Abstract
Aims:
The aim of this study is to estimate the associations of early and current socio-economic position (SEP) on adult cardiometabolic risk factors in the Indian Migration Study (N = 7,067).
Methods and Results:
Linear mixed models were used to estimate associations between early and current SEP and cardiometabolic risk factors: systolic blood pressure (SBP), body fat and Homeostasis Model Assessment (HOMA) score. In males, high current SEP was associated with higher SBP. In both genders, high early and current SEP were associated with higher body fat, current SEP dominating the associations. High early SEP was associated with higher HOMA score in males only, and the effect size halved after adjustment for current SEP. High current SEP was associated with higher HOMA score more strongly in males than in females.
Conclusion:
Higher SEP, more importantly in adulthood than childhood, was associated with cardiometabolic risk factors in an Indian population. The relationship between SEP over the life course and urbanization should be considered in the Indian context when public health interventions to prevent cardiovascular disease are planned.
Keywords: Blood pressure, cardiovascular diseases, insulin resistance, obesity, risk factors, social class
Introduction
The prevalence of cardiovascular disease (CVD) and its risk factors are rising rapidly in India.1 Studies from developed countries suggest that low socio-economic position (SEP) throughout the life course is associated with higher level of cardiometabolic risk factors and CVD risk.2–4 In contrast, studies from developing countries have constantly reported associations between high SEP and cardiometabolic risk, although recently there has been some debate about the direction of association.5–8 The relative importance of early life and adult SEP on CVD risk remain a matter of controversy. There is a lack of studies on CVD risk from developing countries that have SEP recorded in both childhood and adulthood. A study of South Asian men that had migrated to the UK but had recall data on childhood (pre-migration) SEP suggested a cumulative protective effect of longer education and non-manual occupation in relation to CVD mortality.9 However, a recent study in rural Indian adolescents aged 13–18 years found that higher SEP was associated with greater adiposity but not with other CVD risk factors, suggesting that the role of early SEP on later CVD risk may be dependent on later SEP or lifestyle changes (Sanjay Kinra and co-authors, unpublished manuscript).
This study aims to assess how early and current SEP in conjunction are associated with cardiometabolic risk factors in adulthood in a large Indian population. Based on previous research, we hypothesize that early and current SEP will have an effect on cardiometabolic risk factors, the former being mediated and potentially also modified by the latter. If the Indian experience reflects that found in developed countries, we would expect to see adverse patterns in less affluent subjects, but we suspect that this pattern may be reversed in the Indian setting.
Methods
Study population
The Indian Migration Study (IMS) is nested within a cardiovascular risk factor screening study conducted in factories in north, central and south India. The design and sampling methodology of the IMS has been described previously.10, 11
Briefly, the study was conducted in four factories located in cities representing the northern (Lucknow), central (Nagpur) and southern (Hyderabad, Bangalore) regions of India. Factory workers and their co-resident spouses were asked about rural-to-urban migration, and those responding positively, together with a 25% random sample of urban non-migrants, were recruited into the study. Each participant was asked to identify one sibling who was still resident at their place of origin, preferably of the same sex and closest to them in age. As a result of this sampling strategy, a geographically representative cross-sectional sample of participants was established, and comprised urban participants from four major cities in India, their rural-dwelling sibling and a subsample of urban factory workers and their urban dwelling siblings (N = 7,067). The fieldwork took place in 2005–2007.
Measurements
The participants were invited to a clinical examination in the factory for the outcome data collection. An interviewer-administered questionnaire was used to collect socio-demographic, health and lifestyle data. Three continuous cardiometabolic risk factors were examined: systolic blood pressure (SBP), percent body fat and Homeostasis Model Assessment (HOMA) score. Early and current SEP were chosen as exposures for these three outcomes in the analyses.
SEP was estimated using the Standard of Living Index (SLI).12 Data on current and early SEP was collected through a subset of questions used to derive the SLI, which is a household-level, asset-based scale devised for use in India.12, 13 Early SEP refers to household circumstances at ages 10–12. This asset-based scale was considered a more appropriate indicator of SEP for these analyses than education, income or occupation. It includes rural and urban material goods items and reflects wealth from crops/trading that may be missed in income measures. Measuring at the household level is appropriate in India, where the joint family structure of the household renders individual’s own SEP less important. A low SLI is associated with tobacco and alcohol use14 and with mortality,13 indicating its validity as a socioeconomic marker.
The full SLI has 29 items, but only a subset of items believed to be most informative for this study population was used to derive the early and current SEP. The 14 items selected to compute the current SEP were quality of house, toilet facilities, source of lighting, drinking water, land ownership, livestock ownership, and possession of clock, radio, television, bicycle, motorcycle, car, tractor, refrigerator and telephone. A subset of 10 questions was used to derive the early SEP (same items excluding source of lighting and possession of car, tractor and telephone).
