Abstract
Trafficking of autoreactive leukocytes across the blood-nerve barrier and into peripheral nerves is an early pathological hallmark of Guillain-Barré syndrome (GBS). Tumor necrosis factor-α (TNF-α), a proinflammatory cytokine, promotes transendothelial migration by up-regulating endothelial expression of inflammatory mediators, including CCL2, a chemokine implicated in GBS. We sought to determine the mechanism by which TNF-α induces expression and secretion of CCL2 from peripheral nerve microvascular endoneurial endothelial cells (PNMECs). Expression of CCL2 mRNA and protein in quiescent PNMEC cultures was minimal. In contrast, cultures treated with TNF-α exhibited increased CCL2 mRNA and protein content, as well as protein secretion. Simvastatin significantly attenuated TNF-α induced CCL2 secretion without affecting CCL2 mRNA or protein expression. Co-incubation with geranylgeranyl pyrophosphate, but not farnesyl pyrophosphate, prevented the effect of simvastatin. By comparison, inhibiting protein isoprenylation with GGTI-298, but not FTI-277, mimicked the effect of simvastatin and significantly attenuated transendothelial migration in vitro. Inhibition of the monomeric GTPase Cdc42, but not Rac1 or RhoA-C, attenuated TNF-α mediated CCL2 secretion. TNF-α mediated trafficking of autoreactive leukocytes into peripheral nerves during GBS may proceed by a mechanism that involves Cdc42 facilitated secretion of CCL2.
Keywords: cytokine, endothelial, GTPases, microvascular, peripheral nerve
Introduction
Endothelial cells of the endoneurial microvasculature are considered the primary interface between circulating blood and peripheral nerves. These peripheral nerve microvascular endothelial cells (PNMECs) establish the protective blood-nerve barrier (BNB) (Poduslo et al., 1994). In many autoimmune inflammatory disorders, including Guillain-Barré syndrome (GBS), emerging evidence strongly supports early activation of the endoneurial microvasculature as an initiating pathological hallmark (Archelos et al., 1993; Zou et al., 1999; Putzu et al., 2000; Orlikowski et al., 2003; Press et al., 2003; Sainaghi et al., 2010). While the etiology of GBS remains unknown, proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) are reported to be elevated in sera of patients with GBS early in disease progression (Exley et al., 1994; Zhu et al., 1998). An increased presence of TNF-α has been detected within affected peripheral nerves of GBS patients (Putzu et al., 2000). Early experimental studies (Stoll et al., 1993) demonstrate an enhanced presence of TNF-α expressing macrophage infiltrates within nerve roots from rats with experimental autoimmune neuritis (EAN), an established and well-characterized animal model of GBS (Hahn, 1996). Neutralizing TNF-α, or its homotrimeric cell surface receptor TNFR1, has been shown to ameliorate the course of EAN (Stoll et al., 1993; Bao et al., 2003), and TNF-α receptor knockout mice exhibit an attenuated course of EAN (Mao et al., 2010). TNF-α is now recognized as playing a pivotal role in the pathogenesis of GBS (Lu and Zhu, 2011).
Migration of leukocytes across the endoneurial microvasculature is a highly regulated process, accomplished by a series of interactions between molecules expressed on circulating leukocytes and by the activated vascular endothelium (Springer, 1994; Hordijk, 2006). Many of these molecules have been implicated in the pathogenesis of inflammatory and autoimmune diseases (Archelos et al., 1993; Zou et al., 1999; Putzu et al., 2000; Orlikowski et al., 2003; Press et al., 2003; Sainaghi et al., 2010). Of particular relevance, chemokine (C-C motif) ligand 2 (CCL2) has been identified as a key participant in GBS disease progression (Putzu et al., 2000; Orlikowski et al., 2003). In patients with GBS, disease severity correlates with enhanced appearance of CCL2 in cerebrospinal fluid, as well as increased expression within endoneurial vascular endothelial cells (Orlikowski et al., 2003; Press et al., 2003). In rats with EAN, marked enhancement of CCL2 expression is reported to precede the onset of clinical deficits and persist through the peak of disease (Fujioka et al., 1999; Kieseier et al., 2000). Antibody-mediated neutralization of CCL2 in vivo ameliorates the course of EAN (Zou et al., 1999).
Emerging evidence demonstrates that TNF-α induces the expression and secretion of CCL2 from microvascular endothelial cells (Harkness et al., 2003; Chui and Dorovini-Zis, 2010), including those derived from the endoneurium of the peripheral nerve (Langert et al., 2013). Although the mechanism by which this occurs is not known, the transcription factor NF-κB has been demonstrated to be involved (Baldwin, 1996; Veillard et al., 2006). Small monomeric Rho GTPases are necessary intermediates in TNF-α mediated activation of NF-κB, and subsequent transcription of target genes such as CCL2 (Gnad et al., 2001; Hippenstiel et al., 2002; McKenzie and Ridley, 2007; Lim et al., 2009). In this study, we sought to elucidate the mechanism by which TNF-α induces expression and secretion of CCL2 from peripheral nerve microvascular endoneurial endothelial cells (PNMECs). Using a novel fully-characterized immortalized PNMEC cell line (Langert et al., 2013) in combination with primary PNMEC cultures, we demonstrate that small monomeric Cdc42 GTPases participate in TNF-α mediated CCL2 release. These findings suggest that TNF-α mediates trafficking of autoreactive leukocytes across the endoneurial microvasculature and into peripheral nerves, in part, by a mechanism involving Cdc42 GTPase-dependent secretion of CCL2.
Materials and Methods
Peripheral nerve microvascular endoneurial endothelial cell culture
This study was conducted using protocols approved by the Edward Hines, Jr. VA Hospital Institutional Animal Care and Use Committee in accordance with the principles of laboratory animal care. Unless otherwise specified, agents used within this study were obtained from Sigma-Aldrich (St. Louis, MO). Primary cultures of microvascular endothelial cells were prepared from sciatic nerves of naïve Lewis rats as described previously (Argall et al., 1994; Sarkey et al., 2007). Briefly, harvested sciatic nerves were desheathed to remove the epineurium and macrovascular endothelium. The remaining perineurium/endoneurium was minced and digested with 0.05% trypsin and 0.25% collagenase at 37°C for 2 h. After incubation, cells were collected, filtered, and sedimented at 700 g×5 min. The cell pellet was resuspended in Endo media containing Ham’s F10 basal media (Life Technologies, Grand Island, NY) supplemented with 10% FBS, 50 µg/ml endothelial cell growth supplement (ECGS; BD Biosciences, San Jose, CA), 0.4 µg/ml heparin, 5.6 µg/ml amphotericin B, 100 U/ml penicillin, and 100 µg/ml streptomycin (Life Technologies) and plated on collagen-coated (6.0 µg/cm2, rat tail collagen type I, Millipore, Billerica, MA) tissue culture flasks under an atmosphere of 5% CO2/95% air. Initial cultures consisted of a mixed population of microvascular endothelial cells, perineurial epithelioid myofibroblasts, and Schwann cells (Yosef et al., 2010). Under these culture conditions, proliferation of microvascular endothelial cells is optimal whereas Schwann cells do not appreciably proliferate (Argall et al., 1994). Myofibroblasts were selectively eliminated by Thy1.1 antibody-mediated complement-dependent cell lysis (Argall et al., 1994). Cultures prepared in this manner were 95 ± 7.5% (N = 3) pure, 96 ± 5% (N = 12, Trypan Blue dye exclusion) viable, and consistently established contact-inhibited monolayers with a distinctive cobblestone-like morphology. Primary PNMEC cultures were restricted to six passages.
