Abstract
Targeted mutagenesis of the retinoic acid receptor α (RARα) gene has revealed its essential role in spermatogenesis. Although cells in all stages of spermatogenesis were detected in RARα−/− testes, there was an increase in degenerating pachytene spermatocytes and a temporary developmental arrest in step 8–9 spermatids in the first wave of spermatogenesis, a delay in the onset of the second wave, and a temporary arrest in preleptotene to leptotene spermatocytes in the first, second, and third waves. A striking aspect of the mutant phenotype was the failure of spermatids to align at the tubular lumen at stage VIII. Furthermore, there were missing or decreased numbers of the predicted cell types in tubules, and they exhibited a profound asynchrony of mixed spermatogenic cell types. In vivo bromodeoxyuridine labeling revealed a significant decrease in germ cell proliferation in both juvenile and adult RARα−/− testes and confirmed the arrest at step 8–9 spermatids. Retinoid signaling through RARα, thus, appears to be critical for establishment of synchronous progression of spermatogenesis and the subsequent establishment of correct cellular associations.
Keywords: RARα, spermatogenic cycle, retinoid signaling, spermatogonia, spermatocytes, spermatids
Introduction
Spermatogenesis is a highly regulated process of differentiation that takes place in the seminiferous tubules, where complex morphologic alterations lead to the formation of sperm. In adult male mammals, spermatogenesis can be subdivided into three main phases: spermatogonial proliferation, meiosis of spermatocytes, and spermiogenesis of haploid spermatids. Within the seminiferous tubules of rodent testes, differentiating spermatogenic cells form defined associations called stages of the cycle of the seminiferous epithelium (stages I to XII in mouse) that can be identified by morphologic criteria (Oakberg, 1956; Russell et al., 1990). During spermatogonial proliferation, undifferentiated type A spermatogonia (subdivided into Aisolated [Aiso], Apaired [Apr], and Aaligned [Aal] spermatogonia according to their topographical arrangement on the basement membrane) divide mitotically. In response to unknown signals, they then form A1 spermatogonia, which are the first generation of differentiating spermatogonia (Russell et al., 1990; de Rooij, 1998, 2001). These differentiated diploid A1 spermatogonia synchronously go through a series of six divisions, forming sequentially A2, A3, A4, intermediate, and type B spermatogonia. After the last mitosis of type B spermatogonia, preleptotene spermatocytes are formed in tubules at stage VII–VIII. They then initiate meiosis and give rise to leptotene and zygotene spermatocytes at stage IX–XII. These cells differentiate into pachytene and diplotene spermatocytes at stage I–X and XI, respectively, followed by two meiotic divisions at stage XII and formation of haploid step 1 spermatids at stage I. Thereafter, spermatids undergo spermiogenesis, during which the nucleus of germ cell is remodeled and compacted into the form that is found in mature spermatozoa. Haploid spermatids are morphologically classified into 16 steps in the mouse (Russell et al., 1990). Spermatogenesis culminates in spermiation, when mature spermatozoa are released from Sertoli cells into the lumen of the seminiferous epithelium at stage VIII–IX.
The length of the cell cycle and pattern of cell associations, as well as the time necessary to produce spermatozoa, all vary among species (Russell et al., 1990). For example, in the rat, recruitment of committed cells from stem cells occurs every 12.9 days (Clermont and Harvey, 1965), whereas the spermatogenic cycle length is 8.6 days in the mouse (Clermont and Trott, 1969). The onset of differentiation of stem cells that initiates the spermatogenic process is cyclical, and it is followed by an orderly stepwise differentiation of progeny cells into mature spermatozoa. The cell cycle of male germ cells is fixed and cell-autonomous. That is, genetic control of the timing appears to be intrinsic to the germ cell, as evidenced by the rat-specific timing observed in rat germ cells transplanted into mouse testes (Franca et al., 1998). It was not intuitively obvious that this would be the case, given the intimate associations of germ cells with Sertoli cells. Indeed, studies on cell junction dynamics in the testis suggested extensive interactions between Sertoli and germ cells (Byers et al., 1993; Cheng and Mruk, 2002). Intermittent disassembly and reassembly of Sertoli cell adherens junctions and tight junctions and Sertoli–germ cell adherens junctions occur so as to facilitate the movement of germ cells across the epithelium.
During each cycle of the seminiferous epithelium, the proliferative activity of Aiso, Apr, and Aal spermatogonia produces a new cohort of Aal spermatogonia. This cohort of Aal spermatogonia is largely quiescent from stage III onward. At some time during stage III–VIII, probably during stages VII–VIII, virtually all Aal spermatogonia that were formed during the preceding period of proliferative activity differentiate into A1 spermatogonia (de Rooij, 1998, 2001; de Rooij and Grootegoed, 1998; Schrans-Stassen et al., 1999). The A1 spermatogonia all enter S phase in stage VIII and divide into A2 spermatogonia in stage IX. The differentiation of Aal into A1 spermatogonia marks the transition from the randomly cycling population of undifferentiated spermatogonia to the rigidly controlled series of six synchronous divisions of differentiating spermatogonia (de Rooij, 1998, 2001). This step has been shown to be rather vulnerable, because it can be blocked in several different situations, including vitamin A deficiency (VAD; de Rooij, 1998, 2001). Upon administration of vitamin A or reti-noic acid (RA), there is a massive differentiation of Aal into A1 spermatogonia, even after very long periods of arrest. It is not yet known whether the action of RA in inducing differentiation of Aal into A1 is direct, or indirect by means of Sertoli cells. Both spermatogonia and Sertoli cells possess nuclear receptors for retinoids (Akmal et al., 1997; Gaemers et al., 1998; Cupp et al., 1999).
It had been reported previously that testes of RARα-deficient mice at 4–5 months of age showed severe degeneration of the germinal epithelium, with some tubules containing few or no germ cells, while adjacent tubules in the same testis contained almost all the characteristic cell types, from spermatogonia to fully elongated spermatids (Lufkin et al., 1993). However, the epididymis of mutant animals contained only a few abnormal spermatozoa and the animals were sterile (Lufkin et al., 1993; Chung, Wang, and Wolgemuth, manuscript submitted for publication). Furthermore, no information was provided as to when the abnormalities in spermatogenesis were first observed, further complicating our understanding the primary site of action of RARα in the testis. That is, it is possible that RARα is required at only a few steps of differentiation, perhaps even in only the germ cell or somatic cell lineages, but that the severe degeneration results from the disruption of the overall process. For example, mutation of the gene encoding cyclin A1 results in a specific arrest just before the first meiotic division in spermatocytes (Liu et al., 1998). However, with time, the seminiferous tubules degenerate until Sertoli cell-only tubules are observed (Liu et al., 1998; Salazar et al., 2003), very much assembling those reported by Lufkin et al. (1993). In this case, the loss of other germ cell types, thus, is not a primary result of the loss of the cell cycle protein, but rather occurs as the key cell–cell interactions between various stages of germ cells and the supporting Sertoli cells are disrupted.
We therefore wished to determine when during spermatogenic differentiation in RARα−/− mice abnormalities in testicular cells would first be observed. To accomplish this, we took advantage of the relative synchrony in spermatogenesis that occurs during the initial waves of differentiation during postnatal development (Oakberg, 1956). We systematically examined the morphology and association of cell types within tubules at specific postnatal developmental stages in mutant and control mice. We also assayed for the kinetics of progression through spermatogenesis and examined cellular proliferation. This analysis revealed striking changes in testicular cell associations that appear to be primary as opposed to secondary events. The observations also provided important insight as to the particular cell types most obviously affected as the direct result of loss of normal RARα-mediated signaling.
Results
Chronology of Appearance of Abnormalities of Spermatogenic Differentiation in RARα−/− Mouse Testes
Lufkin and colleagues (1993) had reported the presence of a proportion of severely degenerated tubules containing essentially only Sertoli cells and occasional germ cells in testes from RARα-deficient mice at 4–5 months of age. We confirmed this observation by examining histological sections from mice at 16 weeks of age and, furthermore, observed the presence of such tubules at 12 weeks of age as well (data not shown). To distinguish primary vs. secondary effects on germ cell degeneration in the mutant testes, we undertook a systematic investigation of the progression of the first wave of spermatogenic differentiation, which occurs during early postnatal development. The goal of this approach was twofold. First, we wished to identify the first cell types in the spermatogenic pathway that exhibited abnormalities. Second, we wished to discriminate between primary (due to lack of RARα) versus secondary (due to progressive breakdown of cellular associations, etc.) causes of the abnormalities.
