Abstract
Germline mutation at eight human minisatellite loci has been studied among families from rural areas of the Kiev and Zhitomir regions of Ukraine, which were heavily contaminated by radionuclides after the Chernobyl accident. The control and exposed groups were composed of families containing children conceived before and after the Chernobyl accident, respectively. The groups were matched by ethnicity, maternal age, parental occupation, and smoking habits, and they differed only slightly by paternal age. A statistically significant 1.6-fold increase in mutation rate was found in the germline of exposed fathers, whereas the maternal germline mutation rate in the exposed families was not elevated. These data, together with the results of our previous analysis of the exposed families from Belarus, suggest that the elevated minisatellite mutation rate can be attributed to post-Chernobyl radioactive exposure. The mechanisms of mutation induction at human minisatellite loci are discussed.
Introduction
Experimental evidence for radiation-induced germline mutation in humans still remains highly controversial. Because of a lack of experimental data on the effects of radiation exposure on germline mutation in humans, germline mutation induction in mice remains the main source of experimental data used to evaluate the genetic risk of human exposure to ionizing radiation (UNSCEAR 1993; Sankaranarayanan and Chakraborty 2000). Given that such an extrapolation currently cannot be verified, it remains clear that reliable estimates of the genetic risk of human exposure to ionizing radiation should be derived from relevant experimental data on germline mutation induction in human populations.
We have previously reported that minisatellite mutation rates in families inhabiting rural areas of the Mogilev region of Belarus, heavily contaminated with radionuclides after the Chernobyl accident, were unusually high (Dubrova et al. 1996, 1997). We also suggested that this increase could be attributed to the influence of environmental factors, including ionizing radiation. However, the results of this pilot study, in which mutation rates in the exposed group were compared with those in the nonexposed white families of different ethnic origins, do not provide enough evidence for induction of germline mutations by radiation. To verify the results of our previous study and to determine whether mutation rate in the germline of other post-Chernobyl cohorts is also elevated, we have extended this analysis to the group of exposed families inhabiting rural areas of the Kiev and Zhitomir regions of Ukraine.
Subjects and Methods
Blood samples were collected in rural areas of Ukraine. Informed consent was obtained from all families included in this study. The detailed characteristic of families included in this study is given in the “Results” section.
DNA was purified from frozen blood using phenol-chloroform extraction. Four-microgram samples of DNA were digested to completion with AluI, were electrophoresed through a 40-cm-long 0.8% agarose gel (SeaKem, type LE, FMC Products) in 1xTBE buffer (89 mM Tris-borate, pH 8.3, 2 mM EDTA), were transferred to a nylon membrane (Magna Nylon, Osmonics), and were hybridized to 32P-labeled probes, as described elsewhere (Dubrova et al. 1996, 1997). All parents and offspring were profiled using eight hypervariable minisatellite probes CEB1, CEB15, CEB25, CEB36, MS1, MS31, MS32 (loci D2S90, D1S172, D10S180, D10S473, D1S7, D7S21, and D1S8), and B6.7 (located on chromosome 20q13), chosen for their high spontaneous mutation rate (Jeffreys et al. 1988; Tamaki et al. 1999; Vergnaud and Denoeud 2000). These probes were previously used for the analysis of human families from Belarus who were exposed to the Chernobyl radioactive fallout (Dubrova et al. 1996, 1997), as well as families from Kazakhstan who were exposed to fallout from nuclear-weapon tests (Dubrova et al. 2002).
All autoradiographs were scored for the region between 1 and 22 kb, and new mutant bands were identified as offspring bands present in neither parent. DNA fragment sizes were estimated by the method of Southern (1979), using a 1-kb DNA ladder (Invitrogen) included on all gels. By use of the Chakraborty algorithm for parentage testing (Chakraborty et al. 1996), correct paternity for all offspring was validated. The likelihood ratio of paternity to nonpaternity in all families, including those with nonpaternal mutant bands ranged from 102 to 1011.
Results
Population Groups
Blood samples were collected from 256 Ukrainian families inhabiting the rural areas of the Kiev and Zhitomir regions of Ukraine, which were radioactively contaminated following the Chernobyl accident (table 1; fig. 1). The control group was composed of 98 children conceived before the Chernobyl accident and born between 1976 and 1986. The exposed group contained 240 children conceived after the Chernobyl accident and born between 1987 and 1996. Fifty-four families included children conceived before the Chernobyl accident; 171 families included children conceived after the accident. An additional group of 27 families contained children conceived before and after the accident.
Table 1.
Groups Studied
|
No. of Families with Children Conceived |
No. of Children Conceived |
||||
| Location | BeforeChernobyl | AfterChernobyl | Before andAfter Chernobyl | BeforeChernobyl | AfterChernobyl |
| Ovruch | 15 | 94 | 21 | 42 | 146 |
| Borodyanka | 11 | 35 | 5 | 20 | 45 |
| Gornostaipol | 5 | 15 | 1 | 6 | 21 |
| Vasylkov | 23 | 0 | 0 | 30 | 0 |
| Kovalevka | 0 | 19 | 0 | 0 | 20 |
| Ustimovka | 0 |
8 |
0 |
0 |
8 |
| Total | 54 | 171 | 27 | 98 | 240 |
Figure 1.