A stably immortalized PNMEC cell line was produced from purified rat primary PNMEC cultures using a replication-deficient SV40 retrovirus encoding a temperature sensitive, non-SV40-origin binding mutant of the large T antigen and a selectable neomycin resistance gene (generous gift from Dr. P. Jat, University College of London, London, UK). Briefly, semi-confluent primary PNMEC cultures were incubated for 36 h at 37°C in the presence of 8 µg/ml polybrene with undiluted filtered viral supernatant collected from SVU19.5 producer cells (Jat and Sharp 1986; Jat et al., 1986). Selection was achieved by passage into Endo media supplemented with 200 µg/ml gentamycin (G418; Life Technologies). Antibiotic resistant clones were isolated by dilution into 96-well plates at a theoretical density of 0.33 cells/well. Several clones exhibiting phenotypic (contact-inhibition), morphologic (cobblestone-like appearance), and biochemical (expression of von Willebrand Factor/Factor VIII and PECAM-1) properties characteristic of primary microvascular endothelial cells were expanded, and a single clone (clone 4.3) was arbitrarily selected for use throughout this study. Both primary and immortalized PNMEC cultures express tight junction molecules (JAM-1, ZO-1, claudin-5, and occludin) and exhibit increased transendothelial electrical resistance, as we have previously demonstrated (Langert et al., 2013). Immortalized PNMEC cultures were maintained on collagen-coated flasks in Endo media at 37°C under an atmosphere of 5% CO2/95% air.
Unless otherwise specified, confluent cultures of primary or immortalized cells were pretreated overnight with vehicle (0.01% ethanol) or activated (Von Zee et al., 2009) lovastatin, simvastatin, or pravastatin (Calbiochem, Billerca, MA). To determine the role of specific isoprenoids in TNF-α mediated changes in CCL2 expression or secretion, some cell cultures were pretreated with simvastatin in the absence or presence of farnesyl pyrophosphate (FPP) or geranylgeranyl pyrophosphate (GGPP), or with farnesyl transferase inhibitor (FTI-277) or geranylgeranyl transferase inhibitor (GGTI-298). The role of specific Rho family GTPases in facilitating TNF-α mediated CCL2 secretion was determined using selective inhibitors of RhoA-C (C3 exoenzyme, Cytoskeleton, Denver, CO), Cdc42/Rac1 (ML141, Calbiochem) or Rac1 (NSC23766, Calbiochem) signaling. In each case, following pretreatment, cell cultures were stimulated with TNF-α (10 ng/ml) for 2 h (CCL2 mRNA expression) or 4 h (CCL2 intracellular protein expression or secretion).
Real time RT-PCR
Total RNA was extracted from rat primary and immortalized PNMEC cultures with TRIzol reagent, and 5 µg was reverse-transcribed using Super Script III First Strand Synthesis system (Life Technologies) as previously described (Von Zee et al., 2009). Rat-specific CCL2 cDNA sequences were amplified by real-time PCR on a Mini-Opticon PCR detection system using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) with forward: 5'-ATGCAGGTCTCTGTCACG and reverse: 5'-CTAGTTCTCTGTCATACT primers. For each sample, GAPDH (forward, 5'-TCCCTCAAGATTGTCAGCAA; reverse, 5'-AGATCCACAACGGATACATT) was used as a reference control. Optimized amplification steps of 94°C×5 min; 94°C×15 s, 55°C×30 s, 72°C×1 min were used. Reaction efficiencies were typically >90%. For each sample, the specificity of the real-time reaction product was determined by melting curve analysis. The endogenous expression of GAPDH was unaltered by TNF-α treatment (data not shown). Relative fold-changes in mRNA content in each sample were therefore normalized to expressed levels of GAPDH.
Enzyme linked immunosorbent assay
Relative changes in intracellular CCL2 content were quantified using a cell-based ELISA. Briefly, rat primary or immortalized PNMEC cultures were seeded on collagen-coated 96-well plates and confluent cultures fixed with phosphate-buffered (pH 7.4) 4% paraformaldehyde×10 min at 23°C. Fixed cells were permeabilized with 0.1% Triton X-100 and blocked×1 h at 23°C with 1% BSA. Fixed PNMEC cultures were incubated overnight at 4°C in the presence of a 1:2000 dilution of rabbit anti-rat CCL2 polyclonal IgG antibody (Serotec, Raleigh, NC). Immunostained cells were washed and incubated×1 h at 23°C in the presence of a 1:5000 dilution of horseradish peroxidase-conjugated goat anti-rabbit secondary IgG antibody (Jackson ImmunoResearch, West Grove, PA). Color development was initiated with SigmaFast OPD substrate×30 min at 23°C, and stopped with the addition of 3N HCl. Samples were read at 492 nm and nonspecific binding (secondary antibody only control) was subtracted. Data are reported as percent increase over quiescent vehicle-treated cells.
The content of CCL2 released from treated PNMEC cultures was determined using a commercially-available ELISA kit (ThermoScientific, Rockford, IL). Briefly, PNMECs were cultured at a density of 2×105 cells per well (24-well collagen-coated) and media from treated cultures were collected, clarified by centrifugation (700 g ×5 min), and stored at −80°C until use. According to the manufacturer’s instructions, samples were typically diluted with Endo media (1:50 for primary PNMEC cultures, 1:200 for immortalized PNMEC cultures), and aliquots (50 µl) were assayed. Samples were read at 450 nm with a 550 nm correction and results expressed as a percentage of vehicle-pretreated TNF-α treated controls.
Transendothelial migration assay
For migration experiments, a CCR2-expressing human acute monocytic leukemia cell line (THP-1; a generous gift from Dr. E. Kovacs, Loyola University Chicago) was maintained in RPMI 1640 containing 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, and 5 mM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO). PNMEC cultures were grown to confluency on Transwell® microporous (8.0 µm pore size) inserts in 24-well tissue culture plates. An aliquot (1 × 105 cells) of THP-1 monocytes was added to the upper compartment while conditioned media harvested from vehicle- or TNF-α treated (10 ng/ml, 4 h) immortalized PNMEC cultures was added to the bottom compartment. After 4 h at 37°C, cells migrating into the lower chamber were collected and counted using a hemocytometer.
Statistical analysis
Data are expressed as the mean ± SEM of N observations unless noted otherwise. Statistical significance between multiple experimental groups was determined by one-way ANOVA with a Bonferroni post-hoc analysis. In each case, p < 0.05 was considered statistically significant.
Results
TNF-α induces CCL2 expression and secretion
The endogenous content of CCL2 mRNA and intracellular protein expressed in quiescent (media-treated) PNMEC cultures used in this study was minimal, at the level of detection (Fig. 1). In agreement with our previous findings (Langert et al., 2013), quiescent PNMEC cultures responded to TNF-α treatment (10 ng/ml) by robustly increasing CCL2 mRNA (50-fold) and protein (1.5-fold) content (Fig. 1). By comparison, the absolute content of CCL2 protein constitutively secreted from quiescent PNMEC cultures (N=3) was 24.6 ± 4.0 ng/ml (Fig. 2). TNF-α treatment (10 ng/ml) elicited significant enhancement of CCL2 protein secreted into the PNMEC culture medium (Fig. 2).
