Abstract

The total synthesis of an analog of the marine alkaloid kealiiquinone has been completed through application of an intramolecular Diels-Alder reaction of an imidazole-containing enyne. Oxidative aromatization of the lactone adduct and N-methylation facilitates C2-oxidation via the imidazolium salt. Conversion of the lactone to the phthalaldehyde derivative and then to the dihydroxybenzoquinone was achieved via a reaction with glyoxal in the presence of KCN. Esterification of the vinylogous diacid and deprotection provided 7′-desmethylkealiiquinone.
Marine sponges have produced and continue to provide a large number of architecturally unique and biologically active natural products.1,2 These natural products have provided a fertile testing ground for the development and refinement of new synthetic methods and strategies.1 Our group has been particularly interested in the development of synthetic approaches to the growing number of imidazole-containing natural products from marine sponges.3,4 Sponges of the Leucetta and Clathrina genus produce a remarkable number of diverse secondary metabolites in which an imidazole is a characteristic structural motif.5 Recently, our attention has been directed to two structurally related imidazonaphthoquinone natural products, kealiiquinone (1)6 and its 2-amino congener (2-amino-2-deoxykealiiquinone (2)),7 which were isolated by the Clardy and Schmitz groups, respectively, from two Leucetta sponges. Although these compounds were not reported to possess exceptional biological activity, they are cytotoxic. An isomer of 18 has been shown to exhibit fairly potent anti-cancer activity which based on its profile is thought to be mediated through a novel mode of action. More recently, several naturally occurring analogs of kealiiquinone, kealiinines A-C (3–5), have been reported and have been suggested as possible biosynthetic precursors to 1 and 2.9
To date, one synthesis of kealiiquinone (1) has been described in the literature by Ohta and co-workers,10 and involves the sequential metallation and electrophilic trapping of a 2-thiosubstituted imidazole. Subsequent Friedel-Crafts alkylation and adjustment of the oxidation state around the oxygenated benzimidazole provides 1. Our approach to this system involves a different and complementary strategy centered on de novo quinone assembly and intramolecular Diels-Alder reaction of a 4-vinylimidazole derivative; chemistry which we have extensively investigated in our labs.11,12
This strategy, which is depicted retrosynthetically in Figure 2, involves the late-stage introduction of the C2 imidazole functionality, thereby potentially allowing access to both 1 and 2 from an advanced and common intermediate. The precursor imidazonaphthoquinone would be obtained by an interesting and rarely used annulation reaction for incorporation of the dihydroxyquinone moiety involving a masked glyoxal equivalent.13 The requisite phthalaldehyde precursor 6 would be obtained from lactone 7 which in turn would be constructed through the Diels-Alder-oxidation sequence involving the enyne 8.
Figure 2.
Retrosynthetic analysis of kealiiquinones 1 and 2
In a forward sense, our synthesis began with the construction of the enyne 8 which was obtained from the DCC-mediated esterification of the 4-vinylimidazole 9 and the aryl propiolic acid derivative 10 (Scheme 1). The vinylimidazole can be obtained in three steps from urocanic acid by methods previously described by us in excellent yield.11 The enyne undergoes a smooth Diels-Alder reaction leading to the formation of the dihydrobenzimidazole 11 in 89% yield. Subjection of the cycloadduct to oxidation by treatment with MnO2 provided the benzimidazole 7 in 95% yield.14 Benzimidazole 7 was nicely crystalline, and so the structure of the oxidized cycloadduct was confirmed through an X-ray structure determination (inset Scheme 1).
Scheme 1.
Assembly of the benzimidazole framework and X-ray structure of compound 7.
Our choice of the benzyl-protected 4-vinylimidazole was guided by experience with vinylimidazoles which indicated that the 4-isomers are better substrates in Diels-Alder reactions, and we have developed a number of efficient methods for construction of such derivatives.11 As a result, our approach required an “isomerization” step in order to properly position the N1-methyl group. It proved quite convenient to accomplish this tactic with 7. Specifically, it was found that treatment of 7 with methyl iodide provided the imidazolium salt 12. Catalytic hydrogenation led to efficient debenzylation leaving the methyl group in the correct position affording 13 (Scheme 2). However, the presence of aqueous base resulted in variable levels of lactone hydrolysis during this reaction, thus treatment of the reduction product with acid prior to purification was necessary to reproducibly provide 13 in good yields. The lactone was then reduced to the corresponding diol 14 on treatment with DIBAL-H.