Map showing the study area
The control group contained 51 boys and 47 girls; the exposed groups consisted of 129 boys and 111 girls. The paternal age in the exposed group exceeded that for the control group (25.6 ± 0.4 and 26.8 ± 0.3 years for control and exposed groups, respectively; t=2.38, P=.0179), which was mainly attributed to the families containing children conceived before and after the accident. The maternal ages in the control and exposed groups did not significantly differ (23.8 ± 0.4 years and 24.3 ± 0.3 for control and exposed group, respectively; t=0.97, P=.3324). Table 2 gives the parental occupations for the three groups of families with children conceived before and after the Chernobyl accident, both paternal and maternal occupations were similar for all three groups. The number of smokers was also similar for these groups (table 2).
Table 2.
Parental Occupation and Smoking Habits in Control and Exposed Families[Note]
|
No. (%) of Fathers from |
No. (%) of Mothers from |
|||||
| Families with Children Conceived |
Families with Children Conceived |
|||||
| Characteristic | BeforeChernobyl | AfterChernobyl | Before andAfter Chernobyl | BeforeChernobyl | AfterChernobyl | Before andAfter Chernobyl |
| Occupation (Codea): | ||||||
| Management (1) | 0 | 1 (.6) | 0 | 1 (1.8) | 11 (6.4) | 1 (3.7) |
| Education and health (2) | 5 (9.3) | 14 (8.2) | 2 (7.4) | 24 (44.4) | 77 (45.0) | 11 (40.7) |
| Literary, artistic, and sport (3) | 0 | 1 (.6) | 0 | 0 | 1 (.6) | 0 |
| Science, engineering, technology (4) | 2 (3.7) | 7 (4.1) | 2 (7.4) | 1 (1.8) | 7 (4.1) | 4 (14.8) |
| Managerial (5) | 5 (9.3) | 11 (6.4) | 1 (3.7) | 1 (1.8) | 1 (.6) | 0 |
| Clerical and related (6) | 0 | 2 (1.2) | 1 (3.7) | 6 (11.1) | 11 (6.4) | 1 (3.7) |
| Selling (7) | 1 (1.8) | 0 | 0 | 7 (13.0) | 6 (3.5) | 1 (3.7) |
| Security and protective service (8) | 3 (5.6) | 9 (5.3) | 0 | 0 | 1 (.6) | 1 (3.7) |
| Catering, cleaning, and hairdressing (9) | 0 | 2 (1.2) | 0 | 5 (9.3) | 14 (8.2) | 4 (14.8) |
| Farming (10) | 3 (5.6) | 13 (7.6) | 0 | 0 | 7 (4.1) | 0 |
| Material processing (11,12) | 12 (22.2) | 34 (19.9) | 6 (22.2) | 5 (9.3) | 11 (6.4) | 1 (3.7) |
| Painting, assembling, and packaging (13) | 1 (1.8) | 0 | 1 (3.7) | 1 (1.8) | 2 (1.2) | 0 |
| Construction (14) | 4 (7.4) | 13 (7.6) | 2 (7.4) | 1 (1.8) | 3 (1.8) | 1 (3.7) |
| Transport (15) | 12 (22.2) | 52 (30.4) | 12 (44.4) | 0 | 3 (1.8) | 0 |
| Miscellaneous (16) | 1 (1.8) | 3 (1.8) | 0 | 1 (1.8) | 6 (3.5) | 0 |
| Inadequately described (17) | 5 (9.3) | 9 (5.3) | 0 | 0 | 1 (.6) | 0 |
| Housewives | … | … | … | 1 (1.8) | 9 (5.3) | 2 (7.4) |
| Smoking Status: | ||||||
| Non-smokers | 24 (44.4) | 53 (31.0) | 7 (25.9) | 53 | 166 | 27 |
| Smokers | 30 (55.6) | 118 (69.0) | 20 (74.1) | 1 | 5 | 0 |
Note.— For data on fathers' occupations, χ2=6.78; 6 df; P=.3421; for data on mothers' occupations, χ2=5.52; 6 df; P=.4795; and for data on fathers' smoking status, χ2=5.52; 2 df; P=.1404.
Occupational codes are taken from Office of Population Censuses and Surveys (1980).
The data for the exposed group from Ukraine was also compared with our previous data on 125 families from the rural areas of the Mogilev region of Belarus who were affected by the Chernobyl fallout (Dubrova et al. 1996, 1997). The Belarus exposed group contained 61 boys and 64 girls born between February and September 1994 (for the sex ratio in the exposed groups from Ukraine and Belarus, χ2=0.80, 1 df, P=.3703). The paternal (27.6 ± 0.5 years) and maternal (24.5 ± 0.5 years) ages in the exposed group from Belarus did not significantly differ from those in the exposed group from Ukraine (for paternal age, t=1.31 and P=.1914; for maternal age, t=0.28 and P=.7770). The parental occupations in the exposed groups from Ukraine and Belarus were also similar (for paternal occupation, χ2=6.77, 6 df, P=.3426; for maternal occupation, χ2=9.31, 5 df, P=.0972).
