Abstract
Objective
To identify design elements of clinical trials leading to US Food and Drug Administration approval of drugs for neurological diseases with and without orphan indications.
Methods
We used publicly available information to identify approvals for drugs for neurological diseases with an orphan indication (n = 19) and compared them with recent approvals for drugs for neurological diseases without an orphan indication (n = 20). We identified “pivotal trials” from drug labels and drug approval packages, and assessed them on four elements of clinical trial design: control, blinding, randomization, and size.
Results
All drugs for neurological diseases (100%) approved without an orphan indication included at least two randomized, double-blind, placebo-controlled trials. In comparison, 32% of drugs with an orphan indication had at least two such trials (p < 0.001) and 74% had at least one (p = 0.02). Thirty-three pivotal trials were conducted for the 19 drugs approved with an orphan indication. Of the 33 trials, 11 (33%) did not use a placebo control, 9 (27%) were not double blind, and 4 (12%) were not randomized. Drugs approved without an orphan indication had more pivotal trials per drug (3.8 vs 1.7 trials; p < 0.001) and a larger mean trial size (506 vs 164 trial participants; p < 0.001).
Interpretation
The US Food and Drug Administration has approved orphan drugs for neurological diseases without randomized, doubled-blind, placebo-controlled pivotal trials. As orphan drug development grows, demand will likely increase for alternative designs for conducting adequate and well-controlled studies to demonstrate drug efficacy.
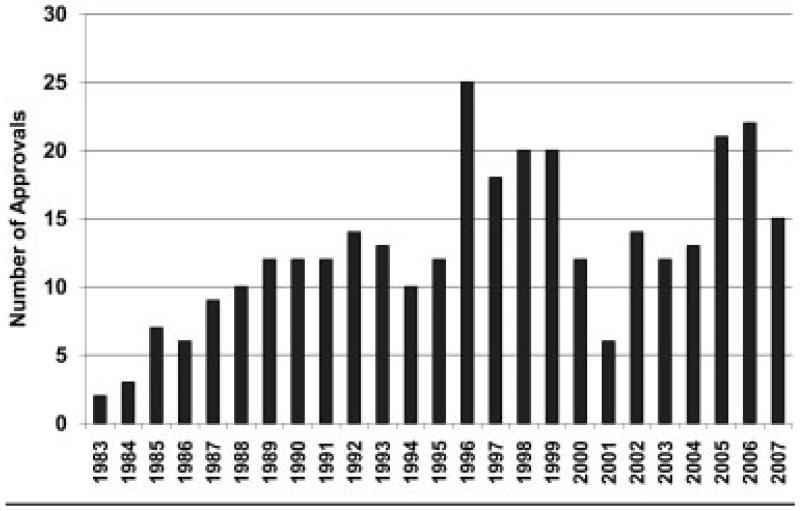
Since the signing of the Orphan Drug Act in 1983, there have been more than 300 US Food and Drug Administration (FDA) market approvals for treatment indications for rare diseases in the United States. The rate of approvals has been increasing despite substantial year-to-year variability (Fig).1 The number of drugs garnering orphan designation status is also on the rise: 608 designations from 2003 to 2007 versus 343 designations from 1998 to 2002.2 Therapies for rare diseases are important to drug development; from 2003 through 2007, 26% of new molecular and biological entities approved by the FDA had an orphan indication.3 The success of the Orphan Drug Act in promoting the development of therapies for rare diseases is widely accepted.4–7 However, the policy has been critiqued for concerns of misuse by drug developers,4,5 providing too few financial incentives,5,6 providing financial incentives unfavorable to society,8 creating an unsustainable market for rare disease drugs,4 and for being resistant to critical evaluation.9
Fig.
US Food and Drug Administration (FDA) orphan indication approvals, 1983 to 2007.
The FDA Office of Orphan Products Development states that “approval of orphan designation does not alter the standard regulatory requirements and process of obtaining marketing approval,” and that “safety and efficacy of a compound must be established through adequate and well-controlled studies.”10 Adequate and well-controlled studies are defined according to Section 314.126 of Title 21 of the Code of Federal Regulations (21 CFR 314.126).11 The regulation requires a “design that permits a valid comparison with a control.”11 The control may be a placebo, dose comparison, active treatment, or historically derived. Concurrent controls should be randomized. The Director of the Center for Drug Evaluation and Research at the FDA may “waive in whole or in part any of the criteria” regarding adequate and well-controlled studies. However, “uncontrolled studies or partially controlled studies are not acceptable as the sole basis for the approval of claims of effectiveness.”