The performance of the short SLI has previously been examined by the IMS group against the full SLI using the National Family Health Survey 2 (NFHS-2; the national demographic survey of India involving 91,117 households) dataset for adults. The short SLI classified 98.5% (N = 89,716) of the NFHS-2 participants in the same or adjacent quintile of the full SLI (66% in exactly the same quintile), with only 1.5% (N = 1,401) falling outside this range (kappa statistic = 0.58); there was no evidence for an urban–rural classification bias.
Blood pressure was measured on the right upper arm in the sitting position, after a 5-minute rest. Two readings were taken using an appropriate sized cuff connected to a digital device (Model M5-I, Omron, Matsusaka Co, Japan). Skinfold thickness was measured three times at triceps and subscapular areas using Holtain callipers. Fasting (>8 hours) venous samples were centrifuged within 45 minutes, stored locally at −20℃, and transported monthly to the Cardiac Biochemistry laboratory at the All India Institute of Medical Sciences (New Delhi) for biochemical assays.
Quality assurance of measurements
All instruments and protocols were piloted before the start of the study. All fieldworkers were trained together and standardized at the outset, and subsequently every 6 months. The anthropometric equipment was calibrated at the start of every clinic. The Cardiac Biochemistry Lab was part of the UK National External Quality Assessment (UKNEQAS) program for quality assurance of the biochemical assays.
Variables and their transformations
Weights of items for the SLI were developed by the International Institute of Population Sciences in India,12 and were based on a priori belief about the relative significance of the items. The full SLI uses weights to give a total score of 67. For the short versions, the total score is 38 for the current and 25 for the early SLI. To analyse permutations of SEP levels at the two time points, a binary indicator of SEP was created by dichotomizing the standardized SLI by the median, and assigning values 1/0 for high/low SEP. The early and current binary SEP indicators (SEP1 and SEP2) were then combined into a four-class variable, and those having low early and current SEP were chosen as a reference group.
SBP was based on a mean of two readings. HOMA scores to estimate insulin resistance were calculated from fasting blood glucose and serum insulin levels using a standard formula:15
(plasma glucose (mmol/l) × plasma insulin (mU/l))/22.5.
HOMA has been validated by comparison with biochemical markers of insulin resistance in healthy Indian people, yielding moderate correlations.16 Subscapular and triceps skinfolds were averaged and used to calculate percent body fat using an equation previously validated in Indian populations.17 SBP and body fat distributions were approximately normal, whereas the HOMA score distribution was log-normal (medians, geometric means and percentage differences are reported for HOMA score).
Statistical analyses
The distributions of demographic characteristics and of cardiovascular risk factors were studied by gender for descriptive purposes. Pearson’s Chi-Square test and t-tests were used to examine gender differences in the sample.
Linear mixed models were fitted between SEP1, SEP2 and the three cardiometabolic risk factors (SBP, percent body fat and HOMA). SEP1 and SEP2 were included in the model at first individually and then simultaneously. The latter model was fitted with and without an interaction term SEP1*SEP2. Subsequently, the combined SEP was analysed as an exposure. All models included random effects of sib-pair and factory site to account for clustering.
The estimates and adjusted means were calculated using the following model for the combined SEP and each of the risk factors (Y):
where β is the parameter of interest, and the combined SEP has four classes: early and current low (0–0), early high and current low (1–0), early low and current high (0–1) and early and current high (1–1) SEP. Adjustment for age is by the fixed effect γ whereas effects of sib-pair and factory are included as normally distributed random effects η1 and η2 with variances τ12 and τ22, respectively. Since gender interactions were observed in preliminary analyses, statistical analyses were stratified by gender. STATA version 11 was used for all analyses.
Ethical approval and role of the study sponsor
This study complies with the Declaration of Helsinki. Ethical approval for the study was obtained from the ethics committee of the All India Institute of Medical Sciences, and also from the local institutional review boards. Written informed consent (witnessed thumb print if illiterate) was obtained from the participants. The study sponsor played no part in the design, collection or analysis of data, interpretation of findings, writing of the manuscript or the decision to submit it for publication.
Results
Distributions of demographic characteristics and cardiovascular risk factors are given in Table 1. Overall, the numbers of rural, migrant and urban participants were similar, although a higher proportion of females were urban dwelling or migrants to the urban environment compared with males. This difference is also reflected in SEP; a higher proportion of females had a high early and current SEP. About 69% of participants with low early and current SEP were rural dwellers, whereas 71% of participants with low early and high current SEP were migrants and 60% of those with both high early and current SEP were urban dwellers. The age range in the sample was wide (15–76 years). Males had higher SBP than females but females had higher body fat and HOMA levels than males.