Scheme 2.
Site selective methylation and oxidation state adjustment.
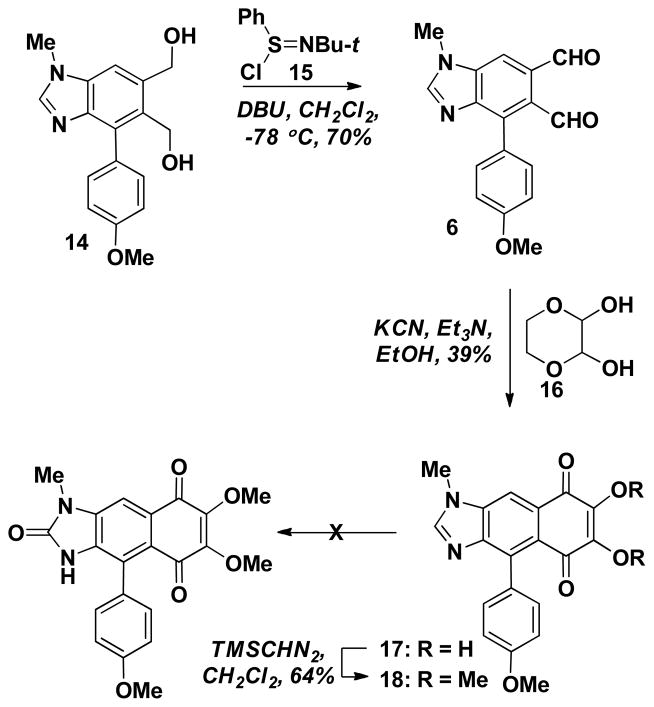
With diol 14 in hand, we were in position to introduce the final ring and to accomplish this the diol required oxidation to the dialdehyde. However, this transformation proved to be a more challenging undertaking than anticipated. Out of the intial set of common oxidants that we evaluated, Swern conditions were found to be most encouraging, but there were some reproducibility issues. Therefore, we searched for Swern-like oxidations with improved reliability. In the process of this search, we came across the use of S-chloro sufinimines reported by Mukaiyama and co-workers, and found this procedure to be the most reproducible providing the dialdehyde 6 in ~70% yield (Scheme 3).15 The phthalaldehyde 6 was then reacted with the masked glyoxal derivative 16 to arrive at the 2,3-dihydroxyquinone 17 in moderate, but reproducible yield.13 Treatment of 17 (effectively a vinylogous diacid) with TMS-diazomethane led to esterification and formation of the dimethyl derivative 18. We have routinely functionalized imidazoles and benzimidazoles via lithiation and electrophilic trapping to incorporate either the 2-oxo or 2-amino group. Unfortunately, C2-metalation of 18 with LDA and trapping with an appropriate electrophile (e.g., (PhCO2)2 or TsN3) was not successful.16 Although we do not know the precise fate of the imidazole-containing species, a brightly-colored and water soluble material was produced, suggesting some sort of electron transfer process. Clearly, in order to move this project forward we had to overcome this roadblock. A general solution to functionalizing at the C2-position of imidazoles has been reported recently by our lab through the use of hypochlorite or N-chloro amides with imidazolium salts. This leads to the direct formation of 2-imidazolones and 2-imino imidazoles, respectively.17
Scheme 3.
