Abstract
Background
The purpose of the study is to review the CT findings associated with ventriculostomy placement in regards to the safety of an EVD plus recombinant tissue plasminogen activator (rt-PA) for IVH.
Methods
A retrospective review was conducted for patients receiving intraventricular rt-PA for IVH from January 2004 to September 2009. Safety was assessed by the presence of EVD tract hemorrhage by CT at baseline after EVD placement, worsening hemorrhage after rt-PA, and CSF infection. IVH volumetrics were assessed by the Le Roux score and outcomes by Glasgow Outcome Scale and modified Rankin Scale.
Results
Twenty-seven patients received rt-PA for IVH. Median dose was 2 mg (range 0.3–8) and a median of two doses (range 1–17) were given. Worsening EVD catheter tract hemorrhage after rt-PA was 46.7 %, with a significantly higher incidence of worsening tract hemorrhage seen with incorrectly placed EVDs (p = 0.04). IVH hematoma burden decreased by a median Le Roux score of 10 (range 3–16) prior to rt-PA to 4 (range 0–16) after rt-PA. There were no central nervous system bacterial infections.
Conclusion
Intraventricular rt-PA appears to be relatively safe especially when all EVD fenestrations are within the ventricle and reduces IVH burden similar to other studies. We describe a CT-based EVD tract hemorrhage grading scale to evaluate EVD tract hemorrhage before and after thrombolysis, and a bone-window technique to evaluate EVD fenestrations prior to IVH thrombolysis. Further research is needed evaluating these imaging techniques in regard to intraventricular thrombolytic safety and EVD tract hemorrhage.
Keywords: Intracerebral hemorrhage, Intraventricular hemorrhage, Cerebrovascular disease, Stroke, Critical care
Introduction
Intraventricular hemorrhage (IVH) is a neurological emergency that often results in poor outcomes for patients with high mortality rates if left untreated [1–5]. Mechanisms that play a role in the morbidity and mortality of IVH include elevation of intracranial pressure (ICP), ischemic encephalopathy from intracranial hypertension, hydrocephalus, and pro-inflammatory effects in response to the presence of blood in the ventricles [6–9].
The primary goal of treatment is removing blood from the ventricles. The current standard of care for IVH is placement of an external ventricular drain (EVD). The benefits of an EVD are reduction in ICP and cerebral spinal fluid (CSF) diversion, which can be offset by complications including: intracranial bleeding such as EVD tract hemorrhage, CSF infection (meningitis, ventriculitis), CSF leak, and EVD malposition and need for re-operation [10–12]. Use of an EVD alone to resolve IVH is likely inadequate due to the presence of clotted blood within the ventricle which often blocks the drainage of CSF, the EVD catheter, and more rapid removal of the IVH inflammatory blood products [13]. Controversy remains whether EVD alone improves the overall outcome in patients [2, 13].
Prior studies have shown that the intervention combining an EVD plus thrombolytic therapy has shown some benefit in patients with IVH [2, 6, 14, 15]. The introduction of thrombolytics into the intraventricular space has demonstrated faster clot removal, lowering of ICP, reduced duration of the EVD, and decreased edema and hydrocephalus [13, 16]. These additive effects of thrombolytic therapy have shown a trend towards better patient outcomes in relation to neurologic recovery [2, 6, 13–15]. There is also an ongoing international, randomized, placebo controlled trial investigating intraventricular rt-PA versus saline placebo (CLEAR IVH Phase III, NCT00784134) to help answer the scientific question whether rt-PA is superior to saline injections for IVH clearance and eventual clinical outcomes.
The purpose of this study is to evaluate the safety of an EVD plus recombinant tissue plasminogen activator (rt-PA) for the treatment of IVH. There was an additional emphasis on reviewing the computed tomography (CT) scan findings for the incidence of EVD tract hemorrhage before and after thrombolysis in relation to the EVD fenestration’s location within the brain or ventricle.