Table 1.
Distributions of demographic characteristics and cardiometabolic risk factors
| Variable | Male (N = 4,123) | Female (N = 2,944) | Total (N = 7,067) |
|---|---|---|---|
| Migration status, % (N) | |||
| Rural | 38.5 (1,459) | 23.9 (652) | 32.4 (2,111) |
| Migrant | 29.8 (1,127) | 36.2 (985) | 32.4 (2,112) |
| Urban | 31.7 (1,201) | 39.9 (1,086) | 35.1 (2,287) |
| Early SEP, % (N) | |||
| Low (0) | 51.2 (2,112) | 43.2 (1,271) | 47.9 (3,383) |
| High (1) | 48.9 (2,011) | 56.8 (1,673) | 52.1 (3,684) |
| Late SEP, % (N) | |||
| Low (0) | 47.2 (1,945) | 40.9 (1,203) | 44.6 (3,148) |
| High (1) | 52.8 (2,178) | 59.1 (1,741) | 55.4 (3,919) |
| Age (years), mean (SD) | 41.5 (10.6) | 39.8 (9.9) | 40.8 (10.4) |
| SBP (mmHg), mean (SD) | 124.4 (16.9) | 119.2 (17.2) | 122.2 (17.4) |
| Body fat (%), mean (SD) | 23.8 (7.3) | 31.2 (7.5) | 26.9 (8.2) |
| HOMA score, median (IQR) | 1.25 (0.68 – 2.09) | 1.34 (0.75 – 2.14) | 1.28 (0.72 – 2.12) |
HOMA, Homeostasis Model Assessment; IQR, inter-quartile range; SBP, systolic blood pressure; SD, standard deviation; SEP, socio-economic position.
Systolic blood pressure
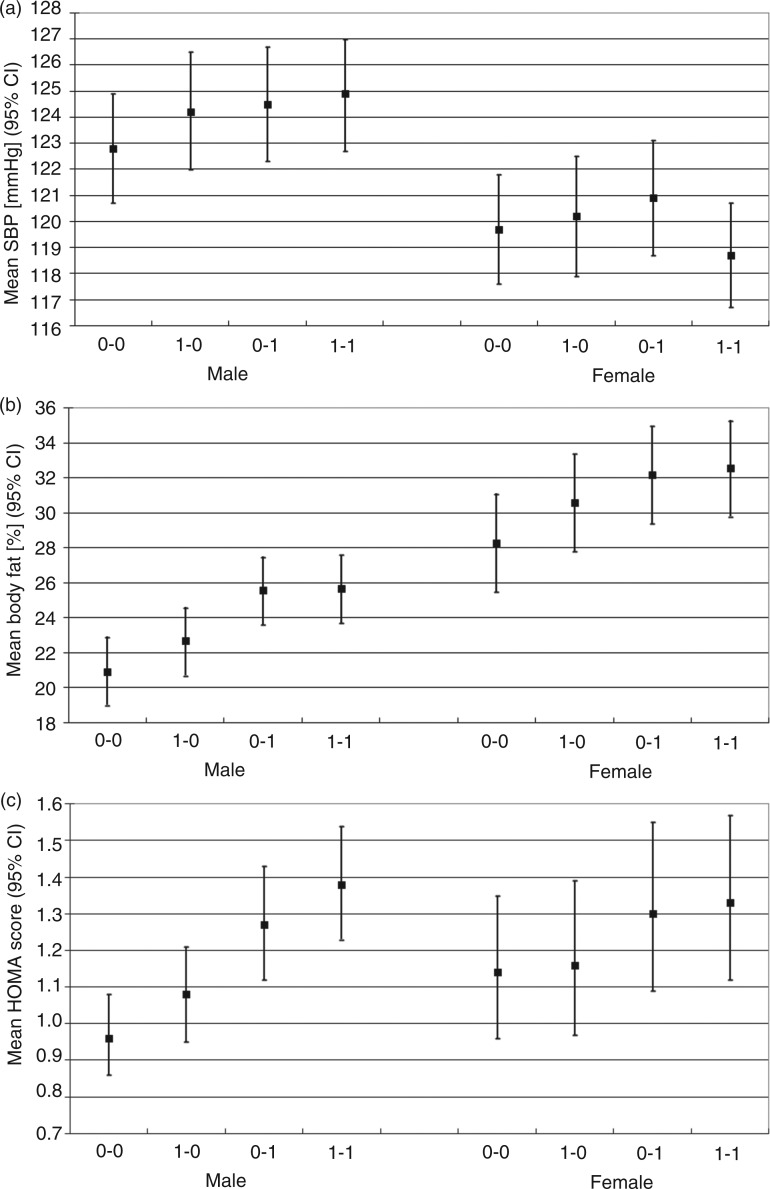
In males, high early SEP was associated with higher SBP but adjustment for current SEP attenuated this association by a third (Table 2); however, the association between high current SEP and SBP did not attenuate markedly after adjustment for early SEP. In females, early SEP was not associated with SBP on its own, but an interaction between early and current SEP on SBP was observed (p = 0.028): in females with low early SEP, high current SEP was associated with higher SBP, whereas in females with high early SEP, high current SEP was associated with lower SBP. In the combined SEP analysis, males with high early and current SEP had on average 2 mmHg higher SBP (p = 0.002) compared with males with low early and current SEP (Table 3, Figure 1a). In males with low early SEP, current SEP was associated with 1.7 mmHg higher SBP (p = 0.013).
