Abstract
Tuberous sclerosis complex (TSC) is a familial hamartoma syndrome in which renal involvement is common and, at times, life threatening. We have investigated the potential effect of a non-TSC gene on renal disease in a cohort of 172 TSC patients with TSC2 mutations. Patients were genotyped for an interferon-γ (IFN-γ) microsatellite polymorphism, within intron 1, for which one common allele (allele 2, with 12 CA repeats) has been shown to have a higher expression of IFN-γ. A χ2 analysis was used to examine the association between IFN-γ allele 2 and the development of kidney angiomyolipomas (KAMLs) in this TSC2 cohort. Because of the age-dependent development of KAMLs in TSC, we initially focused on the 127 patients who were >5 years old. Additional subgroup analyses were done to investigate the influence of age and gender. The transmission/disequilibrium test (TDT) was also performed in a subset of this cohort (46 probands) for whom parent and/or sibling samples were available for analysis. Both χ2 analysis and TDT suggested an association between IFN-γ allele 2 and the absence of KAMLs in patients who have known TSC2 mutations. Among the 127 patients who were >5 years old, KAMLs were present in 95 (75%) and were absent in 32 (25%). In the group with KAML present, the frequency of IFN-γ allele 2 was 56%; in the group with KAML absent, the frequency of IFN-γ allele 2 was significantly higher, at 78% (P=.02, by χ2 analysis). The family-based TDT analysis gave similar results, with a TDT statistic (TDT χ2=5.45) corresponding to a P value of .02. Subgroup analyses show that both age and gender may influence the impact of this association. Although these results should be replicated in other populations with TSC, the present study suggests that modifier genes play a role in the variable expression of TSC and also suggests a potential therapy for KAMLs in patients with TSC.
Introduction
Tuberous sclerosis complex (TSC [MIM 605284 and MIM 191092]) is a multisystem familial tumor syndrome with autosomal dominant inheritance. Two causative genes (TSC1 and TSC2) have been identified (European Chromosome 16 Tuberous Sclerosis Consortium 1993; van Slegtenhorst et al. 1997). The tumors that develop are hamartomas, and the organs most commonly affected are the brain, skin, and kidneys (Kwiatkowski and Short 1994; Gomez et al. 1999; Cheadle et al. 2000). Although there is high penetrance, the expression is variable even among individuals with identical mutations (Smalley et al. 1994; Dabora et al. 2001). There is a wide range of severity of TSC manifestations, with some individuals so severely affected they are unable to care for themselves, while others are only mildly affected and may not be aware of their diagnosis until adulthood. Since many TSC lesions result from a two-hit mechanism, some of the variability is likely due to the stochastic nature of second-hit events (Henske et al. 1996, 1997; Niida et al. 2001). It is also possible that environmental exposures or modifier genes influence severity of disease in TSC.
Renal disease in TSC is a source of significant morbidity and mortality, since 17%–25% develop cysts and 55%–80% develop kidney angiomyolipomas (KAMLs) (Ewalt et al. 1998; Gomez et al. 1999; Jozwiak et al. 2000; Dabora et al. 2001). Transgenic mice and Eker rats in which the Tsc2 gene had been inactivated also developed significant renal disease in the form of kidney cystadenomas and carcinomas (Yeung et al. 1994; Kobayashi et al. 1999; Onda et al. 1999). Although the lesions are not identical to the cysts and angiomyolipomas that develop in humans with TSC, the frequency of tumors is similar. Strain differences observed in the mouse model of TSC2 suggest that modifier genes may influence severity of certain lesions (Onda et al. 1999).
Human interferon-γ (IFN-γ) is a homodimeric 34-kD peptide secreted by T lymphocytes and natural killer cells. It plays an important role in the coordinated regulation of expression of the immune response via the stimulation or repression of key genes (Farrar and Schreiber 1993). Human IFN-γ is encoded by a single gene on chromosome 12q24.1 and contains four exons with three introns. The first intron contains a CA microsatellite repeat that is highly polymorphic, with up to six alleles. Allele 2, with 12 CA repeats, has been shown to be associated with high levels of IFN-γ production in vitro (Pravica et al. 1999), and this may be due to its association with a nearby SNP within a putative NF-kappa B binding site (Pravica et al. 2000). This allele has been associated with higher or lower risk of a variety of diseases, including rheumatoid arthritis, allograft fibrosis in lung-transplant recipients, and acute graft-versus-host disease in bone-marrow–transplant recipients (Awad et al. 1999; Khani-Hanjani et al. 2000; Cavet et al. 2001).
Because IFN-γ has been shown to be a useful mediator of tumor regression in animal models of kidney tumors (Lee et al. 2000; Becker et al. 2001) and because there is a known high-expressing allele of IFN-γ in humans, we examined the relationship between the IFN-γ genotype and the severity of renal disease in patients with TSC who had TSC2 mutations.