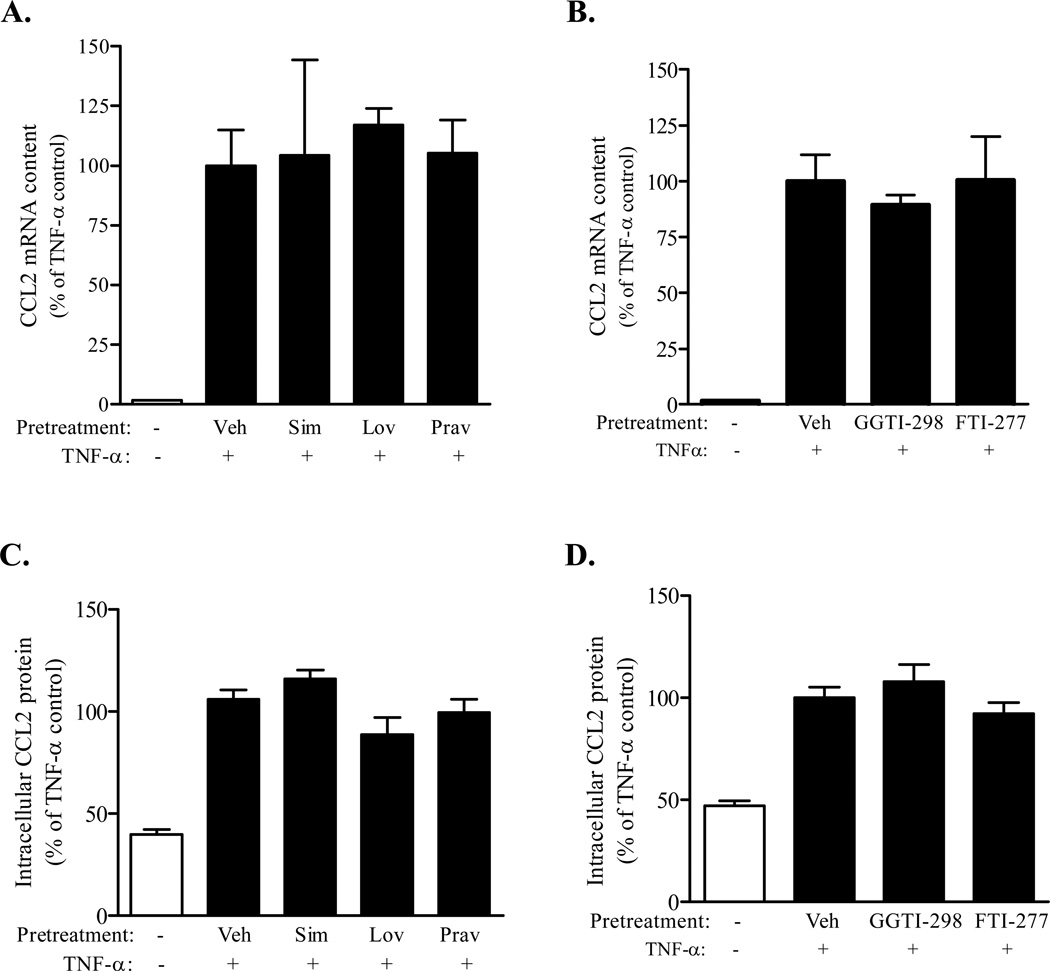
Figure 1. TNF-α mediated increases in CCL2 expression occur independently of protein prenylation.
Confluent immortalized PNMEC cultures were incubated overnight (16 h) (A, C) in the absence (Veh: 0.01% ethanol (A, C), 0.6% DMSO (B, D)) or presence (10 µM) of activated simvastatin (Sim), lovastatin (Lov), or pravastatin (Prav) or (B, D) GGTI-298 or FTI-277, as indicated. Pretreated cultures were subsequently incubated without (media) or with TNF-α (10 ng/ml; 2 h, mRNA; 4 h, protein). Relative changes in CCL2 mRNA or protein content were quantified by qRT-PCR and cell-based ELISA, respectively. Data shown are the means ± SEM (N = 3–9) from two independent experiments. Relative changes in CCL2 mRNA content were normalized to GAPDH expression. Data are expressed as a percentage change from vehicle pretreated TNF-α stimulated controls. In each case (A–D), no statistically significant differences were observed between vehicle- and inhibitor-pretreated TNF-α stimulated cultures.
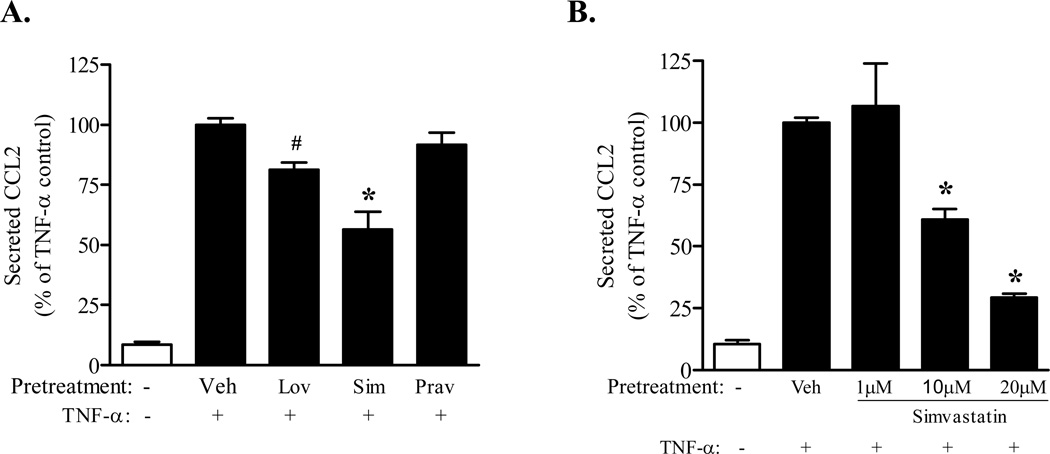
Figure 2. Simvastatin attenuates TNF-α mediated CCL2 secretion.
Confluent immortalized PNMEC cultures were incubated overnight (16 h) (A) in the absence (Veh: 0.01% ethanol) or presence (10 µM) of activated simvastatin (Sim), lovastatin (Lov), or pravastatin (Prav) or (B) activated simvastatin (1, 10, 20 µM), as indicated. Pretreated cultures were subsequently incubated without (media) or with TNF-α (10 ng/ml, 4 h). Relative changes in CCL2 protein released into the culture media were quantified by ELISA. Data shown are the means ± SEM (N = 3–6) from two independent experiments. Data are expressed as a percentage change from vehicle pretreated TNF-α stimulated controls. #, p < 0.05; *, p < 0.01 compared with vehicle-pretreated TNF-α control; one-way ANOVA with Bonferroni’s post-hoc analysis.
Protein isoprenylation facilitates TNF-α mediated CCL2 secretion
To address the role of GTPases in regulating TNF-α mediated CCL2 expression and release, PNMEC cultures were pretreated with various statins to limit the endogenous isoprenylation and activation of GTPases (Von Zee et al., 2009; Stubbs and Von Zee, 2012). In these experiments, statins serve as indirect inhibitors of Rho signaling. PNMEC cultures pretreated overnight (10 µM, 16 h) with activated lovastatin, simvastatin, or pravastatin responded to TNF-α (10 ng/ml, 2–4 h) by increasing CCL2 mRNA and CCL2 protein content in a manner that was statistically indistinguishable from vehicle pre-treated controls (Fig. 1A, 1C). Pretreatment with GGTI-298 or FTI-277, selective inhibitors of protein prenylation, similarly had no effect on TNF-α induced increases in CCL2 expression (Fig. 1B, 1D). In contrast, PNMEC cultures pretreated with lovastatin exhibited a modest (~20%), but significant, reduction in TNF-α mediated CCL2 secretion (Fig. 2A). By comparison, pretreatment with simvastatin (a highly lipophilic statin) attenuated TNF-α mediated CCL2 secretion by as much as 75% at the highest dose (20 µM) studied (Fig. 2B). In contrast, pravastatin (a hydrophilic statin) did not alter TNF-α mediated CCL2 secretion (Fig. 2A).