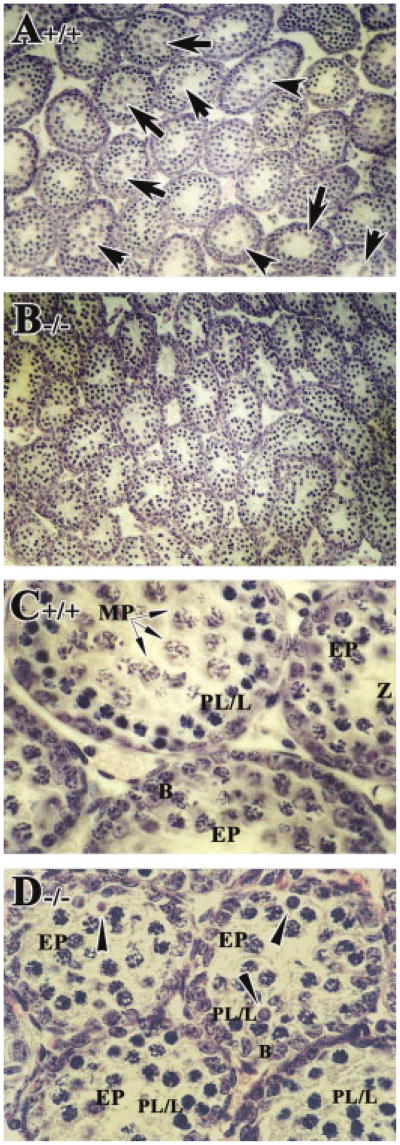
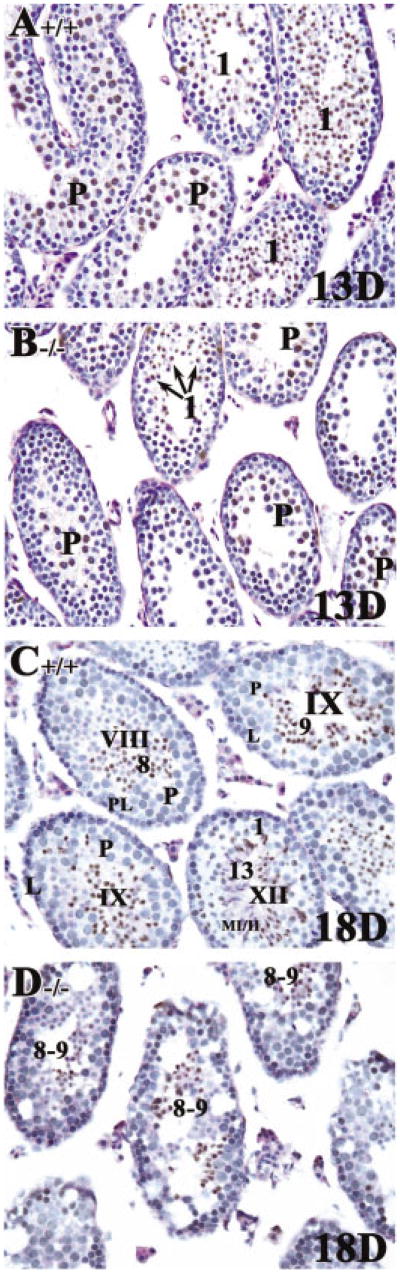
In control mice at 2 weeks of age, cells in the first wave of spermatogenesis had progressed to mid-prophase of meiosis I and mid-pachytene spermatocytes (arrows in Fig. 1A,C), as expected (Bellve et al., 1977; Sung et al., 1986; Russell et al., 1990). Spermatogonia and preleptotene spermatocytes in the second wave of spermatogenesis were also present. In RARα−/− testes, there appeared to be fewer tubules with early or mid-pachytene spermatocytes (∼13%, Fig. 3B) compared with controls (∼48%, Fig. 3B), and many of these cells appeared to be degenerating (arrowheads in Fig. 1D). Concomitantly, there appeared to be an increase in tubules containing preleptotene, leptotene, and zygotene spermatocytes accumulated from both the first and second wave of spermatogenesis (∼87%, Fig. 3B; Fig. 1B,D). In fact, some of the tubules that should have entered the second wave of spermatogenesis had only spermatogonia, suggesting a delay in the onset of the second wave (Fig. 1B,D).
Fig. 1.
Increased numbers of degenerating pachytene spermatocytes from the first wave of spermatogenesis and delay in the development of the second wave. A–D: Histological sections of 2-week-old testis from RARα+/+ (A,C) and RARα−/− mice (B,D). Arrows in A and C point to mid-pachytene spermatocytes. Arrowheads in D point to degenerating early pachytene spermatocytes. A,B: Original magnification, ×20. C,D: Original magnification, ×40. EP, early pachytene spermatocytes; PL/L, preleptotene or leptotene spermatocytes; B, type B spermatogonia; Z, zygotene spermatocytes; MP, mid-pachytene spermatocytes.
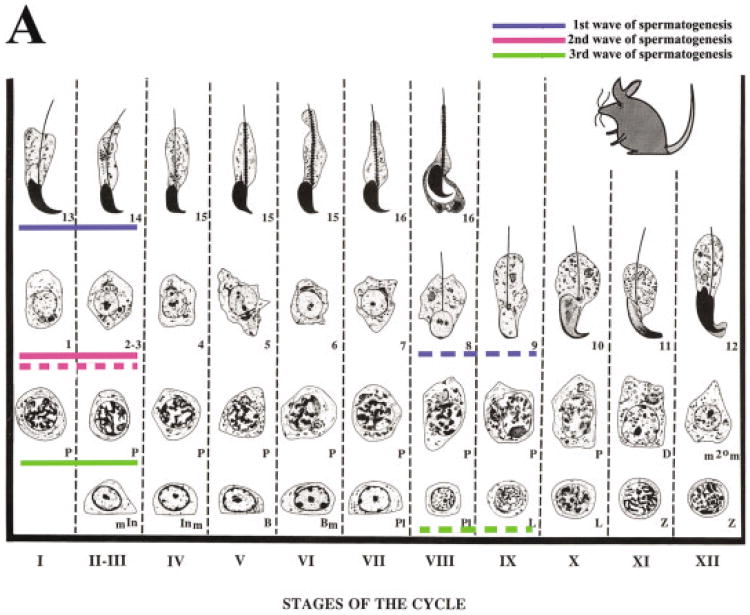
Fig. 3.
A: Diagrammatic representation of the spermatogenic cycle illustrating a profound asynchrony of spermatogenic progression in RARα−/− testes. Details of the symbols used in the staging map shown can be found in Russell etal. (1990). The solid blue line indicates the most advanced cell types and cellular associations of first wave of spermatogenesis in 4-week-old RARα+/+ (step 13–14 spermatids). In RARα−/− testes, step 8–9 spermatids are observed (dashed blue line). The solid pink line indicates the most advanced cell types of the second wave of spermatogenesis (step 1-3 spermatids) in 4-week-old RARα+/+. The dashed pink line indicates the most advanced cell types of the second wave (step 1–3 spermatids) in RARα−/−. The solid green line indicates the most advanced cell types from the third wave of spermatogenesis in 4-week-old RARα+/+ (early pachytene spermatocytes), and the dashed green line indicates the most advanced cell types from the third wave in the RARα−/− (preleptotene and leptotene spermatocytes). B: Spermatogenic cell distribution during early postnatal testicular development in RARα+/+ and RARα−/− testes. Bar graph indicating the number of various spermatogenic cells at 2 and 4 weeks of age after birth in RARα+/+ and RARα−/− mice (100 tubules scored per testis: three mice for each time point). The total number of various spermatogenic cells (preleptotene and leptotene spermatocytes [PL/L]; early and mid-pachytene spermatocytes [E-& M-P]; step 1 or 2 spermatids [Step 1 or 2]; Step 8–9 spermatids [Step 8–9]) per 100 seminiferous tubules were counted. Error bars represent the mean ± SD of the counts.
At 4 weeks of age, tubules from control testes showed an organized progression of cell types from spermatogonia at the periphery to step 13–14 spermatids at the innermost layers (Fig. 2A,C; arabic numerals in C). Different steps of round spermatids were radily observed in the seminiferous tubules, from step 1 to 13–14. In striking contrast, tubules from 4-week-old RARα−/− testes lacked this clear organization. One of the most consistent observations was the presence of an excess of tubules containing step 8–9 spermatids (Fig. 2B,D,E). This distinctive feature of the spermatids was readily seen with periodic acid-Schiff (PAS) staining (note the smaller cells with the clearly visible acrosome caps; arrows in Fig. 2B,D–F). Quantification of this phenotype is presented in Table 1. We found that approximately 91% (Fig. 3B) of the tubules contained step 8–9 spermatids in 4-week-old RARα−/− testes, when compared with controls (∼52%, Fig. 3B). Another feature was an increase in the proportion of tubules (∼72%, Fig. 3B) with preleptotene to leptotene spermatocytes (Fig. 2D,E).
Fig. 2.
Presence of an excess of tubules containing step 8–9 spermatids in 4-week-old RARα−/− testes. A–H: Histological sections of 4-week-old testes from RARα+/+ (A,C,G) and RARα−/−mice (B,D–F,H). Arrows in B point to vacuolated regions. Arrows in D–F point to step 8–9 spermatids. G,H: γ-H2AX was used to confirm the presence of preleptotene/leptotene spermatocytes in both RARα+/+ (G) and RARα−/− testes (H). A,B: Original magnification, ×20. C–H: Original magnification, ×40. P, pachytene spermatocytes; PL/L, preleptotene or leptotene spermatocytes; B, type B spermatogonia; Z, zygotene spermatocytes; DSG, degenerating spermatogonia; ES, elongated spermatids. Arabic numerals indicate the step of spermatid differentiation. Roman numerals in C and G indicate the stage of the seminiferous tubules.