Subjects and Methods
Patients and TSC Mutation Analysis
We studied 172 patients with TSC for whom we have identified TSC2 mutations and for whom we have data on renal phenotype; 121 have been described elsewhere (Dabora et al. 2001), and 51 are patients with new mutations found by similar methods. We included all available patients with both a known pathogenic TSC2 mutation and clinical information on the presence or absence of KAMLs. Most (150) patients were referred by pediatric neurology practices (80 from Warsaw, 49 from Cincinnati, and 21 from Boston), and the remainder (22) came from mail requests. Informed consent was obtained from all participants, and this study was approved by the institutional review boards at all participating institutions. The presence or absence of KAMLs was determined by ultrasound, computed tomography (CT), or magnetic-resonance imaging (MRI) and was graded on a scale of 0–3, as described elsewhere (Dabora et al. 2001). Grade 0 indicates that no KAMLs were detected; grade 1 indicates that one or more KAMLs <1 cm were detected; grade 2 indicates that one or more KAMLs >1 cm but <4 cm were detected; and grade 3 indicates that one or more KAMLs >4 cm were detected. The age used for each patient in this analysis was the age of the patient at most recent complete clinical evaluation. Kidney imaging was performed within 1 year of this age in most (132/172 [77%]) patients, within 2 years in 16/172 (9%) patients, and within ⩾3 years in 11/172 (6%) and could not be confirmed in 13/172 (8%).
The method of kidney imaging was determined by medical record review in 169/172 (98%) patients, and consisted of ultrasound in nearly all of these (162/169 [96%]); in 16/162 (10%), CT or MRI was done in addition to ultrasound. In the remaining seven patients, kidneys were imaged by CT (five patients) or MRI (two patients) scan. Of the 22 patients that had CT or MRI scans, most (21/22) had grade 2 or 3 KAMLs, and 1 had grade 1 KAMLs. Kidney-imaging data was collected prior to IFN-γ genotyping in all cases. Most (129/172 [75%]) of the kidney-imaging studies were read by radiologists at centers with specific expertise in the care of patients with TSC.
IFN-γ Genotyping
Genotyping for the intron 1 CA repeat of the IFN-γ gene was performed by modification of the fluorescent PCR method described by Khani-Hanajani et al. (2000). The following primers were obtained from Research Genetics: forward, 5′-6-FAM AGACATTCACAATTGATTTTATTCTTAC-3′, and reverse, 5′-GTGTCTTCCTTCCTGTAGGGTATTATTATACG-3′. The underlined portion of the reverse primer is a 7-bp clamp that was added to reduce stutter artifact. PCR was performed using AmpliTaqR Gold (Perkin Elmer); 20-μl reactions were used with 10–50 ng of genomic DNA, 0.5 μM of each primer, 10 mM deoxyribonucleoside triphosphates, 0.2 μl of AmplitaqR gold (1 U), and the manufacturer's recommended buffers. PCR cycling was performed on an MJ Research PTC-100 thermal cycler, at 95°C for 12 min, followed by 35 cycles at 94°C for 30 s; 57°C for 30 s, for annealing; 72°C for 45 s, for extension; and a final extension step at 72°C for 4 min. The 6-FAM–labeled PCR products were run on an ABI 3100 capillary sequencer with HD 400 as a size standard. Genescan 3.7 software (ABI) was used to determine the size of the amplicons, to assign the IFN-γ alleles present in each sample, and was confirmed by manual review.
Statistical Analysis
χ2-, Fisher-, and t-test analyses were done with Statview 5.0 software. The χ2 or Fisher test was used for nominal variables, and the one-tailed t test was used for quantitative variables. The transmission/disequilibrium test (TDT) statistic was calculated, as described by Spielman et al. (1993), as follows: TDT χ2=(b-c)2/(b+c), where TDT χ2 is used to calculate the P value from a χ2 table with 1 df, b represents the number of times that the allele of interest is transmitted, and c represents the number of times that it is not transmitted.
Results
IFN-γ Allele 2 Is Associated with Absence of KAMLs in TSC2 Patients >5 Years Old
We studied 172 patients with known TSC2 mutations and for whom clinical data on the presence or absence of KAMLs were available, to investigate the association of IFN-γ allele 2 with the development of KAMLs in TSC2 patients. Because of the age-dependent development of KAMLs in TSC (Kwiatkowski and Short 1994; Ewalt et al. 1998; Jozwiak et al. 2000), we initially considered only the 127 individuals who were >5 years old. Mutation analysis was performed as described elsewhere (Dabora et al. 2001), and KAMLs were detected by renal ultrasound, CT, or MRI scan. As shown in table 1, there were 95 (75%) TSC2 patients with KAMLs present and 32 (25%) TSC2 patients with KAMLs absent in this cohort. This includes 44/59 (75%) from Warsaw, 30/35 (86%) from Cincinnati, 11/13 (85%) from Boston, and 10/20 (50%) from mail requests. Gender and age distribution were similar in the KAML-present and KAML-absent groups. However, the frequency of IFN-γ allele 2 was 78% (25/32) in the KAML-absent group and 56% (53/95) in the KAML-present group. This difference is statistically significant (P=.02, by χ2 analysis). Our data do not demonstrate a trend of lower frequency of IFN-γ allele 2 in patients with higher-grade KAMLs, but this analysis is limited by relatively small numbers of patients with KAMLs of grades 2 and 3. The frequency of IFN-γ allele 2 for KAMLs of grades 1–3 was 57% (31/54 patients) for grade 1, 42% (10/24 patients) for grade 2, and 71% (12/17 patients) for grade 3. Viewed from the perspective of allelic risk, 68% of TSC2 patients with allele 2 have KAMLs, and 32% do not. In contrast, 86% of TSC2 patients without allele 2 have KAML, and 14% do not. Thus, TSC2 patients with IFN-γ allele 2 are approximately twice as likely to be free of KAMLs as are TSC2 patients without IFN-γ allele 2 (table 2).