Installation of quinone and attempted elaboration of C2
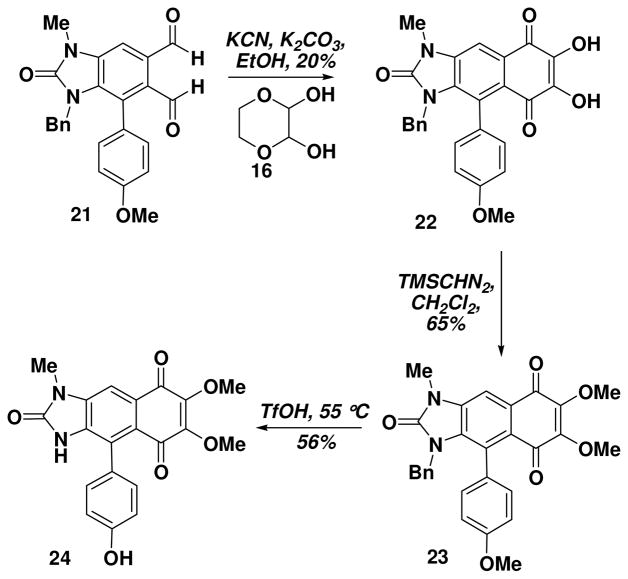
Specifically, we found that treating a THF solution of 12 with commercial bleach solutions provided the 2-benzimidazolone 19 in an excellent 75% yield (Scheme 4).17 Lactone reduction to the diol was accomplished with DIBAL (confirmed by X-ray crystallography), and oxidation to the dialdehyde 21 was performed as above with the thioimidoyl chloride.15 Subsequent treatment of the phthalaldehyde 21 with the glyoxal derivative 16 and KCN provided the dihydroxy quinone 2213 which upon treatment with TMS-diazomethane provided 3-benzyl kealiiquinone 23 (Scheme 5). It is well recognized that the removal of benzyl groups from amides can be a challenging proposition with many of the standard debenzylation techniques failing to result in cleavage. We also found this to be the case with imidazolone 23. We subsequently determined that the benzyl moiety could be removed upon treatment with TfOH and heating (55 °C), but this also resulted in the loss of the methyl group on the p-anisyl moiety. A number of attempts have been made to remethylate the phenolic OH but these have not been successful due to either bismethylation or selective N-methylation.
Scheme 4.
Successful C2-Oxidation and X-ray crystal structure of compound 20.
Scheme 5.
In summary, we have developed a convenient 10-step synthesis of a close analog of the Leucetta-derived alkaloid kealiiquinone via an intramolecular Diels-Alder reaction from the known vinylimidazole 9. Introduction of the C2-oxo moiety was accomplished through a novel hypochlorite-mediated oxidation. Incorporation of the quinone fragment was accomplished through the use of a masked glyoxal system. Efforts to improve the efficiency of the quinone forming step and to obtain the 2-deoxy-2-amino congener 2 are underway.
Supplementary Material
Figure 1.
Structures of the kealiinine-family of alkaloids
Acknowledgments
This work was supported by the Robert A. Welch Foundation (Y-1362) and in part by the NIH (GM065503). The NSF (CHE-0234811 and CHE-0840509) is thanked for partial funding of the purchases of the NMR spectrometers used in this work.
Footnotes
Supporting Information Available Detailed experimental procedures and copies of 1H and 13C NMR spectra for all new compounds. CIFs for compounds 7 and 20. This information is free of charge from the internet at http://pubs.acs.org.
References
- 1.Morris JC, Phillips AJ. Nat Prod Rep. 2011;28:269. doi: 10.1039/c0np00066c. [DOI] [PubMed] [Google Scholar]
- 2.Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR. Nat Prod Rep. 2011;28:196. doi: 10.1039/c005001f. [DOI] [PubMed] [Google Scholar]
- 3.Jin Z. Nat Prod Rep. 2011;28:1143. doi: 10.1039/c0np00074d. [DOI] [PubMed] [Google Scholar]
- 4.For a summary of some of our efforts see Du H, He Y, Sivappa R, Lovely CJ. Synlett. 2006:965.
