M, Fünsterer C, Pulst SM, Baser ME, Kluwe L, Zanella FE (1995) Spinal tumors in patients with neurofibromatosis type 2: MR imaging study of frequency, multiplicity, and variety. AJR Am J Roentgenol 165:951–955 [DOI] [PubMed] [Google Scholar]
- McCarthy BJ, Davis FG, Freels S, Surawicz TS, Damek DM, Grutsch J, Mench HR, Laws ER Jr (1998) Factors associated with survival in patients with meningioma. J Neurosurg 88:831–839 [DOI] [PubMed] [Google Scholar]
- Mérel P, Hoang-Xuan K, Sanson M, Bijlsma E, Rouleau G, Laurent-Puig P, Pulst S, Baser M, Lenoir G, Sterkers JM, Philippon J, Resche F, Mautner VF, Fisher G, Hulsebos T, Aurias A, Delattre O, Thomas G (1995) Screening for germ-line mutations in the NF2 gene. Genes Chromosomes Cancer 12:117–127 [DOI] [PubMed] [Google Scholar]
- Mosteller F, Tukey JW (1977) Data analysis and regression: a second course in statistics. Addison-Wesley, Reading, MA [Google Scholar]
- Narod SA, Parry DM, Parboosingh J, Lenoir GM, Ruttledge M, Fischer G, Eldridge R, Martuza RL, Frontali M, Haines J, Gusella JF, Rouleau GA (1992) Neurofibromatosis type 2 appears to be a genetically homogeneous disease. Am J Hum Genet 51:486–496 [PMC free article] [PubMed] [Google Scholar]
- Parry DM, Eldridge R, Kaiser-Kupfer MI, Bouzas EA, Pikus A, Patronas N (1994) Neurofibromatosis 2 (NF2): clinical characteristics of 63 affected individuals and clinical evidence for heterogeneity. Am J Med Genet 52:450–461 [DOI] [PubMed] [Google Scholar]
- Parry DM, MacCollin MM, Kaiser-Kupfer MI, Pulaski K, Nicholson HS, Bolesta M, Eldridge R, Gusella JF (1996) Germ-line mutations in the neurofibromatosis 2 gene: correlations with disease severity and retinal abnormalities. Am J Hum Genet 59:529–539 [PMC free article] [PubMed] [Google Scholar]
- Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, Hoang-Xuan K, Demczuk S, Desmaze C, Plougstel B, Pulst SM, Lenoir G, Bijlsma E, Fashold R, Dumanski J, de Jong P, Parry D, Eldridge R, Aurias A, Delattre O, Thomas G (1993) Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature 363:515–521 [DOI] [PubMed] [Google Scholar]
- Ruttledge MH, Andermann AA, Phelan CM, Claudio JO, Han F-y, Chretien N, Rangaratnam S, MacCollin M, Short P, Parry D, Michels V, Riccardi VM, Weksberg R, Kitamura K, Bradburn JM, Hall BD, Propping P, Rouleau GA (1996) Type of mutation in the neurofibromatosis type 2 gene (NF2) frequently determines severity of disease. Am J Hum Genet 59:331–342 [PMC free article] [PubMed] [Google Scholar]
- Scoles DR, Huynh DP, Morcos PA, Coulsell ER, Robinson NGG, Tamanoi F, Pulst SM (1998) Neurofibromatosis 2 tumour suppressor schwannomin interacts with βII-spectrin. Nat Genet 18:354–359 [DOI] [PubMed] [Google Scholar]
- Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, Eldridge R, Kley N, Menon AG, Pulaski K, Haase VH, Ambrose CM, Munroe D, Bove C, Haines JL, Martuza RL, MacDonald ME, Seizinger BR, Short MP, Buckler AJ, Gusella JF (1993) A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell 72:791–800 [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD (1997) Modern applied statistics with S-Plus. 2nd ed. Springer, New York [Google Scholar]
- Welling DB, Slater PW, Thomas RD, McGregor JM, Goodman JE (1999) The learning curve in vestibular schwannoma surgery. Am J Otol 20:644–648 [PubMed] [Google Scholar]
- Zucman-Rossi J, Legoix P, Sarkissian HD, Cheret G, Sor F, Bernardi A, Cazes L, Giraud S, Ollagnon E, Lenoir G, Thomas G (1998) NF2 gene in neurofibromatosis type 2 patients. Hum Mol Genet 7:2095–2101 [DOI] [PubMed] [Google Scholar]