Table 1.
IFN-γ Allele Frequencies in TSC2 Patients >5 Years Old
| All | KAMLAbsent | KAMLPresent | P | |
| N (%) | 127 | 32 (25) | 95 (75) | |
| Male (%) | 58 (46) | 15 (47) | 43 (45) | |
| Female (%) | 69 (54) | 17 (53) | 52 (55) | |
| Mean age (range) [in years] | 15.2 (6–49) | 14.1 (6–34) | 15.6 (6–49) | .37a |
| No. with allele 2 present (female/male) | 78 (40/38) | 25 (12/13) | 53 (28/25) | .02b |
| No. with allele 2 absent (female/male) | 49 (29/20) | 7 (5/2) | 42 (24/18) | |
| % with allele 2 present | 61 | 78 | 56 | |
| % with allele 2 absent | 39 | 22 | 44 |
By t test; result not significant.
By χ2 analysis.
Table 2.
KAML Frequency in the Presence or Absence of IFN-γ Allele 2 in TSC2 Patients >5 Years Old[Note]
|
No. (%) with IFN-γ Genotype |
||
| 2,2 or 2,X | X,X | |
| KAML present | 53 (68) | 42 (86) |
| KAML absent | 25 (32) |
7 (14) |
| Overall | 78 | 49 |
Note.— Allele X represents any allele other than 2.
IFN-γ Allele and Genotype Frequencies
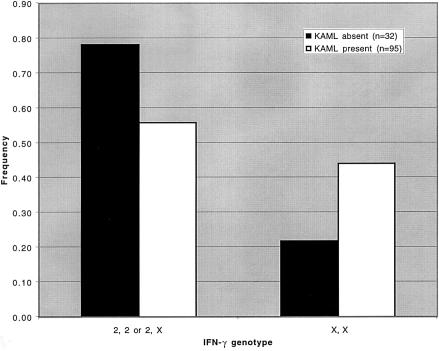
The allele and genotype frequencies in this study and those reported in the literature (Perrey et al. 1998) are shown in table 3. Although up to six alleles for the CA repeat within intron 1 of IFN-γ have been reported, two common alleles (2 and 3) account for 85%–95% of alleles in several studies (Perrey et al. 1998; Awad et al. 1999; Pravica et al. 1999; Khani-Hanjani et al. 2000). In the cohort of 127 patients that we studied, alleles 2 and 3 account for 88% of the alleles. Although the frequencies of alleles in this entire cohort are similar to those in other studies, the frequency of allele 2 in the KAML-present group (36%) was lower than in the KAML-absent group (50%). The results were more striking when genotype frequencies were compared (table 3 and fig. 1). In control cohorts that have been described elsewhere, the frequency of a genotype that contains at least one allele 2 is 75%. We found that, although the frequency of a genotype that contains allele 2 in the entire cohort was fairly close to this, at 61.4%, there was a difference in this frequency when the KAML-present group was compared with the KAML-absent group, with a significantly lower frequency of allele 2 in the KAML-present group (56% vs. 78%; P=.02), as stated above.
Table 3.
IFN-γ Allele and Genotype Frequencies in TSC2 Patients >5 Years Old[Note]
|
Frequency in(%) |
||||
| KAML Absent(n = 32) | KAML Present(n = 95) | Entire Cohort(n = 127) | Perrey et al. 1998(n = 164) | |
| Allele: | ||||
| 2 | 50.0 | 36.3 | 39.8 | 48.2 |
| 3 | 42.2 | 50.5 | 48.4 | 42.7 |
| 4 | 6.3 | 6.8 | 6.7 | 4.3 |
| 5 | 1.6 | 5.8 | 4.7 | 4.9 |
| 6 | .0 | .5 | .4 | .0 |
| Genotype: | ||||
| 2,2 | 21.9 | 16.8 | 18.1 | 20.7 |
| 2,3 | 46.9 | 29.5 | 33.9 | 47.0 |
| 2,4 | 6.3 | 5.3 | 5.5 | 3.0 |
| 2,5 | 3.1 | 4.2 | 3.9 | 4.3 |
| 3,3 | 15.6 | 27.4 | 24.4 | 14.6 |
| 3,4 | .0 | 8.4 | 6.3 | 4.3 |
| 3,5 | 3.1 | 7.4 | 6.3 | 5.5 |
| 3,6 | .0 | 1.1 | .8 | .0 |
| 4,4 | 3.1 | .0 | .8 | .6 |
| 2,2 or 2,Xa | 78.1 | 55.8 | 61.4 | 75.0 |
| X,X | 21.9 | 44.2 | 38.6 | 25.0 |
Note.— Allele X represents any allele other than 2.
All genotypes containing allele 2.
Figure 1.
IFN-γ genotype frequencies in TSC2 patients >5 years old. The KAML-absent group (n=32) is denoted by blackened bars, and the KAML-present group (n=95) is denoted by unblackened bars. Allele X refers to any allele other than 2.
