Abstract
The pathogenesis of morphea and other cutaneous sclerosing disorders remain poorly understood. Although they are considered to be autoimmune disorders, abnormal tryptophan metabolism may be involved. Current therapy is directed to supressing the autoimmune response. Demonstration of a therapeutic response to manipulation of the kynurenine pathway would both support a role for abnormal tryptophan metabolism and offer additional targets for therapy. Tranilast is a 3-hydroxyanthranilic acid derivative known to target the kynurenine pathway. The aim of this study was to see if tranilast lowered the urinary excretion of the kynurenine metabolites kynurenic and quinolinic acid under condition of L tryptophan loading in a volunteer. Mean baseline value for kynurenic acid and quinolinic acid were 1.1 and 2.1 mmol/mol creatinine, respectively. This rose to 5.6 and 3.8 mmol/mol creatinine respectively under conditions of L tryptophan loading 2 grams daily. Adding 1 g of tranilast daily lowered the values to 2.0 and 2.9 mmol/mol creatinine, respectively. These data suggest that tranilast acts as a competitive inhibitor of either indoleamine 2, 3-dioxygenase (IDO), tryptophan 2, 3-di-oxygenase (TDO) or both. As it involved only 1 subject, the results may not be representative of the larger population and must be considered preliminary.
Keywords: tranilast, kynurenic acid, quinolinic acid, morphea, tryptophan
Introduction
Morphea is a localized variant of scleroderma. Its variants include plaque, bullous, linear, frontoparietal, subcutaneous, generalized and pan-sclerotic morphea of childhood. Eosinophilic fasciitis was recently included in the disease spectrum.1 It is considered to be an auto-immune disease possibly related to microchimerism of fetal cells.2 Abnormal tryptophan metabolism has been reported in other sclerosing cutaneous diseases.3 L-tryptophan supplementation was responsible for an outbreak of eosinophilia myalgia syndrome, which resulted in cutaneous sclerosis resembling eosinophilic fasciitis.4 Although initially reported in association with contaminated L-tryptophan, later reports linked the condition to L-tryptophan from other suppliers.5
Abnormalities in L-tryptophan metabolism were also reported in the toxic oil syndrome reported in Spain from contaminated rapeseed (canola) oil.6 This resulted in cutaneous sclerosis similar to eosinophilic fasciitis.
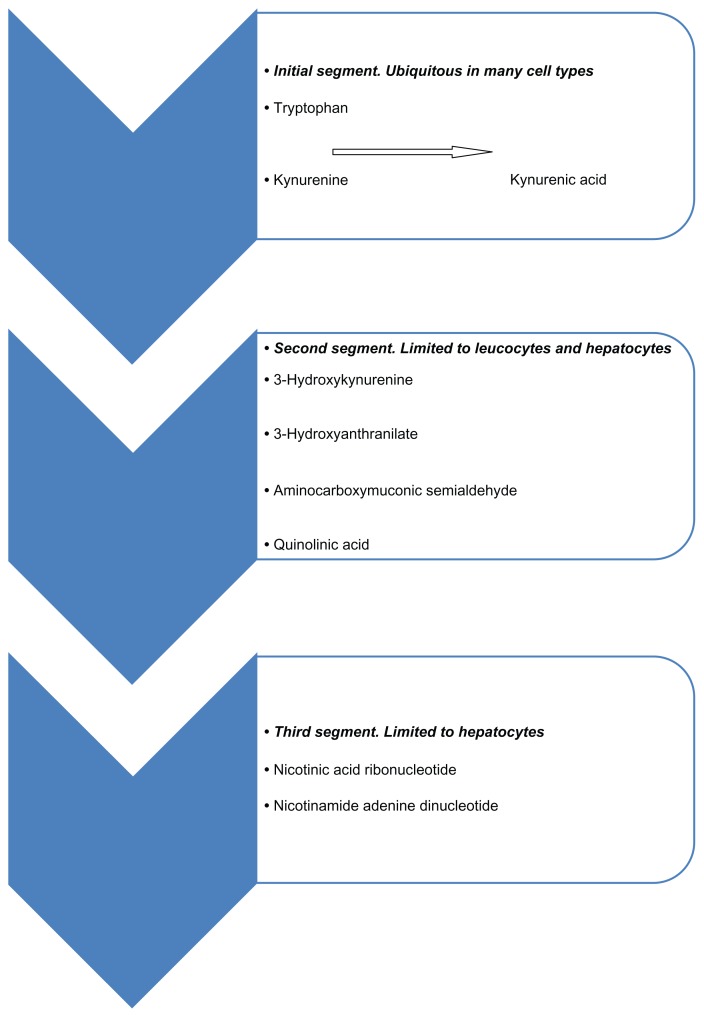
The kynurenine pathway is shown in Figure 1. Several drugs known to act on the kynurenine pathway have been reported to produce cutaneous sclerosis as side-effects. These are listed in Table 1.
