Abstract
Humans have the ability to synthesize vitamin D during the action of ultraviolet (UV) radiation upon the skin. Apart from the regulation of calcium and phosphate metabolism, another critical role for vitamin D in immunity and respiratory health has been revealed, since vitamin D receptors have also been found in other body cells. The term “vitamin D insufficiency” has been used to describe low levels of serum 25-hydroxyvitamin D that may be associated with a wide range of pulmonary diseases, including viral and bacterial respiratory infection, asthma, chronic obstructive pulmonary disease, and cancer. This review focuses on the controversial relationship between vitamin D and asthma. Also, it has been found that different gene polymorphisms of the vitamin D receptor have variable associations with asthma. Other studies investigated the vitamin D receptor signaling pathway in vitro or in experimental animal models and showed either a beneficial or a negative effect of vitamin D in asthma. Furthermore, a range of epidemiological studies has also suggested that vitamin D insufficiency is associated with low lung function. In the future, clinical trials in different asthmatic groups, such as infants, children of school age, and ethnic minorities are needed to establish the role of vitamin D supplementation to prevent and/or treat asthma.
Keywords: vitamin D, asthma, immunomodulation, anti-inflammation
Introduction
According to the Global Initiative for Asthma, bronchial asthma is a chronic inflammatory disorder that is related with hyperresponsiveness of the airways and leads to repeated episodes of wheezing, dyspnea, chest tightness, and cough, particularly at night or early in the morning. These episodes are usually associated with extensive but variable bronchial obstruction, which is often reversible, either spontaneously or after treatment. Medical therapy involves two different classes of medication – inhaled corticosteroids used as daily controller and beta-adrenergic agonists used for bronchodilation.1
Asthma has become one of the most prevalent diseases worldwide causing a major public health concern. While there is evidence that the condition of asthma is multifactorial in etiology, changing environmental factors may underlie the rising prevalence of asthma, such as atmospheric pollution, dietary changes, allergens, improvements in health and hygiene, and lifestyle changes. Among nutritional hypotheses, vitamin D status is of particular interest regarding the controversial beneficial effects in non-skeletal disorders, such as cardiovascular disease, cancer, schizophrenia, multiple sclerosis, and asthma.2–6
Recently, the governments of the United States (USA) and Canada supported the initiative of the Institute of Medicine (IOM) to update the nutrient reference values, known as Dietary Reference Intake (DRI), including vitamin D status. The DRI for vitamin D was evidence based, as carried out by the US Department of Health and Human Services Agency for Healthcare Research and Quality,7,8 selecting bone health as a composite indicator. Dose (intake)-response, values for UL, and serum 25-hydroxyvitamin D [25(OH)D] were considered as the best indicators for setting the DRI. Due to variations in sunlight exposure and the variable response to that exposure, as well as the concept against sun exposure to reduce the risk of skin cancer, the establishment of DRI for vitamin D was based on the assumption of minimal or no sunlight exposure. However, inconsistent data suggest that sun exposure currently contributes meaningful amounts of vitamin D.6 Therefore, determining intake levels for vitamin D is somewhat more complicated.
It is recommended that the vitamin D status, assessed by the serum 25(OH)D concentration, be measured by a reliable assay.9,10 The 2011 DRI for vitamin D associated <30 nmol/L with an increased risk of deficiency, <40 nmol/L with risk of inadequacy, and ≥50 nmol/L but <125 nmol/L with adequacy and safety.6 IOM for Canada and the USA specifies that – in the absence of sunshine – a recommended dietary allowance (RDA) of 600 International Units (IU)/day (15 μg/day) of vitamin D will provide a serum 25(OH)D concentration of at least 50 nmol/L. Similarly, The Endocrine Society’s guidelines recommend 400–1,000 IU for children and 1,500–2,000 IU for adults for the maintenance of serum 25(OH)D levels above 30 ng/mL for preventing and treating vitamin D deficiency.10 Vitamin D deficiencies might cause rickets and insufficiency might be associated with immune dysfunction.11 It is also a fact that serum concentrations of 25(OH)D above 30 ng/mL (75 nmol/L) are not consistently associated with increased benefit, and 25(OH)D levels above 50 ng/mL (125 nmol/L) might be responsible for some disease risks, challenging the thought that “more is better.”12
Currently, a growing body of literature supports an association between serum vitamin D levels and respiratory infections attributable to the immunomodulatory properties of vitamin D.13 This interaction is important for individuals with asthma, as respiratory infections may influence the frequency, severity, and duration of asthmatic symptoms.14 In this study, we review the existing relevant literature focusing on epidemiological and interventional studies over the past decade and propose directions for future research.
Vitamin D overview: in vitro and in vivo models
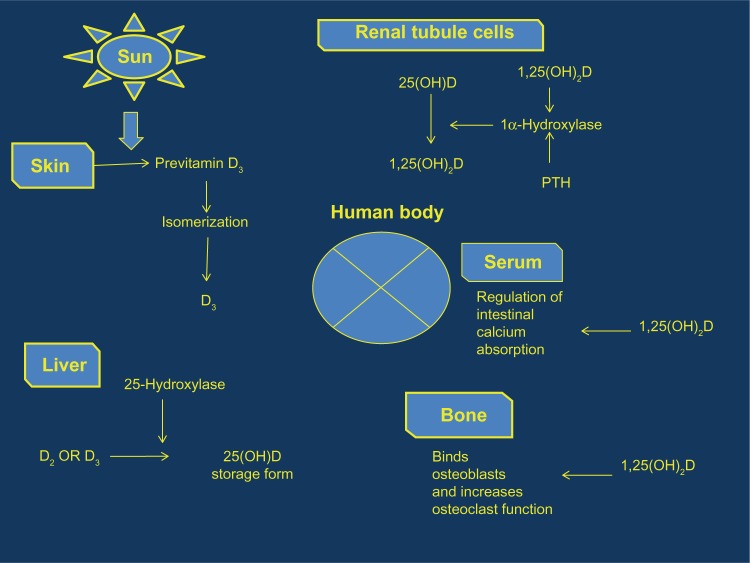
Humans have a combination of vitamins D2 (ergocalciferol) and D3 (cholecalciferol), both produced by exposure to ultraviolet (UV) radiation. Vitamin D2 is synthesized during UV radiation of ergosterol, whereas vitamin D3 is produced by UV radiation (290–315 nm) exposure of the skin by converting 7-dehydrocholesterol in the skin to previtamin D3 and, subsequently, to vitamin D3.6,15,16 Both vitamins are converted in the liver to 25(OH)D, via the action of 25-hydroxylases. Then, 1α-hydroxylase converts 25(OH)D to 1,25-dihydroxyvitamin D [1,25(OH)2D], primarily generated in the kidneys. 1,25(OH)2D binds to vitamin D receptors on cell membranes and becomes part of the steroid hormone nuclear receptor complex, verifying its classification as a steroid hormone.15 The biologically active form of vitamin D [1,25(OH)2D], also known as calcitriol, binds to the nuclear vitamin D receptor (VDR), a ligand-regulated transcription factor to mediate its effect17 (Figure 1).