Similar Spectrum of TSC2 Mutation Types across Subgroups
We also considered the possibility that the type of TSC2 mutation might influence the development of KAMLs. Although there is some variation in the frequency of mutation type in the KAML-present versus the KAML-absent groups (table 4), it was not dramatic. Moreover, when the frequency of all mutations predicted to cause premature truncation (splice, nonsense, frameshift insertions or deletions, and larger rearrangements) versus those predicted to change one or a few amino acids (missense and in-frame insertions or deletions), there is no difference in the KAML-present group versus the KAML-absent group (table 4).
Table 4.
TSC2 Mutation Spectrum in KAML and IFN-γ Allele Subgroups in TSC2 Patients >5 Years Old
|
No. (%) in Subgroup |
||
| Mutation Type(s) | KAML Present | KAML Absent |
| In-frame deletion | 7 (7) | 2 (6) |
| In-frame insertion | 2 (2) | 0 |
| Missense | 16 (17) | 6 (19) |
| Frameshift deletion | 8 (8) | 7 (22) |
| Frameshift insertion | 9 (9) | 2 (6) |
| Nonsense | 29 (31) | 3 (9) |
| Splice | 17 (18) | 7 (22) |
| Large deletion | 7 (7) | 3 (9) |
| Duplication | 0 |
2 (6) |
| Overall | 95 | 32 |
| IF-I, IF-D, missensea | 25 (26) | 8 (25) |
| Truncatingb | 70 (74) | 24 (75) |
In-frame insertion, deletion, or missense.
Nonsense, splice, frameshift, or large deletion/duplication.
Age Dependence of IFN-γ Allele 2 and KAML Association
To investigate the possibility that age influences the association between IFN-γ allele 2 and the absence of KAMLs in TSC2, we did additional subset analyses on different age groups in the cohort that we studied (table 5). We divided the cohort into three age groups: 1–5-year-olds, 6–20-year-olds, and 21–49-year-olds. We observed an association between the IFN-γ allele 2 and the absence of KAMLs in the 6–20-year-old group (P=.02, by χ2 analysis) but not in the other age groups. We also observed a puzzling result in the 1–5-year-olds. Although the 1–5-year-olds have an expected low rate of KAMLs (27%), the frequency of IFN-γ allele 2 in the 1–5-year-old group with KAMLs present (12 patients) is unexpectedly high (92%). Our age-subgroup data suggest that the association between IFN-γ allele 2 and the absence of KAMLs is strongest in 6–20-year-olds. The high frequency of IFN-γ allele 2 in the 1–5-year-olds with KAMLs present is discussed below.
Table 5.
Distribution of KAMLs, Gender, and Allele 2 in Different Age Subgroups of TSC2 Patients
|
% With KAMLs Present |
No. (% with Allele 2 Present) among |
|||||||
| Subgroups by Age (in years) | No. Total | % Female | AmongFemalePatients | AmongMalePatients | Overall | KAML-AbsentGroup | KAML-PresentGroup | KAMLs Presentvs. KAML Absent,P Valuea |
| 1–5 | 45 | 42 | 26 | 27 | 27 | 33 (61) | 12 (92) | .05 |
| 6–20 | 100 | 53 | 75 | 74 | 75 | 25 (84) | 75 (56) | .02 |
| 21–49 | 27 | 63 | 76 | 70 | 74 | 7 (57) | 20 (55) | .30 |
By χ2 analysis.
Because exclusion of the 1–5-year-old group with KAMLs present could favor the demonstration of an association between IFN-γ allele 2 and the absence of KAMLs, we repeated χ2 analysis and included these patients, as shown in table 6. When we compared 1–20-year-olds with KAMLs present to 6–20-year-olds with KAMLs absent, the KAML frequency was 78% (87/112), and the age distribution was similar in both groups (P value not significant [NS], by t test). The frequency of IFN-γ allele 2 was 84% in the KAML-absent group and 61% in the KAML-present group (P=.03, by χ2 analysis). When the older patients were included for comparison between 1–49-year-olds with KAMLs present and 6–34-year-olds with KAMLs absent, the KAML frequency was 77%, and the age distribution was similar in both groups (P value NS, by t test). Including the older patients, the frequency of IFN-γ allele 2 was 78% in the KAML-absent group and 59% in the KAML-present group (P=.06, by χ2 analysis). This data demonstrates that the association between IFN-γ allele 2 is statistically significant in the 1–20-year-old group (P=.03) and approaches statistical significance in the 1–49-year-old group (P=.06).
Table 6.
IFN-γ Allele Frequencies in TSC2 Patients Including 1–5-Year-Olds with KAMLs Present
| All | KAMLAbsent | KAMLPresent | P | |
| Age >20 years excluded: | ||||
| N (%) | 112 | 25 (22) | 87 (78) | |
| Mean age (range) [in years] | 11.5 (6–20) | 10.7 (1–20) | .42a | |
| No. (%) with allele 2 present | 21 (84) | 53 (61) | .03b | |
| No. (%) with allele 2 absent | 4 (14) | 34 (39) | ||
| Age >20 years included: | ||||
| N (%) | 139 | 32 (23) | 107 (77) | |
| Mean age (range) [in years] | 14.1 (6–34) | 14.3 (1–49) | .89c | |
| No. (%) with allele 2 present | 25 (78) | 64 (59) | .06d | |
| No. (%) with allele 2 absent | 7 (22) | 43 (41) |
By t test; result not significant.