Figure 1.
The kynurenine pathway.
Table 1.
Agents known to be active in the kynurenine pathway which have been reported to produce scleroderma like syndromes. Based on sites of action all would appear to increase kynurenine levels.
| L-tryptophan | Tryptophan 2,3 di-oxygenase inducer |
| Benserazide | Kynureninase inhibitor |
| Aromatic hydrocarbons | Bind to the aryl hydrocarbon receptor |
L-Tryptophan is an inducer of tryptophan2, 3 di-oxygenase,7 the first enzyme in the hepatic kynurenine pathway. Benserazide is a potent inhibitor of the enzyme kynurenase8 and a component of levodopa containing preparations used to treat Parkinsonism. Several cases of scleroderma-like illnesses have been reported during treatment of Parkinsonism with L-5-hydroxytryptophan and levodopa/benserazide combinations.9 Aromatic hydocarbons have also has been reported to precipitate scleroderma-like illnesses,10 and these are ligands for the aryl hydrocarbon receptor. Kynurenine is a natural ligand for the aryl hydrocarbon receptor.11
Manipulation of this pathway may provide additional therapeutic options to supplement the traditional immunosuppressive modalities known to have efficacy in this disorder (corticosteroids, methotrexate, mycophenylate mofetil and phototherapy).12 An ideal candidate agent would have known safety, approval for use in human subjects and anecdotal reports of efficacy in this group of disorders.
Tranilast is an agent approved in Japan and Korea for the management of allergic disorders. It is known to inhibit the release of mast cell mediators.13 More recently, it was demonstrated to inhibit the release of transforming growth factor beta 114 and it is now approved for the management of keloids and hypertrophic scars. There have been anecdotal reports of beneficial use in morphea. 15 It is a derivative of 3- hydroxyanthralinic acid, which is a known stimulator of TGF beta production,16 suggesting that it is acting as an antagonist.
The aim of this study was to evaluate whether tranilast had a direct effect on the kynurenine pathway under conditions of tryptophan loading.
Methods and Materials
L tryptophan was obtained from Bioeva Australia (BIOVEA, Hurstville NSW 2220 Australia). Tranilast was obtained from eBiochem (Pudong, Shanghai, China), weighed and capsulated. Early morning urine specimens were obtained, frozen and transported in laboratory-supplied kits. Organic acid profiles were performed at the Great Plains Laboratory (Lenaxa Ks) via gas chromatography/mass spectrometry. A healthy 56-year-old male with no significant co-morbidities acted as the subject. Ethical approval for this research was obtained, and the subject gave his written, informed consent to participate.
Results
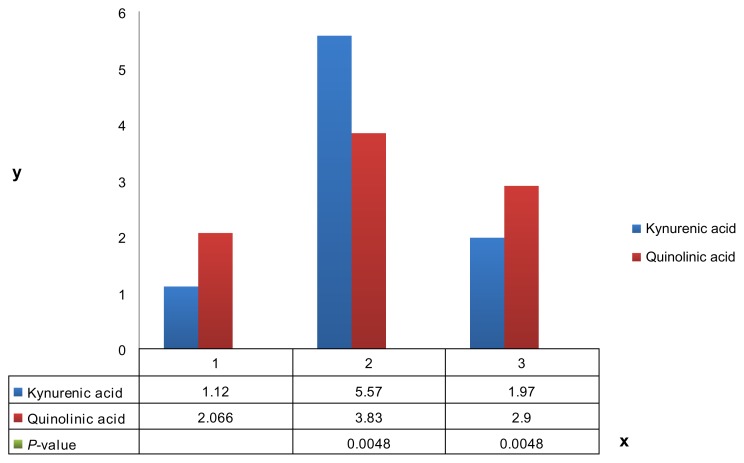
Urinary organic acid profiles were performed commercially at a CLIA certified laboratory (Great Plains Laboratory Lenaxa Ks) via gas chromatography/mass spectroscopy. Results are listed in Table 2. All results are expressed in mmol/mol creatinine. 3 values were obtained at baseline and under conditions of L tryptophan loading 2 grams daily, with 4 values obtained for L tryptophan 2 grams daily with tranilast 1 gram daily. Mean values were derived and are displayed in Figure 2.
