Abstract
Multiminicore disease (MmD) is an autosomal recessive congenital myopathy characterized by the presence of multiple, short core lesions (known as “minicores”) in most muscle fibers. MmD is a clinically heterogeneous condition, in which four subgroups have been distinguished. Homozygous RYR1 mutations have been recently identified in the moderate form of MmD with hand involvement. The genes responsible for the three other forms (including the most prevalent phenotype, termed the “classical” phenotype) remained, so far, unknown. To further characterize the genetic basis of MmD, we analyzed a series of 62 patients through a combined positional/candidate-gene approach. On the basis of clinical and morphological data, we suspected a relationship between classical MmD and the selenoprotein N gene (SEPN1), which is located on chromosome 1p36 (RSMD1 locus) and is responsible for the congenital muscular dystrophy with rigid spine syndrome (RSMD). A genomewide screening, followed by the analysis of 1p36 microsatellite markers in 27 informative families with MmD, demonstrated linkage to RSMD1 in eight families. All showed an axial myopathy with scoliosis and respiratory failure, consistent with the most severe end of the classical MmD spectrum; spinal rigidity was evident in some, but not all, patients. We excluded linkage to RSMD1 in 19 families with MmD, including 9 with classical MmD. Screening of SEPN1 in the 8 families that showed linkage and in 14 patients with classical sporadic disease disclosed 9 mutations affecting 17 patients (12 families); 6 were novel mutations, and 3 had been described in patients with RSMD. Analysis of three deltoid biopsy specimens from patients with typical RSMD revealed a wide myopathological variability, ranging from a dystrophic to a congenital myopathy pattern. A variable proportion of minicores was found in all the samples. The present study represents the first identification of a gene responsible for classical MmD, demonstrates its genetic heterogeneity, and reassesses the nosological boundaries between MmD and RSMD.
Introduction
MmD is an early-onset, autosomal recessive congenital myopathy characterized by the presence of multiple, poorly circumscribed, short areas of sarcomere disorganization and mitochondria depletion (areas that are termed “minicores”) in most muscle fibers (Engel et al. 1971). Typically, no dystrophic signs, such as muscle fiber necrosis or regeneration or significant endomysial fibrosis, are present in MmD. The most common clinical presentations are neonatal hypotonia, delayed motor development, and generalized muscle weakness and amyotrophy, which may progress slowly or remain stable. Nevertheless, MmD is a clinically heterogeneous condition in which four subgroups have been distinguished (Ferreiro et al. 2000; Jungbluth et al. 2000; Ferreiro and Fardeau 2002). The phenotype of the classical form, which is the most prevalent, is characterized by the predominance of axial muscle weakness that leads, in two-thirds of patients, to the development of severe, life-threatening respiratory insufficiency and scoliosis. The moderate form of MmD with hand involvement consists of generalized muscle weakness that affects predominantly the pelvic girdle, and includes hand weakness, amyotrophy, and hyperlaxity; scoliosis and respiratory involvement are mild or absent (Ferreiro et al. 2002). Of the two other forms of the disease, one is characterized by a clinical picture similar to that of the classical form but includes ophthalmoplegia (MIM 255320) (Jungbluth et al. 2000), and the second is characterized by antenatal onset with arthrogryposis (Ferreiro et al. 2000). Because of this wide clinical spectrum, genetic heterogeneity of MmD was long suspected, and it has been recently proved by the identification of homozygous mutations of the skeletal muscle ryanodine receptor gene (RYR1 [MIM 180901]), which are responsible for the moderate form with hand involvement (Ferreiro et al. 2002). The genetic basis of the three remaining MmD forms was, so far, unknown.
We describe here the identification, in 17 patients with classical MmD, of nine different mutations in the selenoprotein N gene (SEPN1 [MIM 606210]), the gene recently recognized as responsible for congenital muscular dystrophy with rigid spine (RSMD [MIM #602771]) (Moghadaszadeh et al. 2001). Interestingly, three of these mutations are identical to those described elsewhere in patients with RSMD; six of them, including a mutation of the first base in the translation start codon ATG (1A→G), are novel changes. Linkage of MmD to the RSMD1 locus was excluded in 19 informative families with MmD, including 9 with the classical form of the disease; this observation demonstrates the existence of genetic heterogeneity, even within the classical group. The present report is the first description of a gene responsible for the classical MmD phenotype, and it indicates the need to reassess the nosological boundaries between MmD and RSMD, which, until now, have been considered two distinct entities.
Subjects and Methods
Patients
Sixty-two patients with MmD, who belonged to 41 families, were included in this study; 27 families were informative, and, of these, 14 were thought to include consanguineous parents (consanguinity documented in 12 and possible in 2), and 13 included ⩾2 affected children. This informative group comprised 48 patients with an initial diagnosis of classical (16 families), ophthalmoplegia (2 families), arthrogryposis-kyphosis (2 families), or atypical (7 families) MmD. Each of the remaining 14 families included a single patient with classical MmD. All parents appeared to be normal during clinical examination. Families F2, F6, F10, and F11 have been described elsewhere (as families F6, F13, F5, and F2, respectively, in the publication by Ferreiro et al. [2000]).
In all patients, the diagnosis of MmD was established after analysis, by light and electron microscopy, of a muscle biopsy specimen from at least one patient per family. Our selection criteria have been described in detail elsewhere (Ferreiro et al. 2002). They included a clinical picture highly suggestive of a congenital myopathy, evidence of autosomal recessive transmission, and the presence of multiple minicores in both type 1 and type 2 muscle fibers as the main morphological abnormality. The presence of any morphological sign of muscular dystrophy, such as a necrosis-regeneration pattern or significant endomysial fibrosis, was an exclusionary criterion. The typical myopathological features of MmD are shown in figure 1.
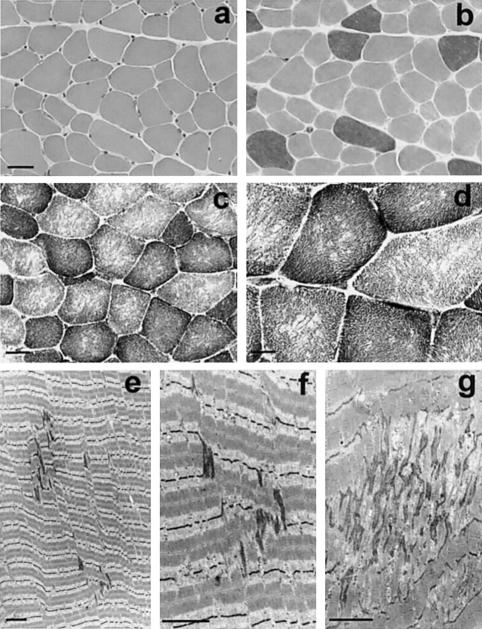
Figure 1.