By χ2analysis.
By t test; result not significant.
By χ2analysis.
Association between IFN-γ Allele 2 and Absence of KAMLs Is Evident in Males but Not in Females
We also considered the possibility that gender is an important variable in the association between IFN-γ allele 2 and the absence of KAMLs. Here we will focus on the 6–49-year-old group and the 6–20-year-old group. The ratio of female:male patients in these age groups was close to 50%, and the KAML frequency (∼75%) is similar in male and female patients (tables 1 and 5). The frequency of IFN-γ allele 2 is similar in male and female patients in the KAML-present group. However, in the KAML-absent group, the frequency of IFN-γ allele 2 is higher in male patients than in female patients (table 7). Furthermore, in the 6–20-year-old group, there is evidence for significant association between IFN-γ allele 2 and the absence of KAMLs in male patients (P=.006, by Fisher test) but not in female patients (P=.52 [NS], by Fisher test). In the 6–49-year-old group, there was a trend toward a significant association in male patients (P=.06, by Fisher test) but not in female patients (P=.28). Because there is evidence that these age groups are associated when male and female patients are combined, it is possible that there is a weaker association in female patients that could not be demonstrated by this relatively small study.
Table 7.
IFN-γ Allele 2 Frequencies in Male and Female Patients
|
No. (% with Allele 2 Present) among |
|||
| Subgroups by Age (in years) and Sex | KAML-AbsentGroup | KAML-PresentGroup | KAML Presentvs. KAML Absent,P Valuea |
| 6–49: | |||
| M | 15 (87) | 42 (57) | .06 |
| F | 17 (71) |
53 (55) |
.28 |
| Overall | 32 (78) | 95 (56) | .03 |
| 6–20: | |||
| M | 12 (100) | 35 (57) | .005 |
| F | 13 (69) |
40 (55) |
.52 |
| Overall | 25 (84) | 75 (56) | .02 |
| 21–49: | |||
| M | 3 (33) | 7 (57) | >.99 |
| F | 4 (75) |
13 (54) |
.60 |
| Overall | 7 (57) | 20 (55) | >.99 |
By Fisher test.
TDT Suggests That IFN-γ Allele 2 Is Associated with Reduced Frequency of KAMLs
We also applied TDT to this set of patients, to confirm the association between IFN-γ allele 2 and the absence of KAMLs. In the present analysis, we included a subset of the same 127 patients (ages 6–49 years) for whom parental DNA samples were available for IFN-γ genotyping. Of the 190 parents of patients with KAMLs, 111 (58%) were genotyped for the IFN-γ polymorphism, yielding information on transmission or nontransmission of allele 2 in 35 of the 95 patients (37%). Of the 64 parents of patients with no KAMLs, 22 (34%) of were genotyped, yielding information on transmission in 11 of the 32 patients (34%). The TDT data are summarized in table 8. Analysis of all informative cases shows that, among TSC2 patients with KAMLs present, allele 2 was transmitted in 17 (40%) and was not transmitted in another 26 (60%) (TDT χ2=1.88; P=.170). Among TSC2 patients with KAMLs absent, allele 2 was transmitted in 12 (75%) and was not transmitted in 4 (25%) (TDT χ2=4.0; P=.045). If we combine the data from the KAML-present and KAML-absent groups for these TSC2 patients, then we can calculate a more significant combined TDT χ2 value (Spielman et al. 1993). The combined TDT χ2 value increases to 4.90, with a more significant P value of .027. Because several investigators (Curtis and Sham 1995; Ewens and Spielman 1999; Knapp 1999) have described certain biases in the TDT if incomplete data are included, we repeated the TDT calculation and excluded single-parent cases that, according to Ewens and Spielman (1999), may introduce bias. The resulting TDT statistic was 5.45 (P<.020). These findings agree with the standard χ2 analysis (table 1) and support our conclusion that there is an association between IFN-γ allele 2 and the absence of KAML development in TSC2 patients.
Table 8.
TDT for IFN-γ Allele 2 and KAML in TSC2 Disease
|
No. (%) of AllelesTransmitted WhenIFN-γ Allele 2 Is |
|||||
| Group | Transmitted | Not Transmitted | ProbandNumber | TDT χ2 | P |
| All informative cases: | |||||
| TSC2 and KAMLs present: | |||||
| Observed | 17 (40) | 26 (60) | 35 | 1.88 | .17 |
| Expected | 21.5 (50) | 21.5 (50) | |||
| TSC2 and KAMLs absent: | |||||
| Observed | 12 (75) | 4 (25) | 11 | 4 | .045 |
| Expected | 8 (50) | 8 (50) | |||
| Combined: | |||||
| Affected (KAMLs present) | 17 (40) | 26 (60) | 35 | 4.9 | .027 |
| Unaffected (KAMLs absent) | 12 (75) | 4 (25) | 11 | ||
| One-parent cases with potential bias excluded:a | |||||
| Affected (KAMLs present) | 14 (38) | 23 (62) | 30 | 5.45 | .02 |
| Unaffected (KAMLs absent) | 12 (75) | 4 (25) | 11 | ||
Combined.