Table 1. Percentages of Tubules Containing Step 8–9 Spermatids in 4-Week-Old RARα−/− Mouse Testesa.
| Animal number | RARα+/+ | Animal number | RARα−/− |
|---|---|---|---|
| 1 | 174/325 = 53.54% | 1 | 63/75 = 84.00% |
| 2 | 181/360 = 50.28% | 2 | 72/75 = 96.00% |
| 3 | 158/309 = 51.13% | 3 | 141/150 = 94.00% |
| Mean ± SD | 513/994 = 51.7 ± 1.7% | 4 | 126/150 = 84.00% |
| 5 | 136/150 = 90.67% | ||
| 6 | 144/150 = 96.00% | ||
| Mean ± SD | 682/750 = 90.9 ± 5.6% |
Significantly different from wild-type testis: P < 0.0001 (n ≥ 3). RARα, retinoic acid receptor α.
The cell types arrested were also confirmed using specific biochemical markers. For example, γ-H2AX, the phosphorylated form of H2AX at serine 139, was used as a marker for preleptotene/leptotene spermatocytes in the RARα−/− testes (Mahadevaiah et al., 2001). By immunostaining, γ-H2AX first appears in preleptotene spermatocytes at stage VII–VIII as expected (Mahadevaiah et al., 2001; Fig. 2G). At stage IX, extensive γ-H2AX staining was found in leptotene spermatocytes (Fig. 2G). During zygotene, the staining of γ-H2AX declined and is restricted to the sex body throughout pachytene and disappeared during the diplotene-metaphase I transition (data not shown; Mahadevaiah et al., 2001). In the RARα−/− testes, there were clearly more γ-H2AX–expressing preleptotene or leptotene spermatocytes, consistent with our morphological assessment of the cell type (see Fig. 2H). Staining for the expression of cyclin A1 was used to mark late pachytene to diplotene spermatocytes (Ravnik and Wolgemuth, 1999), which were not obviously different in control and RARα−/− testes (data not shown).
The net result of these abnormalities was a profound asynchrony of spermatogenic progression. This is illustrated in the photomicrographs of the characteristic mutant tubules shown in Figure 2D–F. For example, in Figure 2F, only a few tubules of 4-week-old RARα−/− testes contained the expected step 13–14 spermatids. Instead, the differentiation of most of round spermatids in the first wave of spermatogenesis was arrested or delayed at step 8–9 (Fig. 2F and highlighted as the dashed blue line in Fig. 3A). Once initiated, progression of some spermatogenic cells through meiotic prophase of spermatocytes from the second wave proceeded relatively normally, with step 1–2 round spermatids being readily observed (Fig. 2F and highlighted as the dashed pink line in Fig. 3A). However, tubules containing step 1–2 round spermatids composed only ∼7% (Fig. 3B) of the tubules in RARα−/− testes, whereas ∼23% (Fig. 3B) were seen in the control testes. Although several tubules contained a few early pachytene spermatocytes from the third wave of spermatogenesis, most had preleptotene–leptotene spermatocytes from the third wave that were arrested or delayed (Fig. 2F and highlighted as the dashed green line in Fig. 3). Degenerating spermatogonia in the third wave were also observed (Fig. 2F).
A further striking observation was the presence of two to three different steps of spermatids within the same plane of a section within a single tubule (Fig. 2F). Step 8–9 spermatids were frequently observed together with spermatocytes from the next wave in diverse stages of spermatogenic maturation states (Fig. 2D,E). In some tubules, profoundly asynchronized spermiogenesis was seen, with a few of these spermatids having passed through step 8–9 and forming step 13–14 elongate spermatids (Fig. 2F). Other frequently seen morphological features included large vacuolated regions within most of the tubules (Fig. 2B,D,E).
By 6 weeks of postnatal development, control testes contained 12 stages of spermatogenesis with their proper cellular associations (Fig. 4A,C). For example, in stage IX, leptotene spermatocytes, late pachytene spermatocytes and step 9 spermatids were found (Fig. 4C), whereas step 2–3 spermatids and step 14 spermatids were found in stage II–III, together with early pachytene spermatoctyes (Fig. 4C). In RARα−/− testes, most tubules contained step 1–3 round spermatids and some elongated spermatids (Fig. 4B,D). Spermiogenesis, arrested or delayed at step 8–9 in the first wave of spermatogenesis, seemed to have been restarted synchronously, and the cells entered into the elongating process. The following wave of spermatogenesis was also being processed synchronously into step 1–3 spermatids. Vacuoles were also readily observed in the mutant testes (Fig. 4B).
Fig. 4.
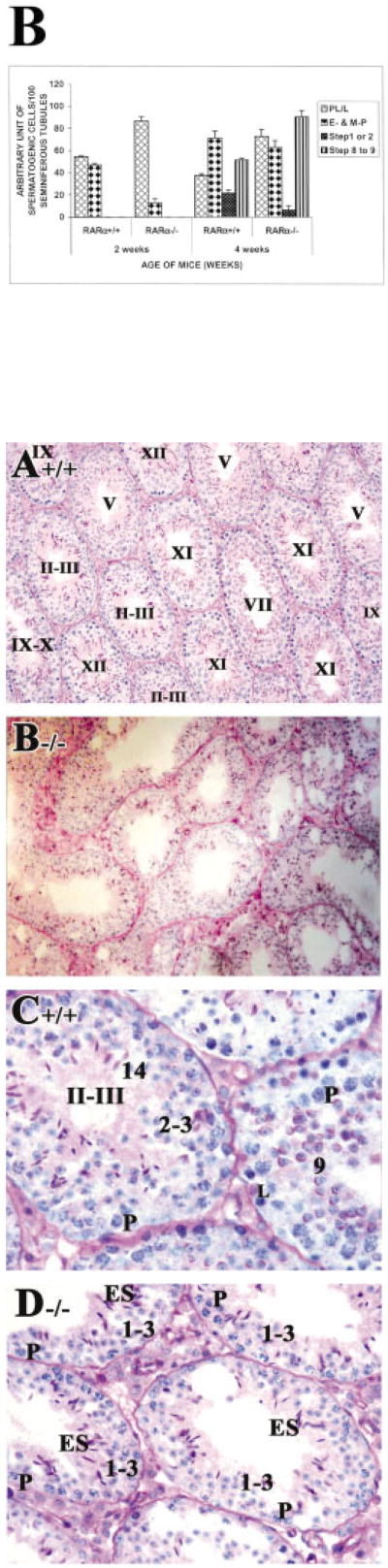
Spermiogenesis restarted synchronously and elongated spermatids observed in most tubules of 6-week-old RARα−/− testes. A–D: Histological sections of 6-week-old testis from RARα+/+ (A,C) and RARα−/− mice (B,D). Most tubules in RARα−/− contained step 1–3 round spermatids and some elongated spermatids (B,D). A,B: Original magnification, ×20. C–F: Original magnification, ×40. P, pachytene spermatocytes; L, leptotene spermatocytes; ES, elongated spermatids. Arabic numerals indicate the step of spermatid differentiation. Roman numerals indicate the stage of the seminiferous tubules.