Methods
An observational, retrospective review of patients admitted to Mayo Clinic and St. Luke’s hospitals in Jacksonville, FL who received open-label intraventricular rt-PA for IVH from January 1, 2004 to September 30, 2009 was conducted. Data for the primary and secondary endpoints was collected from the electronic medical record. Approval to conduct this retrospective analysis was obtained from the Mayo Clinic Institutional Review Board (IRB# 09-007732).
Patient Selection and Institutional Intraventricular rt-PA Protocol
According to our institutional intraventricular rt-PA treatment protocol, patients were considered eligible for open-label intraventricular rt-PA if they were 18 years of age or older; had a diagnostic CT scan indicating IVH prior to rt-PA; had an EVD placed for clinical indications; and had administration of the first rt-PA dose within 72 h from the CT scan diagnosing IVH. Patients did not receive intraventricular rt-PA if they had a ruptured, unsecure intracranial aneurysm or arteriovenous malformation (AVM); uncontrolled intracranial hypertension; choroid plexus malformation; Moyamoya disease; platelet count less than 100,000; ongoing internal bleeding; or were pregnant. All patients who received open-label intraventricular rt-PA by the above inclusion and exclusion criteria were included in the study.
Data Collection
The primary endpoint of safety was the incidence of EVD catheter tract hemorrhage assessed by the EVD tract hemorrhage grading scale (Fig. 1) on serial CT scans. The EVD tract hemorrhage grading scale was defined for this study to quantify the severity of EVD tract hemorrhage since no grading scale exists. Correlation of EVD placement with presence of EVD fenestrations inside the ventricle or the surrounding parenchyma in relation to EVD tract hemorrhage was performed by comparing CT bone and parenchymal windows (Fig. 2). In order to determine if the EVD was placed correctly, the position of the EVD fenestrations were marked using the CT bone-window and then the CT parenchymal window was used to verify that all fenestrations were located within the ventricle. If all EVD fenestrations were within the ventricle then the EVD was defined as correctly placed and if any of the fenestrations were outside of the ventricle in the parenchyma then the EVD was defined as incorrectly placed for the analysis.
Fig. 1.
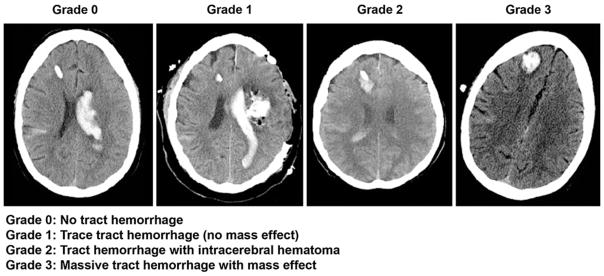
EVD tract hemorrhage grading scale (grading scale was defined for the purpose of this study). Examples of right frontal EVD’s (noncontrast head CT’s). The Grade 0 example is a patient with no tract hemorrhage, who had intraventricular hemorrhage primarily in left lateral ventricle and some right subarachnoid hemorrhage. The Grade 1 example is a patient with a intraparenchymal hematoma (left subcortical) status post decompressive hemicraniectomy and hematoma evacuation, large left lateral ventricular clot and trace cortical subarachnoid hemorrhage. There is trace hemorrhage around the EVD tract. The Grade 2 example is a patient with hematoma formation without midline shift (mass effect) with intraventricular blood and subarachnoid hemorrhage. The Grade 3 example shows sizeable tract hematoma with surrounding hypodense cerebral edema with subtle midline shift of the right parasagittal brain from right to left
Fig. 2.
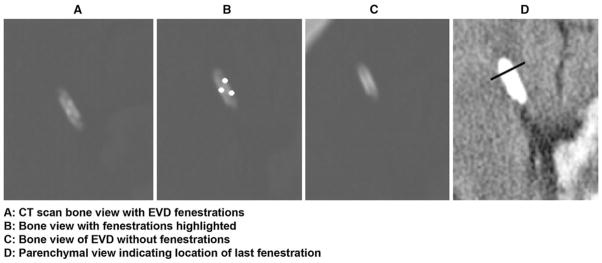
Bone and parenchymal window-technique for visualizing EVD fenestrations
Secondary endpoints included reduction of ventricular hematoma burden (IVH volumetrics) assessed by the Le Roux IVH severity grading system [17] on serial CT scans. The total Le Roux score (0–16) is a composite score grading each of the four ventricles separately (0–4) as follows: 0 = no blood; 1 = trace of blood; 2 = less than half the ventricle is filled with blood; 3 = more than half the ventricle is filled with blood; and 4 = the ventricle is filled with blood and expanded. Secondary clinical outcomes were assessed by comparison of baseline (pre-stroke) functional outcome to that at hospital discharge evaluated by the modified Rankin Scale (mRS) [18, 19] and Glasgow Outcome Scale (GOS) [20].