Discussion
The high degree of variability of TSC clinical manifestations, including those among related and unrelated patients with the same mutation (Smalley et al. 1994; Dabora et al. 2001), suggests the possibility that modifier genes influence disease severity. In the present study, we have examined the relationship between the severity of renal disease and alleles of an IFN-γ microsatellite in patients with TSC. This is the first study to show that genetic modifiers may influence the TSC clinical phenotype.
We found an association between IFN-γ allele 2 and the absence of KAMLs in TSC2 patients. This finding suggests that IFN-γ allele 2 may be a genetic modifier that reduces KAML development or growth. Although this is a relatively small study, the association between IFN-γ allele 2 and the decreased frequency of KAMLs was demonstrated using two independent statistical approaches, strengthening the conclusion that there is a true association. We also showed that the distribution of TSC2 mutation types in the KAML-present group is similar to that in the KAML-absent group, eliminating the possibility that mutation type is a significant confounding variable. Because allele 2 has been shown to be associated with a higher level of IFN-γ expression in mitogen-stimulated mononuclear cells in vitro (Pravica et al. 1999), it is plausible that the association reported here is due to a reduction in KAML development in the presence of higher levels of IFN-γ.
Because both age and gender are known variables that influence the expression of a variety of TSC manifestations, we did subgroup analyses, to investigate the impact that age and gender have in the present study. We initially used an age cutoff of >5 years, because we wished to exclude the youngest age group with a relatively low frequency of KAMLs. When we divided our entire cohort into three age groupings (1–5 years, 6–20 years, and 21–49 years), we found evidence of an association between IFN-γ allele 2 and the absence of KAMLs in the 6–20-year-old group (P=.02) but not in the others (table 5). In the 21–49-year-old group, the frequency of IFN-γ allele 2 was similar in the KAML-absent and KAML-present groups. In the 1–5-year-old group there was an unexpected finding of a very high frequency of IFN-γ allele 2 in the KAML-present group (11 of 12 patients [92%]) compared with a frequency of 61% in the KAML-absent group. We considered the possibility that this group might have a different mutation spectrum, so we investigated this and found a spectrum similar to that of the remainder of the cohort (data not shown). The observation seems likely to have been the result of random chance in a small subgroup of 12 patients. To eliminate potential bias introduced by excluding 1–5-year-olds with KAMLs present, we repeated the χ2 analysis in two age groups that include these cases. As shown in table 6, there is evidence for significant association between IFN-γ allele 2 and the absence of KAMLs in the 1–20-year-old group (P=.03), and there is a trend toward significant association in the 1–49-year-old group (P=.06). These results support the conclusion that the unexpected high frequency of IFN-γ allele 2 in the group of 1–5-year-olds with KAMLs present is due to random chance in a small subgroup.
Although there are no obvious gender differences in the neurologic and skin manifestations of TSC, some studies suggest that sex differences do exist in lung and kidney lesions. Many reports describe lymphangioleiomyomatosis (LAM) in a small percentage of young women with TSC (Gomez et al. 1999). The prevalence of LAM in young women with TSC increases to 34%–39% if high-resolution CT scanning is used to screen women without pulmonary symptoms (Franz et al. 2001; Moss et al. 2001). Gender differences in KAML growth have also been observed. Although Ewalt et al. (1998) reported a similar frequency of KAMLs in male (72%) and female (76%) patients in a study of 60 children (ages 1–18 years) with TSC, when the lesions were followed over time, the tendency for the lesions to grow was higher in female patients than in male patients, suggesting that female hormones play a role in KAML progression. This possibility is further supported by the demonstration of progesterone-receptor immunostaining on a high number of KAMLs in women (Henske et al. 1998). One more example of gender differences in TSC comes from a TSC1 mouse model in which liver hemangiomas are more severe in female littermates than in male littermates and is associated with early lethality in the female littermates (Kwiatkowski et al. 2002). For these reasons, we analyzed our data according to gender (tables 5 and 7). Although there is a similar number of male and female patients with a similar frequency of KAMLs (table 5), the association between IFN-γ allele 2 and the absence of KAMLs can be demonstrated in male 6–20-year-olds and 6–49-year-olds but not in female patients of the same age groups. Because there is evidence of association when male and female patients are combined and also because the frequency of IFN-γ allele 2 is higher in female patients with KAMLs absent than in female patients with KAMLs present, it is possible that there is a weaker association in female patients that could not be demonstrated in this subgroup analysis. These data suggest that the factors that contribute to KAML development may be different in male and female patients with TSC. Because the numbers of patients in the gender subgroups are small, it will be important to verify this gender analysis in a larger cohort.
Renal disease, primarily the development of KAMLs, is a significant cause of morbidity and mortality in TSC. Kidney pathology (renal failure, bleeding, or renal cell carcinoma) was the cause of death in 31% (11/36) patients >10 years old with TSC who died of TSC-related complications (Shepherd et al. 1991). Although further investigation is required, this study suggests a potential role for IFN-γ in controlling the development and/or progression of KAMLs in TSC. Since IFN-γ is already in general clinical use for the treatment of chronic granulomatous disease (Johnston 2001) and malignant osteopetrosis (Key et al. 1995) and has been studied at a variety of doses in clinical trials for the treatment of renal cell carcinoma (Aulitzky et al. 1989; Ellerhorst et al. 1994; Gleave et al. 1998), it could be readily adopted in clinical trials for KAMLs. Since there is currently no known medical therapy for treatment of progressive KAMLs, this is of significant interest. In addition to suggesting a potential novel treatment for KAMLs, the present study is the first to demonstrate the presence of genetic modifiers for manifestations of TSC. Our results indicate that age and gender may be important variables that influence the role of modifiers and that they should be considered in further investigations of modifying factors and as treatments are developed and tested in TSC.