To date, the key characteristics of the clinical trials leading to the FDA approval of drugs with orphan indications have not been described and compared with trials conducted for drugs without orphan indications. The size and phase of clinical trials leading to orphan drug approvals by the European Medicines Agency (EMEA) was shown in a 2008 Lancet essay.12 The author, a former member of the Committee for Orphan Medicinal Products of EMEA, concluded that European regulators “do the best that they can with the limited information available.”12 Other trial designs have occasionally been used to investigate treatments for small patient populations,4 but the rate of use of these alternative strategies has not been shown. Thus, we set out to determine four key elements of clinical study design (control, blinding, randomization, and size) for the trials used to approve drugs for neurological diseases with and without an orphan indication.
Subjects and Methods
Scope of Investigation
Our study population comprised all drugs for neurological diseases approved with an orphan indication through 2007. We created a study sample by examining the FDA listing of all orphan drugs with marketing approval2 for drugs with neurological disease indications. We defined neurological diseases as those for which research was supported by the National Institute for Neurological Disorders and Stroke,13 the National Institute of Mental Health,14 the National Institute on Aging,15 or the National Institute on Drug Abuse.16 Our sample of drugs approved with an orphan indication included two approval classifications: (1) new molecular and biological entities (n = 14), and (2) new indications for drugs with a prior approval (n = 5) (Table 1). New molecular and biological entities are drugs with “structurally unique active ingredients that have never before been marketed.”17 Many orphan drugs are approved compounds for which a rare disease application was later discovered. An existing drug can be approved for an orphan indication through a new drug application (mitoxantrone hydrochloride) or a supplement to an existing approval (temozolomide, topiramate, botulinum toxin type A, lamotrigine). All new drug applications are subject to the regulatory standards set forth in 21CFR 314.126.18 For supplements, however, “information required in the supplement is limited to that needed to support the change.”18
Table 1.
Pivotal Trials Leading to the Approval of Drugs for Neurological Diseases with an Orphan Indication
| Proper Name (Trade Name) | Approval Type | Approval Date | Target Disease | Number of Pivotal Trials | Randomization | Blinding | Control | Study Participants (n) |
|---|---|---|---|---|---|---|---|---|
| Idursulfase (Elaprase) | BLA | 7/24/2006 | Hunter's syndrome | 1 | √ | √ | √ | 96 |
| Alglucosidase alfa (Myozyme) | BLA | 4/28/2006 | Pompe disease | 2 | √ | OL | H | 18 |
| Galsulfase (Naglazyme) | BLA | 5/31/2005 | Mucopolysaccharidosis type VI | 3 | √ | √ | × | 7 |
| × | OL | × | 10 | |||||
| √ | √ | √ | 56 | |||||
| Temozolomide (Temodar) | SNDA | 3/15/2005 | Glioblastoma multiforme | 1 | √ | OL | A | 573 |
| Apomorphine hydrochloride (Apokyn) | NDA | 4/20/2004 | Parkinson's disease | 3 | √ | √ | √ | 29 |
| √ | √ | √ | 17 | |||||
| √ | √ | √ | 62 | |||||
| Miglustat (Zavesca) | NDA | 7/31/2003 | Gaucher's disease type I | 3 | × | OL | × | 28 |
| × | OL | × | 18 | |||||
| √ | OL | A | 36 | |||||
| Laronidase (Aldurazyme) | BLA | 4/30/2003 | Mucopolysaccharidosis type I | 1 | √ | √ | √ | 45 |
| Agalsidase beta (Fabrazyme) | BLA | 4/24/2003 | Fabry's disease | 1 | √ | √ | √ | 58 |
| Sodium oxybate (Xyrem) | NDA | 7/12/2002 | Narcolepsy | 2 | √ | √ | √ | 136 |
| √ | √ | √ | 55 | |||||