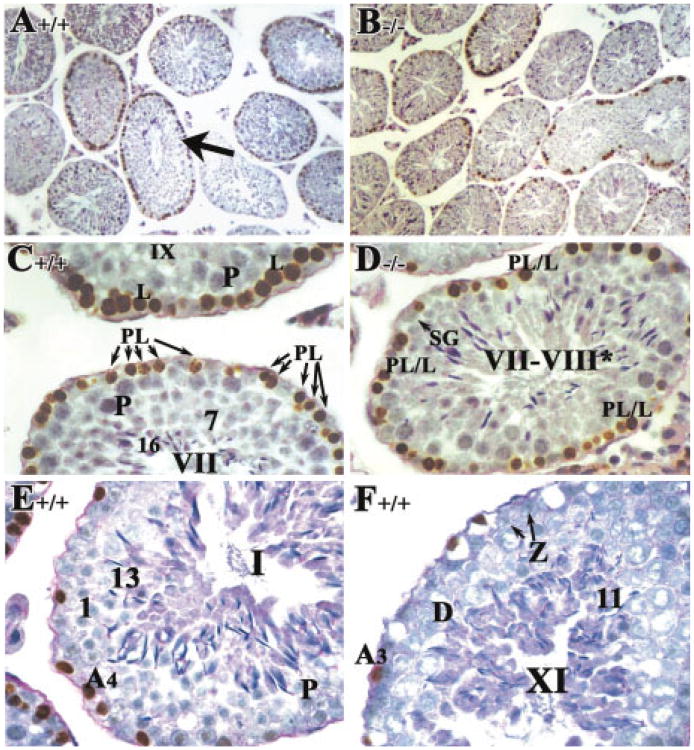
An obvious feature in control young adult testes (8 weeks of age) is stage VIII tubules with mature step 16 spermatids aligned along the tubular lumen of the seminiferous epithelium before spermiation (labeled with an asterisk in Fig. 5A; higher magnification in Fig. 5C). However, in RARα−/− testes at the same age, such characteristic stage VIII tubules were never seen (Fig. 5B,D). Rather, tubules with mixed spermatogenic cell types, reflecting an asynchronous cell association, were observed (Fig. 5B,D–F). A striking observation was the presence of spermatids exemplifying almost the entire range of spermiogenic differentiation, from step 1 to condensed elongated spermatids, within the same plane of a section within a single tubule (Fig. 5F). Also, these spermatids were frequently observed together with spermatocytes with diverse maturation states, from early pachytene spermatocytes to diplotene spermatocytes (Fig. 5F). It was difficult, thus, to “stage” the RARα−/− testes according the criteria developed by Oakberg (1956) and refined by Russell etal. (1990). Nonetheless, we attempted to assess the developmental progression of the cells within the tubules using the acrosomal system (Russell et al., 1990), and we refer to these approximately staged tubules with a roman numeral followed by an asterisk (e.g., stage IX*). In some severely defective tubules, an entire layer of given cell types was missing (Fig. 5E). For example, at stage XII-I*, MI/MII spermatocytes with chromosomes aligned at the metaphase plate, secondary spermatocytes (caret symbols in Fig. 5E) and step 1 round spermatids were readily seen (arrows in Fig. 5E). However, zygotene-early pachytene spermatocytes and spermatogonia were missing (bracket in Fig. 5E). Of interest, although some of the cell types were reduced in number, later stages of spermatogenic differentiation were observed. Finally, it was also observed that approximately 17% of tubules in RARα−/− testes contained prominent vacuolar-like spaces, although the lumens of the tubules were found to be relatively similar in size in RARα−/− and control testes (Fig. 5B, A, respectively).
Fig. 5.

Morphological abnormalities in RARα−/− young adult testes. A–F: Histological sections of 8-week-old (young adult) testes from RARα+/+ (A,C) and RARα−/− mice (B,D–F). Failure of spermatid alignment and release at stage VIII (B,D), tubules lacking entire populations of cells (B, bracket in E), and profoundly asynchronous tubules (B,F) were observed in the young adult RARα−/− testis but not in the RARα+/+ testis (A,C). A,B: ×20. C–F: ×60. Although the asynchronous cell associations complicate staging, an attempt was made to stage the RARα−/− tubules using the acrosomal system. Arabic numerals indicate the step of spermatid differentiation. Roman numerals indicate the stage of the seminiferous tubules, staged as described by Russell et al. (1990). P, pachytene spermatocytes; PL/L, preleptotene or leptotene spermatocytes; D, diplotene spermatocytes; B, type B spermatogonia; In, intermediate spermatogonia; ES, elongated spermatid; MI, meiosis I.
Assessment of Kinetics of Progression Through Spermatogenesis in RARα−/− Mice
The asynchrony of association of particular cell types could result in part from abnormalities in the timing of progression of spermatogenic cells through the cell cycle and stages of differentiation. The timing of the interval between the round of DNA synthesis that occurs before entering meiotic prophase and the appearance of elongated spermatids, therefore, was assessed by administering a single pulse of bromode-oxyuridine (BrdU) and examining the appearance of BrdU-labeled spermatogenic cells at specific subsequent time points. Because of the predictable temporal progression of spermatogenesis, we can detect delays or acceleration of the progression by identifying the most advanced cell type labeled with BrdU.
Initial experiments involved BrdU labeling of testicular cells in 7- to 8-week-old testes, which even in the mutant contain the full range of spermatogenic cell types. In control testes, 24 hr after BrdU injection, A3 and A4 spermatogonia were labeled (Fig. 6F, E, respectively) as were preleptotene spermatocytes in the tubules at stage VII–IX (Fig. 6C). In RARα−/− testes (Fig. 6B), the number of labeled cells per tubule was much lower than that seen in control testes (Fig. 6A). The number of tubules with BrdU-labeled cells, where they populated the periphery of the tubule, also appeared to be lower in the mutants. One hundred tubules in each sample were examined for the presence of labeled cells. Approximately 863 cells in 100 seminiferous tubules were labeled in RARα−/− testes compared with ∼1,300 cells in controls. Even in a tubule with a relatively higher number of labeled preleptotene/leptotene spermatocytes at stage VII–VIII* of RARα−/− testes (Fig. 6D), the absence of mature step 16 spermatids aligned at the tubular lumen before spermiation, compared with stage VIII in normal control (arrow in Fig. 6A), is striking.
Fig. 6.
Reduced germ cell proliferation in RARα−/− young adult mice as shown by in vivo bromodeoxyuridine (BrdU) labeling. A–F: Histological sections of 8-week-old testis from RARα+/+ (A,C,E,F) and RARα−/− mice (B,D) 1 day after BrdU injection (see Experimental Procedures section). Strong BrdU labeling was noted in the proliferating spermatogonia and preleptotene spermatocytes in many of seminiferous tubules of 8-week-old RARα+/+ testes (A,C,E,F). Arrows in C point to preleptotene spermatocytes. Arrow in D points to spermagonia. Arrows in F point to zygotene spermatocytes. A,B: Original magnification, ×20. C–F: Original magnification, ×40. SG, spermatogonia; A3 and A4, A3 and A4 spermatogonia, respectively; P, pachytene spermatocytes; L, leptotene spermatocytes; PL/L, preleptotene or leptotene spermatocytes; D, diplotene spermatocytes; Z, zygotene spermatocytes. Arabic numerals indicate the step of spermatid differentiation. Roman numerals indicate the stage of the seminiferous tubules.
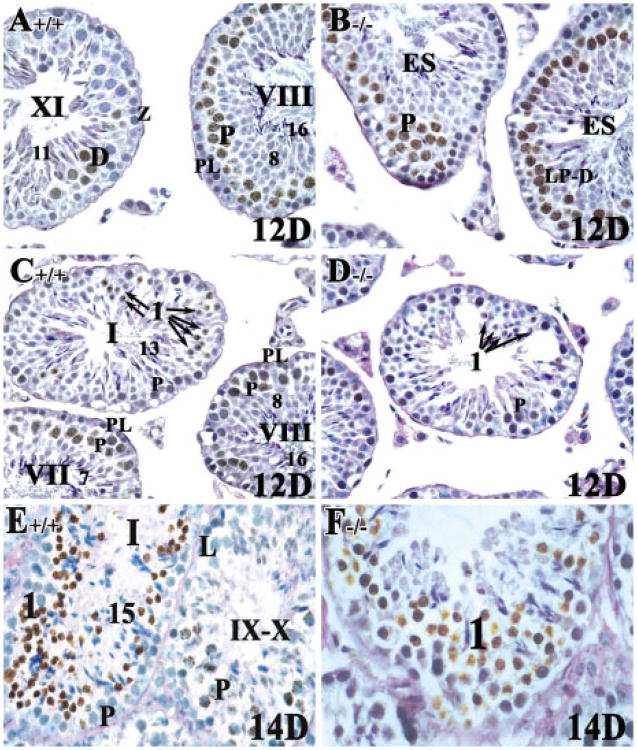
Twelve days after BrdU injection (corresponding to ∼3 weeks of postnatal development), labeled late pachytene and diplotene spermatocytes in stage XI tubules and mid-pachytene spermatocytes at stage VII-VIII from both control and RARα−/− testes were seen (Fig. 7A,B). A few labeled step 1 round spermatids were observed in RARα+/+ testes (∼2%; n = 150 tubules; arrows in Fig. 7C), but only a very few were observed in RARα−/− testes (∼0.8%; n = 150 tubules; arrows in Fig. 7D). This finding suggested that, although once initiated, spermatogenic cells can proceed at a comparable pace from preleptotene spermatocytes to step 1 spermatids in both control and RARα−/− adult testes, fewer do so in the mutant testes. Accordingly, 14 days after BrdU injection, labeled newly formed step 1 spermatids were observed in both control and RARα−/− testes (Fig. 7E,F).
Fig. 7.
Spermatogenesis can proceed at a comparable pace from preleptotene spermatocytes to step 1 spermatids in both RARα+/+ and RARα−/− young adult mice 12 or 14 days after bromodeoxyuridine (BrdU) injection. A–F: Histological sections of 8-week-old testis from RARα+/+ (A,C,E) and RARα−/− mice (B,D,F). A–D: Mid-pachytene spermatocytes at stage VII–VIII were most frequently labeled in both RARα+/+ and RARα−/− testes 12 days after BrdU injection. Arrows in C and D point to step 1 spermatids. D: Very few step 1 spermatids were found in RARα−/− mice testes 12 days after BrdU injection. E,F: Labeled step 1 round spermatids were readily detected in both RARα+/+ and RARα−/− testes after 14 days of BrdU injection. A–F: Original magnification, ×40. Z, zygotene spermatocytes; LP-D, late pachytene spermatocytes to diplotene spermatocytes; PL, preleptotene spermatocytes; L, leptotene spermatocytes; P, pachytene spermatocytes; ES, elongated spermatids. Arabic numerals indicate the step of spermatid differentiation. Roman numerals indicate the stage of the seminiferous tubules.