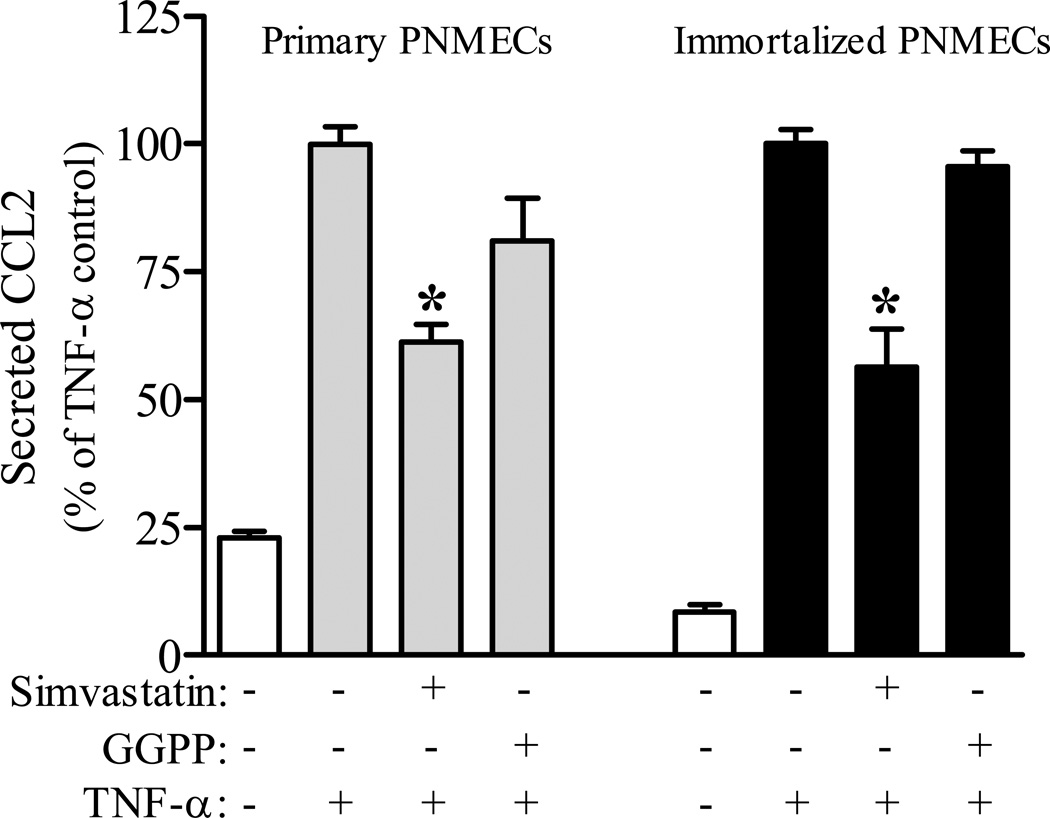
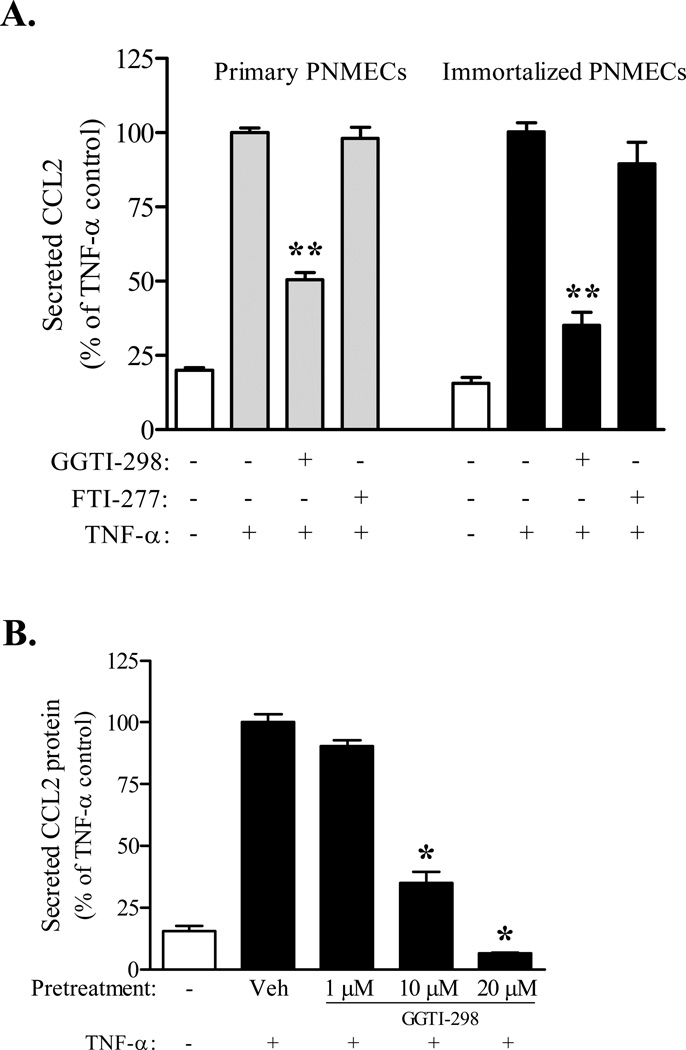
Co-incubation of primary and transformed PNMEC cultures with geranylgeranyl pyrophosphate (GGPP, 10 µM) protects against the inhibitory effects of simvastatin on TNF-α mediated CCL2 secretion (Fig. 3). By comparison, transformed PNMEC cultures (N=5) co-incubated with farnesyl pyrophosphate (FPP, 10 µM) exhibited levels of CCL2 release (200.9 ± 8.6 ng/ml) that were statistically indistinguishable from cultures incubated with simvastatin alone (198.5 ± 8.2 ng/ml). These findings suggest a role for post-translational geranylgeranylation in cytokine-mediated CCL2 release. Consistent with this thesis, PNMEC cultures pretreated with GGTI-298, a selective inhibitor of geranylgeranyl transferase I, but not with an inhibitor of farnesyl transferase (FTI-277), exhibited a marked dose-dependent attenuation of TNF-α mediated CCL2 secretion (Fig. 4A, 4B). These findings were not a unique property of transformed cells, as GGTI-298 pretreatment similarly attenuated TNF-α mediated secretion of CCL2 from primary PNMEC cultures (Fig. 4A).
Figure 3. Geranylgeranyl pyrophosphate protects against simvastatin-attenuation of TNF-α mediated CCL2 secretion.
Confluent primary or immortalized PNMEC cultures were incubated overnight (16 h) in the absence (0.01% ethanol) or presence (10 µM) of activated simvastatin in media supplemented without or with 10 µM geranylgeranyl pyrophosphate (GGPP), as indicated. Pretreated cultures were subsequently incubated without (media) or with TNF-α (10 ng/ml, 4 h). Relative changes in CCL2 protein released into the culture media were quantified by ELISA. Data shown are the means ± SEM (N = 3–6) from two independent experiments. Data are expressed as a percentage change from vehicle pretreated TNF-α stimulated controls. *, p < 0.01 compared with vehicle-pretreated TNF-α control; one-way ANOVA with Bonferroni’s post-hoc analysis.
Figure 4. Geranylgeranylation selectively facilitates TNF-α mediated CCL2 secretion.
Confluent primary (A) or immortalized (A, B) PNMEC cultures were incubated overnight (16 h) in the absence (0.6% DMSO, veh) or presence of GGTI-298 (1–10 µM) or FTI-277 (10 µM), as indicated. Pretreated cultures were subsequently incubated without (media) or with TNF-α (10 ng/ml, 4 h). Relative changes in CCL2 protein released into the culture media were quantified by ELISA. Data shown are the means ± SEM (N = 3–6) from two independent experiments. Data are expressed as a percentage change from vehicle pretreated TNF-α stimulated controls. *, p < 0.05 **, p < 0.01 compared with vehicle-pretreated TNF-α control; one-way ANOVA with Bonferroni’s post-hoc analysis.