Table 2.
Association between early and current socio-economic position, their interaction, and cardiometabolic risk factors (maximum N = 4,120 males, 2,940 females)
| Risk factor | Unadjusted modela | Adjusted modelb | Interaction modelc |
|---|---|---|---|
| SBP (mmHg) | Males | ||
| SEP1 | 1.2 [0.1, 2.2], | 0.8 [–0.2, 1.9], | 1.4 [−0.1, 2.9], |
| p = 0.027 | p = 0.131 | P = 0.068 | |
| SEP2 | 1.4 [0.4, 2.4], | 1.2 [0.2, 2.2], | 1.7 [0.4, 3.0], |
| p = 0.004 | p = 0.017 | p = 0.013 | |
| SEP1*SEP2 | N/A | N/A | −1.1 [−3.0, 0.9], |
| p = 0.287 | |||
| Females | |||
| SEP1 | −1.1 [−2.4, 0.1], | −1.1 [−2.4, 0.2], | 0.5 [−1.4, 2.4], |
| p = 0.084 | p = 0.106 | p = 0.620 | |
| SEP2 | −0.4 [−1.6, 0.8], | −0.2 [−1.4, 1.1], | 1.2 [−0.5, 2.9], |
| p = 0.515 | p = 0.811 | p = 0.180 | |
| SEP1*SEP2 | N/A | N/A | −2.7 [ − 5.0, −0.3], |
| p = 0.028 | |||
| Body fat (%) | Males | ||
| SEP1 | 2.0 [1.6, 2.3], | 0.9 [0.5, 1.2], | 1.7 [1.2, 2.2], |
| p < 0.001 | p < 0.001 | p < 0.001 | |
| SEP2 | 4.1 [3.8, 4.4], | 3.9 [3.5, 4.2], | 4.6 [4.2, 5.1], |
| p < 0.001 | p < 0.001 | p < 0.001 | |
| SEP1*SEP2 | N/A | N/A | −1.6 [−2.3, −1.0], |
| p < 0.001 | |||
| Females | |||
| SEP1 | 1.9 [1.5, 2.4], | 1.2 [0.7, 1.6], | 2.3 [1.7, 2.9], |
| p < 0.001 | p < 0.001 | p < 0.001 | |
| SEP2 | 3.2 [2.8, 3.6], | 2.9 [2.5, 3.3], | 3.8 [3.3, 4.4], |
| p < 0.001 | p < 0.001 | p < 0.001 | |
| SEP1*SEP2 | N/A | N/A | −1.9 [−2.7, −1.1], |
| p < 0.001 | |||
| HOMA score (%-difference) | Males | ||
| SEP1 | 19.4 [12.3, 27.0], | 10.4 [3.6, 17.7], | 11.8 [2.5, 21.9], |
| p < 0.001 | p = 0.002 | p = 0.012 | |
| SEP2 | 33.5 [26.0, 41.5], | 30.1 [22.4, 38.2], | 31.6 [21.2, 42.9], |
| p < 0.001 | p < 0.001 | p < 0.001 | |
| SEP1*SEP2 | N/A | N/A | −2.5 [−13.3, 9.6], |
| p = 0.670 | |||
| Females | |||
| SEP1 | 5.7 [−1.6, 13.5], | 2.1 [−5.1, 9.9], | 2.1 [−8.0, 13.4], |
| p = 0.129 | p = 0.573 | p = 0.694 | |
| SEP2 | 14.7 [7.3, 22.6], | 14.2 [6.6, 22.2], | 14.1 [3.8, 25.5], |
| p < 0.001 | p < 0.001 | p = 0.006 | |
| SEP1*SEP2 | N/A | N/A | 0.0 [−12.4, 14.2], |
| p = 0.999 |
HOMA, Homeostasis Model Assessment; SBP, systolic blood pressure; SD, standard deviation; SEP1, high early socio-economic position; SEP2, high current socio-economic position.
