SUMMARY
SETTING
Patients with cavitary pulmonary tuberculosis (TB) on baseline chest radiograph (CXR) who remain culture-positive after 8 weeks of treatment are at high risk of relapse. The role of end-of-treatment (EOT) CXR in predicting relapse is unclear.
OBJECTIVE
To determine whether EOT CXR independently predicts TB relapse.
DESIGN
We conducted a secondary analysis of a randomized trial of intermittent treatment using rifapentine in the continuation phase of TB treatment among 1004 human immunodeficiency virus seronegative adults with culture-proven pulmonary TB.
RESULTS
Relapse occurred in 17.3% of subjects with persistent cavity on EOT CXR, in 7.6% of subjects with a cavity that resolved by EOT, and 2.5% (P = 0.002 for trend) of subjects who never had a cavity. In multivariable analysis, patients with persistent cavity on EOT CXR were significantly more likely to relapse than patients with no cavity on baseline or 2-month CXR (hazard ratio [HR] 4.22, 95%CI 2.00–8.91), and were more likely to relapse than subjects whose early cavity had resolved by EOT CXR (HR 1.92, 95%CI 1.09–3.39).
CONCLUSION
A persistent cavity after 6 months of TB treatment was independently associated with disease relapse after controlling for other variables. EOT CXR may help predict those likely to relapse.
Keywords: tuberculosis, relapse, chest radiograph
TUBERCULOSIS (TB) is a major global public health problem, with incidence rates ranging from 5 per 100 000 population per year in the United States and other market economies to over 400/100 000 in most of sub-Saharan Africa and much of Asia.1–3 Standard TB treatment requires a minimum of 6 months of four-drug rifampicin (RMP) based treatment for fully drug-susceptible isolates. Even with standard treatment, 5% or more of individuals treated with recommended regimens will either fail therapy or relapse with TB after treatment is completed. Risk factors associated with treatment failure or relapse include persisting organisms visible by microscopy that grow in culture despite 8–12 weeks of TB treatment,4,5 extent of baseline pulmonary disease (e.g., bilateral compared with unilateral disease),4 recognizable cavities on initial chest radiographs (CXRs),4,6 resistance to one or more TB drugs being used,7,8 malabsorption of TB drugs,9 human immunodeficiency virus (HIV) co-infection7,10,11 and patient non-adherence.12
Risk factors associated with TB relapse were identified in the context of a US-based randomized clinical trial—the TB Trials Consortium (TBTC) Study 22—a multicenter study comparing a standard twice-weekly TB treatment regimen during the continuation phase of therapy with a once-weekly regimen substituting a new drug, rifapentine (RFP), for the standard agent, RMP. The final study cohort of 1004 subjects was restricted to HIV-seronegative individuals.13 Five characteristics were found to be independently associated with increased risk of failure or relapse. These included positive sputum cultures for Mycobacterium tuberculosis at 2 months of TB treatment, the presence of a cavity on a CXR taken in the first 2 months of treatment, being underweight, having bilateral pulmonary involvement and being a non-Hispanic white person. After adjusting for the five independent risk factors, random treatment assignment did not significantly predict failure/relapse. The risk for disease relapse was over 20% in the group with both culture-positive sputum after 2 months of treatment plus a cavity on an early CXR, and <2% in the group with neither a cavity on CXR nor positive sputum cultures after 2 months of TB treatment. Based on these findings, the Centers for Disease Control and Prevention (CDC) recommends that TB treatment be extended from 6 to 9 months for individuals who fit into the highest risk group.14
The initial analysis of Study 22 data resulted in important findings with respect to failure/relapse, but the results may be of limited value to TB programs in countries with the highest TB burden because the high-risk group is defined by CXR findings plus 2-month culture data, which are rarely available in low-income countries. TB programs in such countries rely on sputum microscopy to look for the presence of TB bacteria in the sputum as a surrogate for culture. On the other hand, CXRs are available in some resource-poor countries that do not have mycobacterial culture capacity, and might provide a more accessible and efficient way to determine risk for relapse. We hypothesized that Study 22 data—including radiographic findings at the end of treatment (EOT) for pulmonary TB—could predict disease relapse independently from culture results, thus extending the usefulness of the data to resource-limited settings.