Other secondary endpoints were intensive care unit (ICU) and hospital length of stay, and incidence of bacterial central nervous system (CNS) infection from CSF microbiological culture. CSF was not routinely drawn, unless there was clinical suspicion for ventriculitis or meningitis (e.g., fever, headache, unexplained obtundation, leukocytosis), or at each time intraventricular rt-PA was injected a CSF sample was collected. Additionally CSF was obtained prior to ventricular peritoneal (VP) shunting for operative planning.
Statistical Analysis
Data for the primary endpoint was evaluated using the Fisher’s exact statistical test to evaluate if a significant difference in EVD tract hemorrhage existed between patients with correctly placed EVDs versus those with incorrectly placed EVDs. Statistical test was run using the Stats Direct software. Data for the secondary endpoints are presented with descriptive statistics including: means ± standard deviations (SD), medians, ranges, and percentages.
Classification of Evidence
The purpose of this study was to evaluate the safety of an EVD plus rt-PA for the treatment of IVH. This retrospective analysis provides Class IV evidence that EVD plus intraventricular rt-PA administration appears relatively safe with a reduced incidence of EVD tract hemorrhage when the EVD is placed correctly (verified by CT bone-window technique).
Results
Twenty-seven patients received open-label rt-PA for IVH and were included in this safety analysis. Baseline characteristics of the 27 patients are summarized in Table 1. Patients received a median of 2 mg of rt-PA per dose (mean 2.1 ± 1.3, range 0.3–8) and a median of two doses total for treatment (mean 3.7 ± 3.8, range 1–17). The median total amount of rt-PA administered to patients was 4 mg (mean 7 ± 7.0, range 0.5–25) over a median of 2 days (mean 4.2 ± 4.2, range 1–17). Dosing of rt-PA was as frequent as every 8 h but most often administration was once daily. Subsequent doses were administered only after another serial CT scan was performed and rt-PA was deemed appropriate by the neurosurgeon evaluating the CT scan. Administration of rt-PA was stopped when patient had clearance of IVH by CT, or at the discretion of the treating neurosurgeon.
Table 1.
Baseline characteristics of patients who received intraventricular rt-PA for IVHa
| Characteristic | Patients (n = 27) |
|---|---|
| Age, years | 59 (15.0) |
| Weight, kilograms | 82.8 (18.9) |
| Gender | |
| Male | 17 (63.0) |
| Female | 10 (37.0) |
| Ethnicity | |
| White/Caucasian | 16 (59.3) |
| African American | 7 (25.9) |
| Asian/Other | 4 (14.8) |
| Admission IVH category | |
| Primary IVH | 3 (11.1) |
| Secondary IVH (ICH or IPH) | 15 (55.6) |
| Secondary IVH (SAH)b | 9 (33.3) |
| Past medical historyc | |
| Hypertension | 22 (81.5) |
| Hyperlipidemia | 7 (25.9) |
| Previous TIA or stroke | 5 (18.5) |
| Medications prior to admission | |
| Anti-plateletd | 7 (25.9) |
| Warfarin | 2 (7.4) |
| Medications during admission | |
| Anti-plateletd | 8 (29.6) |
| Heparine | 12 (44.4) |
| Pro-coagulantf | 13 (48.2) |
ICH intracranial hemorrhage, IPH intraparenchymal hemorrhage, IVH intraventricular hemorrhage, SAH subarachnoid hemorrhage, TIA transient ischemic attack
Continuous data are presented as mean (one SD); categorical data as number of patients (percentage of sample)
Patients with secured aneurysms only
Risk factors for IVH
Aspirin, cilostazol, or clopidogrel
Heparin 5,000 units subcutaneous every 8 or 12 h; 1 patient received heparin during surgery
Aminocaproic acid, desmopressin, protamine sulfate, or recombinant factor VIIa