Histochemical and ultrastructural features of MmD. a–d, Serial transverse cryostat sections. a and b, Patient 16 at 5 years of age, left deltoid, stained with hematoxylin-eosin (HE) (a) and myosin adenosine triphosphatase, pH 9.4 (b). Note variability of fiber size and predominance of type 1 (lighter) fibers. Although all the type 2 fibers show large diameters, both large and small type 1 fibers are observed, which produces a smaller mean diameter (“type 1 relative hypotrophy”). c and d, Patient 2 at 14 years of age, right deltoid; nicotinamide adenine dinucleotide–tetrazolium reductase (NADH-TR) (c) and succinate dehydrogenase (d). Multiple focal areas lacking oxidative activity are present in most type 1 (darker) and type 2 fibers. As shown here in two different biopsy specimens, there was no significant increase in endomysial connective tissue. e–g, Longitudinal electron micrograph sections from patients 8 (e, f) and 16 (g); left quadriceps and left deltoid at 23 and 5 years of age, respectively. e and f, Mild, early-stage minicore lesions; the sarcomeres appear out of register, Z-line streaming is limited to one sarcomere in length and 3–4 myofibrils in width, and the longitudinal myofilament array is still recognizable. g, Electron-dense material of Z-line origin, forming irregular zones, with severe focal disorganization of the myofibrillar structure. Bar=40 μm in a and b, 25 μm in c, 12.5 μm in d, and 2.5 μm in e–g.
Clinical evaluation, muscle biopsies, and drawing of blood samples were performed after written informed consent was obtained, in accordance with the protocol approved by the local ethics committees.
Morphological Methods
Specimens obtained during open muscle biopsies were immediately frozen or fixed and were processed for light microscopy and ultrastructural studies as described elsewhere (Ferreiro et al. 2000).
Genotyping
Genomic DNA was extracted from blood samples or lymphoblastoid cell lines, using standard procedures. A whole-genome screening was performed in 13 informative families, using a 400-microsatellite marker set (Applied Biosystems) with an average spacing of 10 cM. These fluorescently labeled markers were coamplified (an average of four markers per reaction) in a total reaction volume of 10 μl, using TaqGold polymerase (0.4 U) (Perkin-Elmer) and the GeneAmp 9700 PCR system (Applied Biosystems). After automated purification, the PCR products were separated using a MegaBace 1000 sequencer (Amersham Biosciences), and the allele values were defined using MegaBace Collection software and Genetic Profiler software, v. 1.1 (Amersham Biosciences).
Additional polymorphic markers were used for fine mapping of potential loci. Markers D1S3766, D1S3767, D1S2885, D1S3768, and D1S3769 (Moghadaszadeh et al. 1998) were amplified using forward primers labeled with 6-FAM, HEX, or NED fluorochromes and were placed on gels in an ABI 377 automated sequencer (Applied Biosystems). Results were analyzed using GENOTYPER software, v. 2.0 (Applied Biosystems).
Linkage Analysis
Linkage analysis was performed using the LINKAGE package, v. 5.2, under the assumptions of autosomal recessive inheritance, an equal recombination frequency for female and male subjects, a disease-gene frequency of 0.0001, one liability class, and a penetrance of 0.95. Two-point LOD scores were calculated for equal allele frequencies, using the MLINK program. Results were analyzed globally, family by family, and by groups of families with the same phenotype. Haplotypes were constructed under the assumption of the minimum number of recombinations.
SEPN1 Screening
All 13 SEPN1 exons and the selenocysteine insertion sequence (SECIS) element, a secondary structure located in the 3′ UTR of the transcript that allows selenocysteine incorporation at a UGA codon (Moghadaszadeh et al. 2001), were screened for mutations by PCR-SSCP and sequencing, using primers defined to amplify each exon and exon/intron boundary (Moghadaszadeh et al. 2001). We used the touchdown PCR method, with annealing temperature decreasing from 70°C to 60°C in the first 10 cycles and being fixed at 60°C in the final 20 cycles, except for exon 3 and SECIS, for which the touchdown 60°C–50°C method was used. All PCRs were performed using Platinum Taq polymerase (Gibco BRL/Life Technology), except for exon 1, which required the GC-rich PCR system kit (Roche) for amplification. SSCP analyses were performed on 10% acrylamide/bisacrylamide 37.5:1 gels at 7°C and 25°C. PCR products were sequenced on an ABI 3100 automated sequencer, using a BigDye terminator kit (Applied Biosystems).
Exons 5, 7, 8, 10, and 11 were analyzed by denaturing high-performance liquid chromatography (DHPLC) in 200 control chromosomes, as described elsewhere (Xiao et al. 2001).
Results
Identification of a Potential Linkage to Chromosome 1p36
A genomewide screening was undertaken in 13 informative families with classical MmD. Linkage to the locus on chromosome 19q13.1 that contains the RYR1 gene, which was recently identified as responsible for the moderate form of MmD with hand involvement, was excluded in all the families. We found multiple markers potentially linked to the disease in this set of families, but none of them gave a cumulative LOD score >3.
We then chose a combined positional/candidate-gene approach. A possible relationship between classical MmD and RSMD linked to chromosome 1p36 (RSMD1 locus) was suspected on the basis of the following data: both patients with RSMD and patients with classical MmD share a predominantly axial myopathy, with particular involvement of the respiratory function; four of the patients in our series, two of them siblings (F10), showed spinal rigidity (Ferreiro et al. 2000); the endomysial fibrosis seen in the biopsy specimens of most of the patients with RSMD is very mild, with a general absence of necrosis and regeneration. Marker D1S234, which had been included in the genome screening and is contiguous to RSMD1, was analyzed family by family, because of the suspicion of genetic heterogeneity. We found a potential linkage of this marker to the disease in 5 of the 13 families, including family F10, with a cumulative LOD score of 2.3 at recombination fraction (θ) = 0.0.
We then analyzed contiguous microsatellite markers defining the RSMD1 locus in all 27 informative families in our series. We confirmed linkage to these markers in a total of eight families, including seven with classical MmD and one that showed some atypical findings. The maximum cumulative LOD score in the linked subgroup was 5.55 for D1S3769 at θ=0.0 (table 1).
Table 1.