Selected specimens were then evaluated quantitatively for both the most advanced cell type that was labeled as well as the relative numbers of tubules containing such cells. That is, the proportion of tubules that contained labeled cells from stage IX through XII was assessed (Table 2). The frequency of tubules at stage IX–XII in the RARα+/+ testes was very similar to the data obtained in the classic studies by Oakberg (1956). In contrast, there appeared to be a diminution in the frequency of tubules at such comparably advanced stages in the RARα−/− testes.
Table 2. Percentages of Tubules at Stage IX to XII.
| Genotype | Age of animals + days postinjection | No. of IX–XII tubules/total no. counted | % stage IX–XII |
|---|---|---|---|
| +/+ | 7w + 10d | 292/947 | 30.83% |
| +/+ | 34d + 11d | 217/650 | 33.38% |
| +/+ | 7w + 14d | 92/430 | 21.40% |
| +/+ | 7w + 14d | 518/1417 | 36.56% |
| −/− | 7w + 11d | 4/471 | 0.85% |
| −/− | 7w + 12d | 17/273 | 6.23% |
| −/− | 7w + 14d | 27/440 | 6.14% |
We next evaluated the kinetics of progression during the first wave of spermatogenesis. This involved injecting BrdU at day 8–9 after birth and then harvesting testes at intervals of 13, 18, and 26 days postinjection (p.i.). The expectation is that, if the timing of spermatogenic progression is normal, the most advanced stage at day 8 plus 13 days p.i. would be at the end of meiosis II and steps 1–3 spermatids. At p.i. 18, cells should be at steps 13–14 spermatids; and at p.i. 26, mature sperm from the first wave of spermatogenesis should be present. Thirteen days after administration of BrdU to 8- to 9-day-old mice, ∼50% of the step 1 spermatids in RARα+/+ testes were found to be labeled (Fig. 8A). In most of the RARα−/− testes examined at this age, the number of labeled step 1 spermatids was much lower than that seen in RARα+/+ mice (Fig. 8B). Accordingly, there are relatively higher numbers of tubules with labeled pachytene spermatocytes (∼42%) in the RARα−/− testes (Fig. 8B), when compared with controls (∼29%; Fig. 8A).
Fig. 8.
A decrease in step 1 spermatids and an arrest of step 8–9 spermatids from the first spermatogenic wave in RARα−/− juvenile mouse testes 13 or 18 days after bromodeoxyuridine (BrdU) injection. A–D: Histological sections of 8- to 9-day-old testis from RARα+/+ (A,C) and RARα−/− mice (B,D) 13 or 18 days after BrdU injection, respectively. ∼50% of step 1 spermatids were found to be labeled in 9-day-old RARα+/+ testes 13 days after BrdU injection, whereas the number in RARα−/− mouse testes was much lower. Arrows in B point to step 1 round spermatids. BrdU-labeled spermatids appeared to be arrested at step 8–9 in RARα−/− mouse testes as shown in D. A–D: Original magnification, ×40. P, pachytene spermatocytes; L, leptotene spermatocytes; PL, preleptotene spermatocytes; MI/MII, meiosis I/II. Arabic numerals, the step of spermatid differentiation. Roman numerals indicate the stage of the seminiferous tubules.
In day 8–9 plus 18 days p.i. (∼4 weeks postnatal) control testes, steps 8–9 round and elongating spermatids along with a few step 13–14 spermatids were labeled (Fig. 8C). In contrast, in RARα−/− testes, only labeled steps 8–9 spermatids were detected (Fig. 8D). That is, although some of the cells in the first spermatogenic wave had proceeded at a comparable pace through early spermiogenesis, most of them arrested after they reached step 8–9, consistent with the morphological observations. At p.i. day 26, mature spermatozoa were highly condensed, such that it was impossible to detect the BrdU-labeled sperm in either RARα+/+ or RARα−/− testes (data not shown).
Discussion
In the VAD rat testis, at least three major defects in spermatogenesis have been identified (Ismail et al., 1990; van Pelt and de Rooij, 1990b; de Rooij et al., 1994; Packer and Wolgemuth, 1999). They include failure of the production of A2 spermatogonia from A1 spermatogonia, a delay in the onset of and an abnormality in the progression of meiotic prophase, and spermatid degeneration. At the onset of the growth retardation in rats on a VAD diet, the production of A2 spermatogonia is arrested, and there is also a temporary arrest of preleptotene spermatocytes (de Rooij et al., 1989; Griswold et al., 1989; Ismail et al., 1990; Van Pelt and de Rooij, 1990a; de Rooij et al., 1994). These defects ultimately result in tubules composed of only actively proliferating Aiso, Apr, and Aal spermatogonia and Sertoli cells in the VAD testes (de Rooij, 2001). Administration of either vitamin A (de Rooij et al., 1989; Griswold et al., 1989; Ismail et al., 1990; Van Pelt and de Rooij, 1990a) or intraperitoneal injection of high doses of RA (van Pelt and de Rooij, 1991) causes a mass production of A1 spermatogonia throughout the testis that synchronously re-enter spermatogenesis. RARα mRNA was readily detected by in situ hybridization in Sertoli cells and A spermatogonia 6 hr after injection of RA in VAD mice (de Rooij etal., 1994). The expression of at least two of the RARs (RARα and RARγ) in A spermatogonia indicates that the action of RA in these cells could involve these receptors. Collectively, these several major defects occurred in VAD led to the hypothesis that a possible role of retinoids in both spermatogonial proliferation and differentiation and in the onset of the progression of meiotic prophase and transition of spermatocytes from preleptotene to leptotene (de Rooij et al., 1994; de Rooij and van Dissel-Emiliani, 1997). In normal rat and mouse testes, RARα transcripts and protein have been reported to be expressed in most cell types, suggesting possible roles in processes as diverse as Sertoli cell functioning, meiotic prophase, and spermiogenesis (Kim and Griswold, 1990; Eskild et al., 1991; van Pelt et al., 1992; Kim and Wang, 1993; Lufkin et al., 1993; Akmal et al., 1997).
The timing of spermatogenesis is rigidly controlled, resulting in rodents in a characteristic association of different cell types (Leblond and Clermont, 1952; Russell et al., 1990; Parvinen, 1993). However, the mechanisms initiating the sequence of divisions that begins the synchronous development of cells and controls the cell cycle are not known. In VAD rat testes, examples of temporary arrest in development of spermatogenesis have been demonstrated, suggesting the tightly controlled spermatogenic cell cycle can be altered by retinoids (Huang and Hembree, 1979; de Rooij, 1983; Morales and Griswold, 1987; Ismail et al., 1990; de Rooij et al., 1994). We now note that absence of the RARα receptor alone resulted in a temporary arrest in spermiogenic progression, at step 8–9 spermatids, in the first wave of spermatogenesis. There was also a delay in the onset of the second wave, as well as a temporary arrest and delay in the appearance of preleptotene and leptotene spermatocytes in the first, second, and third waves. In vivo BrdU labeling revealed a notable decrease in germ cell proliferation in both juvenile and adult RARα−/− testes and confirmed the arrest at step 8–9 spermatids observed morphologically in the first wave of spermatogenesis. The net result of these abnormalities was a profound asynchrony of spermatogenic progression in RARα−/− seminiferous tubules. Taking these observations collectively, we speculate that RARα receptor-regulated factors are required for A spermatogonia to initiate a precise series of divisions, for preleptotene spermatocytes to traverse a normal meiotic prophase, and for step 8–9 spermatids to continue to undergo spermiogenesis, respectively. Although there may be other cell types that are also affected, RARα is essential for synchronous development of spermatogenic cells in the testis.