METHODS
We used a subset of TBTC Study 22 data—comprising 1004 HIV-seronegative adult subjects with drug-susceptible pulmonary TB—to conduct an analysis of EOT CXR and its ability to predict disease relapse independently of the 2-month culture results. In Study 22, subjects with fully susceptible pulmonary TB were randomized to one of two study arms after receiving 8 weeks of standard TB treatment, which included isoniazid (INH), RMP, pyrazinamide and ethambutol by directly observed treatment. Half of the subjects received twice-weekly INH and RMP, while the other half received once-weekly INH and RFP. All subjects received 24 weeks of treatment. CXRs were required within 2 weeks of randomization, which occurred after 8 weeks of standard treatment, and at the EOT. CXRs at the time of initial diagnosis were not required for the study, but if available, they were assessed in the same way as the study-required films and included in the study database.
Central reading of CXRs was not performed during Study 22. TB investigators or radiologists at each site reviewed study CXRs and assessed whether each was ‘normal’, had a cavity, or had evidence of bilateral disease, based only on standard postero-anterior and lateral CXRs. Although site investigators were either chest or infectious disease physicians with considerable experience in the diagnosis and management of TB, they did not receive specific training on CXR interpretation prior to Study 22. When subsequent CXRs were obtained, site investigators—who were not blinded to treatment arm or clinical condition—compared CXRs to earlier films and assessed whether there had been disease progression or development of any new lesions. Cavity on ‘early’ CXR was defined as the presence of a cavity on a CXR obtained within 2 weeks of the start of initial TB treatment (initial CXR) and/or cavity on a CXR obtained at the end of 8 weeks of induction therapy, just prior to being randomized to one of the 16-week continuation arms. ‘Persistent’ cavity on EOT CXR was defined as the presence of a cavitary lesion on an early CXR and on the EOT CXR.
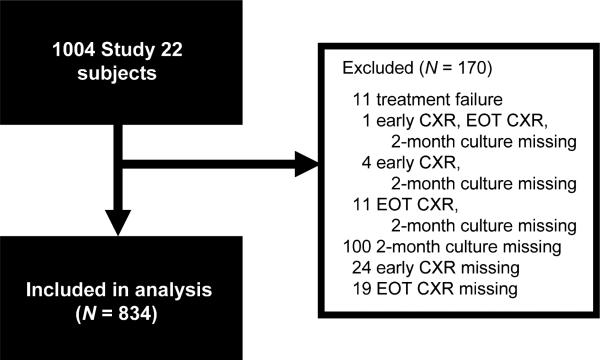
Study 22 subjects included in our analysis met the following inclusion criteria: 1) received at least one dose of study medication; 2) had available 2-month sputum culture results, ‘early’ CXR results and EOT radiograph results; and 3) did not meet the definition for treatment failure. ‘Treatment failure’ was defined as a positive culture at or after 4 months of TB treatment or evidence of progressive TB by clinical and/or radiographic methods without a positive culture. Because treatment failure was defined as occurring before the anticipated EOT, evaluating EOT CXR to predict this pre-EOT event would not be relevant. ‘Relapse’, on the other hand, was defined in Study 22 as a positive culture or clinical symptoms recurring after completion of treatment, and is the outcome variable for this analysis. Sputum was to be submitted for smear and culture on all study subjects at the EOT as well as at four follow-up study intervals over the ensuing 2 years. A general flow diagram for the EOT CXR relapse analysis is shown in Figure 1.
Figure 1.
Flow of data for EOT CXR and relapse analysis showing exclusions based on protocol. CXR = chest radiograph; EOT = end of treatment.
A traditional way to try to quantitate bacillary load is to grade the number of acid-fast bacilli (AFB) seen on direct microscopy of the expectorated sputum. We used a scoring system from 0 (negative) to 4+, corresponding to the number of M. tuberculosis bacteria present per ml of concentrated sputum.15 Because we did not have large numbers of subjects in each group, we grouped 3+ and 4+ smears together as indicating high-level bacillary load, and compared relapse rates to all the others (negative, 1+, and 2+).
Because central reading of CXR was not performed during Study 22, prior to our analyses we randomly sampled CXRs from 10% of the Study 22 subjects to determine whether CXR data recorded during the study were biased. Three investigators (CDH, JES and PCG) reviewed 241 films from 99 subjects across TBTC sites, blinded to the subjects’ treatment arms or outcomes, and developed a consensus reading which was then compared to the sites’ original interpretations. We found no significant bias except for the finding of bilateral disease (P = 0.0004, McNemar's χ2), which the consensus group read more often than the original interpretation. The kappa (κ) statistic was 0.54 for cavity (80% agreement), 0.72 (86% agreement) for bilateral disease and 0.74 (97% agreement) for the presence of new lesions. Based on these findings, we used the original Study 22 data regarding CXR findings for our analyses.