Geranylgeranylation facilitates CCL2-dependent transendothelial migration
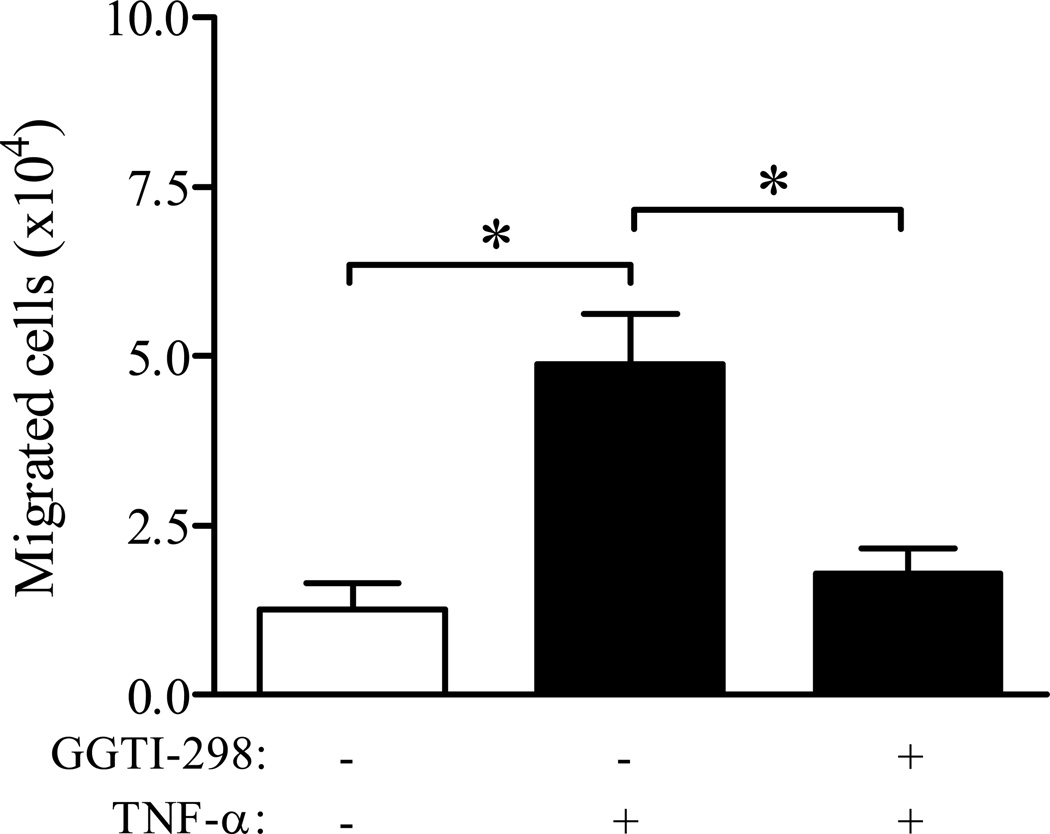
To address the functional consequence of disrupting post-translational protein geranylgeranylation on TNF-α mediated CCL2 secretion, a transwell in vitro migration assay using CCR2-expressing human THP-1 monocytes was utilized (Langert et al., 2013). PNMEC cultures were pretreated overnight (16 h) with vehicle (0.6% DMSO) or GGTI-298 (10 µM) and migration of THP-1 monocytes through monolayers of quiescent PNMEC cultures in response to conditioned media was determined. Chemotaxis of THP-1 monocytes in response to control media was minimal (Fig. 5). By comparison, addition of conditioned media harvested from TNF-α (10 ng/ml, 4 h) treated PNMEC cultures significantly promoted THP-1 monocyte chemotaxis (Fig. 5). In contrast, conditioned media collected from GGTI-298 pre-treated TNF-α (10 ng/ml, 4 h) treated PNMEC cultures elicited a marked reduction in THP-1 monocyte chemotaxis (Fig. 5).
Figure 5. Geranylgeranylation facilitates CCL2-dependent transendothelial migration of THP-1 monocytes.
Conditioned media was collected from confluent immortalized PNMEC cultures incubated overnight (16 h) in the absence (0.6% DMSO, vehicle) or presence of GGTI-298 (10 µM) and subsequently treated without or with TNF-α (10 ng/ml, 4 h), as indicated. Total cell migration across a quiescent PNMEC monolayer was performed and quantified as described in Methods. Data shown are the means ± SEM (N = 6) of total number of cells migrated. *, p < 0.01, one-way ANOVA with Bonferroni’s post-hoc analysis.
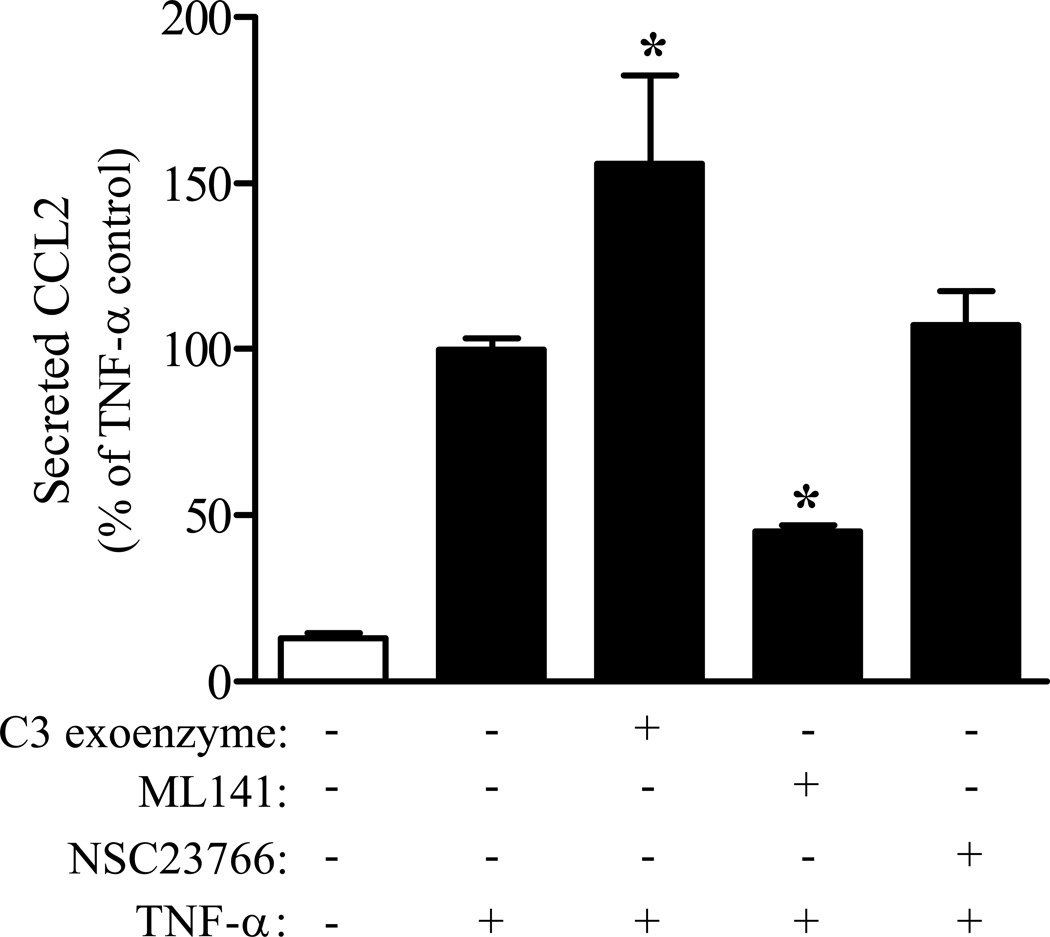
Cdc42 GTPases facilitate TNF-α mediated CCL2 secretion
The role of specific Rho family GTPases in facilitating TNF-α mediated CCL2 secretion was determined using selective inhibitors of RhoA-C (C3 exoenzyme), Cdc42/Rac1 (ML141), or Rac1 (NSC23766) signaling. PNMEC cultures pretreated with C3 exoenzyme exhibited marked enhancement of TNF-α mediated CCL2 secretion (Fig. 6). In contrast, inhibiting Cdc42/Rac1 signaling with ML141 elicited a dose-dependent (data not shown) significant attenuation of TNF-α mediated CCL2 secretion, compared to vehicle pretreated controls (Fig. 6). The role of Rac1 signaling alone was elucidated by pretreating cells with a selective Rac1 inhibitor, NSC23766. In comparison to ML141, NSC23766 did not affect TNF-α mediated CCL2 secretion (Fig. 6), strongly supporting a key role for Cdc42 GTPases in facilitating TNF-α mediated CCL2 secretion.
