Summary
The tyrosine phosphatase PTPN22 regulates T cell receptor signaling. In this issue of Immunity, Wang et al. (2013) show that in myeloid cells PTPN22 potentiates TLR-induced type I IFN production and that autoimmunity-associated allele PTPN22W encodes a reduced-function variant.
Protein tyrosine phosphatase nonreceptor type 22 (PTPN22, also termed Lyp and encoded by PTPN22) is preferentially expressed in hematopoietic and immune cells. The role of PTPN22 and its murine ortholog Ptpn22 (also termed PEP and encoded by Ptpn22) has attracted intense investigation since allelic variants of PTPN22 have been identified as one of the strongest genetic risk factors for a variety of autoimmune diseases, including type I diabetes, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Hashimoto’s thyroiditis and Graves disease (Burn et al., 2011; Zheng et al., 2012). The autoimmune disease-associated risk allele of PTPN22 encodes a substitution of tryptophan (W) for arginine (R) at position 620 (R620W) and is termed PTPN22W, whereas the nonrisk allele is PTPN22R. Many PTPN22W-associated diseases are characterized by dysregulated adaptive immunity and autoantibody production.
PTPN22 is expressed in many immune cell types, including T cells, B cells, NK cells, dendritic cells and monocytes and macrophages (reviewed in (Burn et al., 2011)). Full length PTPN22 contains 807 amino acid residues and several functional domains, including an amino-terminal catalytic domain and carboxy-terminal proline-rich motifs, including a PLPXR motif termed P1 that harbors the R620W substitution and can mediate interactions with SH3-containing proteins. Identified substrates for PTPN22 include multiple activating signaling molecules such as Src family kinases, Syk and ZAP70, T cell receptor components CD3ε and TCRζ, and the E3 ubiquitin ligase Cbl. Although experimental differences have not been fully reconciled, the preponderance of evidence indicates that PTPN22 and its murine ortholog Ptpn22 function as negative regulators of TCR signaling (Figure 1), and suggests that PTPN22W represents a gain-of-function allele with increased phosphatase activity that attenuates TCR signaling. Thus, PTPN22W may predispose to autoimmunity by decreasing negative selection or by increasing differentiation into regulatory rather than effector T cells. It has been difficult to resolve how a change in amino acid residue 620, which is distant from the catalytic domain, affects PTPN22 enzymatic function. The most likely explanation is that the R620W substitution weakens the interaction of PTPN22 with the kinase Csk, with an attendant increase in PTPN22 activity (Fiorillo et al., 2010).
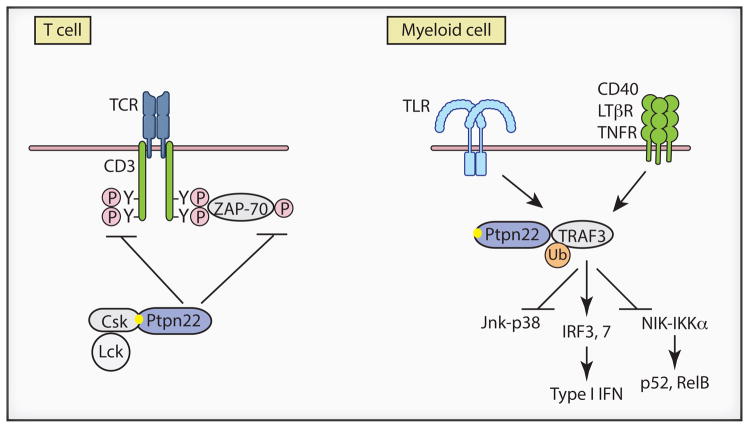
Figure 1.
Different mechanisms of PTPN22 action in T cells and myeloid cells. In T lineage cells PTPN22 negatively regulates T cell receptor (TCR) signaling. PTPN22 dephosphorylates and thereby inactivates signaling intermediates such as immunoreceptor tyrosine-based activation motifs (ITAMs) and tyrosine kinase ZAP-70 (Z-70). PTPN22 interacts with Csk via the P1 proline rich motif PLPXR (shown as yellow dot) whose sequence is altered to PLPXW in autoimmune disease-associated variant PTPN22W. The R620W substitution in PTPN22W destabilizes interactions with Csk and results in increased phosphatase activity (not depicted). In myeloid lineage cells PTPN22 modulates TLR, and possibly TNFR, signaling by interacting with TRAF3 to augment activating lysine 63-linked ubiquitination and thus increase IRF3 and IRF7 activation and type I IFN production. The PTPN22W variant results in lower TRAF3 activation and diminished IFN production (not depicted). Altered interactions of PTPN22W with TRAF3 may also affect Jnk-p38 and noncanonical NF-κB signaling leading to activation of p52 and Rel B.