This project was granted exempt status by the Duke University Health System Institutional Review Board and a non-research determination at the CDC. Sites obtained local institutional review board exemption or approval prior to sending randomly selected CXRs for central review.
Statistical analysis
The objective of our analysis was to examine whether EOT CXRs independently predicted TB relapse, adjusting for other factors associated with TB relapse in the original study. The original Study 22 analysis found five independent risk factors for failure/relapse to be white race, underweight, cavity on early CXR (CXR at diagnosis or in first 8 weeks of treatment), bilateral pulmonary involvement and positive sputum culture after 8 weeks of standard treatment. After adjusting for the identified risk factors, treatment arm was not an independent predictor of failure/relapse.13 However, in order to confirm that treatment arm did not predict relapse in our subset of Study 22 subjects, we forced treatment into our initial multivariable model and confirmed that treatment arm was not a significant predictor of relapse, nor did it confound the estimates of the other predictors, and so it was excluded from the final model. In addition, the analysis of each arm independently identified the same variables to be significantly associated with TB relapse. For our analysis, the treatment arms were therefore combined and TB relapse was modeled as a dependent variable in a Cox proportional hazards model. For the purposes of the Cox model, subjects were censored at the time of death, loss to follow-up or completion of follow-up phase.
Univariate analysis was performed using each independent variable alone in this model. Multivariable analysis was performed by including all independent variables significantly associated with TB relapse in the original Study 22 analysis, plus two other findings on EOT CXR (bilateral disease and cavity). A final model was obtained by backward elimination, retaining variables significantly (P < 0.05) associated with TB relapse. Significant confounding was defined as a >10% change in the hazard ratio (HR) for a particular variable upon removal of another variable from the model;16 variables that were significant confounders were retained in the model regardless of statistical significance. Subjects with missing values for a given variable were removed from analyses involving that variable; no imputation was performed to correct for missing data.
RESULTS
Of the 1004 original Study 22 subjects, 834 met the inclusion criteria for the current analysis (Figure 1). Individuals meeting the inclusion criteria were comparable with those excluded in terms of age, sex, race/ ethnicity and CXR and clinical findings (Table 1).
Table 1.
Clinical characteristics of TBTC Study 22 subjects
| Subjects used for EOT CXR analysis (n = 834) % | Subjects with failure or missing data (n = 170) % | P value for comparison | |
|---|---|---|---|
| Mean age, years | 43.7 | 46.1 | 0.08 |
| Male sex | 75.2 | 73.5 | 0.65 |
| Race | 0.56 | ||
| Non-Hispanic black | 39.3 | 41.8 | |
| Non-Hispanic white | 15.3 | 18.1 | |
| Asian/Pacific Islander | 14.2 | 12.4 | |
| Hispanic | 24.5 | 28.2 | |
| Native American | 4.0 | 2.4 | |
| Underweight | 30.7 | 29.4 | 0.74 |
| Diabetes | 84.4 | 85.3 | 0.77 |
| Alcoholism | 55.6 | 55.3 | 0.95 |
| Early cavity | 53.1 | 57.5* | 0.34 |
| EOT cavity | 23.3 | 19.1† | 0.28 |
| Bilateral disease on enrollment CXR | 55.9 | 57.3‡ | 0.74 |
29 subjects missing data.
34 subjects missing data.
8 subjects missing data.
TBTC = Tuberculosis Trials Consortium; EOT = end of treatment; CXR = chest radiograph.