Unadjusted model: SEP1 and SEP2 included in separate models.
Adjusted model: SEP1 and SEP2 included simultaneously.
Interaction model: SEP1 and SEP2 and their interaction included. All models are adjusted for age and include random effects for sib-pair and factory site.
Table 3.
Association between combined early and current socio-economic position and cardiometabolic risk factors
| N (%) | SBP (mmHg) | Missing | Body fat (%) | Missing | HOMA score (%-difference) | Missing | ||
|---|---|---|---|---|---|---|---|---|
| SEPa | 7,060 | – | 127 | 739 | ||||
| Males | 4,120 | – | 70 | 460 | ||||
| 0–0 | 1,250 (30.3) | Reference | – | Reference | 8 | Reference | 73 | |
| 1–0 | 692 (16.8) | 1.4 [−0.1, 2.9] | – | 1.7 [1.2, 2.2] | 7 | 11.8 [2.5, 21.9] | 39 | |
| 0–1 | 859 (20.8) | 1.7 [0.4, 3.0] | – | 4.6 [4.2, 5.1] | 17 | 31.6 [21.2, 42.9] | 165 | |
| 1–1 | 1,319 (32.0) | 2.0 [0.8, 3.3] | – | 4.7 [4.3, 5.2] | 38 | 43.4 [33.1, 54.5] | 183 | |
| Females | 2,940 | – | 57 | 279 | ||||
| 0–0 | 702 (23.9) | Reference | – | Reference | 10 | Reference | 45 | |
| 1–0 | 498 (16.9) | 0.5 [−1.4, 2.4] | – | 2.3 [1.7, 2.9] | 6 | 2.1 [−8.0, 13.4] | 25 | |
| 0–1 | 568 (19.3) | 1.2 [−0.5, 2.9] | – | 3.8 [3.3, 4.4] | 10 | 14.1 [3.8, 25.5] | 74 | |
| 1–1 | 1,172 (39.9) | −1.0 [−2.6, 0.5] | – | 4.2 [3.7, 4.7] | 31 | 16.6 [6.8, 27.3] | 135 | |
HOMA, homeostasis model assessment; SBP, systolic blood pressure; SEP, socio-economic position.
SEP coding: 0–0 = low early and low current SEP, 1–0 = high early and low current SEP, 0–1 = low early and high current SEP, 1–1 = high early and high current SEP.
Figure 1.
Adjusted means [95% confidence interval] of (a) systolic blood pressure (SBP, mmHg), (b) body fat (%), and (c) HOMA score (geometric mean) by permutations of socio-economic position (SEP). Coding of SEP: 0–0 = low early and low current SEP, 1–0 = high early and low current SEP, 0–1 = low early and high current SEP, 1–1 = high early and high current SEP.
Body fat
In the analysis of body fat, the results were similar in both genders (Table 2). High early SEP was associated with about 2%-units higher body fat and this estimate halved after adjustment for current SEP (p < 0.001 in all analyses). High current SEP was associated with 3–4%-units higher body fat and this was little attenuated after adjustment for early SEP. Current SEP was more weakly associated with body fat in those with high early SEP compared with those with low early SEP (interaction estimates: –1.6%-units in males and −1.9%-units in females, p < 0.001 in both). In the combined SEP analysis (Table 3 and Figure 1b), high early and low current SEP (1–0) group had about 2%-units and the low/high early and high current SEP groups (0–1 and 1–1) had 4–5%-units higher body fat than the reference group (0–0) (p < 0.001 for all comparisons).
HOMA score
Differences in HOMA scores by SEP were more pronounced in males than in females and the patterns of association were also different by gender. High early SEP was associated with higher HOMA score only in males (Table 2) and the effect size halved after adjustment for current SEP. High current SEP was associated strongly with higher HOMA score in both genders but in males the estimated effect size was over double of that observed in females, regardless of early SEP. In the combined SEP analysis in males (Table 3, Figure 1c), high current SEP (0–1) was more strongly associated with a higher HOMA score than high early SEP (1–0), and males with both early and current high SEP (1–1) had the highest score. In females, there was less evidence of such a graded pattern. Instead, it appeared that high current SEP (irrespective of early SEP) was associated with higher HOMA score.