The possibility that retinoid signaling, mediated through the RARs and RXRs, could be important for initiation of and progression through the cell cycle in spermatogonia, spermatocytes, and spermatids (a specialized telophase) is intriguing. Retinoids have been shown to directly affect expression of cell cycle-regulatory genes at the G1 stage (Pestell et al., 1999; Altucci and Gronemeyer, 2001; de Rooij, 2001). Several steroid hormone receptor superfamily members (estrogen receptor, androgen receptor, or progesterone receptor) stimulate expression of cyclin D1 (Chen et al., 1996; Zhuang et al., 2001; Fujita et al., 2002), which interacts with and activates Cdk4. The activated cyclin-Cdk complex phosphorylates pRB, which dissociates from DP-E2F complex, thus allowing transcription of other cell cycle regulatory genes (Altucci and Gronemeyer, 2001). In an opposite regulatory mode, vitamin D and reti-noic acids can induce expression of the p21CIP, which blocks Cdk activity, resulting in G1 arrest of treated U937 cells. The p21CIP gene is a retinoic acid-responsive target gene, and an RA response element in the promoter is required to confer retinoic acid induction through RAR/RXR heterodimers (Liu et al., 1996). The RA-mediated inhibition of cyclin D1 protein in bronchial epithelial cells is regulated at the posttranslational level, likely through increased ubiquitin-dependent proteasome degradation (Langenfeld et al., 1997). In these studies, calpain inhibitor I and lactacystin each prevented the decrease in cyclin D1 protein expression in the presence of RA treatment, suggesting the possible RA-signaled cyclin D1 proteolysis (Langenfeld et al., 1997). Of interest, targeted disruption of Cdk4, which is expressed in spermatogonia and early stage spermatocytes, resulted in smaller testis with reduced numbers of spermatogonia/spermatocytes with perturbed layer formation (Kang etal., 1997; Tsutsui etal., 1999; Zhang et al., 1999). In human lung squamous carcinoma CH27 cells, RA-mediated G1 arrest is associated with induction of p27kIP1 (Hsu et al., 2000). In p27kIp1 knockout mice, aberrations in the spermatogenic process were observed. First, an increase in the number of A spermatogonia was found. Second, abnormal preleptotene spermatocytes were observed, some of which seemingly tried to enter a mitotic division instead of entering the meiotic prophase (Beumer et al., 1999). By immunohistochemistry, p27kIP1 expression was seen in gonocytes on E16.5 to day 2 postnatal, while in adult mouse testis, staining was only found in Sertoli cells (Beumer et al., 1999). This finding suggests a role of p27kIP1 in the regulation of spermatogonial proliferation and/or apoptosis and the onset of meiotic prophase in preleptotene spermatocytes indirectly by Sertoli cells (Beumer et al., 1999). Studies on the possible defects on these cell cycle-regulatory gene expression in the absence of RARα receptor-mediated pathway may help us understand the regulation of synchronization of spermatogenic cycle.
There are four general schemes that may ultimately lead to a diminution in the numbers of sperm produced by the testis in a given round of spermatogenesis: (1) interruption of the pattern of stem cell divisions, (2) degeneration and phagocytosis of germ cells, (3) arrest or retardation of differentiation, and (4) sloughing of germ cells (Russell et al., 1990). These may occur separately or in combination. Both interruption of the pattern of stem cell division and a temporary arrest of spermatogenesis have been proposed to be the primary defects in spermatogenesis in VAD rats (Ismail et al., 1990; Van Pelt and de Rooij, 1990a; de Rooij et al., 1994). By examining the primary site of defects in the early postnatal development of RARα−/− testis, we obtained important insight in the possible function of RARα in maintaining the precisely controlled spermatogenic cell cycles. In the RARα−/− mouse, there was an increased number of degenerating pachytene spermatocytes and a temporary arrest at step 8–9 in the first wave of spermatogenesis, a delay in the onset of the second wave, as well as a delay and temporary arrest in preleptotene and leptotene spermatocytes in the first, second, and third waves. These delays and arrests at progression of meiotic prophase and onset of spermiogenesis in RARα−/− testis resulted in asynchronous tubules, with mixed spermatogenic cell types.
Disappearance of specific spermatogenic cell types is usually due to degeneration of germ cells. Degeneration can occur at anytime during one or more of the many developmental phases in the germ cell's life history and indeed, a certain level of cell death is required for normal spermatogenesis (Rodriguez et al., 1997). The observation of degenerating early pachytene spermatocytes as early as in the first wave in RARα−/− testes led to our hypothesis that RARα receptor-regulated factors are essential for preleptotene spermatocytes to traverse a normal meiotic prophase. Degeneration can also occur in response to failure of germ cells to differentiate properly and to form the proper cellular associations required for the maintenance of spermatogenesis. This degeneration is considered more related to secondary causes of the abnormalities due to progressive breakdown of the required cellular associations. For example, in 8-week-old RARα−/− testes, entire layers of specific cell types were missing. Although the mechanism for this loss is not known, it may involve an arrest of spermatogonia divisions or the degeneration of a specific cell types (or both). Our detection of terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) -positive elongated spermatids situated at the periphery of the seminiferous epithelium suggested the phagocytosis of defective spermatids by Sertoli cells (Chung et al., manuscript submitted for publication).
We also observed large, clear, rounded vacuolar-like spaces within the RARα−/− tubules, a phenotype often associated with Sertoli cell damage (Russell et al., 1990). That is, the presence of such structures is a phenomenon that often occurs early in the histopathological response, before extensive germ cell degeneration, and it is believed to be an early indicator of damage or malfunctioning of the Sertoli cells (Russell et al., 1990). Our detection of vacuolation in RARα−/− testis, which is particularly severe at 4 weeks of age, may indicate a possible defect in the normal function of Sertoli cells. A larger than normal tubular lumen may signify a blockage in the tubules or a blockage in the duct system, while a smaller lumen usually indicates a decrease in fluid secretion by the Sertoli cells (Russell et al., 1990). There were not obvious differences in the lumens of RARα−/− testis, which may suggest normal duct system and fluid secretion by Sertoli cells in the absence of RARα.
In the VAD rat testis model, spermatid degeneration is associated with a delay in spermiation (Mitranond et al., 1979; Sobhon et al., 1979; Huang and Marshall, 1983; Morales and Griswold, 1991) and a disruption of Sertoli cell tight junctions has been reported (Huang et al., 1988). Our RARα mutant model revealed a delay and temporary arrest at step 8–9 spermatids and a failure of spermatids to align next to the lumen for spermiation. The similar findings of defects in spermiation in both VAD rat testis and RARα−/−mouse testis suggested that mechanisms leading to spermiogenesis and completion of spermiation are extremely sensitive to change in the status in retinoic acid, most likely through an RARα receptor-mediated pathway.
It is striking to note that there is a common stage of the cycle of the seminiferous epithelium where the delay or temporary arrest of spermatogenic cells occur in the spermatogenic pathway in the absence of RARα. In the RARα−/− testes, there is a temporary arrest at step 8–9 at stage VIII–IX in the first wave of spermatogenesis, and a delay or arrest in preleptotene and leptotene spermatocytes at stage VIII–IX in the first, second, and third waves. The observation of asynchronous tubules in mutant testis leads to our hypothesis that RA-mediated signaling is implicated in the transition from the randomly cycling population of undifferentiated spermatogonia to the rigidly series of six synchronous divisions of differentiating spermatogonia. This step has also been shown to be rather vulnerable, because it can become blocked in several different situations, including VAD (de Rooij, 1998, 2001). Intriguingly, this step also involves the differentiation of A1 spermatogonia to form A2 spermatogonia, again from stage VIII to IX. Furthermore, the mature spermatozoa failed to align at the tubular lumen at stage VIII for spermiation in RARα−/− testes. Coincidentally, the expression of RARα mRNAs were found to be stage-specific, with the highest expression at stage VIII (Kim and Griswold, 1990; Akmal et al., 1997). Although the mechanisms responsible for this stage specificity remain to be determined, it appears that important developmental events that occur at stage VIII are under rigid control, possibly by means of RARα-mediated signaling.
Experimental Procedures
Source of Animals and Tissues
Testes of RARα−/− (Lufkin et al., 1993) and RARα+/+ mice at 8–9 days, 2, 4, 6, 7, 8, 9, 12, and 16 weeks of age were dissected from anesthetized animals that had been perfused through the heart with phosphate-buffered saline (PBS) and then with 4% paraformaldehyde in PBS or Bouin's fixatives (15 parts of picric acid-aqueous solution, 5 parts of formaldehyde, and 1 part of glacial acidic acid). Tissues were fixed overnight at 4°C. For frozen sections, testes were frozen in liquid nitrogen and sections were cut in a cryostat, mounted on coverslips, and air-dried. They were post-fixed with cold acetone for 3 min at room temperature.
Histological Study
Perfused, fixed tissues were embedded in paraffin, sectioned at 5 μm, and mounted on Superfrost slides (Fisher). Histological sections were deparaffinized in Histoclear, hydrated through an alcohol series, and washed with H2O. Slides were stained with hematoxylin according to standard procedures. They were viewed on a Nikon photomicroscope under brightfield optics. For staging of the mouse seminiferous epithelium according to Oakberg (1956) and Russell et al. (1990), the testis sections were stained for the PAS reaction before hematoxylin counterstaining (Sigma; Leblond and Clermont, 1952).