EVD Placement and Tract Hemorrhage
Twenty-seven patients had EVD placement as standard of care and had CT analyzed in terms of baseline EVD tract hemorrhage, and subsequent hemorrhage. Three patients received bilateral EVD placement making a total of 30 EVDs. Correct placement of the EVD, defined as having all fenestrations on the tip of the EVD fully inside the ventricle, occurred with 21 out of 30 (70 %) EVD placements. Hemorrhage along the EVD tract was assessed by the EVD tract hemorrhage grading scale (Fig. 1). A comparison of EVD tract hemorrhage grading and outcomes between correctly and incorrectly placed EVD’s by CT bone-window technique is depicted in a flow diagram (Fig. 3). Prior to the administration of rt-PA, the EVD tract hemorrhage grading scale was assessed on the pre- injection CT scan which showed 16 (53.3 %) of EVDs had no evidence of tract hemorrhage (Score 0); 5 (16.7 %) EVDs had trace tract hemorrhage with no mass effect (Score 1); 7 (23.3 %) EVDs had tract hemorrhage with intracerebral hematoma (Score 2); and 2 (6.7 %) EVDs had a massive tract hemorrhage with mass effect (Score 3). Evidence of EVD tract hemorrhage, defined by a tract hemorrhage score ≥2, was seen in 9 out of 30 (30 %) EVD placements prior to rt-PA.
Fig. 3.
Comparison of EVD Tract Hemorrhage grading scale and outcomes between correctly and incorrectly placed EVD’s by CT bone-window technique. EVD external ventricular drain, CT computed tomography, GOS Glasgow Outcome Scale. *These 2 patients had bilateral EVDs with both left and right EVDs incorrectly placed. †One patient had bilateral EVDs with the left EVD correctly placed and right EVD incorrectly placed
After rt-PA administration worsening tract hemorrhage on CT scan, defined by a one point increase in the tract hemorrhage score, was seen in 14 out of 30 (46.7 %) of all EVD placements, correct and incorrectly placed. The 2 patients with grade 3 hemorrhage prior to rt-PA were excluded from the worsening tract hemorrhage analysis because the scale has a maximum of 3 points. Incidentally, the 2 EVD’s with grade 3 hemorrhage prior to rt-PA one had no change in hemorrhage and the other had improvement of tract hemorrhage to a grade 2 after rt-PA administration. Of the 7 EVDs either correctly placed or incorrectly placed EVDs that had grade 2 tract hemorrhage prior to rt-PA administration, 4 (57.1 %) had worsening of the tract hemorrhage and 3 (42.9 %) had no change in tract hemorrhage. Of the 14 EVD placements (correct or incorrectly placed) with worsening tract hemorrhage after rt-PA administration, 5 (35.7 %) EVDs had improvement of the tract hemorrhage defined by a score ≤1 (trace tract hemorrhage with no mass effect) on the last CT scan prior to discharge. In regards to the placement of the EVD, worsening tract hemorrhage after rt-PA administration occurred with 7 out of 9 (77.8 %) of incorrectly placed EVDs and with 7 out of 21 (33.3 %) of correctly placed EVDs; p = 0.04.
IVH Volumetrics
The median Le Roux score on admission CT scan was 11 out of 16 (mean 9.7 ± 4.6) and 10 out of 16 (mean 9.9 ± 3.9) on the CT scan prior to the first dose of rt-PA. A reduction in hematoma burden occurred following rt-PA administration as seen by a decrease in the median Le Roux score to 4 out of 16 (mean 5.3 ± 3.9) on CT scan following the last dose of rt-PA. There was a further reduction in median Le Roux score to 2 out of 16 (mean 3 ± 4.3) as seen on the last CT scan prior to discharge. Median time from last dose of intraventricular rt-PA to last CT scan taken was 10 days (mean 12.6; range 0–58).