Discussion
This study in a large Indian sample of adults found that indicators of higher SEP in childhood and adulthood were associated with cardiometabolic risk factors, and that some of the patterns of associations differed by gender. On the whole, we found that current SEP had a greater contribution to the cardiometabolic risk status than early SEP, suggesting that the effect of early SEP may be partly mediated through current SEP. In some of the analyses effect modification was present; for example, current high SEP was less strongly associated with higher body fat in those with high early SEP compared with those with low early SEP. These findings are consistent with the Forsdahl hypothesis which argued that poverty followed by subsequent affluence may be worse for cardiovascular risk than prolonged poverty.18 This suggests early and current SEP may not act independently on cardiometabolic risk factors. In this study, those individuals in the highest SEP group for both time periods had the worst cardiovascular profile, except for SBP in women.
Various conceptual models have been developed to describe the hypotheses on the relationship between exposures and disease outcomes over the life course.19,20 A critical period model is based on the idea that there is a limited time window in which an exposure adversely (or protectively) affects the development of an individual and subsequent disease outcome. A model in which later exposure to physiological or psychological stressors changes the effect of the exposure is called a critical period model with later effect modifiers. A sensitive period model is similar to the critical period model but it allows weaker effects also outside specific time windows, as well as more scope for modification/reversal of the effects. A simpler accumulation-of-risk model can be used to test the cumulative effect of exposures on the outcome, based on the number, duration or the severity of exposures at various times. Some of these models have been tested with respect to life course SEP and CVD risk factors, predominantly in developed countries.2,4 In the present study, SEP was only available at two time points, and therefore formal testing of these models was omitted. However, available data from the present study suggests consistency with an adult sensitive period model rather than a simple accumulation-of-risk model.
Comparisons with previous research
Comparisons between studies on SEP and cardiometabolic risk are challenging for various reasons. There are several dimensions of SEP (e.g. education, occupation, income, place of residence, ownership of household items) that are captured by different SEP definitions to varying degrees. Although many of the dimensions of SEP are interrelated, their associations with health outcomes may differ,6 which limits comparability of studies that have applied different SEP definitions. The timing of SEP measurement varies between studies, and few studies have measured SEP at several time points. Cardiometabolic outcomes measured in each study may also vary, ranging from intermediate risk outcomes (e.g. adiposity, blood pressure, insulin resistance) to CVD morbidity and mortality. The age of the study population strongly influences the choice of the outcomes measured. Furthermore, the study setting and design have to be considered when any comparisons are made.
In developed countries, such as England and Wales, the social gradient in cardiovascular disease has switched from a positive gradient with the affluent more affected in 1931 and 1951 to an inverse gradient of higher rates of heart disease among the poorer social classes by 1961,21 now a consistent finding in contemporary studies in the developed world. In developing countries there is evidence of a positive social gradient in cardiovascular disease.22 The explanation for such gradients and changes is not straightforward, and may depend on access to health care more than social patterning of risk factor distributions.23 In India, using rural dwelling participants from the IMS, we have demonstrated that tobacco and alcohol use, low intake of fruit and vegetables and being underweight were more common in lower current socioeconomic positions, whereas obesity, dyslipidaemia, diabetes (men only) and hypertension (women only) were more prevalent in higher current socioeconomic positions.8
The association between SEP over the life course and cardiometabolic risk factors has been little studied in developing countries. A recent review of five databases covering all countries and languages captured 30 studies on the relationship between childhood SEP and adulthood obesity, most of which also had adulthood SEP available.4 Childhood SEP was inversely associated with adulthood obesity in 70% of the included studies in females but only in 27% of the studies in males, adjusted for age. After an additional adjustment for adult SEP, this association persisted only in 47% of the studies in females and in 14% of the studies in males, suggesting that adult SEP may be one of the mechanisms explaining the observed inverse association between childhood SEP and adulthood obesity. However, all of these study populations were from developed countries (North America, Australia, New Zealand) except for one population from China. No studies in other Asian populations met the inclusion criteria. Our study in the Indian setting shows an opposite pattern compared with the studies reviewed from developed countries, with higher childhood and adulthood SEP associated with a higher body fat and other cardiometabolic risk factors. In both cases adulthood SEP seems to have a larger impact, probably mediating the effect of childhood SEP on the cardiometabolic outcomes. However, more data are needed from developing countries to enable reliable comparisons of the associations between life course SEP and cardiometabolic risk in different settings.