Acknowledgments
We wish to thank the families with TSC who contributed samples for analysis. We also thank Edwin Silverman, Nan Laird, and Benjamin Raby, for assistance with the statistical analyses and for their comments on the manuscript, and members of the Kwiatkowski laboratory, for helpful discussions. This work was supported by National Cancer Institute grant CA86248, National Institute of Neurological Disorders and Stroke grant NS31535, and the Tuberous Sclerosis Alliance.
Electronic-Database Information
Accession numbers and the URL for data presented herein are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for TSC [MIM 605284 and MIM 191092])
References
- Aulitzky W, Gastl G, Aulitzky WE, Herold M, Kemmler J, Mull B, Frick J, Huber C (1989) Successful treatment of metastatic renal cell carcinoma with a biologically active dose of recombinant interferon-gamma. J Clin Oncol 7:1875–1884 [DOI] [PubMed] [Google Scholar]
- Awad M, Pravica V, Perrey C, El Gamel A, Yonan N, Sinnott PJ, Hutchinson IV (1999) CA repeat allele polymorphism in the first intron of the human interferon-γ gene is associated with lung allograft fibrosis. Hum Immunol 60:343–346 [DOI] [PubMed] [Google Scholar]
- Becker C, Pohla H, Frankenberger B, Schüler T, Assenmacher M, Schendel DJ, Blankenstein T (2001) Adoptive tumor therapy with T lymphocytes enriched through an IFN-γ capture assay. Nat Med 7:1159–1162 [DOI] [PubMed] [Google Scholar]
- Cavet J, Dickinson AM, Norden J, Taylor PR, Jackson GH, Middleton PG (2001) Interferon-γ and interleukin-6 gene polymorphisms associate with graft-versus-host disease in HLA-matched sibling bone marrow transplantation. Blood 98:1594–1600 [DOI] [PubMed] [Google Scholar]
- Cheadle JP, Reeve MP, Sampson JR, Kwiatkowski DJ (2000) Molecular genetic advances in tuberous sclerosis. Hum Genet 107:97–114 [DOI] [PubMed] [Google Scholar]
- Curtis D, Sham PC (1995) A note on the application of the transmission disequilibrium test when a parent is missing. Am J Hum Genet 56:811–812 [PMC free article] [PubMed] [Google Scholar]
- Dabora SL, Jozwiak S, Franz DN, Roberts PS, Nieto A, Chung J, Choy YS, Reeve MP, Thiele E, Egelhoff JC, Kasprzyk-Obara J, Domanska-Pakiela D, Kwiatkowski DJ (2001) Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet 68:64–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerhorst JA, Kilbourn RG, Amato RJ, Zukiwski AA, Jones E, Logothetis CJ (1994) Phase II trial of low dose gamma-interferon in metastatic renal cell carcinoma. J Urol 152:841–845 [DOI] [PubMed] [Google Scholar]
- European Chromosome 16 Tuberous Sclerosis Consortium (1993) Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75:1305–1315 [DOI] [PubMed] [Google Scholar]
- Ewalt DH, Sheffield E, Sparagana SP, Delgado MR, Roach ES (1998) Renal lesion growth in children with tuberous sclerosis complex. J Urol 160:141–145 [PubMed] [Google Scholar]
- Ewens WJ, Spielman RS (1999) Disease associations and the transmission/disequilibrium tests (TDT and S-TDT). Curr Protoc Hum Genet 1.12.1–1.12.15 [Google Scholar]
- Farrar MA, Schreiber RD (1993) The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol 11:571–611 [DOI] [PubMed] [Google Scholar]
- Franz DN, Brody A, Meyer C, Leonard J, Chuck G, Dabora S, Sethuraman G, Colby TV, Kwiatkowski DJ, McCormack FX (2001) Mutational and radiographic analysis of pulmonary disease consistent with lymphangioleiomyomatosis and micronodular pneumocyte hyperplasia in women with tuberous sclerosis. Am J Respir Crit Care Med 164:661–668 [DOI] [PubMed] [Google Scholar]
- Gleave ME, Elhilali M, Fradet Y, Davis I, Venner P, Saad F, Klotz LH, Moore MJ, Paton V, Bajamonde A (1998) Interferon gamma-1b compared with placebo in metastatic renal-cell carcinoma. New Engl J Med 338:1265–71 [DOI] [PubMed] [Google Scholar]
- Gomez M, Sampson J, Whittemore V (eds) (1999) The tuberous sclerosis complex. Oxford University Press, Oxford [Google Scholar]
- Henske EP, Ao X, Short MP, Greenberg R, Neumann HP, Kwiatkowski DJ, Russo I (1998) Frequent progesterone receptor immunoreactivity in tuberous sclerosis-associated renal angiomyolipomas. Mod Pathol 11:665–668 [PubMed] [Google Scholar]
- Henske EP, Scheithauer BW, Short MP, Wollmann R, Nahmias J, Hornigold N, van Slegtenhorst M, Welsh CT, Kwiatkowski DJ (1996) Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions. Am J Hum Genet 59:400–406 [PMC free article] [PubMed] [Google Scholar]
