Abstract
Purpose
Quantify incidence of cardiovascular outcomes in patients with advanced breast cancer receiving cardiotoxic and non-cardiotoxic chemotherapy.
Methods
Identified all women at a Midwestern health system with initial diagnosis of AJCC stage III/IV breast cancer (1995–2003) and random sample of 50 women initially diagnosed with stage I/II who progressed to stage III/IV. Calculated rate of new cardiovascular outcomes (heart failure, dysrhythmia and ischemia events) for cardiotoxic (anthracycline or trastuzumab) and non-cardiotoxic agents.
Results
Of 315 patients, 90.5% (N=285) received systemic cancer therapy; 67.7% (n=193) received cardiotoxic drugs. Older patients were less likely to receive cardiotoxic agents (86.4% ≤ 59 years vs. 31.9% aged 70+). Adjusting for age, race, stage, surgery/radiation, ER/PR status and diagnosis year, rate of new cardiac events was higher in patients exposed to cardiotoxic drugs compared to those exposed to non-cardiotoxic drugs (adjusted hazard ratio=2.5, 95% CI 0.9, 7.2). Patients with cardiac event history (relative risk=3.2, 95% CI 2.0–5.1) and those with heart failure history (relative risk=5.9, 95% CI 2.4–14.6) were more likely to receive non-cardiotoxic treatment. Heart failure events occurred steadily over time; after 3 years follow-up 16% exposed to cardiotoxic drugs experienced an event, and 8% of those exposed to non-cardiotoxic drugs experienced an event.
Conclusions
Patients with cardiac comorbidity are less likely to receive cardiotoxic agents. Use of cardiotoxic agents is common, treatment is related to patient and tumor characteristics and is associated with substantial risk of cardiotoxicity that persists during patient’s remaining lifespan.
Keywords: breast cancer, chemotherapy, cardiotoxic agents, cardiotoxicity risk
INTRODUCTION
Heart disease is a significant issue in women treated for breast cancer. In addition to increased age as a risk factor for heart disease and breast cancer,1,2 commonly used chemotherapeutic agents, particularly anthracyclines, have cardiotoxic effects.3,4 Although many cited estimates come from older studies, the incidence of chronic cardiotoxicity, specifically congestive heart failure (CHF), is reported to range up to 50% depending on cumulative anthracycline dose received.5,6,7,8 In a clinical trial of women with advanced breast cancer, decreases in left ventricular ejection fraction (LVEF), CHF, and histologic changes were noted in 31% of patients, suggesting that a large proportion of women exposed to anthracyclines experience subclinical cardiac changes.9
Most studies assessing chemotherapy and cardiotoxicity were conducted in clinical trial settings, with strict inclusion/exclusion criteria. There are limited data on early and longer-term effects in patients treated in clinical practice. Doyle et al.10 conducted a population-based cohort study using the Surveillance Epidemiology and End Results (SEER)-Medicare database in women ≥65 years diagnosed with stage I-III breast cancer and found that the relative rate of cardiotoxicity in patients exposed to anthracyclines remained elevated 5 years after a breast cancer diagnosis. However, the study relied upon an administrative data coding algorithm to identify cardiotoxicity. Pinder and colleagues extended this work by including longer follow-up in the SEER-Medicare database and demonstrated that the rate of CHF in anthracycline-exposed patients increased over time.11 Recent studies have suggested that other commonly used therapies may adversely affect cardiac outcomes. 12,13,14
Given limited information on treatments received in clinical practice and the fact that studies conducted using only administrative data may not accurately classify cardiotoxicity, we conducted a population-based study in a large Midwestern health system. We used medical record abstraction to identify cardiovascular risk factors and outcomes in women with advanced breast cancer.
METHODS
This retrospective cohort study was conducted within the Henry Ford Health System (HFHS). The HFHS is an integrated health system serving residents in southeastern Michigan. HFHS maintains a tumor registry that includes cancer site, tumor characteristics and initial treatment received, allowing for record linkage and confirmation of tumor site. The cancer registry employs a thorough case finding system that includes review of all pathology and cytology reports, as well as radiation and oncology consultations. The registry uses the AJCC scheme, staging cancer by examination of tumor size, invasion, nodal involvement and presence of metastases (TNM classification). HFHS automated data include comprehensive information on chemotherapy exposure, using internal service codes that record all infusions. This study was approved by the HFHS Institutional Review Board.
Study Population
To capture the spectrum of patients treated in clinical practice, we included women with advanced breast cancer at the time of diagnosis and those whose tumor progressed after being diagnosed at an earlier stage. This ensured that our sample would include women who had received prior breast cancer treatment. Using the tumor registry, we identified all patients with an initial diagnosis of AJCC stage III or IV breast cancer from 01/01/1995 through 12/31/2003. To include a sample of 50 patients that progressed from early stage disease, we also identified all cases of newly diagnosed AJCC stages I or II diagnosed from 01/01/1996 through 12/31/2002 and manually reviewed electronic medical records to identify women who subsequently developed metastases. These women were included a priori in order to fully represent patients with advanced disease in the real-world setting, not limited to those initially diagnosed with advanced disease. Progression to advanced disease was based on AJCC staging criteria; chemotherapy exposure began at initial diagnosis for this group. We obtained automated enrollment and encounter data to limit the population to women 18 years or older, enrolled for at least one year before diagnosis. The final study population (91% of patients) included women who, after detailed medical record review, were determined to have sufficient medical record documentation (i.e. medical records indicated that treatment was not received outside the health system). All women in this study were treated with chemotherapy.