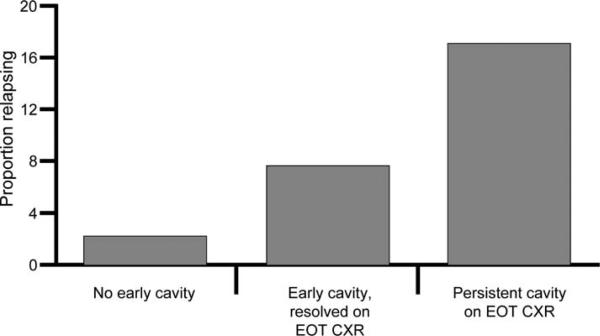
The first two columns of Table 2 show the results of univariate and multivariable analyses from the original Study 22 data as reference, and the second two columns show the results of our analysis of EOT CXR data. Sixty (7.2%) individuals relapsed—39 (9.3%) in the RFP (experimental) arm and 21 (5.1%) in the RMP (control) arm. All of the variables originally identified in Study 22 remained significantly associated with TB relapse in the 834 individuals included in our analysis. We found that TB relapse occurred in 17.3% (29/168) of subjects who had a persistent cavity on their EOT CXR, in 7.6% (21/275) of subjects whose early cavity had resolved at EOT CXR, and in 2.5% (9/365) of subjects for whom no cavity was seen on either early or EOT CXR (P = 0.002, Cochran-Armitage test for trend) (Figure 2).
Table 2.
Predictors of failure/relapse in original Study 22 data and in EOT CXR data*
| Original Study 22 data (n = 1004) |
EOT CXR data (n = 834) |
|||
|---|---|---|---|---|
| Variable | Univariate HR (95%CI) | Multivariable HR (95%CI) | Univariate HR (95%CI) | Multivariable HR (95%CI) |
| Treatment arm | 1.86 (1.09–3.16) | NA | 1.86 (1.09–3.16) | NA |
| Positive 2-month sputum culture | 4.73 (2.85–7.85) | 2.80 (1.64–4.77) | 4.73 (2.85–7.85) | 2.61 (1.52–4.47) |
| Cavity on CXR | ||||
| None | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| Cavity on early CXR | 4.55 (2.31–8.96) | 2.97 (1.48–5.96) | — † | — † |
| Cavity on early CXR, resolved | — † | — † | 3.01 (1.42–6.40) | 2.19 (1.02–4.71) |
| Persistent cavity on EOT CXR | — † | — † | 7.23 (3.52–14.85) | 4.22 (2.00–8.91)‡ |
| White race | 2.53 (1.49–4.31) | 1.89 (1.10–3.25) | 2.53 (1.49–4.31) | 1.84 (1.07–3.17) |
| Underweight | 4.13 (2.44–6.98) | 2.97 (1.74–5.06) | 4.13 (2.44–6.98) | 2.92 (1.71–4.99) |
| Bilateral disease (2-month) | 2.90 (1.57–5.36) | NA | 2.90 (1.57–5.36) | NA |
| Bilateral disease (EOT CXR) | — † | — † | 2.79 (1.66–4.69) | NA |
EOT CXR data excludes treatment failure cases and missing CXR or culture data.
Analyses not done.
P value = 0.02 for comparison of subjects with cavity on early CXR vs. subjects with persistent cavity on CXR.
EOT = end of treatment; CXR = chest radiograph; HR = hazard ratio; CI = confidence interval; NA = not available; TB = tuberculosis.
Figure 2.
Proportion of subjects with tuberculosis relapse by findings on early and EOT CXR. EOT = end of treatment; CXR = chest radiograph.
In addition, 26 subjects who were not reported to have a cavity on early CXR were reported to have a cavity on EOT CXR; one (3.9%) of these subjects relapsed. In some cases, this represented an initial infiltrate obscuring an underlying cavity, which then became evident when the infiltrate cleared with treatment. In other such cases, however, the cavity on EOT CXR may have represented over-interpretation by the treating physician.
In multivariable analysis that adjusted for white race, underweight, cavity on early CXR and positive sputum culture at 2 months, patients with a persistent cavity on EOT CXR were significantly more likely to relapse than patients with no cavity on early CXR (HR 4.22, 95% confidence interval [CI] 2.00–8.91, Table 2) and were more likely to relapse than patients whose early cavity had resolved by EOT CXR (HR 1.92, 95%CI 1.09–3.39, not shown in Table 2).
We also analyzed the data removing sputum culture data from the model to simulate the situation in many resource-poor countries where it is not possible to perform culture for TB. Persistent cavity on EOT CXR remained the best predictor of relapse (HR 4.81, 95%CI 2.31–10.03), followed by being underweight (HR 3.19, 95%CI 1.88–5.42), having a cavity on early CXR that resolved (HR 2.23, 95%CI 1.04–4.79), and having high initial bacillary load noted on sputum smear microscopy (3+ or greater) (HR 1.95, 95%CI 1.14–3.34) (Table 3).