Figure 1.
Vitamin D metabolic pathway per system.
Abbreviations: PTH, parathyroid hormone; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, serum 25-hydroxyvitamin D.
Although vitamins D2 and D3 result in the same active metabolite (calcitriol), several studies have shown that they differ in their effectiveness in raising serum 25(OH)D concentrations – the established marker of vitamin D status.12,18–21
Overall, humans have a combination of vitamins D2 and D3 available to them as part of a typical lifestyle from ambient UV exposure (vitamin D3), habitual dietary intakes of vitamin D3-rich foods (egg yolks and oily fish), fortified foods (margarine and breakfast cereals, which generally have vitamin D2 fortification), and vitamin supplements in which both vitamins D2 and D3 are available.6
It has been recently found that genetic variants in the vitamin D receptor (VDR) are variably associated with the risk of asthma development.22 VDR is also a critical molecule in calcium metabolism and bone turnover or other immune and inflammatory disorders.23 Furthermore, the vitamin D pathway has revealed a number of polymorphisms in its components, such as the vitamin D receptor VDR, the major receptor for the bioactive form of 25(OH)D, the microsomal vitamin D hydroxylase CYP2R1, and the vitamin D-binding protein GC.
Several research groups have studied VDR and CYP2R1 variants with contradictory results,24–29 possibly due to linkage disequilibrium between typed markers or the diverse population studied. Recent studies investigated VDR gene polymorphisms, CYP2R1 variants, and susceptibility in Tunisian,30 Chinese,31,32 and African American populations,33 with inconsistent results (Table 1).
Table 1.
Studies investigating VDR gene polymorphisms, CYP2R1 variants, and asthma susceptibility in different populations
| Study | N | Polymorphisms investigated | Population | Outcome |
|---|---|---|---|---|
| Fang et al31 | 101 | VDR SNPs | Chinese | No association between Fok I and Bsm I polymorphisms of VDR gene and asthma development |
| Li et al32 | 467 | Eight exons of VDR and all five exons of CYP2R1 | Chinese Han | Association between GC variants and asthma susceptibility |
| Pillai et al33 | 139 | 12 SNPs in VDR, CYP24A1, and CYP2R1 | Young African Americans | Association between CYP2R1 (SNP rs10766197) homozygous minor genotype and asthma. Association between CYP24A1 (SNP rs2248137) and lower vitamin D levels |
| Maalmi et al30 | 155 | VDR SNPs | Tunisian children | No association between VDR SNPs and serum 25(OH)D levels. Association between VDR gene polymorphisms and susceptibility to asthma |
Abbreviations: VDR, vitamin D receptor; SNP, single-nucleotide polymorphism; 25(OH)D, serum 25-hydroxyvitamin D.
Moreover, several studies have investigated the vitamin D receptor signal pathway in vitro34 or in experimental animal models,35 showing the beneficial effect of vitamin D in asthma. In contrast, the hypothesis that VDR levels play a negative role in asthma was reinforced by Wittke et al, who observed that vitamin D receptor-deficient mice failed to develop experimental allergic asthma.26 Other relevant studies reported that vitamin D3 can potentially increase the therapeutic response to glucocorticoids in steroid-resistant patients36,37 or have identified an additional pathway via which vitamin D can restrain inflammation in the airways to maintain respiratory health.35 Also, vitamin D has been shown to influence the function of cells intrinsic to innate and adaptive immunity.34
Vitamin D action relevant to asthma
Although several studies have reported an association between vitamin D and asthma, to date no study has fully described the mechanisms of vitamin D action relevant to asthma. Examples of vitamin D actions in asthma include improvement of immune function in lung tissues by protecting individuals from developing respiratory infections that could trigger asthma38–40 or overcoming steroid resistance by upregulation of IL-10 production from CD4+ T cells.36 Upregulation of antimicrobial proteins,41 such as beta defensins and cathelicidins (eg, hCAP-18 or LL-37),42 and anti-inflammatory,37 antiproliferative43 effects on airway smooth muscle, decelerating cell cycling, and decreasing hyperplasia44 also include examples of the important role of vitamin D in asthma. More specifically, cathelicidin, which is regulated by 1,25(OH)D, is a peptide active against Mycobacterium tuberculosis, gram positive and negative bacteria, viruses, and fungi.45 It is a fact that people deficient in cathelicidin are more susceptible to mucosal infections. Furthermore, 1,25(OH)2D3 induces secretion of antimicrobial activity against Pseudomonas aeruginosa.46 Even though the mechanisms of vitamin D action in asthma are not clear, it is possible that vitamin D could decrease acute asthma severity and reduce lung remodeling.
Maternal/infant vitamin D status
Many cross-sectional studies have focused on dietary etiology of asthma with inconsistent results47 (Table 2). Urban living environments,48 obesity,49 and poor nutrition,50,51 include risk factors responsible for both hypovitaminosis D and asthma. It is a fact that more women from different races are deficient in vitamin D, both before and during pregnancy.52
Table 2.