Cell Proliferation Study
For BrdU labeling, adult mice (7–8 weeks) were injected intraperitoneally with 100 μg/g body weight of 10 mg/ml PBS of BrdU (Sigma–Aldrich). To label preleptotene spermatocytes in the first wave of postnatal spermatogenesis, 8- to 9-day-old pups were injected intraperitoneally with 200 μg/g body weight of 10 mg/ml PBS of BrdU (Sigma–Aldrich). The testis tissues were then collected at various intervals after BrdU administration, and the presence of label in specific cell types was detected by immunohistochemistry by using BrdU staining kit (Oncogene, Research Products), according to the manufacturer's instructions. Diaminobenzidine (DAB) -stained sli dures. They were viewed on a Nikon photomicroscope under brightfield optics. Photomicrographs were taken by using Fujicolor 400 film or digital camera (Spot advanced software, Diagnostic instruments, Inc.). For staging of the mouse seminiferous epithelium according to Oakberg (1956) and Russell et al. (1990), the testis sections were stained for the PAS reaction before hematoxylin counterstaining.
Immunohistochemistry
Perfused, fixed tissues were embedded in paraffin, sectioned at 5 μm, and mounted on Superfrost slides (Fisher). Immunohistochemical analyses were performed by using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA) as previously described (Liu et al., 1998). Briefly, histological sections were deparaffinized in Histoclear, hydrated through an alcohol series, and washed with H2O. Antigen unmasking was performed by boiling slides in a microwave for 10 min in 0.01 M citrate buffer, pH 6 (Shi et al., 1991). After treatment with 0.3% H2O2 in methanol for 10 min, sections were washed with 1× PBS. Slides were blocked for 1 hr at room temperature with 2.5% rabbit serum in PBS. The primary antibody incubation was carried out overnight at 4°C in a humidified chamber. Anti-phosphorylated histone H2AX (γ-H2AX; obtained from TREVIGEN, MD; Mahadevaiah et al., 2001) were diluted 1:100 in 1× PBS. For controls, the slides were incubated with corresponding IgG instead of primary antibody. After incubation, slides were washed for 30 min in PBS and processed by using the Vectastain ABC kit following the manufacturer's instructions. DAB-stained slides were counterstained with hematoxylin according to standard procedures. They were viewed on a Nikon Eclipse 800 photomicroscope under bright-field optics. Photomicrographs were taken by using digital camera. For staging of the mouse seminiferous epithelium according to Oakberg (1956) and Russell et al. (1990).
Acknowledgments
We thank Dr. Pierre Chambon for the generous gift of RARα mutant mice and Core B of Program Project, P01 DK54057, under the direction of Dr. Cathy Mendelsohn and staff, for help in breeding some of the RARα mutant mice. D.J.W. was funded by the National Institutes of Health. S.S.W.C. received a fellowship from the Croucher Foundation, Hong Kong.
Grant sponsor: National Institutes of Health; Grant number: P01 DK54057; Grant sponsor: The Croucher Foundation.
References
- Akmal KM, Dufour JM, Kim KH. Retinoic acid receptor alpha gene expression in the rat testis: potential role during the prophase of meiosis and in the transition from round to elongating spermatids. Biol Reprod. 1997;56:549–556. doi: 10.1095/biolreprod56.2.549. [DOI] [PubMed] [Google Scholar]
- Altucci L, Gronemeyer H. Nuclear receptors in cell life and death. Trends Endocrinol Metab. 2001;12:460–468. doi: 10.1016/s1043-2760(01)00502-1. [DOI] [PubMed] [Google Scholar]
- Bellve AR, Cavicchia JC, Millette CF, O'Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beumer TL, Kiyokawa H, Roepers-Gajadien HL, van den Bos LA, Lock TM, Gademan IS, Rutgers DH, Koff A, de Rooij DG. Regulatory role of p27kip1 in the mouse and human testis. Endocrinology. 1999;140:1834–1840. doi: 10.1210/endo.140.4.6638. [DOI] [PubMed] [Google Scholar]
- Byers S, Pelletier RM, Suarez-Quian C. Sertoli-Sertoli cell junctions and the seminiferous epithelium barrier. In: Griswold MD, Russell LD, editors. The Sertoli cell. Clearwater, FL: Cache River Press; 1993. pp. 365–390. [Google Scholar]
- Chen Y, Robles AI, Martinez LA, Liu F, Gimenez-Conti IB, Conti CJ. Expression of G1 cyclins, cyclin-dependent kinases, and cyclin-dependent kinase inhibitors in androgen-induced prostate proliferation in castrated rats. Cell Growth Differ. 1996;7:1571–1578. [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- Clermont Y, Harvey SC. Duration of the cycle of the seminiferous epithelium of normal, hypophysectomized and hypophysectomized hormone treated albino rats. Endocrinology. 1965;76:80–89. doi: 10.1210/endo-76-1-80. [DOI] [PubMed] [Google Scholar]
- Clermont Y, Trott M. Duration of the cycle of the seminiferous epithelium in the mouse and hamster determined by means of 3H-thymidine and radioautography. Fertil Steril. 1969;20:805–817. doi: 10.1016/s0015-0282(16)37153-9. [DOI] [PubMed] [Google Scholar]
- Cupp AS, Dufour JM, Kim G, Skinner MK, Kim KH. Action of retinoids on embryonic and early postnatal testis development. Endocrinology. 1999;140:2343–2352. doi: 10.1210/endo.140.5.6720. [DOI] [PubMed] [Google Scholar]
- de Rooij DG. Proliferation and differentiation of undifferentiated spermatogonia in the mammalian testis. In: Potten CS, editor. Stem cells Their identification and characterization. Edinburg: Churchill Livingstone; 1983. pp. 89–117. [Google Scholar]
- de Rooij DG. Stem cells in the testis. Int J Exp Pathol. 1998;79:67–80. doi: 10.1046/j.1365-2613.1998.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121:347–354. doi: 10.1530/rep.0.1210347. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Grootegoed JA. Spermatogonial stem cells. Curr Opin Cell Biol. 1998;10:694–701. doi: 10.1016/s0955-0674(98)80109-9. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, van Dissel-Emiliani FMF. Regulation of proliferation and differentiation of stem cells in the male germ line. In: Potten CS, editor. Stem cells. London: Academic Press; 1997. pp. 283–313. [Google Scholar]
- de Rooij DG, Van Dissel-Emiliani FM, Van Pelt AM. Regulation of spermatogonial proliferation. Ann N Y Acad Sci. 1989;564:140–153. doi: 10.1111/j.1749-6632.1989.tb25894.x. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, van Pelt AMM, Van de Kant HJG, van der Saag PT, Peters AHFM, Heyting C, de Boer P. Role of retinoids in spermatogonial proliferation and differentiation and the meiotic prophase. In: Bartke A, editor. Function of somatic cells in the testis. Berlin, Heidelberg, New York: Springer; 1994. p. 345. [Google Scholar]
- Eskild W, Ree AH, Levy FO, Jahnsen T, Hansson V. Cellular localization of mRNAs for retinoic acid receptor-alpha, cellular retinol-binding protein, and cellular retinoic acid-binding protein in rat testis: evidence for germ cellspecific mRNAs. Biol Reprod. 1991;44:53–61. doi: 10.1095/biolreprod44.1.53. [DOI] [PubMed] [Google Scholar]
- Franca LR, Ogawa T, Avarbock MR, Brinster RL, Russell LD. Germ cell genotype controls cell cycle during spermatogenesis in the rat. Biol Reprod. 1998;59:1371–1377. doi: 10.1095/biolreprod59.6.1371. [DOI] [PubMed] [Google Scholar]
- Fujita M, Urano T, Horie K, Ikeda K, Tsukui T, Fukuoka H, Tsutsumi O, Ouchi Y, Inoue S. Estrogen activates cyclin-dependent kinases 4 and 6 through induction of cyclin D in rat primary osteoblasts. Biochem Biophys Res Commun. 2002;299:222–228. doi: 10.1016/s0006-291x(02)02640-2. [DOI] [PubMed] [Google Scholar]
- Gaemers IC, van Pelt AM, van der Saag PT, Hoogerbrugge JW, Themmen AP, de Rooij DG. Differential expression pattern of retinoid × receptors in adult murine testicular cells implies varying roles for these receptors in spermatogenesis. Biol Reprod. 1998;58:1351–1356. doi: 10.1095/biolreprod58.6.1351. [DOI] [PubMed] [Google Scholar]
- Griswold MD, Bishop PD, Kim KH, Ping R, Siiteri JE, Morales C. Function of vitamin A in normal and synchronized seminiferous tubules. Ann N Y Acad Sci. 1989;564:154–172. doi: 10.1111/j.1749-6632.1989.tb25895.x. [DOI] [PubMed] [Google Scholar]
- Hsu SL, Hsu JW, Liu MC, Chen LY, Chang CD. Retinoic acid-mediated G1 arrest is associated with induction of p27(Kip1) and inhibition of cyclin-dependent kinase 3 in human lung squamous carcinoma CH27 cells. Exp Cell Res. 2000;258:322–331. doi: 10.1006/excr.2000.4933. [DOI] [PubMed] [Google Scholar]