Table 3.
Predictors of relapse when culture data are not available
| Variable | Univariate HR (95%CI) | Multivariable HR (95%CI) |
|---|---|---|
| Treatment arm | 1.86 (1.09–3.16) | NA |
| Initial sputum smear | ||
| Negative or <2+ AFB | 1.0 (referent) | 1.0 (referent) |
| High bacillary load ( AFB) | 2.74 (1.61–4.66) | 1.95 (1.14–3.34) |
| Cavity on CXR | ||
| None | 1.0 (referent) | 1.0 (referent) |
| Cavity on early CXR, resolved | 3.01 (1.42–6.40) | 2.23 (1.04–4.79) |
| Persistent cavity on EOT CXR | 7.23 (3.52–14.85) | 4.81 (2.31–10.03) |
| White race | 2.53 (1.49–4.31) | 2.24 (1.31–3.81) |
| Underweight | 4.13 (2.44–6.98) | 3.19 (1.88–5.42) |
HR = hazard ratio; CI = confidence interval; NA = not available; AFB = acid-fast bacilli; CXR = chest radiograph; EOT = end of treatment.
DISCUSSION
A cavity on CXR is reported in 40–50% of patients with newly diagnosed pulmonary TB.17 Similarly, nearly half of the subjects enrolled in TBTC Study 22 had a cavity at the time of their diagnosis. The 16.9% of subjects who had a persistent cavity at the end of 6 months of treatment had more than twice the risk of TB relapse (17.3% vs. 7.6%) than subjects whose cavity had closed by the end of treatment. After adjusting for other known predictors of TB relapse, persistent cavity on EOT CXR was independently associated with a higher TB relapse rate. This important finding notably extends the original findings of Study 22 and provides evidence that, even when 2-month sputum cultures cannot be obtained, TB programs could identify patients at highest risk for relapse and intervene with extended treatment or close post-treatment follow-up. Even programs that do not routinely obtain initial CXRs might choose to obtain EOT CXRs for individuals who were still AFB smear-positive after 8 weeks of treatment.
Our analysis had several limitations. The most significant limitation is that Study 22 included only HIV-seronegative individuals and our results may not be generalizable to persons with advanced HIV/acquired immune-deficiency syndrome (AIDS), who have a lower rate of cavitary disease.18–21 In addition, each site was responsible for interpreting its patients’ CXRs in a simple, standard format, but there was no central reading of CXRs, no pre-set definitions for reading cavities and no blinding to the patients’ treatment arm or clinical course. However, we did not find significant bias in local readings compared with our central readings, except for the finding of bilateral disease. The level of agreement between the original site and the centralized interpretations was good (κ 0.54), and consistent with other published reports.22 Nevertheless, we have tried to standardize CXR readings in subsequent TB treatment trials, to improve our precision. Finally, Study 22 employed intermittent TB treatment during the continuation phase of treatment; extrapolating our findings to patients receiving daily treatment throughout should be done with caution.
In conclusion, our results show that despite supervised state-of-the-art treatment, HIV-seronegative individuals with a persistent cavity at the end of therapy were at significantly higher risk of disease relapse. Our analysis provides evidence that EOT CXRs might serve as an alternative to sputum culture conversion for identifying HIV-seronegative populations at high risk for relapse, which could be useful to TB treatment programs where EOT CXRs are more accessible or affordable than documenting culture conversion after 2 months of treatment.
Acknowledgements
The US Public Health Service/TBTC Study 22 was sponsored by the US CDC and was funded in part through a Memorandum of Understanding between the CDC and the Washington DC Veterans Affairs Medical Center. Hoechst Marion Roussel, the manufacturer of RFP, provided RFP and contributed to the cost of three investigator meetings, but did not participate in original or secondary analysis study design, data collection, data analysis, data interpretation or writing of the report. CDH was supported by NIAID grant K24-AI001833. JES was supported by NIH/NIAID grant AI51409.