| Topiramate (Topamax) | SNDA | 8/28/2001 | Lennox–Gastaut syndrome | 1 | √ | √ | √ | 112 |
| Botulinum toxin type A (Botox) | SNDA | 12/21/2000 | Cervical dystonia | 1 | √ | √ | √ | 170 |
| Botulinum toxin type B (Myobloc) | BLA | 12/8/2000 | Cervical dystonia | 2 | √ | √ | √ | 109 |
| √ | √ | √ | 77 | |||||
| Mitoxantrone hydrochloride (Novantrone) | NDA | 10/13/2000 | Multiple sclerosis | 2 | √ | O | √ | 194 |
| √ | OL | A | 42 | |||||
| Modafinil (Provigil) | NDA | 12/24/1998 | Narcolepsy | 2 | √ | √ | √ | 285 |
| √ | √ | √ | 273 | |||||
| Lamotrigine (Lamictal) | SNDA | 8/24/1998 | Lennox–Gastaut syndrome | 1 | √ | √ | √ | 169 |
| Glatiramer acetate (Copaxone) | NDA | 12/20/1996 | Multiple sclerosis | 2 | √ | √ | √ | 50 |
| √ | √ | √ | 251 | |||||
| Fosphenytoin sodium (Cerebyx) | NDA | 8/5/1996 | Epilepsy | 2 | √ | √ | A | 112 |
| √ | √ | A | 240 | |||||
| Interferon-β 1a (Avonex) | NDA | 5/17/1996 | Multiple sclerosis | 1 | √ | √ | √ | 301 |
| Riluzole (Rilutek) | NDA | 12/12/1995 | Amyotrophic lateral sclerosis | 2 | √ | √ | √ | 155 |
| √ | √ | √ | 959 |
√ = randomized or double blind or placebo controlled; OL = open label; H = historical; × = none/not conducted; NDA = new drug application; A = active comparator; SNDA = supplemental new drug application; BLA = biologic license application; O = observer blind.
Our comparison population comprised all drugs for neurological diseases approved without an orphan indication through 2007. We created a comparison sample by examining all approvals for new molecular and biological entities reported by the FDA Center for Drug Evaluation and Research.3 We identified drugs for neurological diseases using the same criteria described for our study sample. We identified the 20 most recent new molecular and biological entity approvals for drugs for neurological diseases without an orphan indication (Table 2).
Table 2.
Randomized, Double-Blind, Placebo-Controlled Pivotal Trials Leading to the Approval of Drugs for Neurological Diseases without an Orphan Indication
| Proper Name (Trade Name) | Approval Type | Approval Date | Target Disease | Number of Pivotal Trials | Average Participants per Trial |
|---|---|---|---|---|---|
| Rotigotine (Neupro) | NDA | 5/9/2007 | PD | 3 | 385 |
| Lisdexamfetamine dimesylate (Vyvanse) | NDA | 2/23/2007 | ADHD | 2 | 171 |
| Paliperidone (Invega) | NDA | 12/19/2006 | Schizophrenia | 3 | 555 |
| Rasagiline mesylate (Azilect) | NDA | 5/16/2006 | PD | 3 | 521 |
| Varenicline (Chantix) | NDA | 5/10/2006 | Smoking cessation | 6 | 802 |
| Ramelteon (Rozerem) | NDA | 7/22/2005 | Insomnia | 4 | 456 |
| Pregabalin (Lyrica) | NDA | 12/30/2004 | Neuropathic pain | 3 | 243 |
| Ziconotide (Prialt) | NDA | 12/28/2004 | Severe chronic pain | 3 | 152 |
| Eszopiclone (Lunesta) | NDA | 12/15/2004 | Insomnia | 5 | 354 |
| Natalizumab (Tysabri) | BLA | 11/23/2004 | MS | 2 | 1,057 |
| Duloxetine hydrochloride (Cymbalta) | NDA | 8/3/2004 | Major depressive disorder | 4 | 265 |
| Memantine hydrochloride (Namenda) | NDA | 10/16/2003 | AD | 2 | 328 |
| Eletriptan hydrobromide (Relpax) | NDA | 12/26/2002 | Migraine | 7 | 852 |
| Atomoxetine hydrochloride (Strattera) | NDA | 11/26/2002 | Attention-deficit hyperactivity disorder | 6 | 216 |
| Aripiprazole (Abilify) | NDA | 11/15/2002 | Schizophrenia | 4 | 413 |
| Frovatriptan succinate (Frova) | NDA | 11/8/2001 | Migraine | 5 | 934 |
| Almotriptan malate (Axert) | NDA | 5/7/2001 | Migraine | 3 | 773 |
| Galantamine hydrobromide (Razadyne)a | NDA | 2/28/2001 | AD | 4 | 663 |
| Ziprasidone hydrochloride (Geodon) | NDA | 2/5/2001 | Schizophrenia | 5 | 271 |
| Rivastigmine tartrate (Exelon) | NDA | 4/21/2000 | AD | 2 | 712 |
Original brand name, Reminyl, changed to Razadyne in July 2005.