Studies relating vitamin D with respiratory disorder
| Author | Year | Country–population | Study subjects | Sample size | Methods | Respiratory disorder | Association between vitamin D and risk of disorder |
|---|---|---|---|---|---|---|---|
| Devereux et al59 | 2007 | Scotland | Mother–child pairs (early childhood) | 2,000 | Maternal food-frequency questionnaire | Wheezing in children | Inversely |
| Camargo et al60 | 2007 | USA | Mother–child pairs (early childhood) | 1,194 | Maternal food-frequency questionnaire | Wheezing in children | Inversely |
| Erkkola et al62 | 2009 | Finland | 5 years | 1,669 | Maternal food-frequency questionnaire | Asthma, allergic rhinitis in children | Inversely |
| Camargo et al72 | 2010 | New Zealand | Newborns to 5 years | 929 | Cord blood 25(OH)D | Asthma, wheezing | Inversely in wheezing, none in asthma |
| Carroll et al73 | 2011 | Canada | Mother–infant pairs | 340 | Maternal whole blood 25(OH)D | Asthma in mothers; bronchiolitis in children | Inversely in mothers, none in children |
| Camargo et al74 | 2010 | USA | Newborns | 922 | Cord blood 25(OH)D | Asthma, respiratory infection, wheezing | None in asthma, inversely in respiratory infection, wheezing |
| Morales et al76 | 2012 | Spain | Offspring 1–4 years 4–6 years | 1,724 | Maternal plasma 25(OH)D | Asthma, wheezing in offspring | None |
| Pike et al77 | 2012 | UK | 6-year-old children | 860 | Maternal serum 25(OH)D | Asthma, wheeze or atopy | None |
| Brehm et al82 | 2009 | Costa Rica | Children | 616 | Serum 25(OH)D levels | Asthma, allergy | Inversely |
| Brehm et al83 | 2010 | African American | Children | 1,024 | Serum 25(OH)D levels | Asthma | Inversely |
| Brehm et al84 | 2012 | Puerto Rican | Children | 560 | Serum 25(OH)D levels | Asthma | Inversely |
| Freishtat et al85 | 2010 | African American | Youth | 113 | Serum 25(OH)D levels | Asthma | Inversely |
| Chinellato et al87 | 2011 | Italy | Children | 75 | Serum 25(OH)D levels | Asthma | Inversely |
| Alyasin et al90 | 2011 | Iran | Children | 100 | Serum 25(OH)D levels | Asthma | Inversely |
| Li et al101 | 2011 | People’s Republic of China | Adults | 435 | Serum 25(OH)D levels | Asthma–lung function | Inversely |
Abbreviation: 25(OH)D, 25-hydroxyvitamin D.
Recently, given the early age of asthma onset, several studies focused their investigation on the role of maternal diet on risk of asthma in offspring.53,54 Maternal diet during pregnancy is a crucial factor, as it was recently hypothesized that the increased prevalence of asthma might be related to changing diet.55 It is also a fact that higher maternal dietary vitamin D, E, and zinc intakes in pregnancy are associated with decreased risks of wheezing illnesses and asthma in young children.56–59 Specifically, a higher maternal intake of vitamin D during pregnancy might decrease the risk of recurrent wheezing in early childhood.59,60 However, another study did not find any evidence regarding the associations between maternal intake of most foods during pregnancy and asthma, respiratory, and allergic outcomes in 5 year old children – except for apples and fish – which might reduce the risk of children developing asthma or atopic disease.61 In a population-based birth cohort study of 1,669 children in Finland, it was observed that maternal vitamin D intake from foods during pregnancy might be negatively associated with risk of asthma and allergic rhinitis in childhood.62
Given the maternal vitamin D deficiency and the potential impact on child skeletal health,63 some countries have recommended supplementations.64 In the UK, women who are pregnant or breastfeeding were advised to take 10 μg vitamin D per day;65 whereas, recently the 2011 DRI for the US and Canada established 15 μg vitamin D per day.6 The correct amount of supplementation has been a contradictory subject as the risk to the fetus during fetal development is uncertain.52,66
In many countries, early vitamin supplementation is given routinely to infants; therefore, several studies have reported the association between vitamin D supplementation in infancy and increased risk of atopy and allergic rhinitis later in life.67–69 The DRI for the US and Canada established infant supplementation at 10 μg vitamin D per day (http://books.nap.edu/openbook.php?record_id=13050&page=1106). In a study conducted in northern Finland, it was found that the prevalence of asthma, atopy, and allergic rhinitis at age 31 years was higher in participants who had received vitamin D supplementation (>2,000 IU/day) regularly during the first year of their lives compared to others.67
The majority of the studies have included serum 25(OH)D level as the indicator of overall vitamin D status, since 25(OH)D is the major circulating form of vitamin D.70 One of the first relevant studies concluded that in a pregnant woman, her serum concentration of 25(OH)D was strongly predictive of her child’s 25(OH)D concentration at birth; infants of women who were deficient in vitamin D experienced depleted vitamin D concentrations in utero and were born with low stores.71 High (>75 nmol/L) maternal serum vitamin D levels during late pregnancy have been associated with an increased likelihood of childhood eczema at 9 months and asthma at 9 years.52
Another population-based study, in which serum levels of 25(OH)D in the cord blood were measured in newborns of New Zealand (N = 929), resulted in determinants of low vitamin D status, such as winter month of birth, non-European ethnicity, longer gestational age, younger maternal age, and a parental history of asthma.72 Cross-sectional analyses concluded that higher maternal vitamin D levels were associated with decreased odds of maternal asthma but not associated with infant bronchiolitis.73 However, Camargo et al,74 in a study on cord blood 25(OH)D and risk of early childhood infection, concluded that cord-blood levels of 25(OH)D had inverse associations with risk of respiratory infection and childhood wheezing but no association with incident asthma.
Furthermore, a population birth cohort study in Australia explored the associations between perinatal conditions and the risk of hospital admission with asthma between the second and fifth birthday.75 The researchers reported that the season-associated risk of childhood asthma was consistent with early pregnancy exposures, such as the winter flu season or low vitamin D. Recently, it was reported that higher maternal circulating 25(OH)D concentrations in pregnancy were independently associated with lower risk of lower respiratory tract infections in offspring in the first year of life and not with wheezing or asthma in childhood.76 Additionally, measuring serum 25(OH)D at 34 weeks’ gestation in the mothers of 860 children born at term, showed no evidence that exposure to higher concentrations of 25(OH)D in maternal serum during late pregnancy increased the risk of childhood asthma, wheeze, or atopy.77 Therefore, despite the therapeutic option for vitamin D supplementation in mothers during pregnancy or the lactating period, further research is needed to explore the risks of such development.