References
- 1.Corbett EL, Charalambous S, Fielding K, et al. Stable incidence rates of tuberculosis (TB) among human immunodeficiency virus (HIV)-negative South African gold miners during a decade of epidemic HIV-associated TB. J Infect Dis. 2003;188:1156–1163. doi: 10.1086/378519. [DOI] [PubMed] [Google Scholar]
- 2.Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362:887–899. doi: 10.1016/S0140-6736(03)14333-4. [DOI] [PubMed] [Google Scholar]
- 3.Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938–940. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]
- 4.Aber VR, Nunn AJ. Factors affecting relapse following short-course chemotherapy. Bull Int Union Tuberc. 1978;53:276–280. [PubMed] [Google Scholar]
- 5.Tripathy SP. Relapse in tuberculosis. Indian J Tuberc. 1981;28:45–57. [Google Scholar]
- 6.Mallory KF, Churchyard GJ, Kleinschmidt I, De Cock KM, Corbett EL. The impact of HIV infection on recurrence of tuberculosis in South African gold miners. Int J Tuberc Lung Dis. 2000;4:455–462. [PubMed] [Google Scholar]
- 7.Vernon A, Burman W, Benator D, Khan A, Bozeman L, for the Tuberculosis Trials Consortium Acquired rifamycin mono-resistance in patients with HIV-related tuberculosis treated with once-weekly rifapentine and isoniazid. Lancet. 1999;353:1843–1847. doi: 10.1016/s0140-6736(98)11467-8. [DOI] [PubMed] [Google Scholar]
- 8.Quy HT, Lan NT, Borgdorff MW, et al. Drug resistance among failure and relapse cases of tuberculosis: is the standard retreatment regimen adequate? Int J Tuberc Lung Dis. 2003;7:631–636. [PubMed] [Google Scholar]
- 9.Mitchison DA, Nunn AJ. Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. Am Rev Respir Dis. 1986;133:423–430. doi: 10.1164/arrd.1986.133.3.423. [DOI] [PubMed] [Google Scholar]
- 10.Driver CR, Munsiff SS, Li JH, Kundamal N, Osahan SS. Relapse in persons treated for drug-susceptible tuberculosis in a population with high coinfection with human immunodeficiency virus in New York City. Clin Infect Dis. 2001;33:1762–1769. doi: 10.1086/323784. [DOI] [PubMed] [Google Scholar]
- 11.Nettles RE, Mazo D, Alwood K, et al. Risk factors for relapse and acquired rifamycin resistance after directly observed tuberculosis treatment: a comparison by HIV serostatus and rifamycin use. Clin Infect Dis. 2004;38:731–736. doi: 10.1086/381675. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JL, Okwera A, Vjecha MJ, et al. Risk factors for relapse in human immunodeficiency virus type 1 infected adults with pulmonary tuberculosis. Int J Tuberc Lung Dis. 1997;1:446–453. [PubMed] [Google Scholar]
- 13.The Tuberculosis Trials Consortium Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet. 2002;360:528–534. doi: 10.1016/s0140-6736(02)09742-8. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1–77. [PubMed] [Google Scholar]
- 15.American Thoracic Society/Centers for Disease Control and Prevention Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 16.Kleinbaum DG, editor. Logistic regression: a self-learning text. Springer-Varlay; New York City: 1994. pp. 191–226. [Google Scholar]
- 17.Leung AN. Pulmonary tuberculosis: the essentials. Radiology. 1999;210:307–322. doi: 10.1148/radiology.210.2.r99ja34307. [DOI] [PubMed] [Google Scholar]
- 18.Perlman DC, el-Sadr WM, Nelson ET, et al. Variation of chest radiographic patterns in pulmonary tuberculosis by degree of human immunodeficiency virus-related immunosuppression. Clin Infect Dis. 1997;25:242–246. doi: 10.1086/514546. [DOI] [PubMed] [Google Scholar]
- 19.Eriki PP, Okwera A, Aisu T, Morrissey AB, Ellner JJ, Daniel TM. The influence of human immunodeficiency virus infection on tuberculosis in Kampala, Uganda. Am Rev Respir Dis. 1991;143:185–187. doi: 10.1164/ajrccm/143.1.185. [DOI] [PubMed] [Google Scholar]
- 20.Elliott AM, Luo N, Tembo G, et al. Impact of HIV on tuberculosis in Zambia: a cross-sectional study. BMJ. 1990;301:412–415. doi: 10.1136/bmj.301.6749.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman PC. Tuberculosis and AIDS. Radiol Clin North Am. 1995;33:707–717. [PubMed] [Google Scholar]
- 22.Graham S, Das GK, Hidvegi RJ, et al. Chest radiograph abnormalities associated with tuberculosis: reproducibility and yield of active cases. Int J Tuberc Lung Dis. 2002;6:137–142. [PubMed] [Google Scholar]