A reduction in intraventricular hematoma burden, defined by a decrease in Le Roux score ≥4 points, was observed in 21 (77.8 %) patients after treatment with rt-PA. Of the 6 patients who did not have a CT-measured reduction in hematoma burden (≥4 points), 3 patients died during hospitalization, 1 patient was discharged in a vegetative state, and 2 patients were discharged with moderate to severe disability. The lack of subsequent CT scans in these 6 patients was due to death or lack of follow-up CT scans, which prevented subsequent IVH hematoma burden measurements. The 2 patients discharged with moderate to severe disability both had a total Le Roux score of 4 out of 16 prior to rt-PA and at discharge had total scores of 1 and 2. Complete or near-complete removal of IVH, defined as a Le Roux score ≤1 (trace of blood) for each of the four ventricles at discharge, was seen in 18 (66.7 %) patients.
Clinical Outcomes
For the secondary endpoint of clinical outcome the mRS [18, 19] and GOS [20] were used. According to patient and family reports on admission, 22 (81.5 %) patients had no neurological deficits prior to hemorrhage (mRS 0); 2 (7.4 %) patients had symptoms without disability (mRS 1); 2 (7.4 %) patients had slight disability (mRS 2); and 1 (3.7 %) patient had severe disability from prior strokes (mRS 5). Outcomes for patients at discharge using the mRS scoring system are as follows: 3 (11.1 %) patients with moderate disability (mRS 3); 7 (25.9 %) patients with moderate/severe disability (mRS 4); 10 (37.1 %) patients with severe disability (mRS 5); and 7 (25.9 %) patients died during hospitalization (mRS 6). Outcomes for patients at discharge using the GOS scoring system are as follows: 7 (25.9 %) patients died during hospitalization (GOS 1); 2 (7.4 %) patients were in a vegetative state (GOS 2); 14 (51.9 %) patients with severe disability (GOS 3); and 4 (14.8 %) patients with moderate disability (GOS 4).
Secondary Outcomes
Median length of stay for patients was 16 days (mean 18.3 ± 11.4, range 2–63) in the ICU and 22 days (mean 24.6 ± 14.3, range 2–72) total in the hospital. There were no patients who developed a bacterial CNS infection, defined by a positive CNS culture for bacterial growth during hospital stay, in a total of 101 intraventricular injections of rt-PA. Five (18.5 %) patients required the placement of a permanent VP shunt prior to discharge from the hospital due to consistent elevations in ICP.
Discussion
Treatment of IVH with EVD plus rt-PA aims to expedite IVH clot removal and reverse the inflammatory mechanisms of IVH from the ventricles. Thrombolytics work by enhancing the conversion of plasminogen to plasmin in the presence of fibrin [21]. This expedites dissolution of the blood clot allowing the EVD to function properly. By administering thrombolytics directly into the intraventricular space, the goal is to achieve a localized effect of clot dissolution without systemic adverse effects.
One of the adverse effects noted in this study with intraventricular rt-PA administration was the presence of EVD tract hemorrhage. Tract hemorrhage was seen in 9 out of 30 (30 %) EVD’s placed prior to rt-PA administration by the EVD tract hemorrhage grading scale (Fig. 1), which may be caused by EVD insertion or coagulopathy. Prior to the development of a CT EVD tract hemorrhage scale, it is possible that subtle EVD tract hemorrhage may be under-appreciated in clinical practice and potentially exacerbated by subsequent intraventricular thrombolysis. It is also possible that a 1-point increase on the EVD tract hemorrhage scale did not have an appreciable clinical effect. Future studies are needed to measure clinical and EVD tract hemorrhage with clinical changes. Overall, worsening tract hemorrhage was associated with rt-PA administration in 46.7 % of patients, and with 4 out of the 7 (57.1 %) patients with grade 2 tract hemorrhage prior to rt-PA administration. This high percentage could largely be due to incorrect placement of the EVD with fenestrations being located in the parenchyma surrounding the ventricles allowing for rt-PA to be directly injected into the parenchyma. There was a significantly higher percentage of worsening tract hemorrhages occurring after rt-PA in EVDs that were incorrectly placed versus those correctly placed in this study, 77.8 % versus 33.3 %, respectively; p = 0.04.