- Huang HF, Hembree WC. Spermatogenic response to vitamin A in vitamin A deficient rats. Biol Reprod. 1979;21:891–904. doi: 10.1095/biolreprod21.4.891. [DOI] [PubMed] [Google Scholar]
- Huang HF, Marshall GR. Failure of spermatid release under various vitamin A states - an indication of delayed spermiation. Biol Reprod. 1983;28:1163–1172. doi: 10.1095/biolreprod28.5.1163. [DOI] [PubMed] [Google Scholar]
- Huang HF, Yang CS, Meyenhofer M, Gould S, Boccabella AV. Disruption of sustentacular (Sertoli) cell tight junctions and regression of spermatogenesis in vitamin-A-deficient rats. Acta Anat (Basel) 1988;133:10–15. doi: 10.1159/000146606. [DOI] [PubMed] [Google Scholar]
- Ismail N, Morales C, Clermont Y. Role of spermatogonia in the stagesynchronization of the seminiferous epithelium in vitamin-A-deficient rats. Am J Anat. 1990;188:57–63. doi: 10.1002/aja.1001880107. [DOI] [PubMed] [Google Scholar]
- Kang MJ, Kim MK, Terhune A, Park JK, Kim YH, Koh GY. Cytoplasmic localization of cyclin D3 in seminiferous tubules during testicular development. Exp Cell Res. 1997;234:27–36. doi: 10.1006/excr.1997.3590. [DOI] [PubMed] [Google Scholar]
- Kim KH, Griswold MD. The regulation of retinoic acid receptor mRNA levels during spermatogenesis. Mol Endocrinol. 1990;4:1679–1688. doi: 10.1210/mend-4-11-1679. [DOI] [PubMed] [Google Scholar]
- Kim KH, Wang ZQ. Action of vitamin A on testis: role of the Sertoli cell. In: Griswold MD, Rusell LD, editors. The Sertoli cell. Clearwater, FL: Cache River Press; 1993. pp. 514–535. [Google Scholar]
- Langenfeld J, Kiyokawa H, Sekula D, Boyle J, Dmitrovsky E. Posttranslational regulation of cyclin D1 by retinoic acid: a chemoprevention mechanism. Proc Natl Acad Sci U S A. 1997;94:12070–12074. doi: 10.1073/pnas.94.22.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CP, Clermont Y. Spermiogenesis of rat, mouse, hamster, and guinea pig as revealed by the periodic acid-fuchin sulfurous acid technique. Am J Anat. 1952;90:167–210. doi: 10.1002/aja.1000900202. [DOI] [PubMed] [Google Scholar]
- Liu M, Iavarone A, Freedman LP. Transcriptional activation of the human p21(WAF1/CIP1) gene by retinoic acid receptor. Correlation with retinoid induction of U937 cell differentiation. J Biol Chem. 1996;271:31723–31728. doi: 10.1074/jbc.271.49.31723. [DOI] [PubMed] [Google Scholar]
- Liu D, Matzuk MM, Sung WK, Guo Q, Wang P, Wolgemuth DJ. Cyclin A1 is required for meiosis in the male mouse. Nat Genet. 1998;20:377–380. doi: 10.1038/3855. [DOI] [PubMed] [Google Scholar]
- Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, LeMeur M, Chambon P. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci U S A. 1993;90:7225–7229. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah SK, Turner JM, Baudat F, Rogakou EP, de Boer P, Blanco-Rodriguez J, Jasin M, Keeney S, Bonner WM, Burgoyne PS. Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet. 2001;27:271–276. doi: 10.1038/85830. [DOI] [PubMed] [Google Scholar]
- Mitranond V, Sobhon P, Tosukhowong P, Chindaduangrat W. Cytological changes in the testes of vitamin-A-deficient rats. I. Quantitation of germinal cells in the seminiferous tubules. Acta Anat (Basel) 1979;103:159–168. doi: 10.1159/000145007. [DOI] [PubMed] [Google Scholar]
- Morales C, Griswold MD. Retinol-induced stage synchronization in seminiferous tubules of the rat. Endocrinology. 1987;121:432–434. doi: 10.1210/endo-121-1-432. [DOI] [PubMed] [Google Scholar]
- Morales CR, Griswold MD. Variations in the level of transferrin and SGP-2 mRNAs in Sertoli cells of vitamin A-deficient rats. Cell Tissue Res. 1991;263:125–130. doi: 10.1007/BF00318407. [DOI] [PubMed] [Google Scholar]
- Oakberg EF. A description of spermiogenesis in the mouse and its use in an analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am J Anat. 1956;99:391–414. doi: 10.1002/aja.1000990303. [DOI] [PubMed] [Google Scholar]
- Packer AI, Wolgemuth DJ. Genetic and molecular approaches to understanding the role of retinoids in mammalian spermatogenesis. In: Nau H, Blaner WS, editors. Retinoids: the biochemical and molecular basis of vitamin A and retinoid action. Berlin, Heidelberg, New York: Springer-Verlag; 1999. pp. 347–368. [Google Scholar]
- Parvinen M. Cyclic functions of Sertoli cells. In: Russell LD, Griswold MD, editors. The Sertoli cell. Clearwater, FL: Cache River Press; 1993. pp. 331–347. [Google Scholar]
- Pestell RG, Albanese C, Reutens AT, Segall JE, Lee RJ, Arnold A. The cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of proliferation and differentiation. Endocr Rev. 1999;20:501–534. doi: 10.1210/edrv.20.4.0373. [DOI] [PubMed] [Google Scholar]
- Ravnik SE, Wolgemuth DJ. Regulation of meiosis during mammalian spermatogenesis: the A-type cyclins and their associated cyclin-dependent kinases are differentially expressed in the germ-cell lineage. Dev Biol. 1999;207:408–418. doi: 10.1006/dbio.1998.9156. [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P. An early and massive wave of germinal cell apoptosis is required for the development of functionalspermatogenesis. EMBOJ. 1997;16:2262–2270. doi: 10.1093/emboj/16.9.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, SinhaHikim AP, Clegg ED. Histological and histopathological evaluation of the testis. Clear-water, FL: Cache River Press; 1990. [Google Scholar]
- Salazar G, Liu D, Liao C, Batkiewicz L, Arbing R, Chung SS, Lele K, Wolgemuth DJ. Apoptosis in male germ cells in response to cyclin A1-deficiency and cell cycle arrest. Biochem Pharmacol. 2003;66:1571–1579. doi: 10.1016/s0006-2952(03)00513-6. [DOI] [PubMed] [Google Scholar]
- Schrans-Stassen BH, van de Kant HJ, de Rooij DG, van Pelt AM. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 1999;140:5894–5900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Sobhon P, Mitranond V, Tosukhowong P, Chindaduangrat W. Cytological changes in the testes of vitamin-A-deficient rats. II. Ultrastructural study of the seminiferous tubules. Acta Anat (Basel) 1979;103:169–183. doi: 10.1159/000145008. [DOI] [PubMed] [Google Scholar]
- Sung WK, Komatsu M, Jagiello GM. The kinetics of the first wave of spermatogenesis in the newborn male mouse. Gamete Res. 1986;14:245–254. [Google Scholar]
- Tsutsui T, Hesabi B, Moons DS, Pandolfi PP, Hansel KS, Koff A, Kiyokawa H. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27(Kip1) activity. Mol Cell Biol. 1999;19:7011–7019. doi: 10.1128/mcb.19.10.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pelt AM, de Rooij DG. The origin of the synchronization of the seminiferous epithelium in vitamin A-deficient rats after vitamin A replacement. Biol Reprod. 1990a;42:677–682. doi: 10.1095/biolreprod42.4.677. [DOI] [PubMed] [Google Scholar]
- van Pelt AM, de Rooij DG. Synchronization of the seminiferous epithelium after vitamin A replacement in vitamin A-deficient mice. Biol Reprod. 1990b;43:363–367. doi: 10.1095/biolreprod43.3.363. [DOI] [PubMed] [Google Scholar]
- van Pelt AM, de Rooij DG. Retinoic acid is able to reinitiate spermatogenesis in vitamin A-deficient rats and high replicate doses support the full development of spermatogenic cells. Endocrinology. 1991;128:697–704. doi: 10.1210/endo-128-2-697. [DOI] [PubMed] [Google Scholar]
- van Pelt AM, van den Brink CE, de Rooij DG, van der Saag PT. Changes in retinoic acid receptor messenger ribonucleic acid levels in the vitamin A-deficient rat testis after administration of retinoids. Endocrinology. 1992;131:344–350. doi: 10.1210/endo.131.1.1319320. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wang X, Wolgemuth DJ. Developmentally regulated expression of cyclin D3 and its potential in vivo interacting proteins during murine gametogenesis. Endocrinology. 1999;140:2790–2800. doi: 10.1210/endo.140.6.6756. [DOI] [PubMed] [Google Scholar]
- Zhuang YH, Sarca D, Weisz A, Altucci L, Cicatiello L, Rollerova E, Tuohimaa P, Ylikomi T. Cell type-specific induction of cyclin D and cyclin-dependent kinase inhibitor p27(kip1) expression by estrogen in rat endometrium. J Steroid Biochem Mol Biol. 2001;78:193–199. doi: 10.1016/s0960-0760(01)00087-5. [DOI] [PubMed] [Google Scholar]