A review of Indian studies suggests that over time the association between high SEP and CVD outcomes has started showing reversal, and as the CVD epidemic matures, socio-economically disadvantaged groups are becoming increasingly vulnerable to CVD risk.6 However, studies from representative samples of Indian population do not support this conclusion.7, 24 Furthermore, nearly all studies included in this review have information available only on adulthood SEP, and are therefore unable to take into account the contribution of early life SEP on CVD risk. A migrant study that had recall data on pre-migration childhood SEP suggested that low SEP in both childhood and adulthood is associated with CVD mortality.9 However, the design of this study and the SEP measurement used (years of education) limit comparison with the present study. Nevertheless, it is possible that a similar reversal of the SEP associations on cardiometabolic risk that took place in Europe after the mid-1900s could happen in India in the next few decades, if the higher SEP groups gain better access to healthy foods and exercise facilities, and the lower SEP groups continue smoking, eat high-calorie, saturated fat foods and become more sedentary as occupational opportunities change.
Some studies suggest that subjects from urban middle-socioeconomic status have a high burden of cardiovascular risk factors in low-income countries.25 Migration to an urban environment often results in improvement in SEP; in the present study, 71% of participants with low early and high current SEP were migrants. Previous research within the IMS has also shown that migration to an urban environment rapidly increases body fat and gradually affects other cardiometabolic risk factors.26 The study also found an interaction between urban life-years and current SEP on adiposity, indicating a stronger effect on adiposity in recent migrants (≤10 urban life-years) from lower SEP groups. This interrelation between migration status and SEP is of importance when targeted public health interventions are planned in the Indian setting and suggest that recent migrants may be a vulnerable group.
Strengths and limitations
The present study is one of the first large studies to analyse SEP in two different time points in a low-income setting. The study design ensured a good representation of different SEPs and urban and rural sub-populations in the data. Although the design of the study was rather complex, involving sib-pairs, this was appropriately accounted for in the data analysis.
Some of the limitations of this study are possible measurement error and recall bias in the SEP measures. In particular, the participants may have found difficulty in recalling socio-economic circumstances from childhood, on average 30 years prior to the administration of the questionnaire. In addition, SEP was available in only two time points, which limited a detailed exploration of different life course models in this study.
Migration status, a characteristic closely related to SEP, was included only in a descriptive analysis and in a sensitivity analysis. Its association with cardiometabolic risk factors in the IMS has been analysed previously in detail.26 Sensitivity analyses (not shown) suggest that migration status may partly mediate the observed association between early SEP and SBP in males. Adjustment for migration status did not further attenuate the association between early SEP and body fat already adjusted for current SEP, but it explained part of the association between current SEP and body fat in both genders. A similar pattern was seen for HOMA score as for body fat.
Mediation of SEP associations through lifestyle factors such as diet and physical activity were not explored in this paper, since a separate manuscript is being prepared for that purpose. However, the relationship between migration and diet has already been analysed in the IMS.27 This study showed that migrant and urban participants had a higher overall energy intake. They reported up to 80% higher fruit and vegetable intake and up to 35% higher sugar intake. These groups represent to an extent the high current SEP groups in the present study. The overall energy consumption in these groups is of concern and could be considered as a target for interventions, while ensuring the levels of fruit and vegetable consumption will remain high.
Conclusions
This study found that high SEP in childhood and more strongly in adulthood is associated with cardiometabolic risk factors in an Indian population. The population-level increase in SEP in the Indian setting is closely related to lifestyle changes brought about by urbanization, and therefore life course SEP needs to be taken into consideration for designing interventions that aim for CVD prevention.
Acknowledgements
The authors thank the field staff and local investigators. The Indian Migration Study group comprises Professor K. Srinath Reddy, Dr Dorairaj Prabhakaran, Professor Tulsi Patel, Dr Lakshmy Ramakrishnan, Dr Ruby Gupta and Dr Tanica Lyngdoh (New Delhi); Professor R. C. Ahuja and Professor R. K. Saran (Lucknow); Dr Prashant Joshi and Dr N. M. Thakre (Nagpur); Dr K. V. R. Sarma, Professor S. Mohan Das, Dr R. K. Jain and Dr S. S. Potnis (Hyderabad); Professor Anura V. Kurpad, Dr Mario Vaz, A.V. Barathi and Dr Murali Mohan (Bangalore); Dr Chittaranjan Yajnik (Pune); Professor George Davey Smith and Professor Yoav Ben-Shlomo (Bristol); and Professor Shah Ebrahim and Dr Sanjay Kinra (London School of Hygiene and Tropical Medicine).
Funding
This work was supported by the Wellcome Trust (grant number GR070797MF). George Davey Smith works in a centre supported by the MRC (grant number G0600705) and University of Bristol.
Conflict of interest
The authors declare no conflicts of interest in preparing this article.