Reduction in ventricular hematoma burden was seen after treatment with rt-PA with a decrease in median Le Roux score from 10 to 4 and further improvement to a median score of 2 by discharge. There were no reports of bacterial CNS infections in this study. Several factors contributing to the low incidence of infection include the use of a sterile field for rt-PA administration into the EVD and mixing the rt-PA using sterile technique over the sterile field. Also 5 (18.5 %) patients required VP shunt placement prior to discharge for consistent ICP elevations.
Outcomes of this study were compared to the outcomes of Coplin et al. [2] which evaluated patients with EVD alone and EVD plus urokinase plasminogen activator (u-PA); to Vereecken et al. [6] which evaluated EVD plus rt-PA; and to Naff et al. [15] which evaluated EVD plus rt-PA and EVD plus saline placebo (Table 2). The median initial GCS score was higher in our study compared to patients from the other three studies, Coplin et al. [2]; Vereecken et al. [6]; and Naff et al. [15] This may indicate that patients in our study may not have been as sick as the other patients or that there may be differences in the initial IVH burden or other pre-existing medical conditions. Mortality after treatment with EVD plus a thrombolytic agent appears to be similar among the four treatment groups with the highest percentage seen in the Coplin et al. [2] EVD plus u-PA and the lowest percentage seen in he Naff et al. [15] EVD plus rt-PA. The study by Vereecken et al. [6] also used the IVH severity grading system by Le Roux et al. [17] to evaluate reduction in ventricular hematoma burden. Comparing the mean Le Roux scores for patients in this study and Vereecken et al. [6], there appears to be a similar reduction in ventricular hematoma burden with EVD plus rt-PA administration.
Table 2.
Comparison of results with other published studies
| Jackson et al. n = 27 | Coplin et al. n = 18 | Coplin et al. n = 22 | Vereecken et al. n = 18 | Naff et al. n = 26 | Naff et al. n = 22 | |
|---|---|---|---|---|---|---|
| Intervention | EVD + rt-PA | EVD Alone | EVD + u-PA | EVD + rt-PA | EVD + NS placebo | EVD + rt-PA |
| Median Initial GCS | 10.0 | 5.0 | 5.5 | 4.0 | 7.0 | 8.0 |
| Favorable Prognosis (GOS 3–5) | 66.7 % | 22.2 % | 36.4 % | 72.2 % | – | – |
| Mortality | 25.9 % | 66.7 % | 31.8 % | 27.8 % | 23 % | 19 % |
| IVH Volumetrics (Le Roux score) | ||||||
| Prior to 1st rt-PA dose | 9.9 ± 3.9 | – | – | 11.8 | – | – |
| After last rt-PA dose | 5.3 ± 3.9 | – | – | 4.3 | – | – |
EVD external ventricular drain, NS normal saline, GCS Glasgow Coma Score, GOS Glasgow Outcome Scale, rt-PA recombinant tissue plasminogen activator, u-PA urokinase plasminogen activator, (–) indicates not reported in study
Limitations of this study include the retrospective design, lack of placebo control group for comparative outcome analysis, and small sample size. Another limitation includes the heterogeneous nature of the rt-PA dosing (amount, frequency, duration) which makes it difficult to assess ‘dose–response’ relationship. Future studies including CLEAR IVH Phase III are evaluating a standardized dose and subsequent CT imaging that can ascertain dose–response relationship. However, to our knowledge this is the largest retrospective safety study of IVH patients, outside the prospective CLEAR IVH trial and the trial by Naff et al. [15], treated with EVD plus intraventricular administration of a thrombolytic agent. The current study may be the first to evaluate the incidence of tract hemorrhage due to thrombolytic administration as well as the correlation of EVD fenestration position on CT bone window with the incidence of tract hemorrhage after rt-PA. With such a high percentage of tract hemorrhage associated with incorrect EVD placement, verification of correct EVD placement by CT scan should be considered prior to administration of rt-PA for IVH. We found the bone window method of finding EVD fenestrations (Fig. 2) to be simple and useful. This may be helpful in future IVH studies evaluating EVD plus thrombolytics since incorrect EVD fenestration position was associated with a significant increase in EVD tract hemorrhage.