Medical Record Review
Medical record abstractors collected detailed data on tumor characteristics (including estrogen receptor (ER) and progesterone receptor (PR) and HER-2 neu protein expression) and cancer treatment (systemic therapy, radiation, surgery). Cardiac conditions existing within one year before first breast cancer diagnosis date and conditions requiring medical intervention that occurred after first cancer diagnosis were abstracted from the records. These conditions included CHF, myocardial infarction (MI), coronary artery disease (CAD), cardiac ischemia, cardiac arrest, cardiomyopathy, angina, heart block, myocarditis, or arrhythmia, indicated in the medical record. Abstractors obtained information on LVEF for both baseline and prospective measures, by collecting reports from all echocardiograms (ECHO), angiograms, and multigated acquisition (MUGA) tests 3 years prior to cancer diagnosis as well as all subsequent tests. Ten percent of medical records were double abstracted for quality control purposes. All patients were followed until death, last known visit, disenrollment from health system, or the end of the study period (March 31, 2005).
Classification of Cardiotoxic Agents
Treatment data were obtained from automated HFHS databases that include comprehensive information on the administration of chemotherapy through the use of internal service codes that record all infusions with detailed drug-specific information. These internal service codes have the ability to map to more general claims-based coding that relies on ICD-9/CPT4/HCPCS codes. Using these data, we formed two mutually exclusive categories of pharmacotherapy exposure.3,15
Exposed to Cardiotoxic Drugs: Patients ever exposed to doxorubicin, epirubicin mitoxantrone, or trastuzumab (exposure window began at first exposure to cardiotoxic drugs); and,
Exposed to Non-cardiotoxic drugs: Patients ever exposed to systemic cancer therapy, but not exposed to cardiotoxic agents, as defined above (exposure window began at first exposure to non-cardiotoxic drugs).
Definition of Derived Study Variables
Initially, we intended to use automated data to identify cardiovascular outcomes. However, cursory review indicated that automated data resulted in a sensitivity of 89%, specificity of 18%, and positive predictive value of 31%. Therefore, we conducted medical record review to ascertain cardiovascular events, in particular CHF. Using the data collected from medical record review, we derived the following variables:
Baseline left ventricular ejection fraction (LVEF): Most recent LVEF measurement before first exposure to a cancer drug.
Decreased LVEF: LVEF measurement less than (<) 50%.
Pre-existing/concurrent CV comorbidity: Medical record documentation that any of the following conditions were present on or before the date of first breast cancer diagnosis: congestive heart failure (CHF), cardiomyopathy, arrhythmia, angina, coronary artery disease (CAD), cardiac ischemia, myocardial infarction (MI), cardiac arrest, heart block, decreased LVEF <50%, or myocarditis. If a condition was not mentioned in the medical record, the patient was considered not to have it.
We used medical record abstraction data to create the following outcome categories for documentation of clinical events that occurred after exposure to systemic cancer therapy:
Heart failure event: CHF, cardiomyopathy, decreased LVEF, or myocarditis.
Dysrhythmia event: Cardiac arrest, heart block, or arrhythmia.
Ischemia event: CAD, MI, angina, or cardiac ischemia.
Overall cardiac event: Heart failure, dysrhythmia, and ischemia events combined.
In addition to these cardiovascular outcomes, we merged the entire study cohort with the state of Michigan Death Registry to identify date of death.
Data Analysis
We tabulated frequencies of demographic, clinical characteristics, and outcomes, overall and stratified by receipt of cardiotoxic drugs (yes/no) and calculated the rate of newly diagnosed cardiovascular outcomes (overall cardiac events, heart failure events, dysrhythmia events, and ischemia events). The study sample was not large enough to conduct stable analyses of patients with pre-existing/concurrent cardiac comorbidity. To calculate rates of new events, we limited the analytic sample for each outcome to patients without a history of outcomes in that particular category. As a result, the denominators for each analysis differ. For example, patients with a history of CHF were excluded from the analysis of overall cardiac events and heart failure events, but were included in the analysis of dysrhythmia events. Patients were followed from first cancer drug exposure until death, disenrollment, cardiac event, or end of the study period (whichever came first). To control for confounding, we fit a Cox Proportional hazards model to compare the rate of cardiovascular outcomes in patients receiving cardiotoxic drugs to the rate in those receiving non-cardiotoxic drugs, adjusting for age category, race, stage at advanced diagnosis, surgery (yes/no), left-sided radiation therapy (yes/no), ER/PR status, year of diagnosis of advanced stage breast cancer, and Charlson comorbidity index; therefore differences in follow-up time after exposure are accounted for in the analysis. To evaluate the robustness of our findings, we repeated the analysis with decreased LVEF defined as <40%; using this alternative approach did not materially change the findings (results not presented).
Analyses comparing history of cardiac events occurring after diagnosis but before treatment according to receipt of cardiotoxic versus non-cardiotoxic treatment were conducted by calculating the proportion of patients with a history of cardiac events for each treatment group and dividing these proportions to obtain the relative risk (RR) with corresponding 95% confidence intervals (CI).
While not an a priori objective of this study, in addition to examination of cardiac endpoints, we fit a Cox Proportional hazards model to compare the rate of death in patients receiving cardiotoxic drugs to the rate in those receiving non-cardiotoxic drugs, adjusting for age category, race, stage at advanced diagnosis, surgery (yes/no), left-sided radiation therapy (yes/no), ER/PR status, Charlson comorbidity index, and year of diagnosis of advanced stage breast cancer.