References
- 1.Goenka S, Prabhakaran D, Ajay VS, Reddy KS. Preventing cardiovascular disease in India – translating evidence to action. Curr Sci 2009; 97: 367–377 [Google Scholar]
- 2.Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health 2005; 5: 7–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Power C, Graham H, Due P, et al. The contribution of childhood and adult socioeconomic position to adult obesity and smoking behaviour: an international comparison. Int J Epidemiol 2005; 34: 335–344 [DOI] [PubMed] [Google Scholar]
- 4.Senese LC, Almeida ND, Fath AK, Smith BT, Loucks EB. Associations between childhood socioeconomic position and adulthood obesity. Epidemiol Rev 2009; 31: 21–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subramanian SV, Perkins JM, Ozaltin E, Davey Smith G. Weight of nations: a socioeconomic analysis of women in low- to middle-income countries. Am J Clin Nutr 2011; 93: 413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeemon P, Reddy KS. Social determinants of cardiovascular disease outcomes in Indians. Indian J Med Res 2010; 132: 617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subramanian SV, Subramanyam MA, Davey Smith G. Discrepancy between data and interpretation. Prev Med 2011; 52: 468–469; author reply 470 [DOI] [PubMed] [Google Scholar]
- 8.Kinra S, Bowen LJ, Lyngdoh T, et al. Sociodemographic patterning of non-communicable disease risk factors in rural India: a cross sectional study. BMJ 2010; 341: c4974–c4974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tillin T, Chaturvedi N, Forouhi NG, Davey Smith G, McKeigue PM. Cardiovascular disease mortality in relation to childhood and adulthood socioeconomic markers in British South Asian men. Heart 2008; 94: 476–481 [DOI] [PubMed] [Google Scholar]
- 10.Lyngdoh T, Kinra S, Ben-Shlomo Y, et al. Sib-recruitment for studying migration and its impact on obesity and diabetes. Emerg Themes Epidemiol 2006; 3: 2–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebrahim S, Kinra S, Bowen L, et al. The effect of rural-to-urban migration on obesity and diabetes in India: a cross-sectional study. PLoS Med 2010; 7(4): e1000268–e1000268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. International Institute for Population Sciences (IIPS) and ORC Macro. In. National Family Health Survey (NFHS-2), 1998-99. Mumbai: IIPS; 2000.
- 13.Subramanian SV, Nandy S, Irving M, Gordon D, Lambert H, Davey Smith G. The mortality divide in India: the differential contributions of gender, caste, and standard of living across the life course. Am J Public Health 2006; 96: 818–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramanian SV, Davey Smith G, Subramanyam M. Indigenous health and socioeconomic status in India. PLoS Med 2006; 3(10): e421–e421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998; 21: 2191–2192 [DOI] [PubMed] [Google Scholar]
- 16.Duseja A, Thumburu KK, Das A, et al. Insulin tolerance test is comparable to homeostasis model assessment for insulin resistance in patients with nonalcoholic fatty liver disease. Indian J Gastroenterol 2007; 26: 170–173 [PubMed] [Google Scholar]
- 17.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 1974; 32: 77–97 [DOI] [PubMed] [Google Scholar]
- 18.Forsdahl A. Living conditions in childhood and subsequent development of risk factors for arteriosclerotic heart disease. The cardiovascular survey in Finnmark 1974–75. J Epidemiol Community Health 1978; 32: 34–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C. Life course epidemiology. J Epidemiol Community Health 2003; 57: 778–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra G, Nitsch D, Black S, De Stavola B, Kuh D, Hardy R. A structured approach to modelling the effects of binary exposure variables over the life course. Int J Epidemiol 2009; 38: 528–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marmot MG, Adelstein AM, Robinson N, Rose GA. Changing social-class distribution of heart disease. Br Med J 1978; 2: 1109–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation 1998; 97: 596–601 [DOI] [PubMed] [Google Scholar]
- 23.Song YM, Ferrer RL, Cho SI, Sung J, Ebrahim S, Davey Smith G. Socioeconomic status and cardiovascular disease among men: the Korean national health service prospective cohort study. Am J Public Health 2006; 96: 152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian SV, Perkins JM, Khan KT. Do burdens of underweight and overweight coexist among lower socioeconomic groups in India? Am J Clin Nutr 2009; 90: 369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gupta R, Guptha S, Gupta VP, Agrawal A, Gaur K and Deedwania PC. Twenty-year trends in cardiovascular risk factors in India and influence of educational status. Eur J Cardiovasc Prev Rehabil 2011; in press. [DOI] [PubMed]
- 26.Kinra S, Andersen E, Ben-Shlomo Y, et al. Association between urban life-years and cardiometabolic risk. Am J Epidemiol 2011; 174: 154–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowen L, Ebrahim S, De Stavola B, et al. Dietary intake and rural-urban migration in India: a cross-sectional study. PLoS One 2011; 6(6): e14822–e14822 [DOI] [PMC free article] [PubMed] [Google Scholar]