Table 2.
Urinary organic acid profile for kynurenic and quinolinic acid. All results are expressed in mmol/mol creatinine. A two tail, two sample equal variance t test was performed. The P value is listed.
| Kynurenic acid | Quinolinic acid | |
|---|---|---|
| Baseline | ||
| Day 0 | 0.45 | 1.3 |
| Day 7 | 1.6 | 2.8 |
| Day 35 | 1.3 | 2.1 |
| Mean | 1.1 | 2.1 |
| L tryptophan 2 g daily | ||
| Day 10 | 5.9 | 4.5 |
| Day 17 | 6.3 | 3.4 |
| Day 26 | 4.5 | 3.6 |
| Mean | 5.6 | 3.8 |
| P value | 0.002 | 0.03 |
| L tryptophan 2 g daily/Tranilast 1 g daily | ||
| Day 10 | 1.9 | 2.9 |
| Day 25 | 3.4 | 3.3 |
| Day 61 | 1.2 | 3 |
| Day 66 | 1.4 | 2.4 |
| Mean | 2.0 | 2.9 |
| P value | 0.0048 | 0.048 |
Figure 2.
Graph of mean urinary kynurenic and quinolinic acids under baseline conditions (1), L-tryptophan 2 g/day loading (2) and L-tryptophan 2 grams per day with tranilast 1 gram per day (3). Values on the Y axis are expressed in mmol/mol creatinine.
To improve reliability, specimens were collected over a several-week period at variable time intervals. Tryptophan loading was commenced 10 days prior to the collection of the initial specimen and continued throughout the trial period. The timeline for data collection is included.
Mean baseline value for kynurenic acid and quinolinic acid were 1.1 and 2.1 mmol/mol creatinine, respectively. This rose to 5.6 and 3.8 mmol/mol creatinine, respectively, under conditions of L tryptophan loading 2 grams daily. Adding 1 gram of tranilast daily lowered the values to 2.0 and 2.9 mmol/mol creatinine, respectively. A 2 tail, 2 sample equal variance t test indicated statistical significance (P < 0.05) for all values.
The main limitation of this study was the involvement of a single individual only. Although reaching statistical significance in this individual, the results may not automatically be applicable to a larger population.
Discussion
A number of agents have been reported to exhibit activity on the kynurenine pathway. As drugs known to promote cutaneous sclerosis as an adverse reaction would be anticipated to increase kynurenine levels, any potential therapeutic agent needs to be shown to produce statistically significant lowering of both kynurenine and quinolinic acid, presumably by acting at the level TDO/IDO. The data does not allow us to determine which enzyme or both is being acted on. From a therapeutic perspective, the aim is to lower levels of kynurenine as the evidence suggests this is the most relevant metabolite.
The agent initially used to demonstrate the role of the kynurenine pathway in the maintenance of immunological tolerance during pregnancy, 1-methyl-D-tryptophan, 17 is undergoing trials in patients with advanced malignancy.18 Until these are available, there remains little data on its use in human subjects.
Indoleamine 2, 3-dioxygenase is involved in immune regulation. Expression varies at the body site, being maximal in interface areas19 including the gastrointestinal tract, lung and skin where exposure to foreign antigens occurs, in the spleen and thymus where it presumably plays a role in the regulation of self-antigens, and in the epididymis and prostate where it presumably has a protective role against intracellular infectious organisms.
Tranilast is a 3-hydroxyanthranilic acid derivative. As it inhibits TGF beta activity, which is normally enhanced by 3 hydroxyanthranilic acid, it would appear to be acting as an antagonist to 3 hydroxyanthranilic acid. These data suggest that tranilast may act as a competitive inhibitor at the level of tryptophan 2, 3 di-oxygenase/indoleamine 2, 3-dioxygenase. However as the trial involved only single individual, results must be considered preliminary.
Footnotes
Author Contributions
Conceived and designed the experiments: RRN. Analyzed the data: RRN. Wrote the first draft of the manuscript: RRN. Contributed to the writing of the manuscript: RRN. Agree with manuscript results and conclusions: RRN. Jointly developed the structure and arguments for the paper: RRN. Made critical revisions and approved final version: RRN. The author reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the author has provided signed confirmation of compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
Author(s) disclose no funding sources.