RESULTS
We identified 345 women with newly diagnosed advanced breast cancer and excluded 30 women (8.7%) who received breast cancer treatment outside the health system. Overall, 285 (90.5%) patients received systemic cancer therapy; 67.7% were exposed to cardiotoxic drugs. The demographic and clinical characteristics of the final sample (N=285) are presented in Table 1. Of the 285 patients receiving systemic cancer therapy, 56.8% were 59 years or younger. Older patients were less likely to be exposed to cardiotoxic agents; 86.4% of patients 59 years or younger received cardiotoxic drugs (versus 31.9% of patients 70 years or older). In terms of race, approximately half (51.6%) of the patients were White, and 44.5% were African American. Among these racial groups, 63.9% of Whites and 70.1% of African Americans received cardiotoxic agents. Slightly over one third (35.1%) of patients had ER and PR negative tumors; of those patients, 89.0% received cardiotoxic therapy.
Table 1.
Demographic and Clinical Characteristics of Study Population, Overall2 and Stratified by Receipt of Cardiotoxic Agents (N=285)
| VARIABLE | TOTAL | CARDIOTOXIC1 | NOT CARDIOTOXIC3 |
|---|---|---|---|
|
| |||
| N | N (%)2 | N (%)2 | |
|
| |||
| ALL PATIENTS | 285 (100) | 193 (67.7) | 92 (32.3) |
|
| |||
| Age Category | |||
| <50 | 84 (29.4) | 76 (39.4) | 8 (8.7) |
|
| |||
| 50–59 | 78 (27.4) | 64 (33.2) | 14 (15.2) |
|
| |||
| 60–69 | 51 (17.9) | 30 (15.5) | 21 (22.8) |
|
| |||
| 70–79 | 53 (18.6) | 21 (10.9) | 32 (34.8) |
|
| |||
| 80+ | 19 (6.7) | 2 (1.0) | 17 (18.5) |
|
| |||
| Race | |||
| White | 147 (51.6) | 94 (48.7) | 53 (57.6) |
|
| |||
| African American | 127 (44.5) | 89 (46.1) | 38 (41.3) |
|
| |||
| Other | 11 (3.9) | 10 (5.2) | 1 (1.1) |
|
| |||
| AJCC 3 Stage at First Diagnosis | |||
| I | 12 (4.2) | 7 (3.6) | 5 (5.4) |
|
| |||
| II | 32 (11.2) | 22 (11.4) | 10 (10.9) |
|
| |||
| III | 154 (54.1) | 115 (59.6) | 39 (42.4) |
|
| |||
| IV | 87 (30.5) | 49 (25.4) | 38 (41.3) |
|
| |||
| AJCC Stage at Advanced Diagnosis | |||
| III | 156 (54.7) | 116 (60.1) | 40 (43.5) |
|
| |||
| IV | 129 (45.3) | 77 (39.9) | 52 (56.5) |
|
| |||
| HER-2 neu4 positive | |||
| Yes | 86 (30.2) | 67 (34.7) | 19 (20.6) |
|
| |||
| No | 91 (31.9) | 66 (34.2) | 25 (27.2) |
|
| |||
| Unknown5 | 108 (37.9) | 60 (31.1) | 48 (52.2) |
|
| |||
| ER/PR6 status | |||
| ER positive and/or PR positive | 170 (59.6) | 96 (49.7) | 74 (80.4) |
|
| |||
| ER negative and PR negative | 100 (35.1) | 89 (46.1) | 11 (12.0) |
|
| |||
| Unknown | 15 (5.3) | 8 (4.2) | 7 (7.6) |
|
| |||
| Treatment7 (ever treated) | |||
| Surgery plus radiation plus cancer drug | 116 (40.7) | 97 (50.3) | 19 (20.7) |
|
| |||
| Surgery plus cancer drug | 86 (30.2) | 56 (29.0) | 30 (32.6) |
|
| |||
| Radiation plus cancer drug | 14 (4.9) | 10 (5.2) | 4 (4.3) |
|
| |||
| Cancer drug only | 69 (24.2) | 30 (15.5) | 39 (42.4) |
|
| |||
| Mean Follow-up (years) (± SD) | 2.9 (2.4) | 3.3 (2.4) | 2.1 (2.1) |
Cardiotoxic agents: doxorubicin (Adriamycin), mitoxantrone (Novantrone), trastuzumab (Herceptin); no patients were exposed to epirubicin.
Percentages based on column total; except for ALL PATIENTS row.
American Joint Commission on Cancer
Human Epidermal growth factor Receptor 2.
Note that among the 126 patients with missing HER-2 neu status, 106 (84.1%) were diagnosed in or before 1998; 118 (93.7%) were diagnosed in or before 1999.
Estrogen Receptor/Progesterone Receptor
Treatment is defined as exposure to any cancer drug.
Radiation is defined as radiation therapy to the left breast or left chest wall.
Overall, 18.6% of patients (53/285) had pre-existing/concurrent cardiovascular comorbidity (Table 2). Among the 53 patients with pre-existing/concurrent cardiovascular comorbidity, 37.7% received cardiotoxic agents, while 74.6% of the 232 patients without cardiovascular comorbidity received cardiotoxic therapy (Table 2). Twenty-six percent (6/23) of patients with a history of a heart failure event (CHF, cardiomyopathy, decreased LVEF <50%, or myocarditis) received cardiotoxic chemotherapy. Among the 57 patients for whom LVEF was not evaluated prior to first breast cancer treatment and who had a cardiotoxic outcome, 56 did not have a history of cardiac disease, heart failure, or ischemia according to medical record review. Only 1 patient had a history of dysrhythmia.
Table 2.