NDA = new drug application; BLA = biologic license application.
Our investigation focused on pivotal studies. We defined pivotal studies as the clinical trials used to support the efficacy of a drug. Pivotal studies are predominantly identified on the drug label19; however, labels can also present information on trials that did not support efficacy. We verified pivotal studies by examining the medical review section of the drug approval package of each agent.19 The drug approval package is the full documentation of the FDA's review, and contains information and comments on clinical data, chemistry, pharmacology, statistics, and administrative issues. Although most drug approval packages explicitly identified pivotal studies, we erred on the side of inclusion when it was not clear in the drug approval package. Original labels and drug approval packages were not available for three older orphan drugs: glatiramer acetate, fosphenytoin sodium, and riluzole. In these cases, we examined the most recent label from the drug's manufacturer20–22 and verified the information in the original journal articles on the pivotal studies of these drugs.23–28 Although botulinum toxin type A did not have an original label available, we were able to cross-reference information from the most recent label29 with the drug approval package.
For each pivotal trial, we determined four key elements of study design: control, blinding, randomization, and size. Control methods included placebo, active treatment, historical controls, and no control. Blinding methods included double blind, observer blind, and open label. In controlled studies, participants were randomly assigned to either treatment or a control. In uncontrolled studies, participants were assigned to different doses of active treatment or no randomization was conducted. We defined study size as the number of participants in a trial who were randomized to a treatment assignment. In trials that were not randomized, we used the number of participants who were enrolled into the study. Some randomized, double-blind, placebo-controlled trials were, in fact, multiphase studies involving both open-label and randomized trial segments. We used only the number of patients randomized to define the study size.
Drug labels can have clinical data for more than one indication, each one separately approved by the FDA. We referenced approval letters and the FDA list of orphan drugs with marketing approval1 to confirm that we were examining clinical trial data specific to the approval of the orphan indication.
Statistics
Our analysis uses a subset of drugs, those for neurological diseases approved with an orphan indication, to produce information that can be potentially generalized to the larger set of drugs for other conditions approved with orphan indications.
We conducted three primary statistical analyses comparing the two drug groups. First, we used Fisher's exact test to compare the proportion of drugs approved without at least one pivotal randomized, double-blind, placebo-controlled trial. Second, we used an exact Wilcoxon rank-sum test to compare mean number of pivotal trials per drug. Third, we used another Wilcoxon test to compare mean pivotal trial sizes. These three analyses were repeated after excluding the four supplemental new drug applications in the orphan drug group.
We conducted three secondary analyses within the group of drugs for neurological diseases with an orphan indication. First, we compared biologics, drugs approved under a biologic license application, with nonbiologics, drugs not approved under a biologic license application. We used Fisher's exact test to compare the proportion approved without at least one pivotal randomized, double-blind, placebo-controlled trial, and a Wilcoxon test to compare the mean pivotal trial sizes. Second, we used Fisher's exact test to test for an association between new indication approvals and approval without at least one randomized, double-blind, placebo-controlled trial. Third, we used Fisher's exact test to test for an association between supplemental new drug applications and FDA approval with only one pivotal trial. We performed all statistical tests at a two-sided significance level of 5% and did not make any correction for multiple testing.