Pairwise Cumulative LOD Scores for Linkage between the Disease Phenotype and Markers on Chromosome 1p36 in the Eight Families Showing Linkage
| LOD Score at θ = |
|||||||
| Marker | .0 | .01 | .05 | .1 | .2 | .3 | .4 |
| D1S3766 | 4.80 | 4.65 | 4.06 | 3.34 | 2.02 | .97 | .29 |
| D1S3767 | 5.08 | 4.92 | 4.31 | 3.55 | 2.13 | 1.00 | .28 |
| D1S2885 | 4.34 | 4.21 | 3.69 | 3.05 | 1.84 | .87 | .25 |
| D1S3768 | 3.96 | 3.84 | 3.37 | 2.78 | 1.71 | .85 | .27 |
| D1S3769 | 5.55 | 5.39 | 4.74 | 3.95 | 2.45 | 1.22 | .38 |
Linkage to 1p36 was excluded in the remaining 19 families, including 9 classical ones, all the families presenting with arthrogryposis (2 families) or definite ophthalmoplegia (2 families), and 6 of the 7 families showing atypical features, such as congenital ptosis, swallowing problems, or later onset of the disease.
Identification of SEPN1 Mutations
The coding sequence and the SECIS element (Moghadaszadeh et al. 2001) of the SEPN1 gene were analyzed in the eight families showing linkage to 1p36 and in 14 additional patients with classical, sporadic disease. We identified nine different significant sequence alterations in 17 patients belonging to 12 families. The geographic origin of these families is summarized in table 2, and their clinical phenotype is summarized in table 3. We failed to find any mutation in one of the eight families with potential linkage to RSMD1.
Table 2.
SEPN1 Mutations in Patients with MmD
| Family | No. ofAffectedChildren | Origin | KnownConsanguinity | Exon | NucleotideChange | Homozygous | Amino AcidChangea |
| F1 | 1 | Germany | Yes | 1 | 22dup10bpb | Yes | Frameshift at Q8 |
| F2 | 2 | Italy | No | 1 | 1A→G | Yes | Unknown |
| F3 | 1 | Belgium | No | 1 | 1A→G | Yes | Unknown |
| F4 | 1 | Germany | Yes | 1 | 1A→G | Yes | Unknown |
| F5 | 1 | France | No | 5 | 713-714insA | Yes | Frameshift at N238 |
| F6 | 1 | France | No | 5 | 713-714insA | No | Frameshift at N238 |
| 11 | 1397G→A | No | R466Q | ||||
| F7 | 2 | Belgium | No | 7 | 943G→A | Yes | G315S |
| F8 | 2 | United Kingdom | No | 7 | 943G→A | Yes | G315S |
| F9 | 1 | Germany | No | 7 | 943G→A | No | G315S |
| 11 | 1397G→A | No | R466Q | ||||
| F10 | 2 | France | No | 7 | 878A→G | No | H293R |
| 8 | 1019A→T | No | N340I | ||||
| F11 | 2 | Portugal | No | 10 | 1384T→G | Yes | U462G |
| F12 | 1 | France | No | 10 | 1358G→C | No | W453S |
| 11 | 1397G→A | No | R466Q |
U = selenocysteine.
The insertion/duplication found in F1 (22dup10bp), causing a frameshift at Q8, has been reported elsewhere as G7fs in North African patients with RSMD (Moghadaszadeh et al. 2001).
Table 3.
Clinical Features of Patients with MmD
|
Respiratory Failurec |
|||||||||||
| FamilyandPatient(Sex) | PresentAge(years) | FirstSignsa | Walkedat Age(mo) | FacialWeaknessb | Rigid SpinePreviouslyRemarked | Age at Onsetof Scoliosis(Age atSpinal Fusion)(years) | Age atOnset(years) | % VCat Age(years) | Type of Ventilation (Age at Start)(years) | NightApnea | Progression of Weaknessd |
| F1: | |||||||||||
| 1 (M) | 18 | NH, poor HC | 20 | ++ | Yes | 12 | 14 | 32 (14) | Nasal NV (14) | Yes | Stable, AG |
| F2: | |||||||||||
| 2 (M) | 29e | NH, resp failure at birth | 13 | ++ | No | 10 | Birth | NA | Tracheo and NV (14) | NA | Stable |
| 3 (F) | 30 | NH, resp failure at birth | 15 | ++ | No | 10 | Birth | NA | Tracheo and NV (12) | NA | Stable |
| F3: | |||||||||||
| 4 (F) | 26 | DMM, feeding difficulties | 18 | +++ | Yes | 7 (11) | 10 | 28 (22) | Negative P NV (11), nasal NV (14) | Yes | Stable, AG |
| F4: | |||||||||||
| 5 (M) | 15 | Lack of HC, DMM | 18 | +++ | No | 10 (13) | 7 | 13 (14) | Nasal permanent V (7), tracheo (8) | Yes | Slowly progressive, wheelchair after age 9 years |
| F5: | |||||||||||
| 6 (F) | 17 | NH, weak suckling, lack of HC | 15 | + | No | 1.33 (12) | 11 | 28 (13) | Nasal NV (12) | Yes | Stable, AG |
| F6: | |||||||||||
| 7 (M) | 30 | Poor HC, DMM, failure to thrive | 15 | − | No | 15 | 8 | 28 (22) | Nasal NV (15), tracheo (16) | NA | Stable, unlimited gait at age 23 years |
| F7: | |||||||||||
| 8 (M) | 24 | Lack of HC, PMP, frequent falls | 18 | + | Yes | 14 (17) | 17 | 39 (17) | Nasal NV (17) | Yes | Stable, AG |
| 9 (M) | 21 | Lack of HC, PMP, frequent falls | 20 | + | Yes | 14 | 15 | 47 (17) | Nasal NV (16) | Yes | Stable, AG |
| F8: | |||||||||||
| 10 (F) | 17 | Failure to thrive | 18 | + | Yes | 12 | 13 | 30 (17) | Nasal NV (13) | No | Stable, AG |
| 11 (M) | 10 | Failure to thrive | 13 | + | No | Absent | NA | 40 (9) | None | No | Stable, AG |
| F9: | |||||||||||
| 12 (F) | 6 | NH, weak suckling, lack of HC | 16 | ++ | No | 5 | Absent | NA | None | No | Stable, AG |
| F10: | |||||||||||
| 13 (F) | 23 | NH, poor HC | 14 | ++ | Yes | 7 (13) | 7 | 37 (22) | Nasal NV (8) | Yes | Stable, AG |
| 14 (M) | 19 | NH, poor HC | 14 | +++ | Yes | 10 (14) | 6 | 38 (18) | Nasal NV (8) | Yes | Stable, AG |
| F11: | |||||||||||
| 15 (F) | 37 | Poor HC, DMM, resp insufficiency | 24 | + | No | 7 (13) | 9 | 23 (33) | Tracheo and NV (10) | NA | Slowly progressive, walks 50 meters |
| 16 (F) | 24 | Congenital cervical scoliosis | 14 | − | No | Birth (13) | 11 | 27 (19) | Nasal NV (12) | Yes | Stable, walks indoors |
| F12: | |||||||||||
| 17 (M) | 41 | DMM, thorax deformity | 36 | ++ | No | 3 (10) | 18 | 25 (20) | Tracheo and NV (20) | Yes | Slowly progressive after age 18 years |
HC = head control; NH = neonatal hypotonia; DMM = delayed motor milestones; PMP = poor motor performance; resp = respiratory.