Cardiovascular History, Overall and Stratified by Receipt of Cardiotoxic Agents
| VARIABLE | TOTAL | CARDIOTOXIC1 | NOT CARDIOTOXIC |
|---|---|---|---|
|
| |||
| N (%)2 | N (%)2 | N (%)2 | |
|
| |||
| ALL PATIENTS: N (%) | 285 (100) | 193 (67.7) | 92 (32.3) |
|
| |||
| Any Pre-existing/concurrent CV comorbidity3 | |||
| Yes | 53 (100) | 20 (37.7) | 33 (62.3) |
|
| |||
| No | 232 (100) | 173 (74.6) | 59 (25.4) |
|
| |||
| Decreased LVEF4 before first breast cancer treatment5 | |||
| Yes | 11 (100) | 6 (54.5) | 5 (45.5) |
|
| |||
| No | 158 (100) | 130 (82.3) | 28 (17.7) |
|
| |||
| Not evaluated6 | 116 (100) | 57 (49.1) | 59 (50.9) |
| History of Events before first breast cancer treatment5 | |||
| Overall Cardiac Event7 | 55 (100) | 22 (40.0) | 33 (60.0) |
|
| |||
| Heart Failure Event8 | 23 (100) | 6 (26.1) | 17 (73.9) |
|
| |||
| Dysrhythmia Event9 | 21 (100) | 7 (33.3) | 14 (66.7) |
|
| |||
| Ischemia Event10 | 32 (100) | 14 (43.8) | 18 (56.2) |
Cardiotoxic agents: doxorubicin (Adriamycin), mitoxantrone (Novantrone), trastuzumab (Herceptin); no patients were exposed to epirubicin.
Percentages based on row total.
Medical record documentation that any of the following conditions were present on or before the date of first breast cancer diagnosis: CHF, cardiomyopathy, decreased LVEF < 50%, myocarditis, cardiac arrest, heart block, arrhythmia, CAD, MI, angina or cardiac ischemia.
Decreased Left Ventricular Ejection Fraction is defined as ejection fraction <50%.
Exposure to drug category (i.e. cardiotoxic or not cardiotoxic). Patients exposed to both cardiotoxic and non-cardiotoxic drugs included in the cardiotoxic column of this table.
Among the 57 patients who received cardiotoxic chemotherapy and did not have LVEF measured before treatment initiation, 56 did not have a history of cardiac disease, heart failure, or ischemia according to medical record review. Only 1 patient had a history of dysrhythmia.
Overall Cardiac Event defined as history of Heart Failure, Dysrhythmia or Ischemia Events combined
Heart Failure Event defined as history of CHF, cardiomyopathy, decreased LVEF <50%, or myocarditis
Dysrhythmia Event defined as history of cardiac arrest, heart block, or arrhythmia.
Ischemia Event defined as history of CAD, MI, angina or cardiac ischemia.
Almost 30% of patients receiving cardiotoxic treatment did not have medical record documentation that LVEF was evaluated before treatment initiation (57/193). Among the 136 patients with LVEF evaluated before receipt of cardiotoxic therapy, 96% had normal LVEF (130/136); 63% had stage III disease, and 37% had stage IV breast cancer (data not shown in tabular format). While 11.4% of patients receiving cardiotoxic chemotherapy had a history of any cardiac event (22/193), almost 36% of patients exposed to non-cardiotoxic chemotherapy had a cardiac event history (33/92) (unadjusted RR=3.2, 95% CI 2.0–5.1). In terms of heart failure events, 3% of patients receiving cardiotoxic cancer treatment had a history of heart failure events (6/193), versus 18.5% of those exposed to non-cardiotoxic treatment (17/92) (unadjusted RR= 5.9, 95% CI 2.4–14.6).
The distribution of specific agents received by those exposed to systemic therapy is shown in Table 3. Most of the patients were exposed to cyclophosphamide (67.4%) or doxorubicin (63.5%).
Table 3.
Distribution of Specific Drug Exposure Among Patients Exposed to a Cancer Drug (N=285 Unique Patients)
| Agent* | Number of Patients Exposed To Specific Drug n (%) |
|---|---|
| TOTAL | 285 (100) |
| Aminoglutethimide | 1 (0.4) |
| Anastrozole (Arimidex) | 75 (26.3) |
| Capecitabine (Xeloda) | 69 (24.2) |
| Carboplatin (Paraplatin) | 34 (11.9) |
| Cisplatin (DDP) | 1 (0.4) |
| Cyclophosphamide (Cytoxan) | 192 (67.4) |
| Docetaxel (Taxotere) | 121 (42.5) |
| Doxorubicin (Adriamycin)** | 181 (63.5) |
| Etoposide (VePesid) | 7 (2.5) |
| Exemestane (Aromasin) | 20 (7.0) |
| Florouracil | 89 (31.2) |
| Fluoxymesterone (Halotestin) | 2 (0.7) |
| Fulvestrant (Faslodex) | 13 (4.6) |
| Gefitinib (Iressa) | 1 (0.4) |
| Gemcitabine (Gemzar) | 27 (9.5) |
| Goserelin (Zoladex) | 9 (3.2) |
| Irinotecan (Camptosar) | 5 (1.8) |
| Letrozole (Femara) | 30 (10.5) |
| Leuprolide acetate (Lupron) | 1 (0.4) |
| Megestrol acetate (Megace) | 43 (15.1) |
| Methotrexate | 59 (20.7) |
| Mitoxantrone (Novantrone) | 2 (0.7) |
| Paclitaxel (Taxol) | 58 (20.4) |
| Tamoxifen | 148 (51.9) |
| Temozolomide (Temodar) | 3 (1.1) |
| Thiopeta (Thioplex) | 12 (4.2) |
| Toremifene (Fareston) | 1 (0.4) |
| Trastuzumab (Herceptin) | 35 (12.3) |
| Vincristine | 3 (1.1) |
| Vinorelbine (Navelbine) | 43 (15.1) |
Drugs Classified as Cardiotoxic Listed In Boldface Type
No Patients were Exposed to Epirubicin
The rate of new cardiac events overall was 8.2 per 100 person-years (95% CI 6.0–11.1) in women exposed to cardiotoxic therapy and 4.4 per 100 person-years (95% CI 1.6–9.8) in those exposed to non-cardiotoxic treatment (Table 4). After adjusting for age, race, stage at advanced diagnosis, receipt of surgery or left-sided radiation, procedure type (lumpectomy vs. mastectomy), ER/PR status, year of diagnosis, and Charlson comorbidity index, the rate of new cardiac events was higher in patients exposed to cardiotoxic drugs compared with those exposed to non-cardiotoxic drugs (adjusted hazard ratio (HR)=2.5, 95% CI 0.9–7.2). In terms of specific event categories, the rate of heart failure events was 6.9 per 100 person-years (95% CI 5.0–9.4) in patients exposed to cardiotoxic therapy: a rate 3 times higher than that seen for patients exposed to non-cardiotoxic treatment (adjusted HR=3.0, 95% CI 1.0–9.5). When the subset of women who progressed to stage III or IV breast cancer from early stage disease were excluded from the analysis, we found no material difference in the rate of heart failure events (adjusted HR=3.5, 95% CI 1.0–13.0). The rate of dysrhythmia events was 2.1 per 100 person-years (95% CI 1.2–3.6) in those exposed to cardiotoxic drugs, with no large differences compared to those exposed to non-cardiotoxic chemotherapy (adjusted HR=2.0, 95% CI 0.5–7.6). The rate of ischemia events was 1.0 per 100 person-years (95% CI 0.4–2.1) in patients exposed to cardiotoxic treatment; this rate, although imprecise, was 20% lower than that for patients exposed to non-cardiotoxic agents (adjusted HR= 0.8, 95% CI 0.1–4.4).