Vitamin D status in asthmatic children
As bronchial asthma is still the most common chronic disease of childhood78 and one of the leading causes of morbidity in children worldwide,79 the number of studies concerning the association between vitamin D deficiency and asthma and allergies has recently increased significantly. Epidemiologic data in most of the reported studies suggest that low serum vitamin D (defined as circulating levels of 25(OH)D of <30 ng/mL) in children with asthma is associated with more symptoms, exacerbations, reduced lung function, increased medication usage, and severe disease.80
The first study to examine the effect of fish oil supplementation on the clinical progress of mild and severe asthma in children was conducted by Machura et al.81 They reported that after the eighth week, only slight improvement in the case of mild asthma was observed; the changes in lipids were within the normal range, although there was a significant increase in the 25(OH)D level. Other studies, which were conducted in diverse populations,82–84 demonstrated that lower vitamin D levels were associated with increased markers of allergy and asthma severity in Costa Rican children.82 In the study by Brehm in 2010, in North American children with mild-to-moderate persistent asthma who had an insufficient vitamin D status were found to be associated with higher odds of severe exacerbation over a 4-year period.83 Also, recently in Puerto Rican children, vitamin D insufficiency was associated with severe asthma exacerbations, independently of racial ancestry, atopy, or markers of disease severity or control.84
Several cross-sectional studies of vitamin D status in asthmatic children were conducted by several research groups in different populations. As vitamin D deficiency is more common among African American (AA) individuals, in particular from urban environments or with obesity,85 Freishtat et al investigated urban AA youth with or without persistent asthma, comparing levels between asthmatic and control groups. They reported that AA youth with persistent asthma were vitamin D deficient or insufficient, suggesting a routine vitamin D testing in this population, especially those with asthma.
A survey in Spain indicated that sunny hours had a protective effect on the prevalence of asthma in schoolchildren.86 In another cross-sectional study in Italian children, it was found that hypovitaminosis D was frequent in children with asthma living in a Mediterranean country, regardless of the sun exposure.87 Moreover, lower levels of vitamin D were associated with reduced asthma control in those children.
An Australian multicenter study concluded that oral supplementation with cod liver oil in childhood increased the odds of a history of having asthma.88 Additionally, vitamin D supplementation in children appears to prevent asthma exacerbation triggered by acute respiratory infection.89
As far as lung function is concerned, serum 25(OH)D levels were shown to be inversely associated with asthma, and there was a direct and significant relationship between vitamin D levels and pulmonary function test outcomes in asthmatic children.90 In addition, the first epidemiological study in the Middle East region concluded that vitamin D deficiency was the major predictor of asthma in Qatari children,91 while the age-specific effects of vitamin D in asthmatic patients have also been examined.92 The results demonstrated significant associations between serum vitamin D status and steroid requirement and in vitro responsiveness to corticosteroids in the pediatric, but not in the adult asthma group. Published studies that investigate the interaction of vitamin D with corticosteroid-mediated anti-inflammatory responses have started to accumulate. More recently, the same group of researchers92 concluded that corticosteroid use and worsening airflow limitation were associated with lower vitamin D serum levels in asthmatic children.93 They reported that vitamin D enhanced glucocorticoid action in peripheral blood mononuclear cells from asthmatic patients. In a randomized, double-blind, two-period crossover trial of asthmatic children, the researchers concluded that the administration of 25(OH)D did not affect short-term growth or markers of bone turnover in children with asthma treated with inhaled dry-powder budesonide (400 μg daily).94 However, the beneficial effect of oral corticosteroids on allergen-specific immunotherapy was not confirmed. It was observed that the combined administration of a corticosteroid drug and allergen extract suppressed the early clinical and immunological effects of specific immunotherapy (SIT) and that vitamin D3 prevented this adverse influence of steroids.95 Additionally, in children with asthma treated with inhaled corticosteroids, vitamin D deficiency (defined as 25(OH)D < 20 ng/mL) was associated with poorer lung function than in children with vitamin D insufficiency or sufficiency.96
As vitamin D insufficiency has been related to decreased bone mineral density which is adversely affected by corticosteroids, Tse et al97 sought to determine whether children with asthma who have lower vitamin D levels are more susceptible to the negative effects of corticosteroids on bone mineral density over time. They concluded that vitamin D levels significantly modified the effect of corticosteroids on bone mineral accretion (BMA) in boys. In spite of the inconsistency and the need for additional studies, the therapeutic benefit of vitamin D supplementation in asthmatic children has been revealed.
Vitamin D status in asthmatic adults
Some studies investigated the effects of vitamin D on the inception and severity of asthma on adults. The first study was conducted in patients with allergic bronchial asthma and found that when calcium was given orally in combination with calciferol (vitamin D2) there was a decrease in airway obstruction.98 Similarly, one of the first prospective, controlled, and randomized clinical trials in asthmatics undergoing long-term treatment with systemically applied corticosteroids reported that the combination of ethane-1-hydroxy-1,1-diphosphonate (EHDP), calcium, and vitamin D appeared to be a useful regimen for the management of steroid-induced bone loss in adult asthmatics.99
Since then, apart from the increased number of studies in childhood asthma, there are also a sufficient number of studies in asthmatic adults concerning their relation to vitamin D status. Regarding the treatment of steroid osteoporosis, calcium–D3 Nycomed (Nycomed, Zürich, Switzerland) was suggested to achieve a high clinical efficiency and absence of side effects in patients with hormone-dependent bronchial asthma.100
The findings of a cross-sectional study including Chinese patients with newly diagnosed asthma concluded that vitamin D deficiency was highly prevalent and was associated with decreased lung function in this population.101 Also, in asthmatic adult patients, reduced vitamin D levels were associated with impaired lung function, increased airway hyperresponsiveness, and reduced glucocorticoid response, suggesting that supplementation of vitamin D levels in these patients might improve multiple parameters of asthma severity and treatment response.9 The first study to treat successfully a patient with reactive airways dysfunction syndrome was conducted by Varney et al.102 Symptoms included ones that mimic asthma and, even though the patient appeared unresponsive to conventional asthma treatments, the patient responded to high-dose oral vitamin D supplements.
Several studies have suggested the active involvement of vascular endothelial growth factor (VEGF), a potent proangiogenic cytokine, in the pathogenesis of isocyanate occupational asthma, which might be mediated by 1,25-dihydroxychole-calciferol [1,25(OH)2D3], the active form of vitamin D.103,104 It was recently suggested that the serum vitamin D-binding protein level might be used as a serological marker for the detection of isocyanate-induced occupational asthma among workers exposed to isocyanate.105 It was concluded that the toluene diisocyanate-induced VEGF production/secretion was reversed by 1,25(OH)2D3 treatment, which might provide also a potential therapeutic strategy for these patients. Another study focused on the relationship between serum concentrations of 25(OH)D and pulmonary function, using data from the Third National Health and Nutrition Examination Survey (N = 14,091).106 It was demonstrated that there was a strong relationship between serum concentrations of 25(OH)D, forced expiratory volume 1 (FEV1), and forced vital capacity (FVC). However, Shaheen et al did not confirm a positive association between blood 25(OH)D concentrations and adult lung function,107 reinforcing the inconsistency concerning the hypothesis of vitamin D ’s beneficial effects. Finally, a cross-sectional study in asthmatics and in nonasthmatics (N = 23), determining the correlation between serum 25(OH)D levels and pneumococcal antibody levels, reported that 25(OH)D might enhance humoral immunity against Streptococcus pneumoniae in subjects with atopic conditions, but not without atopic conditions.108
Conclusion
Despite the growing evidence of the role of vitamin D in incident asthma and asthma exacerbation, studies are not all consistent. Some studies have supported the view that vitamin D deficiency is the cause of the global asthma epidemic.2 On the other hand, another study109 has proposed that vitamin D supplements are the cause of the asthma epidemic. In fact, the concept “more is better” has been challenged, given that higher levels of vitamin D status have not been shown to greatly benefit asthmatic patients, but they have been associated with diverse health problems.