Results
All drugs for neurological diseases approved without an orphan indication included at least two randomized, double-blind, placebo-controlled trials. In comparison, 32% (p < 0.001) of drugs with an orphan indication had at least two such trials, and 74% had at least one (p = 0.02). Five drugs did not include a randomized, double-blind, placebo-controlled trial among their pivotal trials: alglucosidase alfa, temozolomide, miglustat, mitoxantrone hydrochloride, and fosphenytoin sodium. Many of the pivotal trials for these drugs were neither double-blind nor placebo-controlled. Fosphenytoin was approved for bioequivalence to phenytoin, requiring the use of the active comparator. Temozolomide was approved under a supplemental drug application because prior data on safety and efficacy were available. There were 33 pivotal studies for the 19 drugs approved with an orphan indication. Of these 33 studies, 11 (33%) did not use a placebo control, 9 (27%) were not double-blind, and 4 (12%) were not randomized. There were 76 pivotal studies for the 20 drugs approved without an orphan indication. All 76 studies were double-blind, placebo-controlled, and randomized.
Drugs with an orphan indication were approved, on average, with 1.7 pivotal studies as compared with 3.8 pivotal studies for drugs without an orphan indication (p < 0.001). Pivotal studies for drugs without an orphan indication had a mean trial size of 506 participants as compared with 163 participants in pivotal studies for drugs with an orphan indication (p < 0.001).
We included four drugs (temozolomide, topiramate, botulinum toxin type A, and lamotrigine) approved with an orphan indication under a supplemental new drug application. Supplemental applications can refer to preclinical and safety data from the same drug's previous approvals. Therefore, these applications may require fewer pivotal studies for approval than new drug applications. After excluding these four drugs from the analysis, the mean number of pivotal trials for drugs with an orphan indication increased slightly to 1.9 but remained less than drugs without an orphan indication (3.8; p < 0.001). In addition, the mean trial size for drugs with an orphan indication decreased to 138, resulting in a larger difference between drugs with versus without an orphan indication (506; p < 0.001). The proportion of drugs with an orphan indication that had at least two randomized, double-blind, placebo-controlled trials increased to 40% after excluding the drugs using supplemental new drug applications, but this proportion was still much lower than drugs without an orphan indication (100%; p < 0.001).
Biological therapies for neurological diseases comprised 6 (32%) of the 19 drugs approved with an orphan indication and 1 (5%) of the 20 drugs approved without an orphan indication. Among the drugs approved with an orphan indication, the proportion approved without at least one pivotal randomized, double-blind, placebo-controlled trial was not different between biologics (17%) and nonbiologics (31%; p = 1.00). Biologics approved with an orphan indication had, on average, 1.7 pivotal trials with a mean pivotal trial size of 56 participants. Natalizumab (Tysabri), the 1 neurological biologic (in this sample) approved without an orphan indication, had 2 pivotal randomized, double-blind, placebo-controlled trials with a mean size of 1,057 participants.
Discussion
Pivotal studies of drugs for neurological diseases approved with an orphan indication are different from those for drugs approved without an orphan indication. All drugs without an orphan indication included at least two randomized, double-blind, placebo-controlled trials. Only 32% of drugs with an orphan indication included two such trials. Further, drugs with an orphan indication were approved with fewer pivotal trials with smaller numbers of participants. These differences held when controlling for the inclusion of supplemental drug applications among those drugs approved with an orphan indication.
Conducting pivotal efficacy trials in orphan conditions has its own challenges. Recruiting a sufficient number of patients into rare disease trials is inherently difficult and made even harder if there is the chance of receiving a placebo. As a result, clinical trials for rare disease therapies tend to be underpowered because of their small size.12 For conditions that are also rapidly fatal, such as infantile-onset Pompe disease, the rigor of a randomized, placebo-controlled clinical trial may not be necessary. Current definitions of rare disease are based on prevalence alone and do not account for severity or mortality rate. Guidelines for interpreting these factors may clarify regulatory decisions on what constitutes an adequate study design. FDA drug approval packages contain detailed information from the sponsor and extensive comments from FDA reviewers, but the motivations for using of a particular study design are not included. We found, for example, that none of the pivotal trials for alglucosidase alfa, temozolomide, miglustat, or mitoxantrone hydrochloride was double-blind. The rationale for the open-label and observer-blinded methods that were used could provide valuable information for evaluating these studies.