Mutation Rate
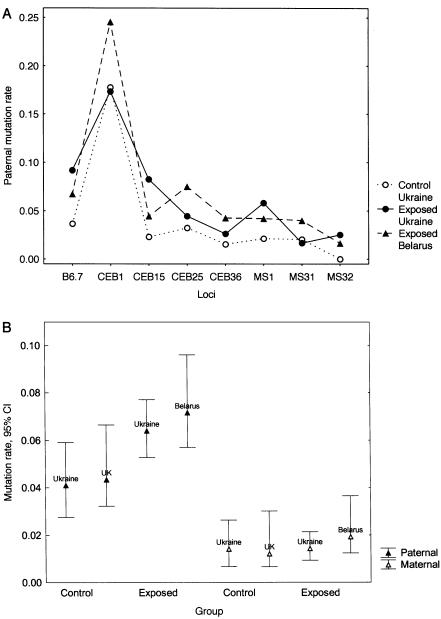
A summary of all mutation data is presented in table 3. A statistically significant 1.6-fold increase in the paternal mutation rate was found in the exposed families from Ukraine, whereas maternal mutation rate in this cohort was not elevated. Most of the minisatellite loci showed an elevated paternal mutation rate in the exposed group (fig. 2A).
Table 3.
Mutation Rates in Control and Exposed Groups
|
Findings in Control Group |
Findings in Exposed Group |
|||||||
| Probe | No. of Mutations | No. of Bands | Rate | No. of Mutations | No. of Bands | Rate | Ratioa | pb |
| Paternal mutations: | ||||||||
| B6.7 | 3 | 82 | .0366 | 18 | 196 | .0918 | 2.51 | … |
| CEB1 | 16 | 90 | .1778 | 38 | 219 | .1735 | .98 | … |
| CEB15 | 2 | 87 | .0230 | 18 | 218 | .0826 | 3.59 | … |
| CEB25 | 3 | 93 | .0323 | 10 | 224 | .0446 | 1.38 | … |
| CEB36 | 1 | 65 | .0154 | 5 | 191 | .0262 | 1.70 | … |
| MS1 | 2 | 94 | .0213 | 13 | 223 | .0583 | 2.74 | … |
| MS31 | 2 | 98 | .0204 | 4 | 239 | .0167 | .82 | … |
| MS32 | 0 |
97 |
0 |
6 |
238 |
.0252 |
… | … |
| Total | 29 | 706 | .0411 | 112 | 1748 | .0641 | 1.56 | .0299 |
| Maternal mutations: | ||||||||
| B6.7 | 0 | 80 | 0 | 4 | 196 | .0204 | … | … |
| CEB1 | 1 | 86 | .0116 | 2 | 208 | .0096 | .83 | … |
| CEB15 | 0 | 91 | 0 | 3 | 211 | .0142 | … | … |
| CEB25 | 1 | 91 | .0110 | 2 | 229 | .0087 | .79 | … |
| CEB36 | 1 | 73 | .0137 | 2 | 179 | .0112 | .82 | … |
| MS1 | 7 | 88 | .0795 | 12 | 226 | .0531 | .67 | … |
| MS31 | 0 | 98 | 0 | 0 | 240 | 0 | … | … |
| MS32 | 0 |
94 |
0 |
0 |
238 |
0 |
… | … |
| Total | 10 | 701 | .0143 | 25 | 1727 | .0145 | 1.01 | 1 |
Exposed:control ratio.
Probability of difference from the control group (Fisher's exact test, two-tailed).
Figure 2.
Minisatellite germline mutation rates in the control and exposed groups from Ukraine, Belarus, and the United Kingdom. A, Single-locus estimates of paternal mutation rates in the control and exposed groups from Ukraine (Wilcoxon matched pairs test, Z=2.10; P=.0357) and exposed group form Belarus (Z=2.52; P=.0117). B, Comparison of paternal and maternal minisatellite germline mutation rates in the exposed and nonexposed families of different origin. Data for the Belarus and U.K. families are taken from previous studies by Dubrova et al. (1996, 1997).
Using the same experimental procedures, we have previously analyzed minisatellite germline mutation rates in the exposed families from Belarus and nonirradiated white families from the United Kingdom (Dubrova et al. 1996, 1997). Figure 2B presents the comparison of our current and previous results, obtained using the same eight minisatellite probes. Despite differences in ethnicity, the nonexposed groups of parents from Ukraine and the United Kingdom showed very similar minisatellite mutation rates in the paternal (P=.8662, Fisher's exact test) and maternal (P=.7903) germlines. Most importantly, the estimates of paternal and maternal mutation rates in the exposed groups from Ukraine and Belarus were indistinguishable (P=.5204 and P=.4938 for paternal and maternal mutation rates, respectively). We therefore conclude that minisatellite mutation rates in the germlines of exposed fathers from Ukraine and Belarus are significantly elevated, whereas maternal mutation rates in these two exposed cohorts do not differ significantly from those in the nonexposed mothers.