References
- 1.Wright DR, Haggins D, Chaffins M, Lokitz M. Eosinophilic fasciitis versus morphea profunda: A spectrum of deep morphea. J Am Acad Dermatol. 2009;60(3 Suppl 1):AB66. [Google Scholar]
- 2.Artlett CM. Microchimerism and scleroderma: an update. Curr Rheumatol Rep. 2003;5(2):154–9. doi: 10.1007/s11926-003-0044-2. [DOI] [PubMed] [Google Scholar]
- 3.Silver RM, McKinley K, Smith EA, et al. Tryptophan metabolism via the kynurenine pathway in patients with the eosinophilia-myalgia syndrome. Arthritis Rheum. 1992;35(9):1097–105. doi: 10.1002/art.1780350916. [DOI] [PubMed] [Google Scholar]
- 4.Freundlich B, Werth VP, Rook AH, et al. L-tryptophan ingestion associated with eosinophilic fasciitis but not progressive systemic sclerosis. Ann Intern Med. 1990;112(10):758–62. doi: 10.7326/0003-4819-112-10-758. [DOI] [PubMed] [Google Scholar]
- 5.Blauvelt A, Falanga V. Idiopathic and L-tryptophan-associated eosinophilic fasciitis before and after L-tryptophan contamination. Arch Dermatol. 1991;127(8):1159–66. [PubMed] [Google Scholar]
- 6.Silver RM, Sutherland SE, Carreira P, Heyes MP. Alterations in tryptophan metabolism in the toxic oil syndrome and in the eosinophilia-myalgia syndrome. J Rheumatol. 1992;19(1):69–73. [PubMed] [Google Scholar]
- 7.Salter M, Pogson CI. The role of tryptophan 2,3-dioxygenase in the hormonal control of tryptophan metabolism in isolated rat liver cells. Effects of glucocorticoids and experimental diabetes. Biochem J. 1985;229(2):499–504. doi: 10.1042/bj2290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith SA, Pogson CI. Effects of benserazide and carbidopa on the metabolism of L-tryptophan by isolated rat liver cells. Biochem Pharmacol. 1981;30(6):623–8. doi: 10.1016/0006-2952(81)90135-0. [DOI] [PubMed] [Google Scholar]
- 9.Joly P, Lampert A, Thomine E, Lauret P. Development of pseudobullous morphea and scleroderma-like illness during therapy with L-5- hydroxytryptophan and carbidopa. J Am Acad Dermatol. 1991;25(2 Pt 1):332–3. doi: 10.1016/s0190-9622(08)80475-6. [DOI] [PubMed] [Google Scholar]
- 10.Bovenzi M, Barbone F, Betta A, Tommasini M, Versini W. Scleroderma and occupational exposure. Scand J Work Environ Health. 1995;4:289–92. doi: 10.5271/sjweh.40. [DOI] [PubMed] [Google Scholar]
- 11.Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 12.Zwischenberger BA, Jacobe HT. A systematic review of morphea treatments and therapeutic algorithm. J Am Acad Dermatol. 2011;65(5):925–41. doi: 10.1016/j.jaad.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Nishigaki T. Mast cell degranulation and its inhibition by an anti-allergic agent tranilast. An electron microscopic study. Virchows Arch, B, Cell Pathol. 1988;55(5):311–22. doi: 10.1007/BF02896590. [DOI] [PubMed] [Google Scholar]
- 14.Yamada H, Tajima S, Nishikawa T, Murad S, Pinnell SR. Tranilast, a selective inhibitor of collagen synthesis in human skin fibroblasts. J Biochem. 1994;116(4):892–7. doi: 10.1093/oxfordjournals.jbchem.a124612. [DOI] [PubMed] [Google Scholar]
- 15.Taniguchi S, Yorifuji T, Hamada T. Treatment of linear localized scleroderma with the anti-allergic drug, tranilast. Clin Exp Dermatol. 1994;19(5):391–3. doi: 10.1111/j.1365-2230.1994.tb02689.x. [DOI] [PubMed] [Google Scholar]
- 16.Stone TW, Stoy N, Darlington LG. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci. 2013;34(2):136–43. doi: 10.1016/j.tips.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 18.ClinicalTrials.gov. 1-Methyl-D-Tryptophan in Treating Patients with Metastatic or Refractory Solid Tumors that cannot be Removed by Surgery. 2013. [Accessed Aug 30, 2013]. Identifier NCT00567931. Available at: http://clinicaltrials.gov/show/NCT00567931.
- 19.Dai X, Zhu BT. Indoleamine 2,3-dioxygenase tissue distribution and cellular localization in mice: implications for its biological functions. J Histochem Cytochem. 2010;58(1):17–28. doi: 10.1369/jhc.2009.953604. [DOI] [PMC free article] [PubMed] [Google Scholar]