- Henske EP, Wessner LL, Golden J, Scheithauer BW, Vortmeyer AO, Zhuang Z, Klein-Szanto AJ, Kwiatkowski DJ, Yeung RS (1997) Loss of tuberin in both subependymal giant cell astrocytomas and angiomyolipomas supports a two-hit model for the pathogenesis of tuberous sclerosis tumors. Am J Pathol 151:1639–1647 [PMC free article] [PubMed] [Google Scholar]
- Johnston RB Jr (2001) Clinical aspects of chronic granulomatous disease. Curr Opin Hematol 8:17–22 [DOI] [PubMed] [Google Scholar]
- Jozwiak S, Schwartz RA, Janniger CK, Bielicka-Cymerman J (2000) Usefulness of diagnostic criteria of tuberous sclerosis complex in pediatric patients. J Child Neurol 15:652–659 [DOI] [PubMed] [Google Scholar]
- Key LL Jr, Rodriguiz RM, Willi SM, Wright NM, Hatcher HC, Eyre DR, Cure JK, Griffin PP, Ries WL (1995) Long-term treatment of osteopetrosis with recombinant human interferon gamma. New Engl J Med 332:1594–1599 [DOI] [PubMed] [Google Scholar]
- Khani-Hanjani A, Lacaille D, Hoar D, Chalmers A, Horsman D, Anderson M, Balshaw R, Keown PA (2000) Association between dinucleotide repeat in non-coding region of interferon-gamma gene and susceptibility to, and severity of, rheumatoid arthritis. Lancet 356:820–825 [DOI] [PubMed] [Google Scholar]
- Knapp M (1999) The transmission/disequilibrium test and parental-genotype construction: the reconstruction-combined transmission/disequilibrium test. Am J Hum Genet 64:861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Minowa O, Kuno J, Mitani H, Hino O, Noda T (1999) Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res 59:1206–1211 [PubMed] [Google Scholar]
- Kwiatkowski DJ, Short MP (1994) Tuberous sclerosis. Arch Dermatol 130:348–354 [PubMed] [Google Scholar]
- Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H (2002) A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet 11:525–534 [DOI] [PubMed] [Google Scholar]
- Lee JK, Sayers TJ, Brooks AD, Back TC, Young HA, Komschlies KL, Wipsgginton JM, Wiltrout RH (2000) IFN-γ-dependent delay of in vivo tumor progression by Fas overexpression on murine renal cancer cells. J Immunol 164:231–239 [DOI] [PubMed] [Google Scholar]
- Moss J, Avila NA, Barnes PM, Litzenberger RA, Bechtle J, Brooks PG, Hedin CJ, Hunsberger S, Kristof AS (2001) Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex. Am J Respir Crit Care Med 164:669–671 [DOI] [PubMed] [Google Scholar]
- Niida Y, Stemmer-Rachamimov AO, Logrip M, Tapon D, Perez R, Kwiatkowski DJ, Sims K, MacCollin M, Louis DN, Ramesh V (2001) Survey of somatic mutations in tuberous sclerosis complex (TSC) hamartomas suggests different genetic mechanisms for pathogenesis of TSC lesions. Am J Hum Genet 69:493–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ (1999) Tsc2+/− mice develop tumors in multiple sites which express gelsolin and are influenced by genetic background. J Clin Invest 104:687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrey C, Pravica V, Sinnott PJ, Hutchinson IV (1998) Genotyping for polymorphisms in interferon-γ, interleukin-10, transforming growth factor-β1 and tumour necrosis factor-α genes: a technical report. Transpl Immunol 6:193–197 [DOI] [PubMed] [Google Scholar]
- Pravica V, Asderakis A, Perrey C, Hajeer A, Sinnott PJ, Hutchinson IV (1999) In vitro production of IFN-γ correlates with CA repeat polymorphism in the human IFN-γ gene. Eur J Immunogenet 26:1–3 [DOI] [PubMed] [Google Scholar]
- Pravica V, Perrey C, Stevens A, Lee JH, Hutchinson IV (2000) A single nucleotide polymorphism in the first intron of the human IFN-γ gene: absolute correlation with a polymorphic CA microsatellite marker of high IFN-γ production. Hum Immunol 61:863–866 [DOI] [PubMed] [Google Scholar]
- Shepherd CW, Gomez MR, Lie JT, Crowson CS (1991) Causes of death in patients with tuberous sclerosis. Mayo Clin Proc 66:792–796 [DOI] [PubMed] [Google Scholar]
- Smalley SL, Burger F, Smith M (1994) Phenotypic variation of tuberous sclerosis in a single extended kindred. J Med Genet 31:761–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman RS, McGinnis RE, Ewens WJ (1993) Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am J Hum Genet 52:506–516 [PMC free article] [PubMed] [Google Scholar]
- van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, et al (1997) Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277:805–808 [DOI] [PubMed] [Google Scholar]
- Yeung RS, Xiao GH, Jin F, Lee WC, Testa JR, Knudson AG (1994) Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc Natl Acad Sci USA 91:11413–11416 [DOI] [PMC free article] [PubMed] [Google Scholar]