There are several confounding factors that could influence the relationship between vitamin D levels and asthma development, such as the fact that asthmatic subjects spend more time indoors, are less physically active, and therefore are exposed less to sunlight.110 Other factors include the insufficient number of subjects studied, or the unequal sex distribution in groups studied.90 As a result, the IOM, after reviewing recent literature regarding vitamin D, concluded that there were insufficient data to recommend vitamin D supplementation for the prevention of nonbone-related diseases.6
To our knowledge, there are no studies available on whether age is a crucial factor in regulating the role of vitamin D in asthma. A study analyzed data from the Third National Health and Nutrition Examination Survey (1988–1994) with linked mortality files through 2006. The researchers divided asthmatics into two groups – younger adults (17–54 years of age) and older adults (55 years or older) – and reported that ethnic differences in asthma mortality and the vitamin D-mortality link merited further investigation.111
Lastly, Litonjua,112 in his review, examined whether the administration of vitamin D enhances corticosteroid actions and potentially reverses steroid resistance, a crucial topic for asthmatic patients. As it has already been mentioned in the present review, several clinical and observational studies have suggested a synergistic effect of vitamin D and corticosteroids in asthma outcomes.83,92,93,96 However, larger studies, including both children and adults are needed to shed light on the circulating level and dose of vitamin D that can result in the greatest potential for synergy with steroids. In spite of some potential mechanisms of this interaction that have been reported, such as the induction of IL-10-secreting T regulatory cells,36 further studies in asthma cell models are required to clarify whether it is the vitamin D or the corticosteroid effects that are primarily operating in asthma.
Future perspectives
In spite of the inconsistency, most of the cross-sectional and prospective studies have revealed the benefit of vitamin D supplementation in asthmatic patients. However, more clinical trials are required to demonstrate the levels of vitamin D, which differ according to individual characteristics. Randomized controlled trials of calcium and vitamin D often fail, raising an important problem.113 The fact that serum 25(OH)D concentrations have a sigmoidal relationship with respect to oral vitamin D intake, meaning that for a given dose, the increase is much larger for people with low initial concentrations than for people with higher concentrations. Thus, it is important that serum 25(OH)D concentrations are measured at least two or three times during the studies, since it is serum 25(OH)D concentration, not vitamin D intake that affects risk of disease.
As vitamin D deficiency is prevalent in sun-replete areas, it is also essential to determine the optimal timing of vit D measurement. Furthermore, dietary vitamin D-mediated intervention in prevention and management of asthma will be enhanced when researchers shed more light on molecular epigenetic mechanism of vitamin D/VDR. All these data suggest that a continuous demand for 25(OH)D measurements will exist for many years to come.
Acknowledgments
Supported by Shanghai Municipal Natural Science Foundation of China (Grant No 13ZR1408900). Figure 1 was created by Paul Zarogoulidis.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Guidelines: GINA Report GSfAMaP Global Initiative for Asthma (GINA) 2012Available from http://www.ginasthma.org/documents/4Accessed August 29, 2013
- 2.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120(5):1031–1035. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toner CD, Davis CD, Milner JA. The vitamin D and cancer conundrum: aiming at a moving target. J Am Diet Assoc. 2010;110(10):1492–1500. doi: 10.1016/j.jada.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Rosen CJ, Adams JS, Bikle DD, et al. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012;33(3):456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 2007;(158):1–235. [PMC free article] [PubMed] [Google Scholar]
- 8.Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep) 2009;(183):1–420. [PMC free article] [PubMed] [Google Scholar]
- 9.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181(7):699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 11.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364(3):248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 12.Vieth R. Implications for 25-hydroxyvitamin D testing of public health policies about the benefits and risks of vitamin D fortification and supplementation. Scand J Clin Lab Invest Suppl. 2012;243:144–153. doi: 10.3109/00365513.2012.682893. [DOI] [PubMed] [Google Scholar]
- 13.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169(4):384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Elden LJ, Sachs AP, van Loon AM, et al. Enhanced severity of virus associated lower respiratory tract disease in asthma patients may not be associated with delayed viral clearance and increased viral load in the upper respiratory tract. J Clin Virol. 2008;41(2):116–121. doi: 10.1016/j.jcv.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borradale D, Kimlin M. Vitamin D in health and disease: an insight into traditional functions and new roles for the ‘sunshine vitamin’. Nutr Res Rev. 2009;22(2):118–136. doi: 10.1017/S0954422409990102. [DOI] [PubMed] [Google Scholar]
- 16.Dixon KM, Tongkao-On W, Sequeira VB, et al. Vitamin d and death by sunshine. Int J Mol Sci. 2013;14(1):1964–1977. doi: 10.3390/ijms14011964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bossé Y, Lemire M, Poon AH, et al. Asthma and genes encoding components of the vitamin D pathway. Respir Res. 2009;10:98. doi: 10.1186/1465-9921-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68(4):854–858. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]