+++ = severe; ++ = moderate; + = mild; − = absent.
NA = not available; NV = night ventilation; P = pressure; tracheo = tracheotomy; V = ventilation; VC = vital capacity in the sitting position (percentage of the expected values).
AG = autonomous gait.
Died at age 29 years.
The identified SEPN1 mutations are distributed throughout the entire gene (table 2). Surprisingly, a homozygous mutation of the start codon (1A→G), which changed the initiator methionine codon to a valine codon, was identified in three unrelated families of Italian, Belgian, and German origin (F2, F3, and F4, respectively), thus representing the most frequent mutation in this series.
A homozygous insertion/duplication of 10 bp in exon 1, which was described elsewhere in North African patients with RSMD (Moghadaszadeh et al. 2001), was identified in a German consanguineous family (F1). We found a novel insertion of 1 bp in exon 5 (713-714insA) in two unrelated French patients, one of them homozygous and the other compound heterozygous. These insertions cause a frameshift at Q8 and N238, respectively, and thus, theoretically, should lead to a truncated protein.
We identified six missense mutations in 11 patients. Two of them (1397G→A and 878A→G), which were described elsewhere in homozygous patients with RSMD (Moghadaszadeh et al. 2001), were found in the heterozygous state in four families with MmD (three French and one German); 1397G→A changes arginine 466 to glutamine, and 878A→G leads to replacement of histidine 293 by arginine. The remaining four were novel missense mutations. Homozygous 943G→A, changing glycine codon 315 to a serine codon (G315S), was identified in exon 7 in two unrelated families from Belgium and the United Kingdom that share identical RSMD1 haplotypes, thus suggesting a founder effect. A German patient with sporadic disease presented the same mutation in a heterozygous state. Interestingly, one homozygous mutation that transformed the selenocysteine codon in exon 10 into a glycine codon (U462G) was found in a Portuguese family. Two other heterozygous missense mutations, 1019A→T (N340I) and 1358G→C (W453S), were identified in two French families; one mutation changed an asparagine to an isoleucine, and the other changed a tryptophan to a serine. All these missense mutations changed evolutionarily conserved amino acids and were absent from 200 control chromosomes.
Segregation analysis showed that all the sequence changes were transmitted by the heterozygous parents; no de novo mutation was found.
Clinical Features
All the patients with MmD and SEPN1 mutations showed quite homogeneous clinical features, consistent with the severe end of the classical MmD spectrum and marked by congenital and severe axial weakness with early scoliosis and respiratory failure, which contrasted with a fairly well-preserved strength of limb muscles.
In all 17 patients (9 male and 8 female), the first signs of the disease were evident during the first year of life; in 8 of them, hypotonia, scoliosis, and/or respiratory failure were noticed at birth. Poor or no head control, revealing a predominantly axial hypotonia and weakness, was invariably an early sign. In contrast, the remaining milestones of motor development were only moderately delayed; 13 patients were able to walk independently at a normal age, and all acquired ambulation at the mean age of 17.7 mo. Because of this relatively good walking ability, a myopathy was not suspected in some patients until their late teens. Nevertheless, most patients have never been able to run and have always had difficulties climbing stairs or walking long distances, showing a certain degree of fatigability.
Although a variable degree of weakness and slenderness was present in most muscles, they were always more marked in axial muscle groups. Muscle weakness was extreme for neck flexion (0 to 2−, according to the Medical Research Council scale) and rotation; sternocleidomastoideus bulk was often particularly reduced. Cervical and dorsal spinal extensors (1 to 2+), trunk flexors (1+ to 2+), and scalene muscles and intercostal muscles were also severely affected. In contrast, limb muscles were relatively preserved, globally scoring between 3 and 4. Quadriceps power was often particularly good. Amyotrophy of the inner thigh led to a particular leg aspect that is shown in figure 2. Most patients had a nasal, high-pitched voice and a variable degree of facial weakness.
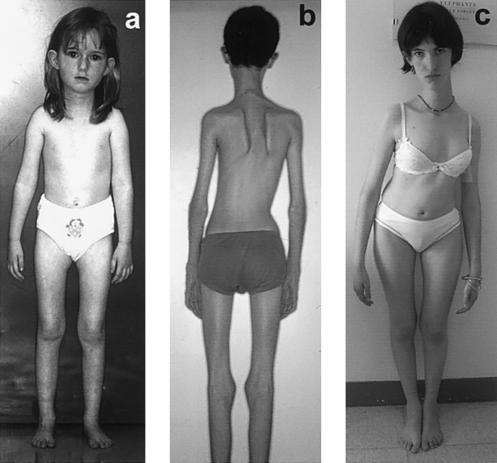
Figure 2.
Clinical features. a, Patient 12 at age 5.5 years. Slender neck with absent sternocleidomastoideus bulk, flat thorax, and moderate deltoid atrophy are present from early stages of the disease, as is an unusual appearance of the lower limbs, characterized by amyotrophic inner thighs, straight calves, and flat feet. Scoliosis also follows a particular pattern, with associated dorsal lordosis (“hollow back”) and a lateral trunk deviation and frequently requires an extensive arthrodesis (b and c). b, Patient 7 at age 15 years, immediately before surgical correction. c, Stabilization of the spinal deformity in patient 6 at age 17 years, 4 years after arthrodesis.