Figure 6. Cdc42 GTPases facilitate TNF-α mediated CCL2 secretion.
Confluent immortalized PNMEC cultures were incubated (1 h) in the absence (media) or presence of C3 exoenzyme (4 µg/ml), ML141 (20 µM), or NSC23766 (20 µM), as indicated. Pretreated cultures were subsequently incubated without (media) or with TNF-α (10 ng/ml, 4 h). Relative changes in CCL2 protein released into the culture media were quantified by ELISA. Data shown are the means ± SEM (N = 3–6) from two independent experiments. Data are expressed as a percentage change from vehicle pretreated TNF-α stimulated controls. *, p < 0.01 compared with vehicle-pretreated TNF-α control; one-way ANOVA with Bonferroni’s post-hoc analysis.
Discussion
In this study, we demonstrate that disrupting endogenous protein geranylgeranylation in primary or immortalized peripheral nerve microvascular endoneurial endothelial cells (PNMEC) attenuates TNF-α mediated CCL2 secretion and leukocyte transendothelial migration, in vitro, without altering intracellular expression of CCL2. Inhibition of the monomeric GTPase Cdc42, but not Rac1 or RhoA-C, significantly attenuated TNF-α mediated CCL2 secretion. We propose that TNF-α mediated recruitment and trafficking of autoreactive leukocytes into peripheral nerves, as seen in GBS, may proceed by a mechanism that involves, in part, Cdc42 GTPase facilitated secretion of CCL2. These findings extend previous in vivo studies from our lab which demonstrate that indirect inhibition of protein prenylation with lovastatin attenuates the development and progression of experimental autoimmune neuritis (EAN), an animal model of GBS, by limiting transendothelial migration of autoreactive leukocytes into the peripheral nerve (Sarkey et al., 2007).
A pivotal pathological hallmark of early onset GBS is the appearance of autoreactive leukocytes within peripheral nerves of affected patients (Hartung et al., 1995; Hughes and Cornblath, 2005). The mechanisms which govern initial recruitment and trafficking of leukocytes into privileged tissue compartments, such as peripheral nerves, remain unclear but may involve pro-inflammatory cytokine mediated localized activation of the vascular endothelium (Yadav et al., 2010; Lu and Zhu, 2011). Phenotypic and functional differences between vascular endothelium from different tissue beds have been recently reported (Yosef et al., 2010; Reijerkerk et al., 2012). Given that recruitment and trafficking of leukocytes across activated vascular endothelial barriers is most likely governed by changes in expression of specific inflammatory mediators unique to the localized vascular bed, we chose in this study to focus on TNF-α mediated activation of microvascular endoneurial endothelial cells harvested from rat peripheral nerve. Although advancements have been made toward the purification and culture of microvascular endothelial cells derived from peripheral nerve (Argall et al., 1994; Sano et al., 2007; Yosef et al., 2010), we address how PNMEC cultures respond to a localized inflammatory challenge such as that experienced during GBS.
TNF-α facilitates leukocyte recruitment and trafficking, in part, by inducing NF-κB-mediated expression and secretion of chemotactic cytokines, including CCL2, from locally activated endothelium (Baldwin, 1996; Veillard et al., 2006; Chui and Dorovini-Zis, 2010). Consistent with this thesis, physiologically relevant doses of TNF-α (Reuben et al., 2002; Radhakrishnan et al., 2004; Lim et al., 2009) elicit marked increases in CCL2 mRNA content in PNMEC cultures (Langert et al., 2013). The concentration of TNF-α (10 ng/ml) used in this study elicits increases in CCL2 mRNA and protein content within PNMECs, as well as induces release of functionally active CCL2 protein. We have previously demonstrated that antibody-mediated neutralization of released CCL2 protein prevents migration of CCR2-expressing monocytes in vitro (Langert et al., 2013). These data are consistent with previous reports demonstrating the ability of PNMECs to form an intact barrier, in vitro, as evidenced by increased transendothelial electrical resistance and expression of tight junction proteins (Sano et al., 2007; Langert et al., 2013). Vascular endothelial cells harvested and purified from rat sciatic nerve therefore represent, in a limited capacity, an in vitro model of a localized vascular bed in which to investigate mechanisms by which pro-inflammatory cytokines, such as TNF-α, mediate recruitment and trafficking of autoreactive leukocytes into peripheral nerves.
In some systems, small monomeric GTPases are necessary intermediates in TNF-α mediated activation of NF-κB and subsequent transcription of target genes (Gnad et al., 2001; Hippenstiel et al., 2002; McKenzie and Ridley, 2007; Lim et al., 2009). Limiting the activation of Rho GTPases with lipophilic statins or with direct inhibitors of protein prenylation has been demonstrated to alter levels of target genes in various endothelial cell types with mixed results, including, in some cases, a potentiation of inflammatory mediators (Schmidt et al., 2002; Bernot et al., 2003; Dunoyer-Geindre et al., 2005). Using human saphenous vein endothelial cells, Veillard and others (2006) demonstrated that simvastatin attenuates NFκB dependent CCL2 mRNA expression. By comparison, simvastatin did not alter TNF-α induced enhancement of CCL2 mRNA or protein content in this study, nor did it affect translocation of NF-κB p65 subunit to the nucleus (data not shown). These findings may best be explained by cell-type specific mechanisms governing cytokine-mediated CCL2 expression during an inflammatory response. Disrupting endogenous geranylgeranylation did, however, elicit a significant reduction in TNF-α mediated CCL2 secretion, suggesting a novel role of small monomeric GTPase in facilitating TNF-α mediated CCL2 secretion in PNMEC cultures.
Chemokines, including CCL2, have been demonstrated in some endothelial cell cultures to be stored in novel regulated secretory granules, or vesicles, that are distinct from von Willebrand factor-containing Weibel-Palade bodies (Knipe et al., 2010). Exposure to an inflammatory mediator such as TNF-α typically initiates rapid mobilization with subsequent exocytosis of storage vesicle content along with de novo synthesis and packaging of newly produced cytokines/chemokines (Oynebraten et al., 2004; Oynebraten et al., 2005; Knipe et al., 2010). In this study, PNMEC cultures responded robustly to TNF-α stimulation by increasing CCL2 mRNA content 50-fold. By comparison, however, only a minor (1.5-fold) increase in TNF-α mediated CCL2 intracellular protein content was observed. Moreover, whereas simvastatin or GGTI-298 significantly attenuated TNF-α mediated CCL2 release (Fig. 2, 4), no further accumulation of CCL2 mRNA or intracellular CCL2 protein was observed in PNMECs responding to TNF-α (Fig. 1). These findings may best be explained by the presence of a novel feedback mechanism governing CCL2 expression in PNMEC cultures. Alternatively, enhanced degradation of newly translated or stored intracellular CCL2 protein must also be considered.