Study designs other than the randomized controlled trial may be considered adequate and well-controlled. Adaptive designs allow investigators to modify trials in progress. Trial procedures such as eligibility criteria, diagnostic procedures, dose, and end points may be altered, as well as some statistical procedures.30 The flexibility of this method is attractive to rare disease researchers, but implementation of these trials is susceptible to confusion and their validity is still in question.31 The n-of-1 trial is another design with potential benefits for rare disease research; it is a randomized multiple crossover trial in which each participant serves as his or her own control. All participants in an n-of-1 trial have the opportunity to receive study drug, and individual level data are used to estimate a population effect. n-of-1 trials suffer from a risk for carryover effects of one period to the next, and they are informative only if the disease is stable and does not fluctuate over time. The FDA is supportive of methodological innovations32 and hosted a 2006 conference on adaptive trial design. Still, there are doubts among drug sponsors and clinical researchers whether these alternatives can be successfully conducted, and concern exists that the FDA is not ready to evaluate them.33
Orphan indication approvals and orphan drug designations have steadily increased since the signing of the Orphan Drug Act in 1983. Current scientific, economic, and regulatory forces suggest that approvals of therapeutics under the Orphan Drug Act will remain an important part of pharmaceutical and biotechnology pipelines in the years to come. First, research in the basic sciences continues to discover more refined disease targets and genetic causative factors. As a result, the number of recognized rare diseases and the spectrum of neurological therapeutics will probably increase, opening avenues for new orphan drug designations. Second, the financial incentives of the Orphan Drug Act are still attractive to pharmaceutical and biotechnology companies. A few blockbuster drugs, those with $1 billion or more in global sales,34 entered the market with an orphan indication approval and have become examples of success, including Epogen, Gleevec, and Cerezyme. Although these are isolated cases among the full cohort of orphan drugs, 16 of the top 100 drugs by 2007 US market sales had orphan indications.35 Finally, the FDA Office of Orphan Products Development has publicized its efforts to make more orphan drugs available. Rare diseases affect an estimated 25 million Americans,36 leaving many patient populations to hope for effective therapeutics to become available soon.
As part of its success, it has been suggested that the Orphan Drug Act has provided a shelter for the development of biologics,5 which is more costly than the development of small molecules.37 Within the two drug groups, we analyzed six biologics with an orphan indication and one without an orphan indication. Two companies focused on developing biologics, Biomarin and Genzyme, each produce two of the six biologics approved for neurological diseases with an orphan indication. These two companies also produce biologics with orphan indications in other therapeutic areas. In the past 8 years, few biologics have been approved for neurological diseases without an orphan indication. Based on a limited sample, the studies conducted for the approval of biologics were not different from nonbiologics for size or study design.
Our study is limited by the scope of drug trials assessed. We restricted our analysis to drugs for neurological diseases. Further research could expand the analysis beyond neurological diseases and provide data on all therapeutics approved with orphan indications. We analyzed only indication approvals after 1995, before which time data were not accessible on the Internet. Our two drug groups, those with and without an orphan indication, were not completely comparable because of the inclusion of drugs with orphan indications that were not new molecular or biological entities. However, even when including only those orphan drugs that were new molecular or biological entities, the principal conclusions remained the same. The difference in time frame of the drug groups was an important source of selection bias because scientific and regulatory procedures change over time. Our comparison of pivotal trials was limited by our ability to determine whether a study was pivotal, but our method was similar to that used by other investigators.38 The term pivotal study is widely used but remains undefined.38 In 1999, an FDA biostatistician included “pivotal study” in a list of terms that lacked clarity.39 He further stated that restricting drug reviews to pivotal studies overestimates drug effects, and thus, all trial data should be evaluated equally. We created an overly inclusive definition of the term pivotal trial to capture the most important clinical trials of efficacy. We did not assess studies that did not support efficacy, such as failed trials or negative trials, or studies that primarily supported safety.
As the number of drugs in development for orphan drugs increases, the demand to design and conduct adequate well-controlled studies to evaluate their effectiveness will increase.7 The results suggest that alternatives other than the traditional randomized, double-blind, placebo-controlled studies are sufficient to gain regulatory approval. Attention to these alternative designs40 and the need to ensure their adequacy in demonstrating efficacy will only grow as science41 and economics42 drive further development of orphan drugs. We thank J. Thompson for his assistance in providing data and Dr R. Katz for his comments.
Acknowledgments
This study was supported by the NIH (National Institute of Neurological Disorders and Stroke, RO1 NS045686; T32 Experimental Therapeutics Training Fellowship, NS 07338-11) and the 2007 Muscle Study Group (1R13N2061458-01) (J.M.).