Conclusions
EVD plus intraventricular rt-PA administration appears relatively safe for the treatment of IVH clot removal especially when all the EVD fenestrations can be identified to be completely within the ventricle by a simple CT bone-window technique. We also describe a useful easy-to-use CT EVD tract hemorrhage grading scale that can be used to assess tract hemorrhage over serial CT scans for research or clinical purposes. Our secondary outcome results appear consistent with other published studies [2, 6, 15] evaluating the use on an EVD plus thrombolytic agent for IVH. Although clinical outcomes were not the primary endpoint, 85 % of patients either died (26 %), were vegetative (7.4 %), or severely disabled (52 %) with only 15 % with moderate disability which reflects the devastating nature of this disease similar to other studies. To reduce infectious complications with intraventricular thrombolytics, administration should be performed using sterile preparation and technique. Further and larger studies are needed to see if there is a true correlation between EVD placement and tract hemorrhage and if intraventricular thrombolytics should not be administered in patients with an existing tract hemorrhage.
Acknowledgments
The authors would like to acknowledge the contributions of the following colleagues: Paula Fuqua, PharmD, CCRC; Jamila Russeau PharmD, BCPS; Leah Ward PharmD; and Amy Swan M.S., PharmD, for guidance and assistance in study protocol development and manuscript review. Michael Heckman, MS for general advice on using appropriate statistics for this study. Tara Brigham, MLIS and Victoria Jackson, MLIS for reviewing and formatting the final manuscript.
Footnotes
Disclosure Dr. Jackson reports no disclosures. Dr. Patel reports no disclosures. Dr. Darracott reports no disclosures. Dr. Hanel serves as a scientific advisor for NeuroVasx, Inc. although it is unrelated to this study. Dr. Freeman receives research support for his time involved as a site investigator in the CLEAR IVH III trial, NIH grant # U01 NS062851, which is a NINDS-Genentech funded study with Dr. Daniel F. Hanley as the principle investigator. Dr. Hanley receives research support for his time involved as principle investigator in the following clinical trials: CLEAR IVH III (NIH grant # U01 NS062851) and MISTIE ICH (NIH grant # U01 PAR 10198), which are NIH-NINDS funded studies; and as Jeffrey & Harriet Legum Professor at Johns Hopkins University.
Contributor Information
Daniel A. Jackson, Department of Pharmacy, Mayo Clinic, Jacksonville, FL, USA
Alden V. Patel, Department of Pharmacy, Mayo Clinic, Jacksonville, FL, USA
Robert M. Darracott, Department of Pharmacy, Mayo Clinic, Jacksonville, FL, USA
Ricardo A. Hanel, Department of Neurosurgery, Mayo Clinic, Jacksonville, FL, USA
William D. Freeman, Email: freeman.william1@mayo.edu, Departments of Neurology and Critical Care, Mayo Clinic, 4500, San Pablo Road, Jacksonville, FL 32224, USA
Daniel F. Hanley, Departments of Neurology, Neurosurgery, and Anesthesiology & Critical Care Medicine, The Johns Hopkins University, Baltimore, MD, USA
References
- 1.Little JR, Blomquist GA, Jr, Ethier R. Intraventricular hemorrhage in adults. Surg Neurol. 1977;8:143–9. [PubMed] [Google Scholar]
- 2.Coplin WM, Vinas FC, Agris JM, et al. A cohort study of the safety and feasibility of intraventricular urokinase for nonaneurysmal spontaneous intraventricular hemorrhage. Stroke. 1998;29:1573–9. doi: 10.1161/01.str.29.8.1573. [DOI] [PubMed] [Google Scholar]
- 3.Steiner T, Diringer MN, Schneider D, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery. 2006;59(4):767–73. doi: 10.1227/01.NEU.0000232837.34992.32. Discussion 773–764. [DOI] [PubMed] [Google Scholar]