Small monomeric GTPases are key regulators of intracellular vesicle trafficking and regulated exocytosis (Burgoyne and Morgan, 2003; Wennerberg et al., 2005). Members of the Rab family of GTPases, in particular, are penultimate participants in vesicle docking and release (Burgoyne and Morgan, 2003). However, the role of Rab GTPases in facilitating TNF-α mediated CCL2 secretion is uncertain, as Rab GTPases are selectively geranylgeranylated by geranylgeranyl transferase II rather than geranylgeranyl transferase I (Leung et al., 2006). These findings suggest that a geranylgeranylated GTPase other than Rab is most likely responsible for facilitating TNF-α mediated CCL2 release from PNMEC cultures seen in this study.
Geranylgeranyl transferase I catalyzes the functional activation of a number of other Ras related GTPases, including members of the Rho family (Rho, Rac, and Cdc42). Pretreating PNMEC cultures with C3 exoenzyme, a specific inhibitor of the Rho subfamily isoforms (RhoA-C), significantly potentiated TNF-α mediated CCL2 secretion. This finding supports a novel role for Rho subfamily GTPases in governing TNF-α mediated CCL2 secretion from PNMEC cultures. The specific Rho GTPase subfamily member (A, B, or C) responsible for this effect was not, however, identified in this study. The mechanism by which Rho subfamily GTPases may regulate CCL2 secretion remains to be established. By comparison, inhibiting Cdc42/Rac1 GTPases significantly attenuated TNF-α mediated CCL2 secretion. NSC23766, a selective inhibitor of Rac1 did not, however, affect TNF-α mediated CCL2 secretion. Collectively, these data support an active role of Cdc42 GTPase in facilitating TNF-α mediated CCL2 secretion from PNMEC cultures. These findings are not without precedence, as transfection of HUVEC cultures with dominant negative Cdc42, but not Rho or Rac, GTPase mutants impaired regulated secretion of von Willebrand factor (Fish et al., 2007).
Acknowledgements
The authors wish to thank Mr. Jonathan Lautz for helpful discussion with this manuscript. This work was supported, in part, by grants from the Department of Veterans Affairs (B3413R & B3756F (EBS); Pre-Doctoral Associated Health Rehabilitation Research Fellowship (KAL)), and NIH (1R03NS061033 (EBS)). We declare that there are no financial or other relationships associated with this study that may result in a conflict of interest.
References
- Archelos JJ, Maurer M, Jung S, Toyka KV, Hartung HP. Suppression of experimental allergic neuritis by an antibody to the intracellular adhesion molecule ICAM-1. Brain. 1993;116:1043–1058. doi: 10.1093/brain/116.5.1043. [DOI] [PubMed] [Google Scholar]
- Argall KG, Armati PJ, Pollard JD. A method for the isolation and culture of rat peripheral nerve vascular endothelial cells. Mol Cell Neurosci. 1994;5:413–417. doi: 10.1006/mcne.1994.1051. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Bao L, Lindgren JU, Zhu Y, Ljunggren HG, Zhu J. Exogenous soluble tumor necrosis factor receptor type I ameliorates murine experimental autoimmune neuritis. Neurobiol Dis. 2003;12:73–81. doi: 10.1016/s0969-9961(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Bernot D, Benoliel AM, Peiretti F, Lopez S, Bonardo B, Bongrand P, Juhan-Vague I, Nalbone G. Effect of atorvastatin on adhesive phenotype of human endothelial cells activated by tumor necrosis factor alpha. J Cardiovasc Pharmacol. 2003;41:316–324. doi: 10.1097/00005344-200302000-00022. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- Chui R, Dorovini-Zis K. Regulation of CCL2 and CCL3 expression in human brain endothelial cells by cytokines and lipopolysaccharide. J Neuroinflammation. 2010;7:1. doi: 10.1186/1742-2094-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunoyer-Geindre S, Dimitrova Y, Fish RJ, Satta N, Reber G, Kruithof EK, de Moerloose P. Fluvastatin increases the expression of adhesion molecules, monocyte chemoattractant protein-1 and tissue factor in HUVEC stimulated by patient IgG fractions containing antiphospholipid antibodies. Thromb Haemost. 2005;93:339–345. doi: 10.1160/TH04-05-0297. [DOI] [PubMed] [Google Scholar]
- Exley AR, Smith N, Winer JB. Tumour necrosis factor-alpha and other cytokines in Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry. 1994;57:1118–1120. doi: 10.1136/jnnp.57.9.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish RJ, Yang H, Viglino C, Schorer R, Dunoyer-Geindre S, Kruithof EK. Fluvastatin inhibits regulated secretion of endothelial cell von Willebrand factor in response to diverse secretagogues. Biochem J. 2007;405:597–604. doi: 10.1042/BJ20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnad R, Kaina B, Fritz G. Rho GTPases are involved in the regulation of NF-kappaB by genotoxic stress. Exp Cell Res. 2001;264:244–249. doi: 10.1006/excr.2001.5165. [DOI] [PubMed] [Google Scholar]
- Hahn AF. Experimental allergic neuritis (EAN) as a model for the immune-mediated demyelinating neuropathies. Rev Neurol. 1996;152:328–332. [PubMed] [Google Scholar]
- Harkness KA, Sussman JD, Davies-Jones GA, Greenwood J, Woodroofe MN. Cytokine regulation of MCP-1 expression in brain and retinal microvascular endothelial cells. J Neuroimmunol. 2003;142:1–9. doi: 10.1016/s0165-5728(03)00251-0. [DOI] [PubMed] [Google Scholar]
- Hartung HP, Pollard JD, Harvey GK, Toyka KV. Immunopathogenesis and treatment of the Guillain-Barre syndrome--Part I. Muscle Nerve. 1995;18:137–153. doi: 10.1002/mus.880180202. [DOI] [PubMed] [Google Scholar]
- Hippenstiel S, Schmeck B, Seybold J, Krull M, Eichel-Streiber C, Suttorp N. Reduction of tumor necrosis factor-alpha (TNF-alpha) related nuclear factor-kappaB (NF-kappaB) translocation but not inhibitor kappa-B (Ikappa-B)-degradation by Rho protein inhibition in human endothelial cells. Biochem Pharmacol. 2002;64:971–977. doi: 10.1016/s0006-2952(02)01162-0. [DOI] [PubMed] [Google Scholar]
- Hordijk PL. Endothelial signalling events during leukocyte transmigration. FEBS J. 2006;273:4408–4415. doi: 10.1111/j.1742-4658.2006.05440.x. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Cornblath DR. Guillain-Barre syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- Jat PS, Cepko CL, Mulligan RC, Sharp PA. Recombinant retroviruses encoding simian virus 40 large T antigen and polyomavirus large and middle T antigens. Mol Cell Biol. 1986;6:1204–1217. doi: 10.1128/mcb.6.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat PS, Sharp PA. Large T antigens of simian virus 40 and polyomavirus efficiently establish primary fibroblasts. J Virol. 1986;59:746–750. doi: 10.1128/jvi.59.3.746-750.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe L, Meli A, Hewlett L, Bierings R, Dempster J, Skehel P, Hannah MJ, Carter T. A revised model for the secretion of tPA and cytokines from cultured endothelial cells. Blood. 2010;116:2183–2191. doi: 10.1182/blood-2010-03-276170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langert KA, Von Zee CL, Stubbs EB Jr. Tumour necrosis factor alpha enhances CCL2 and ICAM-1 expression in peripheral nerve microvascular endoneurial endothelial cells. ASN Neuro. 2013;5:e00104. doi: 10.1042/AN20120048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KF, Baron R, Seabra MC. Thematic review series: lipid posttranslational modifications. geranylgeranylation of Rab GTPases. J Lipid Res. 2006;47:467–475. doi: 10.1194/jlr.R500017-JLR200. [DOI] [PubMed] [Google Scholar]