- 19.Romagnoli E, Mascia ML, Cipriani C, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab. 2008;93(8):3015–3020. doi: 10.1210/jc.2008-0350. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93(3):677–681. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biancuzzo RM, Young A, Bibuld D, et al. Fortification of orange juice with vitamin D(2) or vitamin D(3) is as effective as an oral supplement in maintaining vitamin D status in adults. Am J Clin Nutr. 2010;91(6):1621–1626. doi: 10.3945/ajcn.2009.27972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers AJ, Raby BA, Lasky-Su JA, et al. Assessing the reproducibility of asthma candidate gene associations, using genome-wide data. Am J Respir Crit Care Med. 2009;179(12):1084–1090. doi: 10.1164/rccm.200812-1860OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfeffer PE, Hawrylowicz CM. Vitamin D and lung disease. Thorax. 2012;67(11):1018–1020. doi: 10.1136/thoraxjnl-2012-202139. [DOI] [PubMed] [Google Scholar]
- 24.Hewison M, Freeman L, Hughes SV, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170(11):5382–5390. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 25.Overbergh L, Decallonne B, Waer M, et al. 1alpha,25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524-543) Diabetes. 2000;49(8):1301–1307. doi: 10.2337/diabetes.49.8.1301. [DOI] [PubMed] [Google Scholar]
- 26.Wittke A, Weaver V, Mahon BD, August A, Cantorna MT. Vitamin D receptor-deficient mice fail to develop experimental allergic asthma. J Immunol. 2004;173(5):3432–3436. doi: 10.4049/jimmunol.173.5.3432. [DOI] [PubMed] [Google Scholar]
- 27.van Etten E, Verlinden L, Giulietti A, et al. The vitamin D receptor gene FokI polymorphism: functional impact on the immune system. Eur J Immunol. 2007;37(2):395–405. doi: 10.1002/eji.200636043. [DOI] [PubMed] [Google Scholar]
- 28.Poon AH, Laprise C, Lemire M, et al. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am J Respir Crit Care Med. 2004;170(9):967–973. doi: 10.1164/rccm.200403-412OC. [DOI] [PubMed] [Google Scholar]
- 29.Raby BA, Lazarus R, Silverman EK, et al. Association of vitamin D receptor gene polymorphisms with childhood and adult asthma. Am J Respir Crit Care Med. 2004;170(10):1057–1065. doi: 10.1164/rccm.200404-447OC. [DOI] [PubMed] [Google Scholar]
- 30.Maalmi H, Sassi FH, Berraies A, Ammar J, Hamzaoui K, Hamzaoui A. Association of vitamin D receptor gene polymorphisms with susceptibility to asthma in Tunisian children: A case control study. Hum Immunol. 2013;74(2):234–240. doi: 10.1016/j.humimm.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Fang WL, Gao LB, Liang WB, et al. Association analysis of vitamin D receptor gene polymorphisms in chinese population with asthma. Iran J Allergy Asthma Immunol. 2009;8(3):141–147. [PubMed] [Google Scholar]
- 32.Li F, Jiang L, Willis-Owen SA, Zhang Y, Gao J. Vitamin D binding protein variants associate with asthma susceptibility in the Chinese Han population. BMC Med Genet. 2011;12:103. doi: 10.1186/1471-2350-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pillai DK, Iqbal SF, Benton AS, et al. Associations between genetic variants in vitamin D metabolism and asthma characteristics in young African Americans: a pilot study. J Investig Med. 2011;59(6):938–946. doi: 10.231/JIM.0b013e318220df41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Curr Allergy Asthma Rep. 2011;11(1):29–36. doi: 10.1007/s11882-010-0161-8. [DOI] [PubMed] [Google Scholar]
- 35.Dimeloe S, Nanzer A, Ryanna K, Hawrylowicz C. Regulatory T cells, inflammation and the allergic response-The role of glucocorticoids and Vitamin D. J Steroid Biochem Mol Biol. 2010;120(2–3):86–95. doi: 10.1016/j.jsbmb.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 36.Xystrakis E, Kusumakar S, Boswell S, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116(1):146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerjee A, Damera G, Bhandare R, et al. Vitamin D and glucocorticoids differentially modulate chemokine expression in human airway smooth muscle cells. Br J Pharmacol. 2008;155(1):84–92. doi: 10.1038/bjp.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91(5):1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 39.Brunetti L, Colazzo D, Francavilla R, et al. The role of pulmonary infection in pediatric asthma. Allergy Asthma Proc. 2007;28(2):190–193. doi: 10.2500/aap.2007.28.2964. [DOI] [PubMed] [Google Scholar]
- 40.Ginde AA, Mansbach JM, Camargo CA., Jr Vitamin D, respiratory infections, and asthma. Curr Allergy Asthma Rep. 2009;9(1):81–87. doi: 10.1007/s11882-009-0012-7. [DOI] [PubMed] [Google Scholar]
- 41.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4):2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 42.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4(2):80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damera G, Fogle HW, Lim P, et al. Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br J Pharmacol. 2009;158(6):1429–1441. doi: 10.1111/j.1476-5381.2009.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iqbal SF, Freishtat RJ. Mechanism of action of vitamin D in the asthmatic lung. J Investig Med. 2011;59(8):1200–1202. doi: 10.231/JIM.0b013e31823279f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med (Berl) 2010;88(5):441–450. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 47.McKeever TM, Britton J. Diet and asthma. Am J Respir Crit Care Med. 2004;170(7):725–729. doi: 10.1164/rccm.200405-611PP. [DOI] [PubMed] [Google Scholar]
- 48.Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007;46(1):42–44. doi: 10.1177/0009922806289311. [DOI] [PubMed] [Google Scholar]
- 49.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 50.Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr. 2005;135(10):2478–2485. doi: 10.1093/jn/135.10.2478. [DOI] [PubMed] [Google Scholar]
- 51.Pinto JM, Schneider J, Perez R, DeTineo M, Baroody FM, Naclerio RM. Serum 25-hydroxyvitamin D levels are lower in urban African American subjects with chronic rhinosinusitis. J Allergy Clin Immunol. 2008;122(2):415–417. doi: 10.1016/j.jaci.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 52.Gale CR, Robinson SM, Harvey NC, et al. Princess Anne Hospital Study Group Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62(1):68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gern JE, Lemanske RF, Jr, Busse WW. Early life origins of asthma. J Clin Invest. 1999;104(7):837–843. doi: 10.1172/JCI8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warner JA, Jones CA, Jones AC, Warner JO. Prenatal origins of allergic disease. J Allergy Clin Immunol. 2000;105(2 Pt 2):S493–S498. doi: 10.1016/s0091-6749(00)90049-6. [DOI] [PubMed] [Google Scholar]
- 55.Devereux G. Maternal diet during pregnancy: an emerging risk factor for childhood asthma. Expert Rev Clin Immunol. 2008;4(6):663–668. doi: 10.1586/1744666X.4.6.663. [DOI] [PubMed] [Google Scholar]