Paternal Age, Year of Conception, and Paternal Mutation Rate
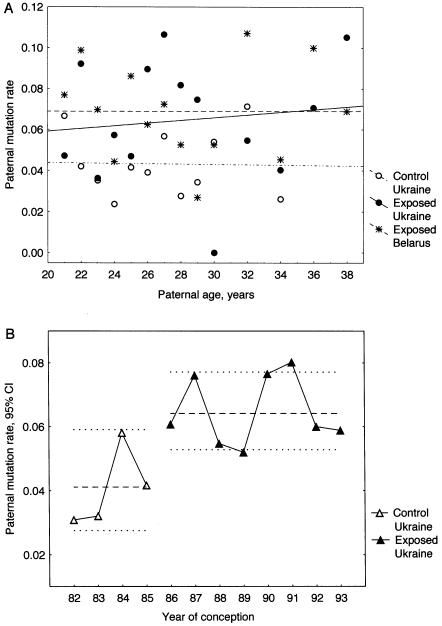
The paternal age in the exposed groups from Ukraine and Belarus exceeded that for the control group from Ukraine. This difference could potentially explain an elevated mutation rate in the exposed cohort from Ukraine. The results of numerous studies show the age-related increase in paternal germline mutation rate at human protein-coding genes (Vogel and Rathenberg 1975), although there has been no data showing such a trend for human minisatellites. To determine whether the increased mutation rate in the exposed fathers might be age-related, a correlation analysis for the control and exposed groups from Ukraine and Belarus was performed, which failed to reveal any significant associations between the paternal age and mutation rate (fig. 3A). Importantly, an elevated mutation rate was found across most of the age cohorts of exposed fathers. We therefore conclude that the relatively small difference in the paternal age between the exposed and control groups cannot explain the increased mutation rate in the exposed fathers.
Figure 3.
Minisatellite paternal mutation rate, paternal age and the year of conception in the control and exposed groups from Ukraine and Belarus. A, The lack of correlation between mutation rate and the paternal age in the control and exposed fathers (Kendall's nonparametric correlation: control Ukrainian fathers, τ=-0.091, P=.6808; exposed Ukrainian fathers, τ=0.033, P=.8695; exposed Belarus fathers, τ=-0.110, P=.5820). B, Paternal mutation rate and the year of conception in the control and exposed group from Ukraine. The dashed lines represent paternal mutation rates (± 95% CI) for the whole control and exposed groups. Data for the Belarus families are taken from previous studies by Dubrova et al. (1996, 1997).
In our previous study, the frequency of minisatellite mutation was evaluated in a cohort of Belarus children born within a narrow interval (February–September 1994), which prevented the analysis of temporal changes in mutation rate following the Chernobyl accident (Dubrova et al. 1996, 1997). The current cohort of children from Ukraine were conceived between 1986 and 1995, which makes such analysis possible. Figure 3B represents the scatter plot of paternal mutation rate. In the control and exposed groups, paternal mutation rates were uniform across the paternal cohorts (for homogeneity of the Poisson distribution, χ2=1.11, 3 df, P=.7747 for control fathers and χ2=2.16, 7 df, P=.9504 for exposed fathers) and did not show any correlation with the year of conception. We can therefore conclude that, within the analyzed period of time, paternal mutation rate in the control and exposed groups from Ukraine remained stable before and after the Chernobyl accident, respectively.
Spectrum of Paternal Minisatellite Mutation
Figure 4A presents the distribution of paternal progenitor alleles in the control and exposed groups from Ukraine and Belarus. The progenitor allele was assumed to be the paternal allele closest in size to the mutant allele (Jeffreys et al. 1988). The sizes of progenitor alleles were similar in the three groups, which allowed further comparison of mutation spectrum (fig. 4B). The results obtained here are in good agreement with the results of previous studies analyzing spontaneous mutation at these minisatellite loci (Jeffreys et al. 1988, 1994; Buard et al. 1998; Tamaki et al. 1999; Vergnaud and Denoeud 2000), most mutation events involved the gain or loss of only few repeat units. The distributions of length changes were indistinguishable between the three groups. We therefore conclude that there is no obvious difference in the spectrum of paternal minisatellite mutations between these groups.
Figure 4.
Spectrum of paternal germline mutations in the control and exposed groups from Ukraine and Belarus. A, Distribution of progenitor allele sizes for mutants (Kolmogorov-Smirnov test for all paired comparisons, P>.10; Kruskal-Wallis ANOVA, H=3.09; P=.2129). B, Distribution of size changes for paternal mutations (Kolmogorov-Smirnov test for all paired comparisons, P>.10; Kruskal-Wallis ANOVA, H=3.27; P=.1953). Data for the Belarus families are taken from previous studies by Dubrova et al. (1996, 1997).