No limb contractures were observed in any patient who was <3 years of age. Indeed, most patients showed a moderate degree of distal hyperextensibility, which especially affected the metacarpal-phalangeal joints. In contrast, retractions of the pectoralis and intercostal muscles and of the costoiliac space were early, common, and severe findings, leading to a poorly developed, flat thoracic cage. Except in patient 16, who presented with congenital cervical scoliosis, cervical contractures were never early findings. In fact, the head dropped forward in some patients—either permanently or when walking—throughout infancy and childhood. In most patients, neck extensor retractions and a variable degree of spinal rigidity developed later; in some instances (seven patients) this was noticed by the clinician referring the patient, whereas in other patients (six) this information could be retrospectively retrieved from the family. Spinal rigidity was never detected in four patients. Hip flexor contractures were common, and, in one instance (patient 4), they resulted in a hyperlordosis that preceded the development of scoliosis by several years. Approximately half of the patients also developed late, mild-to-moderate knee and elbow retractions.
The most striking findings in these patients were early and severe respiratory failure and scoliosis. Scoliosis, present from a mean age of 8.5 years, was generally cervicodorsal, associated with dorsal lordosis and a lateral trunk deviation, and progressive, even in those patients who showed stable weakness or improving motor performance. In spite of early intensive orthopedic treatment, an extensive arthrodesis was required in nine patients, and the procedure resulted in stabilization of scoliosis and improvement of orthopedic and respiratory symptoms. Restrictive respiratory insufficiency (vital capacity 13%–47% of expected values) was the abnormality that led to referral of patients 15 and 17, and it required early respiratory assistance in all but the two youngest patients, who are now aged 10 and 5 years. Six patients underwent tracheotomy. The degree of hypercapnic respiratory insufficiency was always disproportionate to the severity of scoliosis and to the degree of general weakness; all the patients requiring ventilation were still ambulatory. The major determinants of this respiratory involvement were the involvement of accessory respiratory muscles, the presence of diaphragm dysfunction, and the severity of thorax deformities. In each case, the value of vital capacity measured in dorsal decubitus was significantly reduced, by 14%–46% of the value obtained when the same patient was sitting or standing, which clearly indicates diaphragmatic involvement. Diaphragm paralysis was radiologically demonstrated in five instances (patients 5, 10, 11, 15, and 17). Remarkably, episodes of nocturnal desaturation and a high frequency of short apneic periods were detected in 10 of the 13 patients in whom polysomnographic studies were performed, even at early stages of the disease with fairly well-preserved vital capacity (e.g., patient 9, with a vital capacity of 47%).
Failure to thrive, short stature, and low body weight were present in all but one patient and constituted the first referral sign in patients 7, 10, and 11. In early adulthood, the mean body weight was 36.25 kg, and the mean height was 1.54 m. Heart function tests, such as echocardiography, electrocardiography, and/or Holter recordings were routinely performed, and they disclosed no, or only minor, cardiac abnormalities. Intelligence was always normal. Serum creatine kinase levels were, in general, normal and were never more than four times as high as the upper limit of the normal range.
Muscle weakness remained stable in 13 (76%) patients and was slowly progressive in 4. Most patients remain able to walk, at least for short distances (as far as 1.5 km); eight of them lead entirely independent, active lives in adulthood. An exception is patient 5, who was initially considered to have an atypical, particularly severe form of MmD, because of early and marked ankle retractions (which required surgical correction), major hyperlordosis from the age of 1 year, absence of scoliosis before the age of 10 years, steady progression of limb weakness, and obesity. He lost the ability to walk at 8 years of age and is capable of only partial antigravity movements of hands and forearms at age 15 years. He has a facial diplegia and requires permanent ventilation through a tracheotomy, his vital capacity being reduced to 6% in the lying position.
The severity of the disease was defined by its effects on the respiratory function. One patient (patient 2) had severe respiratory insufficiency and died at 29 years of age. Symptoms of the remaining patients are well controlled, after scoliosis stabilization, with intermittent, positive-pressure, mainly nocturnal ventilation.
Morphological Findings and Reevaluation
In a specific search for the presence of dystrophic lesions, we performed a second analysis of the muscle biopsy specimens from eight patients with MmD presenting different mutations. The samples originated from the deltoid (five samples), quadriceps (one sample), or unspecified muscles (two samples). Ages at the time of the diagnostic biopsy were 5–20 years (mean 10.3 years). In patients 1, 4, and 12–15, a previous muscle biopsy had been considered normal or nondiagnostic.
Increased variability of fiber size, predominance and relative hypotrophy of type 1 fiber, and a variable percentage of centrally located nuclei (⩽6% of the fibers) were consistent findings. Typical oxidative-negative lesions affected both fiber types. Only a mild degree of endomysial fibrosis was present in the various samples, and it was entirely equivalent to that observed in any other congenital myopathy. No necrotic fibers were observed; in contrast, we found some sparse, isolated basophilic fibers, probably representing a regeneration process, in the samples from patients 7, 13, and 16 (maximum three regenerating fibers per transverse section of biopsy specimen).
In view of the genetic and clinical overlap, we analyzed, by light and electron microscopy, the muscle biopsy specimens from three patients who were previously diagnosed as having typical RSMD and who had known mutations in SEPN1 (patients RSMD1–RSMD3). Our objective was to reassess the morphological signs of congenital muscular dystrophy (CMD) and to look for the presence of core lesions. Analysis of these specimens, all taken from the deltoid muscle at similar ages, showed an unexpectedly wide myopathological spectrum (fig. 3). Patients RSMD1 and RSMD3 (of Turkish and Iranian origin, respectively) share the same homozygous mutation (G273R) and have been reported elsewhere (Moghadaszadeh et al. 2001). The biopsy from patient RSMD1 is consistent with typical MmD, because of the presence of multiple minicores in most type 1 and type 2 fibers in the absence of any dystrophic feature. In contrast, the sample from patient RSMD3 shows a clearly dystrophic pattern, with severe endomysial fibrosis, some regenerating fibers, and occasional small zones of Z-line streaming. French patient RSMD2 shows a homozygous insertion, 713-174insA, that was also found in some patients with MmD. Her biopsy specimen reveals an intermediate pattern, with abundant minicores, mild endomysial fibrosis, and sparse regenerating fibers.
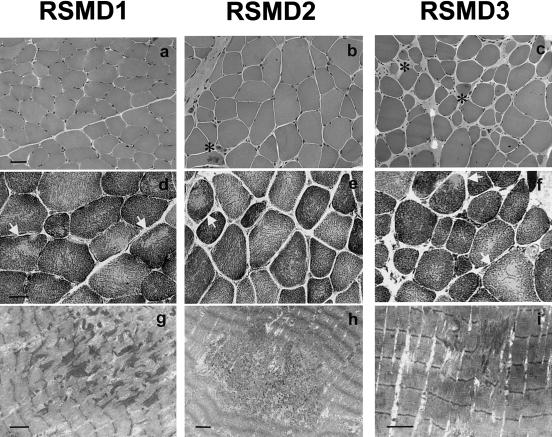
Figure 3.