- 5.Koswatta PB, Lovely CJ. Nat Prod Rep. 2011;28:511. doi: 10.1039/c0np00001a. [DOI] [PubMed] [Google Scholar]
- 6.Akee R, Carroll TR, Yoshida WY, Scheuer PJ, Stout TJ, Clardy J. J Org Chem. 1990;55:1944. [Google Scholar]
- 7.Fu X, Schmitz FJ, Tanner RS, Kelly-Borges M. J Nat Prod. 1998;61:384. doi: 10.1021/np970453q. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura S, Tsuno N, Yamashita M, Kawasaki I, Ohta S, Ohishi Y. J Chem Soc, Perkin Trans 1. 2001;429 [Google Scholar]
- 9.Hassan W, Edrada RA, Ebel R, Wray V, Berg A, van Soest RWM, Wiryowidagdo S, Proksch P. J Nat Prod. 2004;67:817. doi: 10.1021/np0305223. [DOI] [PubMed] [Google Scholar]
- 10.(a) Kawasaki I, Taguchi N, Yamamoto T, Yamashita M, Ohta S. Tetrahedron Lett. 1995;36:8251. [Google Scholar]; (b) Kawasaki I, Taguchi N, Yamashita M, Ohta S. Chem Pharm Bull. 1997;45:1393. [Google Scholar]
- 11.(a) He Y, Krishnamoorthy P, Lima HM, Chen Y, Wu H, Sivappa R, Dias HVR, Lovely CJ. Org Biomol Chem. 2011;9:2685. doi: 10.1039/c0ob00657b. [DOI] [PubMed] [Google Scholar]; (b) Sivappa R, Mukherjee S, Lovely CJ. Org Biomol Chem. 2009;7:3215. doi: 10.1039/b909482b. [DOI] [PubMed] [Google Scholar]; (c) Sivappa R, Hernandez NM, He Y, Lovely CJ. Org Lett. 2007;9:3861. doi: 10.1021/ol0711568. [DOI] [PubMed] [Google Scholar]; (d) Lovely CJ, Du H, Sivappa R, Bhandari MK, He Y, Dias HVR. J Org Chem. 2007;72:3741. doi: 10.1021/jo0626008. [DOI] [PubMed] [Google Scholar]; (e) Lovely CJ, Du H, He Y, Dias HVR. Org Lett. 2004;6:735. doi: 10.1021/ol036403w. [DOI] [PubMed] [Google Scholar]; (f) He Y, Chen Y, Wu H, Lovely CJ. Org Lett. 2003;5:3623. doi: 10.1021/ol0352862. [DOI] [PubMed] [Google Scholar]; (g) Lovely CJ, Du H, Dias HVR. Heterocycles. 2003;60:1. [Google Scholar]; (h) Lovely CJ, Du H, Dias HVR. Org Lett. 2001;3:1319. doi: 10.1021/ol015687m. [DOI] [PubMed] [Google Scholar]
- 12.For other reports of the Diels-Alder reactions of vinylimidazoles see Walters MA, Lee MD. Tetrahedron Letters. 1994;35:8307–8310.Deghati PYF, Wanner MJ, Koomen GJ. Tetrahedron Letters. 1998;39:4561–4564.Poeverlein C, Breckle G, Lindel T. Organic Letters. 2006;8:819–822. doi: 10.1021/ol0526219.Cotterill LJ, Harrington RW, Clegg W, Hall MJ. J Org, Chem. 2010;75:4604–4607. doi: 10.1021/jo100416c.
- 13.Venuti MC. Synthesis. 1982:61. [Google Scholar]
- 14.Fatiadi AJ. Synthesis. 1976:133. [Google Scholar]
- 15.Matsuo J-i, Iida D, Tatani K, Mukaiyama T. Bull Chem Soc Jpn. 2002;75:223. [Google Scholar]
- 16.We have used this strategy extensively in various total syntheses see for example Lima HM, Garcia-Barboza BJ, Khatibi NN, Lovely CJ. Tetrahedron Lett. 2011;52:5725. doi: 10.1016/j.tetlet.2011.08.030.Koswatta P, Lovely CJ. Chem Commun. 2010;46:2148. doi: 10.1039/b926285g.Koswatta P, Lovely CJ. Tetrahedron Lett. 2010;51:164. doi: 10.1016/j.tetlet.2009.10.117.Koswatta PB, Lovely CJ. Tetrahedron Lett. 2009;50:4998. doi: 10.1016/j.tetlet.2009.10.117.Koswatta P, Sivappa R, Dias HVR, Lovely CJ. Synthesis. 2009:2970.Bhandari MR, Sivappa R, Lovely CJ. Org Lett. 2009;11:1535. doi: 10.1021/ol9001762.Koswatta PB, Sivappa R, Dias HVR, Lovely CJ. Org Lett. 2008;10:5055. doi: 10.1021/ol802018r.
- 17.Lima HM, Lovely CJ. Org Lett. 2011;13:5736. doi: 10.1021/ol2022438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.