Discussion
The main results of our study show that the paternal mutation rate at eight minisatellite loci in the exposed families from Ukraine is elevated, and they do not provide evidence for elevated mutation rates in the germline of exposed mothers. In this section, we will compare the Ukrainian data with the results of our previous study of the Belarus families inhabiting the rural areas of the Mogilev region, which was also contaminated after the Chernobyl accident (Dubrova et al. 1996, 1997), and we will address some issues concerning mutation induction at human minisatellite loci.
For this study, the control and exposed groups from Ukraine were matched by ethnicity, maternal age, parental occupation, and smoking habits, and they differed only slightly with respect to paternal age. Analysis of this potentially confounding factor shows that it does not affect minisatellite mutation rates in the germlines of control and exposed fathers (fig. 3A). Such a design provides a much better control population than was used for the previous study of the Belarus families, in which mutation rates in the exposed group were compared with those in the nonexposed white families of different ethnic origins. Importantly, the Ukrainian families do not significantly differ by parental age and occupation from the Belarus families inhabiting geographically similar areas of the Mogilev region, which was also contaminated after the Chernobyl accident.
A statistically significant 1.6-fold increase in mutation rate was found in the germline of exposed fathers from Ukraine. For this set of loci, paternal mutation rate in the exposed families from Belarus was 1.7 times higher than in the control group from Ukraine (P=.0121). In both exposed groups, the mutation rate was elevated over a number of minisatellite loci. Since the control group from Ukraine and both the exposed groups from Ukraine and Belarus were matched for parental age and occupation, the elevated mutation rate found in the exposed families could be attributed to the influence of environmental mutagens.
Over 90% of children from the exposed group were born in Ovruch, Borodyanka, and Gornostaipol, which belong to the most heavily radioactively contaminated areas of Ukraine with a level of surface contamination from cesium-137 of >2 Ci/km2 (IAC 1991). According to gamma spectrometric measurements of radionuclide concentration in soil and measurements of external gamma-exposure rate in air, the whole-body doses from external sources for the rural population of contaminated areas of Ukraine for the period of time from 1986 to 2000 did not exceed 50 mSv (Likhtarev et al. 2002). Similar doses from the ingestion of cesium-137 and cesium-134 for the Ukrainian population were also reported (Likhtarev et al. 2000). These doses are well below all known estimates of the doubling dose for mammalian germline mutation of 1 Sv (UNSCEAR 1993; Sankaranarayanan and Chakraborty 2000) and therefore cannot explain the 1.6-fold increase in mutation rate found in the exposed families. It should be noted, however, that the estimated doses reflect only one component of human exposure and do not take into account internal exposure from the short-lived isotopes.
The Chernobyl accident resulted in an unprecedented release of a wide spectrum of short-lived isotopes with the level of deposition often an order of magnitude higher than that for cesium-137. During the first days after the Chernobyl accident, their contribution to the absorbed dose in air was extremely high (Golikov et al. 1993), and the initial dose rate in air within the 100-km zone was at least 20-fold higher than in the later years (Balonov 1993). The high doses of internal and external exposure from the short-lived radionuclides were also reported for the residents of the 30-km zone around the Chernobyl power plant evacuated within 3–11 d after the accident (Pröhl et al. 2002). It should be noted that, apart from iodine-131, the contribution of short-lived radionuclides to the total exposure of nonevacuated population of Ukraine and Belarus has never been analyzed.
Given the short half-life of unstable radionuclides, their contribution to external and internal exposure can no longer be evaluated by means of physical dosimetry. However, retrospective biological dosimetry provides an alternative approach for the evaluation of the total absorbed doses for the populations of contaminated territories. The analysis of stable and unstable chromosome aberrations has provided an estimate of the mean doses of exposure, for the residents of heavily contaminated areas of the Gomel region of Belarus, ranging between 0.2 and 0.4 Grays (Gy) (Darroudi and Natarajan 1996; Mikhalevich et al. 2000). The reconstructed doses for the evacuees from the 30-km exclusion zone, received within a few days after the Chernobyl accident, were 0.3 and 0.4 Gy for the cohorts from Ukraine and Belarus, respectively (Maznik et al. 1997; Mikhalevich et al. 2000). These estimates are markedly higher than those obtained by physical dosimetry and probably reflect the initial external and internal exposure to the short-lived radionuclides. If we assume that the doubling dose for germline mutation in humans is 1 Gy (UNSCEAR 1993; Sankaranarayanan and Chakraborty 2000), an exposure to 0.2-0.4 Gy could potentially lead to a 1.6-fold increase in minisatellite mutation rate as found in the families from Ukraine and Belarus. Our data also suggest that the elevated paternal mutation rate found in the Ukrainian cohort of exposed families may be attributed to the initial exposure. Thus, within this cohort, the paternal mutation rate remains stable over the period of time from 1986 to 1994 and exceeds that for the control group. If the chronic exposure from cesium-137 and other stable radionuclides caused the elevated mutation rate, then a positive correlation between the year of conception and mutation rate should occur in the Ukrainian cohort. On the contrary, the consistently elevated paternal mutation apparently reflects a relatively high initial exposure for this cohort with a small contribution from the stable nuclides following the decay of the short-lived isotopes.