Histochemical and ultrastructural analysis of deltoid muscle biopsies taken from patients with RSMD at the ages of 11 (RSMD1 and RSMD2) and 14 years (RSMD3). a–f, Transverse cryostat sections; g–i, longitudinal electron micrograph sections. All samples show fiber size variability (a–c, HE) and focal oxidative-negative areas (arrows; d–f, NADH-TR) corresponding, at the ultrastructural level, to short regions of Z-line streaming and sarcomere disarray (g–i). The myopathological pattern in RSMD1 is equivalent to that in patients with typical MmD; absence of necrosis, regeneration, and endomysial fibrosis coexists with the presence of short core lesions in most fibers. In contrast, in RSMD3, there is a predominance of dystrophic features, such as regenerative fibers (asterisks) and marked increase of endomysial connective tissue, while small zones of Z-line streaming were present in less than half of the fibers. Sample RSMD2 presented an intermediate pattern, with some rare regenerative fibers (asterisk), no significant fibrosis, and multiple minicores in approximately two-thirds of the fibers. Bar = 40 μm in a–c, 25 μm in d–f, and 2.5 μm in g–i.
Discussion
Congenital myopathies, such as MmD, are early-onset hereditary muscle disorders defined, on morphological grounds, by particular changes in the architecture of muscle fibers (Fardeau and Tomé 1994) that occur in the absence of necrosis, regeneration, or significant endomysial fibrosis. These last myopathological features are generally referred to as “dystrophic signs,” and, when they coexist with infantile-onset hypotonia and weakness, they are considered typical of another heterogeneous group of congenital muscle disorders, the CMDs (Dubowitz 1995). On the other hand, the presence of a variable degree of spinal rigidity is known to be a nonspecific manifestation of multiple myopathies (Merlini et al. 1989; Topaloglu et al. 1994; Fadic et al. 1997; Kubo et al. 1998). The term “rigid spine syndrome” (RSS) was first proposed by Dubowitz to describe the condition in a subset of patients affected by myopathy with early spinal contractures as a prominent feature (Dubowitz 1973). He referred to it as a nonprogressive “muscle syndrome in search of a name” and stressed the difficulties of classifying it, which made it, for some time, a myopathy of uncertain nosological position (Poewe et al. 1985). Only recently, a subgroup of patients presenting early-onset RSS, together with axial and proximal muscle weakness and severe respiratory failure, have been classified as having a form of CMD, RSMD (Echenne et al. 1983; Banker 1994; Muntoni and Guicheney 2002). A group of these families is linked to the RSMD1 locus, located on chromosome 1p36 (Moghadaszadeh et al. 1998); the gene causing the disease in these patients has recently been identified as SEPN1, which codes for selenoprotein N (Moghadaszadeh et al. 2001).
After independent analysis of a series of patients with MmD, through a combined positional/candidate-gene approach, we found nine SEPN1 mutations in 17 patients from 12 families. All of the patients showed an axial myopathy, with scoliosis and respiratory failure in all but the two youngest; a varying degree of spinal rigidity was a frequent, but not universal, feature. These findings provide definitive evidence that SEPN1 mutations are responsible for a proportion of cases of the classical form of MmD. Some of our patients have mutations previously found in patients with RSMD. In addition, after morphological reevaluation, we found typical minicore lesions in two of three patients with RSMD. Taken together with the clinical similarity, these data support the idea that RSMD and the most severe form of classical MmD are not only allelic disorders but the same entity.
Whether this entity must be considered a CMD or a congenital myopathy is, however, difficult to establish. In most patients with RSMD, both in the literature and in our series, no signs of necrosis or regeneration were observed; and endomysial fibrosis, considered the hallmark of CMD, was absent or mild (Muntoni and Guicheney 2002). Furthermore, minicores have been occasionally described in RSS (Serratrice et al. 1984; Ben Hamida et al. 1987), although the lack of details concerning oxidative staining and electron microscopy in most of the reported cases make the frequency of core lesions difficult to establish. Because minicores are nonspecific lesions verifiable only after extensive histochemical and ultrastructural analysis, which are not routinely performed in patients with CMD, their frequency may have been underestimated. We analyzed the biopsy specimens of the three patients with RSMD available for study in our laboratory and found typical minicore lesions in two of them and small regions of Z-line streaming in the third. Taken together, these data suggest that this entity constitutes a congenital myopathy with minicores, rather than a form of CMD. Nevertheless, the presence of some rare regenerating fibers in the morphological reanalysis of some patients with MmD, as well as the finding of a clearly dystrophic pattern in one of the patients with RSMD and SEPN1 mutations, is not consistent with the classical criteria of congenital myopathies. The patient's age at the time of the muscle biopsy and the type of muscle sampled might play a role in this morphological variability, because axial muscles tend to be more severely affected than those of the limbs. However, these factors cannot explain the differences we found in comparable deltoid samples taken at similar ages, which suggests that the structural changes in muscle probably do not represent a primary developmental abnormality. The nosological situation of this group of patients thus needs further discussion, but it is now clear that SEPN1 mutations are associated with a wide morphological spectrum. We propose that this entity could be tentatively referred to as SEPN-related myopathy.
In contrast with the morphological variability, the clinical features are remarkably homogeneous in all patients. The hallmark of their phenotype is the predominantly axial muscle impairment, leading to life-threatening respiratory involvement and scoliosis, which, if adequately treated, tend to remain stable after the growth spurt. A variable degree of spinal rigidity is evident in many, but not all, patients. The fact that limb-muscle strength remains relatively preserved—and that most patients are ambulatory—deferred the diagnosis of myopathy in some patients until they were in their late teens, when arthrodesis or respiratory assistance became necessary. In some patients, failure to thrive and diffuse amyotrophy, disproportional to weakness, were the referral signs. In fact, most patients in our series had a short stature and, as reported elsewhere (Seay et al. 1977; Goto et al. 1979, 1981; Mussini et al. 1982; Vogel et al. 1982; van Munster et al. 1986), hardly any subcutaneous fat.