- Lim S, Ryu J, Shin JA, Shin MJ, Ahn YK, Kim JJ, Han KH. Tumor necrosis factor-alpha potentiates RhoA-mediated monocyte transmigratory activity in vivo at a picomolar level. Arterioscler Thromb Vasc Biol. 2009;29:2138–2145. doi: 10.1161/ATVBAHA.109.195735. [DOI] [PubMed] [Google Scholar]
- Lu MO, Zhu J. The role of cytokines in Guillain-Barre syndrome. J Neurol. 2011;258:533–548. doi: 10.1007/s00415-010-5836-5. [DOI] [PubMed] [Google Scholar]
- Mao XJ, Zhang XM, Zhang HL, Quezada HC, Mix E, Yang X, Winblad B, Adem A, Zhu J. TNF-alpha receptor 1 deficiency reduces antigen-presenting capacity of Schwann cells and ameliorates experimental autoimmune neuritis in mice. Neurosci Lett. 2010;470:19–23. doi: 10.1016/j.neulet.2009.12.045. [DOI] [PubMed] [Google Scholar]
- McKenzie JA, Ridley AJ. Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. J Cell Physiol. 2007;213:221–228. doi: 10.1002/jcp.21114. [DOI] [PubMed] [Google Scholar]
- Orlikowski D, Chazaud B, Plonquet A, Poron F, Sharshar T, Maison P, Raphael JC, Gherardi RK, Creange A. Monocyte chemoattractant protein 1 and chemokine receptor CCR2 productions in Guillain-Barre syndrome and experimental autoimmune neuritis. J Neuroimmunol. 2003;134:118–127. doi: 10.1016/s0165-5728(02)00393-4. [DOI] [PubMed] [Google Scholar]
- Oynebraten I, Bakke O, Brandtzaeg P, Johansen FE, Haraldsen G. Rapid chemokine secretion from endothelial cells originates from 2 distinct compartments. Blood. 2004;104:314–320. doi: 10.1182/blood-2003-08-2891. [DOI] [PubMed] [Google Scholar]
- Oynebraten I, Barois N, Hagelsteen K, Johansen FE, Bakke O, Haraldsen G. Characterization of a novel chemokine-containing storage granule in endothelial cells: evidence for preferential exocytosis mediated by protein kinase A and diacylglycerol. J Immunol. 2005;175:5358–5369. doi: 10.4049/jimmunol.175.8.5358. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL, Berg CT. Macromolecular permeability across the blood-nerve and blood-brain barriers. Proc Natl Acad Sci U S A. 1994;91:5705–5709. doi: 10.1073/pnas.91.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press R, Pashenkov M, Jin JP, Link H. Aberrated levels of cerebrospinal fluid chemokines in Guillain-Barre syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. J Clin Immunol. 2003;23:259–267. doi: 10.1023/a:1024532715775. [DOI] [PubMed] [Google Scholar]
- Putzu GA, Figarella-Branger D, Bouvier-Labit C, Liprandi A, Bianco N, Pellissier JF. Immunohistochemical localization of cytokines, C5b-9 and ICAM-1 in peripheral nerve of Guillain-Barre syndrome. J Neurol Sci. 2000;174:16–21. doi: 10.1016/s0022-510x(99)00328-7. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan VV, Sumi MG, Reuben S, Mathai A, Nair MD. Serum tumour necrosis factor-alpha and soluble tumour necrosis factor receptors levels in patients with Guillain-Barre syndrome. Acta Neurol Scand. 2004;109:71–74. doi: 10.1034/j.1600-0404.2003.00179.x. [DOI] [PubMed] [Google Scholar]
- Reijerkerk A, Lakeman KA, Drexhage JA, van Het Hof B, van Wijck Y, van der Pol SM, Kooij G, Geerts D, de Vries HE. Brain endothelial barrier passage by monocytes is controlled by the endothelin system. J Neurochem. 2012;121:730–737. doi: 10.1111/j.1471-4159.2011.07393.x. [DOI] [PubMed] [Google Scholar]
- Reuben S, Mathai A, George SM, Nair MD, Radhakrishnan VV. Serum tumor necrosis factor-alpha in Guillain-Barre syndrome and its relation to plasma exchange. Neurologist. 2002;8:7–50. doi: 10.1097/00127893-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Sainaghi PP, Collimedaglia L, Alciato F, Leone MA, Naldi P, Molinari R, Monaco F, Avanzi GC. The expression pattern of inflammatory mediators in cerebrospinal fluid differentiates Guillain-Barre syndrome from chronic inflammatory demyelinating polyneuropathy. Cytokine. 2010;51:138–143. doi: 10.1016/j.cyto.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Sano Y, Shimizu F, Nakayama H, Abe M, Maeda T, Ohtsuki S, Terasaki T, Obinata M, Ueda M, Takahashi R, Kanda T. Endothelial cells constituting blood-nerve barrier have highly specialized characteristics as barrier-forming cells. Cell Struct Funct. 2007;32:139–147. doi: 10.1247/csf.07015. [DOI] [PubMed] [Google Scholar]
- Sarkey JP, Richards MP, Stubbs EB., Jr Lovastatin attenuates nerve injury in an animal model of Guillain-Barre syndrome. J Neurochem. 2007;100:1265–1277. doi: 10.1111/j.1471-4159.2006.04309.x. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Goepfert C, Feitsma K, Buddecke E. Lovastatin-stimulated superinduction of E-selectin, ICAM-1 and VCAM-1 in TNF-alpha activated human vascular endothelial cells. Atherosclerosis. 2002;164:57–64. doi: 10.1016/s0021-9150(02)00053-9. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jung S, Jander S, van der Meide P, Hartung HP. Tumor necrosis factor-alpha in immune-mediated demyelination and Wallerian degeneration of the rat peripheral nervous system. J Neuroimmunol. 1993;45:175–182. doi: 10.1016/0165-5728(93)90178-2. [DOI] [PubMed] [Google Scholar]
- Stubbs EB, Jr, Von Zee CL. Prenylation of Rho G-proteins: a novel mechanism regulating gene expression and protein stability in human trabecular meshwork cells. Mol Neurobiol. 2012;46:28–40. doi: 10.1007/s12035-012-8249-x. [DOI] [PubMed] [Google Scholar]
- Veillard NR, Braunersreuther V, Arnaud C, Burger F, Pelli G, Steffens S, Mach F. Simvastatin modulates chemokine and chemokine receptor expression by geranylgeranyl isoprenoid pathway in human endothelial cells and macrophages. Atherosclerosis. 2006;188:51–58. doi: 10.1016/j.atherosclerosis.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Von Zee CL, Richards MP, Bu P, Perlman JI, Stubbs EB., Jr Increased RhoA and RhoB protein accumulation in cultured human trabecular meshwork cells by lovastatin. Invest Ophthalmol Vis Sci. 2009;50:2816–2823. doi: 10.1167/iovs.08-2466. [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta. 2010;411:1570–1579. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Yosef N, Xia RH, Ubogu EE. Development and characterization of a novel human in vitro blood-nerve barrier model using primary endoneurial endothelial cells. J Neuropathol Exp Neurol. 2010;69:82–97. doi: 10.1097/NEN.0b013e3181c84a9a. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mix E, Link H. Cytokine production and the pathogenesis of experimental autoimmune neuritis and Guillain-Barre syndrome. J Neuroimmunol. 1998;84:40–52. doi: 10.1016/s0165-5728(97)00238-5. [DOI] [PubMed] [Google Scholar]
- Zou LP, Pelidou SH, Abbas N, Deretzi G, Mix E, Schaltzbeerg M, Winblad B, Zhu J. Dynamics of production of MIP-1alpha, MCP-1 and MIP-2 and potential role of neutralization of these chemokines in the regulation of immune responses during experimental autoimmune neuritis in Lewis rats. J Neuroimmunol. 1999;98:168–175. doi: 10.1016/s0165-5728(99)00100-9. [DOI] [PubMed] [Google Scholar]