Our data therefore show that the elevated mutation rate in the germline of exposed fathers is most likely radiation induced and raise the issue of mechanisms of mutation induction at minisatellite loci. We have hypothesized that mutation induction at human minisatellites cannot be attributed to the direct effects of radiation-induced DNA double strand breaks at these small genomic loci (Dubrova et al. 1996, 1997), also similar conclusions have been obtained from studies of somatic and germline mutation rates at other DNA repeat sequences (Sadamoto et al. 1994; Schiestl et al. 1994; Fan et al. 1995; Dubrova et al. 1998, 2000; Barber et al. 2000; Kovalchuk et al. 2000; Yauk et al. 2002). The main argument for nontargeted mechanisms reflects the fact that an unrealistically high number of extra double-strand breaks or other types of damage per genome would be required to explain the mutation induction observed at these loci (details of estimates are given by Schiestl et al. [1994] and Dubrova et al. [1998]). Other evidence for the nontargeted mechanisms comes from the comparison of the spectrum of minisatellite mutation in the germline of control and exposed parents. In contrast to protein-coding genes showing a marked change in the structural basis of spontaneous and radiation-induced mutation (Nelson et al. 1994; Giver at al. 1995), human minisatellite loci and mouse expanded simple tandem repeat loci display indistinguishable mutation spectra in the germline of nonexposed and exposed parents (Dubrova et al. 1996, 1997; Yauk et al. 2002). Our current data also show similar mutation spectra in the control and exposed families, suggesting that there is no obvious difference in mutation process between the two groups.
Current data on the processes of spontaneous mutation at the human GC-rich minisatellites may provide clues to the mechanisms of mutation induction at these loci. Spontaneous mutation at these loci is very complex and almost completely restricted to the germline, with very rare and simple mutational events occurring in the somatic cells (Jeffreys at al. 1994; May et al. 1996; Jeffreys and Neumann 1997; Tamaki et al. 1999; Buard et al. 2000; Stead and Jeffreys 2000). These data also show that minisatellite mutation in the paternal germline most likely occurs at meiosis. If so, then exposure to ionizing radiation could potentially affect the stability of minisatellite loci over a very short interval of meiosis. To judge from the results of our study, this possibility appears to be highly unlikely. Given a relatively high initial exposure from the short-lived radionuclides, the preferential targeting of meiosis should result in a considerably elevated paternal mutation rate during the 1st year after the Chernobyl accident. On the contrary, the mutation rate in the exposed fathers from Ukraine remains stable over the period of time from 1986 to 1994. It therefore appears that premeiotic diploid stem cells and spermatogonia could accumulate radiation-induced damage, elsewhere in the genome, that subsequently affects the stability of minisatellite loci at meiosis. This delayed stimulation of minisatellite mutation in meiotic cells is reminiscent of the phenomenon of radiation-induced genomic instability, in which ionizing radiation can not only induce mutations seen in directly exposed somatic cells but can also lead to delayed effects with new mutations arising many cell divisions after the initial irradiation damage (Morgan et al. 1996).
In contrast to the increased mutation rate in exposed fathers, mutation rate in the germline of exposed mothers from Ukraine and Belarus is not elevated. This may be attributed to the relatively low maternal mutation rates at the most of the minisatellite loci, which do not provide enough statistical power to detect an elevated mutation rate in the exposed cohorts. Indeed, given the wide CI of the ratio of maternal mutation rates in the exposed group to control (95% CI 0.38–2.00), we cannot exclude the possibility of an elevated mutation rate among the irradiated mothers. However, the profound differences in the timing of spermatogenesis and oogenesis may explain the apparent similarity in mutation rates in the germline of exposed and nonexposed mothers. Spermatogenesis is a continuous process of mitotic and meiotic cell divisions, occurring from the beginning of puberty, whereas oocytes are already formed in late embryogenesis and remain arrested until the onset of puberty (Vogel and Motulsky 1997). Most importantly, crossing-over in the maternal germline also occurs in the late embryonic stages. If the mechanisms of minisatellite mutation in females are similar to those in males, then minisatellite mutation in the maternal germline may only occur before birth. All the mothers included in our study were at least 8 years old at the time of the Chernobyl accident, implying that they had been irradiated during the late meiotic stages, where exposure to ionizing radiation may be unable to affect the stability of minisatellite loci in the maternal germline.
In conclusion, the results of our current and previous studies show that mutation rate in the germline of fathers from Ukraine and Belarus is indeed elevated, and these results provide strong evidence that this increase can be attributed to post-Chernobyl radioactive exposure. The main questions remaining concern doses for the population of contaminated territories and the mechanisms of radiation-induced mutation at human minisatellites. Future work should address these important issues.