The coding and genomic sequences of SEPN1 (GenBank accession numbers AJ306399 and AJ306398) have been recently established. The SEPN1 gene contains 13 exons and encodes a 590–amino acid protein whose function and subcellular localization are unknown. On the basis of the protein structure and analogies with other selenoproteins with known function, an enzymatic activity has been hypothesized (Lescure et al. 1999; Fagegaltier et al. 2000). With the exception of selenophosphate synthetase-2, the selenoproteins whose function has been identified so far are catalysts in redox processes (by using thiols as electron donors) or in thyroid hormone processing (Behne and Kyriakopoulos 2001). In humans, selenium deficiency has been related to the cardiomyopathy known as “Keshan” disease (Xu et al. 1997) and to a parenteral nutrition–associated myopathy (Brown et al. 1986; Osaki et al. 1998). In animals, selenium-deficiency diseases include livestock growth depression (“ill-thrift”) and white-muscle disease, a selenium-responsive myopathy of heart and skeletal muscle, principally affecting lambs and calves (Rayman 2000). Although there is not necessarily a strict correlation between selenium deficiency and SEPN abnormalities, some of these entities provide interesting data that, together with the morphological lesions present in SEPN-related myopathy, could provide some clues about the physiopathological mechanisms potentially implicated in this disease.
One of the primary lesions in white-muscle disease is calcification of skeletal and cardiac muscle (Schubert et al. 1961), and it has been demonstrated that the sarcoplasmic reticulum membranes from muscle of affected animals have lost their ability to sequester calcium (Tripp et al. 1993). It is thus interesting to note that the only genetic defect related so far to a minicore myopathy consists of mutations of RYR1, a gene encoding a calcium-release channel of the skeletal muscle sarcoplasmic reticulum (Ferreiro et al. 2002; Jungbluth et al. 2002). On the other hand, SEPN contains a motif that strongly resembles calcium-binding domains found in calcium-binding proteins, such as calmodulin (Moghadaszadeh et al. 2001). Furthermore, mitochondria have been suggested to respond to spatially confined Ca2+ release from sarcoendoplasmic reticulum Ca2+ stores (Rizzuto et al. 1998; Pacher et al. 2002). It is thus attractive to speculate that the focal areas of mitochondria depletion observed in both RYR1- and SEPN1-related myopathies might reflect abnormalities in the calcium-mediated localized interaction between the sarcoplasmic reticulum and the mitochondria. Further studies are necessary to confirm this hypothesis.
In conclusion, mutations in SEPN1 account for a considerable proportion of patients with predominantly axial myopathy, severe scoliosis, and early respiratory failure, whose morphological features range from typical MmD to typical CMD but almost always include minicore lesions. These findings open the way to a conceptual change in the nosological classification of early-onset myopathies, of which SEPN-related myopathy probably represents a much larger proportion than was expected.
Acknowledgments
We thank the patients and their families, for their participation in this study, and all the members of the European Neuromuscular Center MmD Consortium. We thank, as well, the Centre National de Genotypage (France) for technical assistance with the linkage analysis, to which Dr. N. Petit also contributed. Prof. H. Goebel (Germany), Prof. L. Heytens (Belgium), Dr. S. Castedo (Portugal), and Dr. D. Leclaire-Richard (France) contributed to the morphological analysis of muscle samples. Prof. A. Barois and Dr. I. Pagonari (France) helped to collect valuable clinical information. We also thank Prof. F. Hangfeld and Dr. G. Schreiber (Germany) for help with the clinical and pathological examination. This work was supported by funds from INSERM, the Association Française contre les Myopathies, the Fondation Electricité-Santé (to A.F.), EU grant Myo-Cluster QLG1-1999-00870, and the Muscular Dystrophy Campaign of Great Britain and Northern Ireland.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for RSMD1 [MIM 602771], SEPN1 [MIM 606210], minicore myopathy with external ophthalmoplegia [MIM 255320], and RYR1 [MIM 180901])
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for SEPN1 genomic sequence [accession number AJ306398] and SEPN1 cDNA sequence [accession number AJ306399])
References
- Banker BQ (1994) The congenital muscular dystrophies. In: Engel AG, Franzini-Amstrong C (eds) Myology. Vol 2. McGraw-Hill, New York, pp 1275–1289 [Google Scholar]
- Behne D, Kyriakopoulos A (2001) Mammalian selenium-containing proteins. Annu Rev Nutr 21:453–473 [DOI] [PubMed] [Google Scholar]
- Ben Hamida M, Hentati F, Ben Hamida C (1987) Maladie à multiminicores au cours d'un syndrome de la colonne raide. Rev Neurol (Paris) 143:284–289 [PubMed] [Google Scholar]
- Brown MR, Cohen HJ, Lyons JM, Curtis TW, Thunberg B, Cochran WJ, Klish WJ (1986) Proximal muscle weakness and selenium deficiency associated with long term parenteral nutrition. Am J Clin Nutr 43:549–554 [DOI] [PubMed] [Google Scholar]
- Dubowitz V (1973) Rigid spine syndrome: a muscle syndrome in search of a name. Proc R Soc Med 66:219–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ——— (1995) Congenital muscular dystrophy. In: Muscle disorders in childhood. W B Saunders, London, pp 93–105 [Google Scholar]
- Echenne B, Astruc J, Brunel D, Pages M, Baldet P, Martinazzo G (1983) Congenital muscular dystrophy and rigid spine syndrome. Neuropediatrics 14:97–101 [DOI] [PubMed] [Google Scholar]
- Engel AG, Gomez MR, Groover RV (1971) Multicore disease: a recently recognized congenital myopathy associated with multifocal degeneration of muscle fibers. Mayo Clin Proc 46:666–681 [PubMed] [Google Scholar]
- Fadic R, Waclawik AJ, Brooks BR, Lotz BP (1997) The rigid spine syndrome due to acid maltase deficiency. Muscle Nerve 20:364–366 [DOI] [PubMed] [Google Scholar]