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.U.S. Food and Drug Administration [Accessed August 5, 2008];Cumulative list of all Orphan Designated Products that have received marketing approval. Available at: http://www.fda.gov/orphan/designat/allap.rtf.
- 2.US Food and Drug Administration [Accessed July 26, 2008];Orphan designations pursuant to Section 526 of the Federal Food and Cosmetic Act as amended by the Orphan Drug Act (P.L. 97-414) Available at: http://www.fda.gov/orphan/designat/list.xls.
- 3.US Food and Drug Administration [Accessed July 14, 2008];CDER Drug and Biologic Approval Reports: NME and new biologic approvals. Available at: http://www.fda.gov/Cder/rdmt/default.htm.
- 4.Haffner ME. Adopting orphan drugs—two dozen years of treating rare diseases. N Engl J Med. 2006;354:445–447. doi: 10.1056/NEJMp058317. [DOI] [PubMed] [Google Scholar]
- 5.Reaves ND. A model of effective health policy: the 1983 Orphan Drug Act. J Health Soc Policy. 2003;17:61–71. doi: 10.1300/j045v17n04_04. [DOI] [PubMed] [Google Scholar]
- 6.Rin-Laures L-H, Janofsky D. Recent developments concerning the Orphan Drug Act. Harv J Law Technol. 1991;4:269–297. [Google Scholar]
- 7.Yin W. Market incentives and pharmaceutical innovation. J Health Econ. 2008;27:1060–1077. doi: 10.1016/j.jhealeco.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Drummond MF, Wilson DA, Kanavos P, et al. Assessing the economic challenges posed by orphan drugs. Int J Technol Assess Health Care. 2007;23:36–42. doi: 10.1017/S0266462307051550. [DOI] [PubMed] [Google Scholar]
- 9.Rohde DD. The Orphan Drug Act: an engine of innovation? At what cost? Food Drug Law J. 2000;55:125–143. [PubMed] [Google Scholar]
- 10.US Food and Drug Administration . OOPD Program Overview. Rockville, MD: 2008. [Last accessed on May 22, 2008]. http://www.fda.gov/orphan/progovw.htm. [Google Scholar]
- 11.Adequate and well-controlled studies. Title 21 Code of Federal Regulations, pt. 314, sec. 126. 2007. [Google Scholar]
- 12.Buckley BM. Clinical trials of orphan medicines. Lancet. 2008;371:2051–2055. doi: 10.1016/S0140-6736(08)60876-4. [DOI] [PubMed] [Google Scholar]
- 13.National Institute of Neurological Disorders and Stroke [Accessed March 28, 2008];Disorders Index. Available at: http://www.ninds.nih.gov/disorders/disorder_index.htm.
- 14.National Institute of Mental Health [Accessed March 28, 2008];Mental health topics. Available at: http://www.nimh.nih.gov/health/topics/index.shtml.
- 15.National Institute on Aging [Accessed July 20, 2008];About NIA. Available at: http://www.nia.nih.gov/AboutNIA/.
- 16.National Institute on Drug Abuse [Accessed March 28, 2008];Division of Clinical Neuro-science and Behavioral Research: Behavioral and Brain Development Branch. Available at: http://www.nida.nih.gov/about/organization/DCNBR/bbdb.html.
- 17.US Food and Drug Administration [Accessed July 22, 2008];New drug application approvals and receipts, including new molecular entities, 1938 to present. Available at: http://www.fda.gov/oc/history/NDAapprovals.html#data.
- 18.Applicability. Title 21 Code of Federal Regulations, pt. 312, sec. 2. 2003. [Google Scholar]
- 19.U.S. Food and Drug Administration [Accessed August 7, 2008];Drugs@FDA: FDA approved drug products. Available at: http://www.accessdata.fda.gov/Scripts/cder/DrugsatFDA/.
- 20.Pfizer Inc. [Accessed June 23, 2008];Cerebyx (fosphenytoin sodium injection), prescribing information. Available at: http://media.pfizer.com/files/products/uspi_cerebyx.pdf.
- 21. [Accessed June 23, 2008];Sanofi-Aventis U.S. Rilutek (riluzole) tablets, prescribing information. Available at: http://products.sanofi-aventis.us/rilutek/rilutek.html.