Table 4.
Rate of Newly Diagnosed Cardiovascular Events, Crude and Adjusted Hazard Ratios (HR) Comparing Cardiotoxic and Non-cardiotoxic Drugs
| Event Category | Cardiotoxic drugs | Non-cardiotoxic drugs |
|---|---|---|
| First overall cardiac event1 | ||
| Patients in analysis (N) | 171 | 59 |
| Events (N) | 41 | 5 |
| Person-Years (N) | 497.9 | 112.8 |
| Rate (per 100 person-years) (95% CI) | 8.2 (6.0–11.1) | 4.4 (1.6–9.8) |
| Crude HR (95% CI) | 1.9 (0.7–4.8) | 1.0 |
| Adjusted2 HR (95% CI) | 2.5 (0.9–7.2) | 1.0 |
| First heart failure event3 | ||
| Patients in analysis (N) | 187 | 75 |
| Events (N) | 38 | 4 |
| Person-Years (N) | 550.9 | 147.6 |
| Rate (per 100 person-years) (95% CI) | 6.9 (5.0–9.4) | 2.7 (0.9–6.5) |
| Crude HR (95% CI) | 2.6 (0.9–7.3) | 1.0 |
| Adjusted2 HR (95% CI) | 3.0 (1.0–9.5) | 1.0 |
| First dysrhythmia event4 | ||
| Patients in analysis (N) | 186 | 78 |
| Events (N) | 13 | 4 |
| Person-Years (N) | 605.8 | 150.7 |
| Rate (per 100 person-years) (95% CI) | 2.1 (1.2–3.6) | 2.7 (0.8–6.4) |
| Crude HR (95% CI) | 0.8 (0.3–2.4) | 1.0 |
| Adjusted2 HR (95% CI) | 2.0 (0.5–7.6) | 1.0 |
| First ischemia event5 | ||
| Patients in analysis (N) | 179 | 74 |
| Events (N) | 6 | 3 |
| Person-Years (N) | 583.3 | 156.2 |
| Rate (per 100 person-years) (95% CI) | 1.0 (0.4–2.1) | 1.9 (0.5–5.2) |
| Crude HR (95% CI) | 0.5 (0.1–1.8) | 1.0 |
| Adjusted2 HR (95% CI) | 0.8 (0.1–4.4) | 1.0 |
Defined as first occurrence of heart failure, dysrhythmia, or ischemia events
Covariates included age group, race, stage at advanced diagnosis, treatment with surgery (yes/no), treatment with left-sided radiation (yes/no), ER/PR status, year of advanced diagnosis, Charlson comorbidity index.
Defined as first occurrence of CHF, cardiomyopathy, decreased LVEF <50%, or myocarditis.
Defined as first occurrence of cardiac arrest, heart block, or arrhythmia.
Defined as first occurrence of CAD, MI, angina or cardiac ischemia.
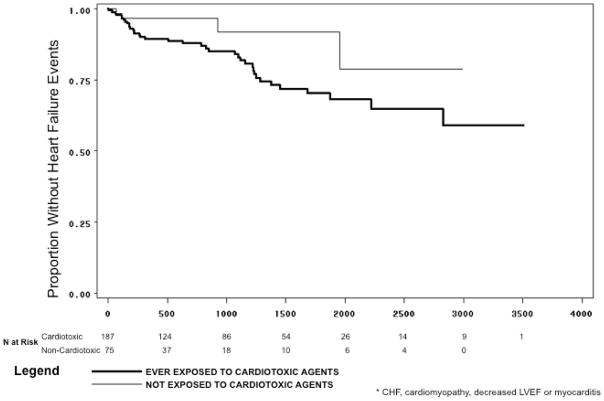
As demonstrated in the Kaplan-Meier curve (Figure 1), heart failure events, among women without a history of such events before treatment, occurred steadily over time; after 3 years of follow-up (1095 days), 16% of patients exposed to cardiotoxic drugs experienced an event, compared with 8% of those exposed to non-cardiotoxic therapy. After 5 years (1825 days), 30% of patients exposed to cardiotoxic therapy experienced a heart failure event, while the rate in those exposed to non-cardiotoxic therapy remained constant. After 8 years of follow-up (2920 days), 41% of patients exposed to cardiotoxic therapy had a heart failure event.