Acknowledgments
We thank Dr. Oksana D. Chernenko from Kyiv Municipal Oncology Center for proving us with blood samples from Ustimovka and Kovalevka. This work was supported by a grant to Y.E.D. from the Wellcome Trust.
References
- Balonov MI (1993) Overview of doses to the Soviet population from the Chernobyl accident and the protective actions applied. In: Mervin SE, Balonov MI (eds) The Chernobyl papers. Vol 1. Research Enterprises, Richland, WA, pp 23–46 [Google Scholar]
- Barber R, Plumb MA, Smith AG, Cesar CE, Boulton E, Jeffreys AJ, Dubrova YE (2000) No correlation between germline mutation at repeat DNA and meiotic crossover in male mice exposed to X-rays or cisplatin. Mutat Res 457:79–91 [DOI] [PubMed] [Google Scholar]
- Buard J, Bourdet A, Yardley J, Dubrova YE, Jeffreys AJ (1998) Influence of array size and homogeneity on minisatellite mutation. EMBO J 17:3495–3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buard J, Collick AJ, Brown J, Jeffreys AJ (2000) Somatic versus germline mutation process at minisatellite CEB1 (D2S90) in humans and transgenic mice. Genomics 65:95–103 [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Strivers DN, Zheng Y (1996) Estimation of mutation rate from parentage exclusion data: application to STR and VNTR loci. Mutat Res 354:41–48 [DOI] [PubMed] [Google Scholar]
- Darroudi F, Natarajan AT (1996) Biological dosimetric studies in the Chernobyl radiation accident, on populations living in the contaminated areas (Gomel regions) and in Estonian clean-up workers, using FISH technique. In: Karaoglou A, Desmet G, Kelly GN, Menzel HG (eds) The radiological consequences of the Chernobyl accident. European Commission, Luxembourg, pp 1067–1072 [Google Scholar]
- Dubrova YE, Nesterov VN, Krouchinsky NG, Ostapenko VA, Neumann R, Neil DL, Jeffreys AJ (1996) Human minisatellite mutation rate after the Chernobyl accident. Nature 380:683–686 [DOI] [PubMed] [Google Scholar]
- Dubrova YE, Nesterov VN, Krouchinsky NG, Ostapenko VA, Vergnaud G, Giraudeau F, Buard J, Jeffreys AJ (1997) Further evidence for elevated human minisatellite mutation rate in Belarus eight years after the Chernobyl accident. Mutat Res 381:267–278 [DOI] [PubMed] [Google Scholar]
- Dubrova YE, Plumb M, Brown J, Fennelly J, Bois P, Goodhead D, Jeffreys AJ (1998) Stage specificity, dose-response and doubling dose for mouse minisatellite germline mutation induced by acute radiation. Proc Natl Acad Sci USA 95:6251–6255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrova YE, Plumb M, Brown J, Boulton E, Goodhead D, Jeffreys AJ (2000) Induction of minisatellite mutations in the mouse germline by low-dose chronic exposure to γ-radiation and fission neutrons. Mutat Res 453:17–24 [DOI] [PubMed] [Google Scholar]
- Dubrova YE, Bersimbaev RI, Djansugurova LB, Tankimanova MK, Mamyrbaeva ZZ, Mustonen R, Lindholm C, Hultén M, Salomaa S (2002) Nuclear weapons tests and human germline mutation rate. Science 295:1037 [DOI] [PubMed] [Google Scholar]
- Fan YJ, Wang Z, Sadamoto S, Ninomiya Y, Kotomura N, Kamiya K, Dohi K, Kominami R, Niwa O (1995) Dose-response of radiation induction of a germline mutation at a hypervariable mouse minisatellite locus. Int J Radiat Biol 68:177–183 [DOI] [PubMed] [Google Scholar]
- Giver CR, Nelson SL, Cha MY, Pongsaensook P, Grosovsky AJ (1995) Mutational spectrum of X-ray induced TK− human cell mutants. Carcinogenesis 16:267–275 [DOI] [PubMed] [Google Scholar]
- Golikov VYu, Balonov MI, Ponomarev AV (1993) Estimation of external gamma radiation doses to the population after the Chernobyl accident. In: Mervin SE, Balonov MI (eds) The Chernobyl papers. Vol 1. Research Enterprises, Richland, WA, pp 247–288 [Google Scholar]
- International Advisory Committee (IAC) (1991) The International Chernobyl Project: assessment of radiological consequences and evaluation of protective measures. Tech rep, International Atomic Energy Agency, Vienna [DOI] [PubMed] [Google Scholar]
- Jeffreys AJ, Royle NJ, Wilson V, Wong Z (1988) Spontaneous mutation rate to new length alleles at tandem-repeat hypervariable loci in human DNA. Nature 332:278–281 [DOI] [PubMed] [Google Scholar]
- Jeffreys AJ, Tamaki K, MacLeod A, Monckton DG, Neil DL, Armour JAL (1994) Complex gene conversion events in germline mutation at human minisatellites. Nat Genet 6:136–145 [DOI] [PubMed] [Google Scholar]