- 4.Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinate of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27:617–21. doi: 10.1097/00003246-199903000-00045. [DOI] [PubMed] [Google Scholar]
- 5.Jayakumar PN, Taly AB, Rao BV, Arya BT, Nagaraja D. Prognosis in solitary intraventricular hemorrhage: clinical and computed tomographic observations. Acta Neurol Stand. 1989;80:1–5. doi: 10.1111/j.1600-0404.1989.tb03833.x. [DOI] [PubMed] [Google Scholar]
- 6.Vereecken KK, Van Havenbergh T, De Beuckelaar W, Parizel PM, Jorens PG. Treatment of intraventricular hemorrhage with intraventricular administration of recombinant tissue plasminogen activator A clinical study of 18 cases. Clin Neurol Neurosurg. 2006;108:451–5. doi: 10.1016/j.clineuro.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Steinke W, Sacco RL, Mohr JP, et al. Thalamic stroke. Presentation and prognosis of infarcts and hemorrhages. Arch Neurol. 1992;49:703–10. doi: 10.1001/archneur.1992.00530310045011. [DOI] [PubMed] [Google Scholar]
- 8.Lee KR, Betz AL, Kim S, Keep RF, Hoff JT. The role of the coagulation cascade in brain edema formation after intracerebral hemorrhage. Acta Neurochir (Wien) 1996;138:396–400. doi: 10.1007/BF01420301. discussion 400–401. [DOI] [PubMed] [Google Scholar]
- 9.Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: a previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke. 1998;29:1352–7. doi: 10.1161/01.str.29.7.1352. [DOI] [PubMed] [Google Scholar]
- 10.Ehtisham A, Taylor S, Bayless L, Klein MW, Janzen JM. Placement of external ventricular drains and intracranial pressure monitors by neurointensivists. Neurocrit Care. 2009;10:241–7. doi: 10.1007/s12028-008-9097-4. [DOI] [PubMed] [Google Scholar]
- 11.Guyot LL, Dowling C, Diaz FG, Michael DB. Cerebral monitoring devices: analysis of complications. Acta Neurochir Suppl. 1998;71:47–9. doi: 10.1007/978-3-7091-6475-4_15. [DOI] [PubMed] [Google Scholar]
- 12.Wiesmann M, Mayer TE. Intracranial bleeding rates associated with two methods of external ventricular drainage. J Clin Neurosci. 2001;8:126–8. doi: 10.1054/jocn.2000.0749. [DOI] [PubMed] [Google Scholar]
- 13.Hanley DF, Naff NJ, Harris DM. Intraventricular hemorrhage: presentation and management options. Semin Cerebrovasc Dis Stroke. 2005;5:209–16. [Google Scholar]
- 14.Hanley DF. Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage Phase III (Clear III Trial) Clinical Trials.gov. : NCT00784134. [PMC free article] [PubMed] [Google Scholar]
- 15.Naff N, Williams MA, Keyl PM, et al. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the intraventricular hemorrhage thrombolysis trial. Stroke. 2011;42(11):3009–16. doi: 10.1161/STROKEAHA.110.610949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naff NJ, Hanley DF, Keyl PM, et al. Intraventricular thrombolysis speeds blood clot resolution: results of a pilot, prospective, randomized, double-blind, controlled trial. Neurosurgery. 2004;54:577–83. doi: 10.1227/01.neu.0000108422.10842.60. discussion 583–584. [DOI] [PubMed] [Google Scholar]
- 17.LeRoux PD, Haglund MM, Newell DW, Grady MS, Winn HR. Intraventricular hemorrhage in blunt head trauma: an analysis of 43 cases. Neurosurgery. 1992;31:678–84. doi: 10.1227/00006123-199210000-00010. discussion 684–685. [DOI] [PubMed] [Google Scholar]
- 18.Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J. 1957;2:200–15. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 19.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–7. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 20.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–4. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 21.Package Insert. San Francisco, CA: Genetech Inc; 2011. Alteplase (rt-PA) [Activase®] [Google Scholar]