Figure 1.
Time from Exposure to Cardiotoxic and Non-Cardiotoxic Agents Until Newly Diagnosed Heart Failure Event*
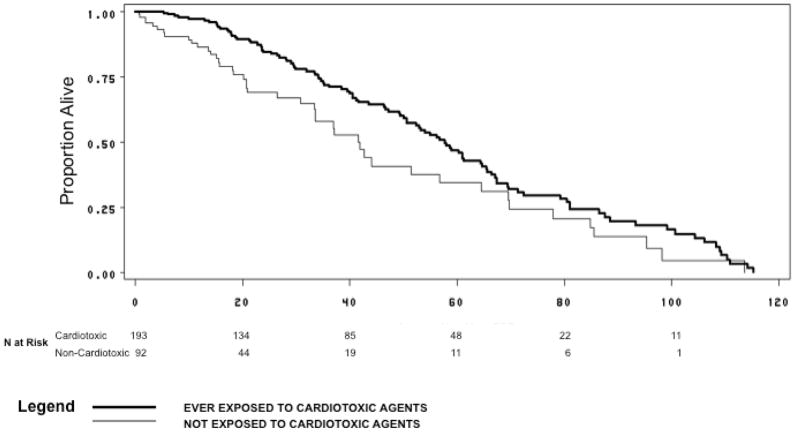
Figure 2 presents the Kaplan-Meier plot comparing rate of death in patients exposed to cardiotoxic and non-cardiotoxic agents. The median time to death from first exposure to cardiotoxic agents was 59 months (95% CI 51–66 months); the median time to death from first exposure to non-cardiotoxic agents was 42 months (95% CI 34–58 months). After adjusting for age category, race, stage at advanced diagnosis, surgery (yes/no), ER/PR status, left-sided radiation therapy (yes/no), Charlson comorbidity index and year of diagnosis of advanced stage breast cancer, the rate of death was lower in those exposed to cardiotoxic agents when compared with those exposed to non-cardiotoxic agents (HR=0.6, 95% CI 0.4–1.0). Patients exposed to cardiotoxic agents had improved survival when stratified by initial stage at diagnosis.
Figure 2.
Time from Exposure to Cardiotoxic and Non-Cardiotoxic Agents Until Death or End of Follow-up
DISCUSSION
During our eight year study period, we found that 18.6% of patients with advanced breast cancer had a history of cardiovascular comorbidity; these patients were about half as likely to have received cardiotoxic chemotherapy as patients without cardiovascular comorbidity (37.7% vs. 74.6%, respectively). Compared with patients exposed to non-cardiotoxic chemotherapy, the rate of newly diagnosed heart failure events (in patients without pre-existing cardiovascular comorbidity) was 3 times higher in patients exposed to cardiotoxic chemotherapy. Although estimates are imprecise, we found no large increases in dysrhythmia and ischemia event rates. Cardiovascular events occurred steadily over the study period, with 30% of patients exposed to cardiotoxic chemotherapy experiencing an event after 5 years and 41% expected to experience an event after 8 years.
It is difficult to directly compare our findings with the results of previous studies, due to substantial differences in study populations, design (randomized trials, observational studies), exposure classification, outcome definition, duration of follow-up, and, notably, breast cancer stage, since little is published in the metastatic setting. Doyle et al. used the Surveillance Epidemiology and End Results (SEER)-Medicare database to study patients diagnosed with stage I to III breast cancer; and they relied upon an administrative data coding algorithm to identify cardiovascular events, chemotherapy exposure and comorbidity.10 Compared with patients who did not receive chemotherapy, after 7 years follow-up, the authors found an increased risk of cardiomyopathy (HR=2.48) and, to a lesser degree, CHF (HR=1.38) and heart disease (HR=1.35) in patients exposed to doxorubicin, with a 7% cumulative incidence of cardiomyopathy at 5 years. A more recent study also used the SEER-Medicare database to study stage I-III breast cancer patients, 66 to 80 years old, without history of CHF.11 These authors found that, among patients 66–70 years old, 14% of those treated with adjuvant anthracyline developed CHF after 3 years of follow-up, 19% developed CHF after 5 years of follow-up, and 29% developed CHF after 8 years of follow-up. In another study, Ganz et al. examined late cardiac effects of adjuvant chemotherapy in breast cancer survivors treated during a Southwest Oncology Group (SWOG) trial by following patients for 13 years after treatment with cyclophosphamide, doxorubicin and fluorouracil (CAF) or cyclophosphamide, methotrexate and fluorouracil (CMF).16 The study included only those women with LVEF measurements and concluded that there was no difference in the proportion of women with decreased LVEF after long term follow-up (after 8 years: 5% of women in the CAF arm and 7% of women in the CMF arm; after 13 years of follow-up: 3% in the CAF arm and 0% in the CMF arm). However, this study is limited by the fact that only 9% of the patients enrolled in the original SWOG trial were included in the analysis, treatment administered after the trial ended was not available, and, by design, patients must have survived (i.e. those who died from CHF were not evaluated). The estimates from the two SEER studies and the SWOG study are lower than the results found in our study in which we found 16% of patients exposed to cardiotoxic therapy experienced a heart failure event at 3 years of follow-up and 41% after 8 years of follow-up.
One prospective observational study monitored cardiotoxicity in 120 patients diagnosed with recurrent metastatic breast cancer treated with anthracycline-based therapy.17 After a median of 3-years follow-up, Jensen et al. found that 59% of patients exposed to anthracycline experienced a 25% relative reduction in LVEF and 20% were diagnosed with CHF. Similar to our study, and unlike the SEER-Medicare studies, analyses included a wider age range (patients < 65 years), were limited to those with advanced disease, and relied upon clinical test results and medical records to determine outcome (including decreased LVEF).