- Fagegaltier D, Lescure A, Walczak R, Carbon P, Krol A (2000) Structural analysis of new local features in SECIS RNA hairpins. Nucleic Acids Res 28:2679–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardeau M, Tomé F (1994) Congenital myopathies. In: Engel AG, Franzini-Armstrong C (eds) Myology. Vol 2. McGraw-Hill, New York, pp 1487–1532 [Google Scholar]
- Ferreiro A, Estournet B, Chateau D, Romero NB, Laroche C, Odent S, Toutain A, Cabello A, Fontan D, dos Santos HG, Haenggeli CA, Bertini E, Urtizberea JA, Guicheney P, Fardeau M (2000) Multi-minicore disease—searching for boundaries: phenotype analysis of 38 cases. Ann Neurol 48:745–757 [PubMed] [Google Scholar]
- Ferreiro A, Fardeau M (2002) 80th ENMC International Workshop on Multi-Minicore Disease: 1st International MmD Workshop, May 12–13, 2000, Soestduinen, The Netherlands. Neuromuscul Disord 12:60–68 [DOI] [PubMed] [Google Scholar]
- Ferreiro A, Monnier N, Romero NB, Leroy J-P, Bönnemann C, Straub V, Haenggeli C-A, Voss WD, Nivoche Y, Jungbluth H, Lemainque A, Voit T, Lunardi J, Fardeau M, Guicheney P (2002) A recessive form of central core disease, transiently presenting as multi-minicore disease, is associated with a homozygous mutation in the ryanodine receptor type 1 gene. Ann Neurol 51:750–759 [DOI] [PubMed] [Google Scholar]
- Goto I, Muraoka S, Fujii N, Ohta M, Kuroiwa Y (1981) Rigid spine syndrome: clinical and histological problems. J Neurol 226:143–148 [DOI] [PubMed] [Google Scholar]
- Goto I, Nagasaka S, Nagara H, Kuroiwa Y (1979) Rigid spine syndrome. J Neurol Neurosurg Psychiatry 42:276–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungbluth H, Müller C, Brockington M, Brown S, Feng L, Chattopadhyay A, Mercuri E, Manzur A, Ferreiro A, Laing N, Davis M, Dubowitz V, Bydder G, Sewry C, Muntoni F (2002) Autosomal-recessive inheritance of RYR1 mutations in a congenital myopathy with cores. Neurology 59:284–287 [DOI] [PubMed] [Google Scholar]
- Jungbluth H, Sewry C, Brown SC, Manzur AY, Mercuri E, Bushby K, Rowe P, Johnson MA, Hughes I, Kelsey A, Dubowitz V, Muntoni F (2000) Minicore myopathy in children: a clinical and histopathological study of 19 cases. Neuromuscul Disord 10:264–273 [DOI] [PubMed] [Google Scholar]
- Kubo S, Tsukahara T, Takemitsu M, Yoon KB, Utsumi H, Nonaka I, Arahata K (1998) Presence of emerinopathy in cases of rigid spine syndrome. Neuromuscul Disord 8:502–507 [DOI] [PubMed] [Google Scholar]
- Lescure A, Gautheret D, Carbon P, Krol A (1999) Novel selenoproteins identified in silico and in vivo by using a conserved RNA structural motif. J Biol Chem 274:38147–38154 [DOI] [PubMed] [Google Scholar]
- Merlini L, Granata C, Ballestrazzi A, Marini ML (1989) Rigid spine syndrome and rigid spine sign in myopathies. J Child Neurol 4:274–282 [DOI] [PubMed] [Google Scholar]
- Moghadaszadeh B, Desguerre I, Topaloglu H, Muntoni F, Pavek S, Sewry C, Mayer M, Fardeau M, Tomé FM, Guicheney P (1998) Identification of a new locus for a peculiar form of congenital muscular dystrophy with early rigidity of the spine, on chromosome 1p35-36. Am J Hum Genet 62:1439–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadaszadeh B, Petit N, Jaillard C, Brockington M, Roy SQ, Merlini L, Romero N, Estournet B, Desguerre I, Chaigne D, Muntoni F, Topaloglu H, Guicheney P (2001) Mutations in SEPN1 cause congenital muscular dystrophy with spinal rigidity and restrictive respiratory syndrome. Nat Genet 29:17–18 [DOI] [PubMed] [Google Scholar]
- Muntoni F, Guicheney P (2002) 85th ENMC International Workshop on Congenital Muscular Dystrophy. 6th International CMD Workshop. 1st Workshop of the Myo-Cluster Project “GENRE,” October 27–28, 2000, Naarden, The Netherlands. Neuromuscul Disord 12:69–78 [DOI] [PubMed] [Google Scholar]
- Mussini JM, Mathe JF, Prost A, Gray F, Labat JJ, Feve JR (1982) Rigid-spine syndrome in a female patient [French]. Rev Neurol 138:25–37 [PubMed] [Google Scholar]
- Osaki Y, Nishino I, Murakami N, Matsubayashi K, Tsuda K, Yokoyama YI, Morita M, Onishi S, Goto YI, Nonaka I (1998) Mitochondrial abnormalities in selenium-deficient myopathy. Muscle Nerve 21:637–639 [DOI] [PubMed] [Google Scholar]
- Pacher P, Thomas AP, Hajnoczky G (2002) Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc Natl Acad Sci USA 99:2380–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W, Willeit H, Sluga E, Mayr U (1985) The rigid spine syndrome: a myopathy of uncertain nosological position. J Neurol Neurosurg Psychiatry 48:887–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280:1763–1766 [DOI] [PubMed] [Google Scholar]
- Schubert J, Muth O, Oldfield J, Remmert L (1961) Experimental results with selenium in white muscle disease of lambs and calves. Fed Proc 20:689–692 [PubMed] [Google Scholar]
- Seay A, Ziter F, Petajan J (1977) Rigid spine syndrome: a type I fiber myopathy. Arch Neurol 34:119–122 [DOI] [PubMed] [Google Scholar]
- Serratrice G, Pellissier JF, Pouget J, Gastaut JL (1984) Rigid spine syndrome and its nosological borders: 2 cases. Presse Med 13:1129–1132 [PubMed] [Google Scholar]
- Topaloglu H, Gogus S, Yalaz K, Kucukali T, Serdaroglu A (1994) Two siblings with nemaline myopathy presenting with rigid spine syndrome. Neuromuscul Disord 4:263–267 [DOI] [PubMed] [Google Scholar]
- Tripp MJ, Whanger PD, Schmitz JA (1993) Calcium uptake and ATPase activity of sarcoplasmic reticulum vesicles isolated from control and selenium deficient lambs. J Trace Elem Electrolytes Health Dis 7:75–82 [PubMed] [Google Scholar]
- van Munster ET, Joosten EM, van Munster-Uijtdehaage MA, Kruls HJ, ter Laak HJ (1986) The rigid spine syndrome. J Neurol Neurosurg Psychiatry 49:1292–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel P, Goebel HH, Seitz D (1982) Rigid spine syndrome in a girl. J Neurol 228:259–265 [DOI] [PubMed] [Google Scholar]
- Xiao W, Stern D, Jain M, Huber CG, Oefner PJ (2001) Multiplex capillary denaturing high-performance liquid chromatography with laser-induced fluorescence detection. Biotechniques 30:1332–1338 [DOI] [PubMed] [Google Scholar]
- Xu GL, Wang SC, Gu BQ, Yang YX, Song HB, Xue WL, Liang WS, Zhang PY (1997) Further investigation on the role of selenium deficiency in the aetiology and pathogenesis of Keshan disease. Biomed Environ Sci 10:316–326 [PubMed] [Google Scholar]