- 22.Teva Pharmaceutical Industries Ltd [Accessed June 23, 2008];Copaxone (glatiramer acetate injection), prescribing information. Available at: http://www.copaxone.com/pdf/PrescribingInformation.pdf.
- 23.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 24.Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 25.Lacomblez L, Bensimon G, Leigh PN, et al. A confirmatory dose-ranging study of riluzole in ALS. ALS/Riluzole Study Group-II. Neurology. 1996;47:S242–S250. doi: 10.1212/wnl.47.6_suppl_4.242s. [DOI] [PubMed] [Google Scholar]
- 26.Ramsay R, Philbrook B, Martinez O, et al. A double-blinded, randomized safety comparison of rapidly infused intravenous loading doses of fosphenytoin vs. phenytoin. Annual Meeting of the American Epilepsy Society; Baltimore, MD. 1995. [Google Scholar]
- 27.Teitelbaum D, Arnon R, Sela M, Abramsky O. Clinical trial of copolymer 1 in multiple sclerosis. Harefuah. 1989;116:453–456. [PubMed] [Google Scholar]
- 28.Wilder BJ, Campbell K, Ramsay RE, et al. Safety and tolerance of multiple doses of intramuscular fosphenytoin substituted for oral phenytoin in epilepsy or neurosurgery. Arch Neurol. 1996;53:764–768. doi: 10.1001/archneur.1996.00550080082016. [DOI] [PubMed] [Google Scholar]
- 29.Allergen Inc. [Last accessed on July 22, 2008];Botox (botulinum toxin type A), prescribing information. 2006 http://www.allergan.com/assets/pdf/botox_cosmetic_pi.pdf.
- 30.Chow SC, Chang M. Adaptive design methods in clinical trials–a review. Orphanet J Rare Dis. 2008;3:11. doi: 10.1186/1750-1172-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coffey CS, Kairalla JA. Adaptive clinical trials: progress and challenges. Drugs R D. 2008;9:229–242. doi: 10.2165/00126839-200809040-00003. [DOI] [PubMed] [Google Scholar]
- 32.Temple R. FDA perspective on trials with interim efficacy evaluations. Stat Med. 2006;25:3245–3249. doi: 10.1002/sim.2631. [DOI] [PubMed] [Google Scholar]
- 33.Gottlieb S. [Accessed July 14, 2008];Speech. Available at: http://www.fda.gov/oc/speeches/2006/trialdesign0710.html.
- 34.Cutler DM. The demise of the blockbuster? N Engl J Med. 2007;356:1292–1293. doi: 10.1056/NEJMp078020. [DOI] [PubMed] [Google Scholar]
- 35.Lamb E. Top 200 prescription drugs of 2007. Pharmacy Times; Plainsboro, NJ: 2008. [Google Scholar]
- 36.Findings and purposes. Rare Diseases Act of 2002, Section 2. 2002. [Google Scholar]
- 37.Grabowski HG, Ridley DB, Schulman KA. Entry and competition in generic biologics. Managerial Economics. 2007;28:439–451. [Google Scholar]
- 38.Lee K, Bacchetti P, Sim I. Publication of clinical trials supporting successful new drug applications: a literature analysis. PLoS Med. 2008;5:e191. doi: 10.1371/journal.pmed.0050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anello C. [Accessed July 9, 2008];Integrated summaries of safety and integrated summaries of efficacy: is this meta-analysis? Available at: http://www.fda.gov/cder/Offices/Biostatistics/Anello_193/index.htm.
- 40.Griggs R, Dunkle M, Batshaw M, et al. Clinical research for rare disease: opportunities, challenges and solutions. Mol Genet Metab. 2009;96:20–26. doi: 10.1016/j.ymgme.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maher PD, Haffner M. Orphan drug designation and pharmacogenomics: options and opportunities. BioDrugs. 2006;20:71–79. doi: 10.2165/00063030-200620020-00001. [DOI] [PubMed] [Google Scholar]
- 42.Samson K. Orphan economics: the downside of supplyside pharmacology. Ann Neurol. 2008;64:A13–A16. doi: 10.1002/ana.21432. [DOI] [PubMed] [Google Scholar]