- Jeffreys AJ, Neumann R (1997) Somatic mutation process at a human minisatellite. Hum Mol Genet 6:129–136 [DOI] [PubMed] [Google Scholar]
- Kovalchuk O, Dubrova YE, Arkhipov A, Hohn B, Kovalchuk I (2000) Wheat mutation rate after Chernobyl. Nature 407:583–584 [DOI] [PubMed] [Google Scholar]
- Likhtarev IA, Kovgan LN, Vavilov SE, Perevoznikov ON, Litvinets LN, Anspaugh LR, Jacob P, Pröhl G (2000) Internal exposure from the ingestion of foods contaminated by 137Cs after the Chernobyl accident—report 2. Ingestion doses of the rural population of Ukraine up to 12 y after the accident (1986–1997). Health Phys 79:341–357 [DOI] [PubMed] [Google Scholar]
- Likhtarev IA, Kovgan LN, Jacob P, Anspaugh LR (2002) Chernobyl accident: retrospective and prospective estimates of external dose of the population of Ukraine. Health Phys 82:290–303 [DOI] [PubMed] [Google Scholar]
- May CA, Jeffreys AJ, Armour JAL (1996) Mutation rate heterogeneity and the generation of allele diversity at the human minisatellite MS205 (D16S309). Hum Mol Genet 5:1823–1833 [DOI] [PubMed] [Google Scholar]
- Maznik NA, Vinnikov VA, Lloyd DC, Edwards AA (1997) Chromosomal dosimetry for some groups of evacuees from Prypiat and Ukrainian liquidators at Chernobyl. Radiat Protec Dosim 74:5–11 [Google Scholar]
- Mikhalevich LS, Lloyd, DC, Edwards AA, Perepetskaya GA, Kartel, NA (2000) Dose estimates made by dicentric analysis for some Belarussian children irradiated by the Chernobyl accident. Radiat Protec Dosim 87:109–114 [Google Scholar]
- Morgan WF, Day JP, Kaplan MI, McGhee EM, Limoli CL (1996) Genomic instability induced by ionizing radiation. Radiat Res 146:247–258 [PubMed] [Google Scholar]
- Nelson SL, Giver CR, Grosovsky AJ (1994) Spectrum of X-ray-induced mutations in the human hprt gene. Carcinogenesis 15:495–502 [DOI] [PubMed] [Google Scholar]
- Office of Population Censuses and Surveys (1980) Classification of occupations and coding index. Her Majesty's Stationery Office, London [Google Scholar]
- Pröhl G, Mück K, Likhtarev I, Kovgan L, Golikov V (2002) Reconstruction of the ingestion doses received by the population evacuated from the settlements in the 30-km zone around the Chernobyl reactor. Health Phys 82:173–181 [DOI] [PubMed] [Google Scholar]
- Sadamoto S, Suzuki S, Kamiya K, Kominami R, Dohi K, Niwa, O (1994) Radiation induction of germline mutation at a hypervariable mouse minisatellite locus. Int J Radiat Biol 65:549–557 [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan K, Chakraborty R (2000) Ionizing radiation and genetic risks. XI. The doubling dose estimates from the mid-1950s to present and the conceptual change to the use of human data on spontaneous mutation rates and mouse data on induced mutation rates for doubling dose calculations. Mutat Res 453:107–127 [DOI] [PubMed] [Google Scholar]
- Schiestl RH, Khogali F, Carls, N (1994) Reversion of the mouse pink-eyed unstable mutation induced by low doses of X-rays. Science 266:1573–1576 [DOI] [PubMed] [Google Scholar]
- Southern E (1979) Measurement of DNA length by gel electrophoresis. Anal Biochem 100: 319–323 [DOI] [PubMed] [Google Scholar]
- Stead JDH, Jeffreys AJ (2000) Allele diversity and germline mutation at the insulin minisatellite. Hum Mol Genet 9:713–723 [DOI] [PubMed] [Google Scholar]
- Tamaki K, May CA, Dubrova YE, Jeffreys AJ (1999) Extremely complex repeat shuffling during germline mutation at human minisatellite B6.7. Hum Mol Genet 8:879–888 [DOI] [PubMed] [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) (1993) Sources and effects of ionizing radiation. United Nations, New York [Google Scholar]
- Vergnaud G, Denoeud F (2000) Minisatellites: mutability and genome architecture. Genome Res 10:899–907 [DOI] [PubMed] [Google Scholar]
- Vogel F, Rathenberg R (1975) Spontaneous mutation in man. Adv Hum Genet 5:223–318 [DOI] [PubMed] [Google Scholar]
- Vogel F, Motulsky AG (1997) Human genetics. Springer, Berlin [Google Scholar]
- Yauk CL, Dubrova YE, Grant GR, Jeffreys AJ (2002) A novel single molecule analysis of spontaneous and radiation-induced mutation at a mouse tandem repeat locus. Mutat Res 500:147–156 [DOI] [PubMed] [Google Scholar]