The findings from this study are consistent with a study in the Netherlands that found that patients with history of cardiac events are substantially less likely to receive cardiotoxic chemotherapy.18 While this finding may not seem surprising, the magnitude of the differential receipt of cardiotoxic chemotherapy in those with history of cardiovascular events should be considered in observational studies examining the effect of cancer treatment on cardiac outcomes. The implications of these findings are that propensity to receive a cardiotoxic regimen is associated with cardiovascular history; therefore, efforts examining outcomes should strive to obtain accurate data on cardiovascular history. At a minimum, our results can be used to quantify the difference in cardiac history in sensitivity analyses. Results including mortality as an end-point are presented for completeness. Survival among women receiving cardiotoxic chemotherapy was substantially improved. However, confounding by indication was not evaluated because survival was not an a priori endpoint in this study.
This study has limitations. Because this study is not randomized, confounding by indication (i.e. patients receive selected treatments based on their inherent or perceived risk for outcome) is possible. However, if, as demonstrated, clinicians preferentially avoided cardiotoxic agents in patients at higher risk for cardiac disease, then our findings represent outcomes as experienced in actual clinical practice. Our outcomes were obtained by review of medical records and medical test results in a real-world setting, and therefore some clinically important endpoints may not have been captured, and evaluation of cardiac outcomes may have been differentially assessed by treatment. In fact, 67% of women exposed to cardiotoxic chemotherapy underwent LVEF measurements during the follow-up period, while 34% of those exposed to non-cardiotoxic chemotherapy underwent LVEF evaluation. While this differential assessment is expected because cardiotoxic chemotherapy is a known risk factor for heart failure events, it requires acknowledgement. In addition, the sample size in this study provided some estimates with limited precision and did not permit analysis of individual treatments or evaluation of patients with history of cardiac comorbidity, and the population was limited to patients with advanced disease. Due to small sample size, we were not able to assess the impact of early stage exposure to chemotherapy among women initially diagnosed at stage I or II but who later progressed to stage III or IV. An additional limitation is that this study did not evaluate dose, schedule or duration of exposure to cardiotoxic agents, and data on comorbidities such as hypertension and diabetes were not available. In this study, almost 30% (n=57) of patients who received cardiotoxic chemotherapy did not have LVEF measured before treatment initiation; it is unlikely that missing LVEF data occurred at random. According to medical record review, among the 57 patients, 56 did not have a history of cardiac disease including heart failure and ischemia. Only 1 patient had a history of dysrhythmia. Therefore, the absence of cardiac disease could potentially explain why these patients did not have an LVEF measurement prior to treatment initiation.
This is one of the first studies to use population-based sampling and detailed medical record review to quantify cardiac comorbid conditions and cardiovascular outcomes in patients with advanced breast cancer treated in clinical practice. We conducted this study in an ethnically diverse health system including a wide range of age groups, with substantial representation of women 70 years and older. In terms of external validity of our findings, other studies conducted in the HFHS population have found results similar to the US population and to other US healthcare systems.19,20 Despite the inherent limitations, observational studies present real-world treatment and outcome data. Although clinical trials provide important information comparing treatment safety and efficacy, trials generally provide limited (short-term) follow-up and highly select patient populations. As a result, translation of trial results into treatment of the actual patient populations for which treatment is intended may not be appropriate. For example, although breast cancer disproportionately affects older women,21 older persons are not adequately represented in cancer or CHF clinical trials.22, 23, 24, 25 Further, some authors have argued that generalizing results from clinical trials with select patient populations may actually cause harm in the heterogeneous populations treated in clinical practice.26,27
CONCLUSION
Our results indicate that, in clinical practice, the use of cardiotoxic agents is common. Receipt of these drugs is related to patient and tumor characteristics and is associated with a substantial risk of cardiotoxicity that persists over time. However, compared with patients exposed to non-cardiotoxic agents, the rate of death was lower in those exposed to cardiotoxic agents. Research that identifies individuals at risk for cardiac toxicity and interventions to minimize this risk would be beneficial to this patient population.
Key points.
Use of cardiotoxic agents in treating advanced breast cancer is common
Treatment is related to patient and tumor characteristics and is associated with a substantial risk of cardiotoxicity that persists during the patient’s remaining lifespan.
The choice to use cardiotoxic agents or not is related to whether the patient has cardiac comorbidity.
Acknowledgments
Financial Support and Disclosure: Dr. Gross’s efforts were supported by a Beeson Career Development Award (1 K08 AG24842). Dr. Hurria’s efforts are supported by a Paul Beeson Career Development Award in Aging Research (K23 AG026749-01), and American Society of Clinical Oncology--Association of Specialty Professors--Junior Development Award in Geriatric Oncology.
This study was funded by GlaxoSmithKline Research & Development Limited.
We are grateful to Robert Lewis, MD, Ulka Campbell, PhD, Rachel Lappin, BS, Syd Phillips, MPH, and Jeanenne J Nelson, PhD for critical input in developing this manuscript.
Footnotes
Supplemental publications: Preliminary results from this study were presented at the 22nd International Conference on Pharmacoepidemiology and Therapeutic Risk Management, Lisbon, Portugal, August 2006.
Conflict of Interest statements: Dr. Beiderbeck was an employee of GlaxoSmithKline at the time of study conduct and draft manuscript preparation; her contributions to the manuscript include: conception and design, financial support, manuscript writing, and final review of the manuscript. Final decisions and authority regarding manuscript content and submission contractually remained with Drs. Ulcickas Yood, Oliveria, Gross, and Hurria.