- 56.Martindale S, McNeill G, Devereux G, Campbell D, Russell G, Seaton A. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am J Respir Crit Care Med. 2005;171(2):121–128. doi: 10.1164/rccm.200402-220OC. [DOI] [PubMed] [Google Scholar]
- 57.Devereux G, Turner SW, Craig LC, et al. Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year-old children. Am J Respir Crit Care Med. 2006;174(5):499–507. doi: 10.1164/rccm.200512-1946OC. [DOI] [PubMed] [Google Scholar]
- 58.Litonjua AA, Rifas-Shiman SL, Ly NP, et al. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am J Clin Nutr. 2006;84(4):903–911. doi: 10.1093/ajcn/84.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Devereux G, Litonjua AA, Turner SW, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85(3):853–859. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 60.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85(3):788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willers SM, Devereux G, Craig LC, et al. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax. 2007;62(9):773–779. doi: 10.1136/thx.2006.074187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erkkola M, Kaila M, Nwaru BI, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy. 2009;39(6):875–882. doi: 10.1111/j.1365-2222.2009.03234.x. [DOI] [PubMed] [Google Scholar]
- 63.Javaid MK, Crozier SR, Harvey NC, et al. Princess Anne Hospital Study Group Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367(9504):36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 64.Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr. 2004;80(6 Suppl):1752S–1758S. doi: 10.1093/ajcn/80.6.1752S. [DOI] [PubMed] [Google Scholar]
- 65.Dietary reference values for food energy and nutrients for the United Kingdom Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy. Rep Health Soc Subj (Lond) 1991;41:1–210. [No authors listed] [PubMed] [Google Scholar]
- 66.Hollis BW, Wagner CL. Nutritional vitamin D status during pregnancy: reasons for concern. CMAJ. 2006;174(9):1287–1290. doi: 10.1503/cmaj.060149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hyppönen E, Sovio U, Wjst M, et al. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. 2004;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- 68.Bäck O, Blomquist HK, Hernell O, Stenberg B. Does vitamin D intake during infancy promote the development of atopic allergy? Acta Derm Venereol. 2009;89(1):28–32. doi: 10.2340/00015555-0541. [DOI] [PubMed] [Google Scholar]
- 69.Kull I, Bergström A, Melén E, et al. Early-life supplementation of vitamins A and D, in water-soluble form or in peanut oil, and allergic diseases during childhood. J Allergy Clin Immunol. 2006;118(6):1299–1304. doi: 10.1016/j.jaci.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 70.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 71.Hollis BW, Pittard WB., 3rd Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J Clin Endocrinol Metab. 1984;59(4):652–657. doi: 10.1210/jcem-59-4-652. [DOI] [PubMed] [Google Scholar]
- 72.Camargo CA, Jr, Ingham T, Wickens K, et al. New Zealand Asthma and Allergy Cohort Study Group Vitamin D status of newborns in New Zealand. Br J Nutr. 2010;104(7):1051–1057. doi: 10.1017/S0007114510001674. [DOI] [PubMed] [Google Scholar]
- 73.Carroll KN, Gebretsadik T, Larkin EK, et al. Relationship of maternal vitamin D level with maternal and infant respiratory disease. Am J Obstet Gynecol. 2011;205(3):215. e1–e7. doi: 10.1016/j.ajog.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Camargo CA, Jr, Ingham T, Wickens K, et al. New Zealand Asthma and Allergy Cohort Study Group Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127(1):e180–e187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 75.Algert CS, Bowen JR, Lain SL, Allen HD, Vivian-Taylor JM, Roberts CL. Pregnancy exposures and risk of childhood asthma admission in a population birth cohort. Pediatr Allergy Immunol. 2011;22(8):836–842. doi: 10.1111/j.1399-3038.2011.01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morales E, Romieu I, Guerra S, et al. INMA Project Maternal vitamin D status in pregnancy and risk of lower respiratory tract infections, wheezing, and asthma in offspring. Epidemiology. 2012;23(1):64–71. doi: 10.1097/EDE.0b013e31823a44d3. [DOI] [PubMed] [Google Scholar]
- 77.Pike KC, Inskip HM, Robinson S, et al. Southampton Women’s Survey Study Group Maternal late-pregnancy serum 25-hydroxyvitamin D in relation to childhood wheeze and atopic outcomes. Thorax. 2012;67(11):950–956. doi: 10.1136/thoraxjnl-2012-201888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma (GINA) Program The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 79.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma – United States, 1980–1999. MMWR Surveill Summ. 2002;51(1):1–13. [PubMed] [Google Scholar]
- 80.Kunisaki KM, Niewoehner DE, Connett JE, COPD Clinical, Research Network Vitamin D levels and risk of acute exacerbations of chronic obstructive pulmonary disease: a prospective cohort study. Am J Respir Crit Care Med. 2012;185(3):286–290. doi: 10.1164/rccm.201109-1644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Machura E, Brus R, Kalacinński W, Lacheta M. The effect of dietary fish oil supplementation on the clinical course of asthma in children. Pediatr Pol. 1996;71(2):97–102. Polish [with English abstract] [PubMed] [Google Scholar]
- 82.Brehm JM, Celedón JC, Soto-Quiros ME, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179(9):765–771. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brehm JM, Schuemann B, Fuhlbrigge AL, et al. Childhood Asthma Management Program Research Group Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. 2010;126(1):52–58. e5. doi: 10.1016/j.jaci.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brehm JM, Acosta-Pérez E, Klei L, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med. 2012;186(2):140–146. doi: 10.1164/rccm.201203-0431OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Freishtat RJ, Iqbal SF, Pillai DK, et al. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr. 2010;156(6):948–952. doi: 10.1016/j.jpeds.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arnedo-Pena A, García-Marcos L, Fernández-Espinar JF, et al. Sunny hours and variations in the prevalence of asthma in schoolchildren according to the International Study of Asthma and Allergies (ISAAC) Phase III in Spain. Int J Biometeorol. 2011;55(3):423–434. doi: 10.1007/s00484-010-0353-x. [DOI] [PubMed] [Google Scholar]