References
- 1. [accessed April 15, 2006];Breast Cancer Facts and Figures. 2003 http://www.cancer.org.
- 2. [accessed April 15, 2006];National Health Interview Survey. 2002 http://www.cdc.gov.
- 3.Fischer D, Durivage H, Knobf M, et al. The Cancer Chemotherapy Handbook. 6. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 4.Shan K, Lincoff A, Young J. Anthracycline-Induced Cardiotoxicity. Ann Intern Med. 1996;125(1):47–58. doi: 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff D, Layard M, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 6.Von Hoff D, Rozencweig M, Piccart M. The cardiotoxicity of anticancer agents. Semin Oncol. 1982;9:23–33. [PubMed] [Google Scholar]
- 7.Allen A. The cardiotoxicity of chemotherapeutic drugs. Semin Oncol. 1992;19:529–542. [PubMed] [Google Scholar]
- 8.Swain S, Whaley F, Ewer M. Congestive heart failure in patients treated with doxorubicin. A retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 9.Wiseman L, Spencer C. Dexrazoxane: A review of its use as a cardioprotective agent in patients receiving anthracycline-based chemotherapy. Drugs. 1998;56(3):385–403. doi: 10.2165/00003495-199856030-00009. [DOI] [PubMed] [Google Scholar]
- 10.Doyle J, Neugut A, Jacobson J, et al. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol. 2005;23:8597–8605. doi: 10.1200/JCO.2005.02.5841. [DOI] [PubMed] [Google Scholar]
- 11.Pinder M, Duan Z, Goodwin J, et al. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25:3808–3815. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 12.Suter T, Cook-Bruns N, Barton C. Cardiotoxicity associated with trastuzumab (Herceptin) therapy in the treatment of metastatic breast cancer. Breast. 2004;13:173–183. doi: 10.1016/j.breast.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Healey Bird B, Swain S. Cardiac toxicity in breast cancer survivors: Review of potential cardiac problems. Clin Cancer Res. 2008;14:14–24. doi: 10.1158/1078-0432.CCR-07-1033. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. NCCN Task Force Report: Breast Cancer in Older Women; Hollywood, FL. May 2008; [accessed on August 11, 2008]. http://www.nccn.org/interactive/podcasts/mp3/annual_conference_2008/Intro_Older%20Woman_Podcast.asp. [Google Scholar]
- 15.Skeel R. Handbook of Cancer Chemotherapy. 6. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 16.Ganz P, Hussey M, Moinpour C, et al. Late Cardiac Effects of Adjuvant Chemotherapy in Breast Cancer Survivors Treated on Southwest Oncology Group Protocol S8897. J Clin Oncol. 2008;26:1223–1230. doi: 10.1200/JCO.2007.11.8877. [DOI] [PubMed] [Google Scholar]
- 17.Jensen B, Skovsgaard T, Nielsen S. Functional Monitoring of anthracycline cardiotoxcity: A prospective, blinded, long-term observational study of outcome in 120 patients. Ann Onco. 2002;13:699–709. doi: 10.1093/annonc/mdf132. [DOI] [PubMed] [Google Scholar]
- 18.Sukel M, Breekveldt-Postma N, Erkens J, et al. Incidence of cardiovascular events in breast cancer patients receiving chemotherapy in clinical practice. Pharmacoepidemiol Drug Saf. 2008;17(2):125–134. doi: 10.1002/pds.1528. [DOI] [PubMed] [Google Scholar]
- 19.Ulcickas Yood M, Oliveria S, Boyer JG, et al. Colon polyp recurrence in a managed care population. Arch Intern Med. 2003;163:422–426. doi: 10.1001/archinte.163.4.422. [DOI] [PubMed] [Google Scholar]
- 20.Ulcickas Yood M, Watkins E, Wells K, et al. The impact of NSAID or COX-2 use on the initiation of antihypertensive therapy. Pharmacoepidemiol Drug Saf. 2006;15:852–860. doi: 10.1002/pds.1327. [DOI] [PubMed] [Google Scholar]
- 21.Ries L, Harkins D, Krapcho M, et al. [accessed April 15, 2006];SEER Cancer Statistics Review, 1975–2003. 2006 http://seer.cancer.gov/csr/1975_2003/
- 22.Murthy V, Krumholz H, Gross C. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 23.Gross C, Wong N, Dubin J, et al. Enrollment of older persons in cancer trials after the Medicare reimbursement policy change. Arch Intern Med. 2005;156(13):1514–1520. doi: 10.1001/archinte.165.13.1514. [DOI] [PubMed] [Google Scholar]
- 24.Masoudi F, Havranek E, Wolfe P, et al. Most hospitalized older persons do not meet the enrollment criteria for clinical trials in heart failure. Am Heart J. 2003;146:250–257. doi: 10.1016/S0002-8703(03)00189-3. [DOI] [PubMed] [Google Scholar]
- 25.Silliman R. What constitutes optimal care for older women with breast cancer? J Clin Oncol. 2003;21(19):3554–3556. doi: 10.1200/JCO.2003.05.083. [DOI] [PubMed] [Google Scholar]
- 26.Gross C, Steiner C, Bass E, et al. Relation between prepublication release of clinical trial results and the practice of carotid endarterectomy. JAMA. 2000;284:2886–2893. doi: 10.1001/jama.284.22.2886. [DOI] [PubMed] [Google Scholar]
- 27.Gross C, Garg P, Krumholz H. The generalizability of observational data to elderly patients was dependent on the research question in a systematic review. J Clin Epidemiol. 2005;58:130–137. doi: 10.1016/j.jclinepi.2004.10.001. [DOI] [PubMed] [Google Scholar]