- 87.Chinellato I, Piazza M, Sandri M, Peroni D, Piacentini G, Boner AL. Vitamin D serum levels and markers of asthma control in Italian children. J Pediatr. 2011;158(3):437–441. doi: 10.1016/j.jpeds.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 88.Hughes AM, Lucas RM, Ponsonby AL, et al. The role of latitude, ultraviolet radiation exposure and vitamin D in childhood asthma and hayfever: an Australian multicenter study. Pediatr Allergy Immunol. 2011;22(3):327–333. doi: 10.1111/j.1399-3038.2010.01099.x. [DOI] [PubMed] [Google Scholar]
- 89.Majak P, Olszowiec-Chlebna M, Smejda K, Stelmach I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127(5):1294–1296. doi: 10.1016/j.jaci.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 90.Alyasin S, Momen T, Kashef S, Alipour A, Amin R. The relationship between serum 25 hydroxy vitamin d levels and asthma in children. Allergy Asthma Immunol Res. 2011;3(4):251–255. doi: 10.4168/aair.2011.3.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bener A, Ehlayel MS, Tulic MK, Hamid Q. Vitamin D deficiency as a strong predictor of asthma in children. Int Arch Allergy Immunol. 2012;157(2):168–175. doi: 10.1159/000323941. [DOI] [PubMed] [Google Scholar]
- 92.Goleva E, Searing DA, Jackson LP, Richers BN, Leung DY. Steroid requirements and immune associations with vitamin D are stronger in children than adults with asthma. J Allergy Clin Immunol. 2012;129(5):1243–1251. doi: 10.1016/j.jaci.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125(5):995–1000. doi: 10.1016/j.jaci.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schou AJ, Heuck C, Wolthers OD. Does vitamin D administered to children with asthma treated with inhaled glucocorticoids affect short-term growth or bone turnover? Pediatr Pulmonol. 2003;36(5):399–404. doi: 10.1002/ppul.10379. [DOI] [PubMed] [Google Scholar]
- 95.Majak P, Rychlik B, Stelmach I. The effect of oral steroids with and without vitamin D3 on early efficacy of immunotherapy in asthmatic children. Clin Exp Allergy. 2009;39(12):1830–1841. doi: 10.1111/j.1365-2222.2009.03357.x. [DOI] [PubMed] [Google Scholar]
- 96.Wu AC, Tantisira K, Li L, Fuhlbrigge AL, Weiss ST, Litonjua A, Childhood Asthma Management Program Research Group Effect of vitamin D and inhaled corticosteroid treatment on lung function in children. Am J Respir Crit Care Med. 2012;186(6):508–513. doi: 10.1164/rccm.201202-0351OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tse SM, Kelly HW, Litonjua AA, Va n Natta ML, Weiss ST, Tantisira KG, Childhood Asthma Management Program Research Group Corticosteroid use and bone mineral accretion in children with asthma: effect modification by vitamin D. J Allergy Clin Immunol. 2012;130(1):53–60. e4. doi: 10.1016/j.jaci.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Utz G, Hauck AM. Oral application of calcium and vitamin D2 in allergic bronchial asthma (author’s transl) MMW Munch Med Wochenschr. 1976;118(43):1395–1398. German [with English abstract] [PubMed] [Google Scholar]
- 99.Worth H, Stammen D, Keck E. Therapy of steroid-induced bone loss in adult asthmatics with calcium, vitamin D, and a diphosphonate. Am J Respir Crit Care Med. 1994;150(2):394–397. doi: 10.1164/ajrccm.150.2.8049820. [DOI] [PubMed] [Google Scholar]
- 100.Ememl’ianov AV, Shevelev SE, Murzin BA, Amosov VI. Efficiency of calcium and vitamin D3 in the treatment of steroid osteoporosis in patients with hormone dependent bronchial asthma. Ter Arkh. 1999;71(11):68–69. Russian [with English abstract] [PubMed] [Google Scholar]
- 101.Li F, Peng M, Jiang L, et al. Vitamin D deficiency is associated with decreased lung function in Chinese adults with asthma. Respiration. 2011;81(6):469–475. doi: 10.1159/000322008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Varney VA, Evans J, Bansal AS. Successful treatment of reactive airways dysfunction syndrome by high-dose vitamin D. J Asthma Allergy. 2011;4:87–91. doi: 10.2147/JAA.S19107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gruber HE, Hoelscher G, Ingram JA, Chow Y, Loeffler B, Hanley EN., Jr 1,25(OH)2-vitamin D3 inhibits proliferation and decreases production of monocyte chemoattractant protein-1, thrombopoietin, VEGF, and angiogenin by human annulus cells in vitro. Spine (Phila Pa 1976) 2008;33(7):755–765. doi: 10.1097/BRS.0b013e3181695d59. [DOI] [PubMed] [Google Scholar]
- 104.Nakagawa K, Kawaura A, Kato S, Takeda E, Okano T. 1alpha,25-Dihydroxyvitamin D(3) is a preventive factor in the metastasis of lung cancer. Carcinogenesis. 2005;26(2):429–440. doi: 10.1093/carcin/bgh332. [DOI] [PubMed] [Google Scholar]
- 105.Kim SH, Choi GS, Nam YH, et al. Role of vitamin D-binding protein in isocyanate-induced occupational asthma. Exp Mol Med. 2012;44(5):319–329. doi: 10.3858/emm.2012.44.5.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128(6):3792–3798. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 107.Shaheen SO, Jameson KA, Robinson SM, et al. Relationship of vitamin D status to adult lung function and COPD. Thorax. 2011;66(8):692–698. doi: 10.1136/thx.2010.155234. [DOI] [PubMed] [Google Scholar]
- 108.Lee J, Zhao H, Fenta Y, Kita H, Kumar R, Juhn YJ. Serum 25-hydroxyvitamin D is associated with enhanced pneumococcal antibody levels in individuals with asthma. Allergy Asthma Proc. 2011;32(6):445–452. doi: 10.2500/aap.2011.32.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wjst M, Dold S. Genes, factor X, and allergens: what causes allergic diseases? Allergy. 1999;54(7):757–759. doi: 10.1034/j.1398-9995.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 110.Weiss ST, Litonjua AA. Maternal diet vs lack of exposure to sunlight as the cause of the epidemic of asthma, allergies and other autoimmune diseases. Thorax. 2007;62(9):746–748. doi: 10.1136/thx.2007.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsai CL, Delclos GL, Huang JS, Hanania NA, Camargo CA., Jr Age-related differences in asthma outcomes in the United States, 1988–2006. Ann Allergy Asthma Immunol. 2013;110(4):240–246. e1. doi: 10.1016/j.anai.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 112.Litonjua AA. Vitamin D and corticosteroids in asthma: synergy, interaction and potential therapeutic effects. Expert Rev Respir Med. 2013;7(2):101–104. doi: 10.1586/ers.12.85. [DOI] [PubMed] [Google Scholar]
- 113.Lappe JM, Heaney RP. Why randomized controlled trials of calcium and vitamin D sometimes fail. Dermatoendocrinol. 2012;4(2):95–100. doi: 10.4161/derm.19833. [DOI] [PMC free article] [PubMed] [Google Scholar]