The majority of previous work has focused on the role of PTPN22 in lymphocytes and on its enzymatic function as a phosphatase (Burn et al., 2011). The study by Wang et al. in this issue of Immunity(Wang et al., 2013) investigated the role of Ptpn22 and the functional consequences of the R620W substitution in PTPN22 in TLR-induced responses in macrophages and DCs. Using complementary biochemical and genetic approaches with Ptpn22−/− mice, the authors provide compelling evidence that Ptpn22 is required for full induction of type I IFNs in response to various TLR ligands, but is dispensable for induction of inflammatory cytokines. The Ptpn22-dependent component of the type I IFN response was biologically important, as it was required for full antigen-induced CD8+ T cell expansion when IFN-inducing poly(IC) was used as adjuvant, and for antiviral responses in vivo. Interestingly, Ptpn22 was required for poly(IC)-induced suppression of arthritis and CpG-induced suppression of DSS-colitis; protection in both models is mediated by type I IFNs whose production was attenuated by Ptpn22 deficiency (Katakura et al., 2005; Yarilina et al., 2007). The protective effect on arthritis occurred independently of lymphocytes, further supporting the functional importance of PTPN22 in myeloid cells. Mechanistic experiments showed that Ptpn22 associates with the TLR-activated signaling molecule TRAF3 to promote its activation via K63-linked polyubiquitination, and thus activation of downstream IRF3 and IRF7 and IFN production (Figure 1). Strikingly, Ptpn22 promoted TLR-mediated type I IFN production independently of its enzymatic function as a phosphatase.
These results establish the importance of Ptpn22 in a new cell type (myeloid cells), identify a key new function of promoting type I IFN induction, and suggest a new molecular mechanism of Ptpn22 action independent of its phosphatase activity. However, interpretation of these results and their relevance for human autoimmunity are subject to several caveats: 1. Most of the aforedescribed results were obtained in murine systems, but as PTPN22 (Lyp) and Ptpn22 (PEP) are only 60% homologous in their noncatalytic regions, it is not clear how these results translate to the role of human PTPN22. 2. The loss of function artificially created by deletion of Ptpn22 is complete and it is not clear how accurately results obtained when Ptpn22 is absent mirror the more subtle effects of the PTPN22W variant. 3. Removal of the Ptpn22 protein will alter the composition Ptpn22-containing signaling complexes and thus the function of proteins contained in these complexes; such effects may not occur in cells that express PTPN22W. The authors resoundingly address these caveats using a transgenic approach to reconstitute Ptpn22−/− mice with human PTPN22R or PTPN22W. Murine Ptpn22-deficient DCs and macrophages expressing PTPN22R produced more IFN (but not TNF) than did cells expressing PTPN22W. Accordingly, expression of the PTPN22R transgene resulted in greater TLR-induced expression of IFNs and interferon-stimulated genes (ISGs) in vivo, and PTPN22R was more effective than PTPN22W in mediating poly(IC)-induced suppression of inflammatory arthritis. Corroborating results obtained in the transgenic system, primary human PBMC from healthy donors who carried the risk variant PTPN22W exhibited lower induction of type I IFNs and ISGs after LPS stimulation than did donors homozygous for PTPN22R. Thus, PTPN22W exhibits reduced function in the most appropriate context of primary human cells from healthy individuals. Mechanistically, PTPN22W showed decreased association with TRAF3, which was associated with decreased TRAF3 K63-linked polyubiquitination and could explain diminished IFN production. These results clearly establish that human PTPN22W functions as a hypomorphic allele in macrophages and DCs, with attendant diminished IFN production and IFN responses on TLR stimulation.
The study by Wang et al. highlights the importance of PTPN22 in innate immune TLR-IFN-mediated responses but raises questions about mechanisms by which a hypomorphic allele that results in lower type I IFN production can promote autoimmunity. The authors observed that PTPN22 was important for increasing type I IFN production in response to exogenous ligands that activate TLR3, Mda5 and TLR9, which is protective in K/BxN serum-induced arthritis and DSS-induced colitis (Katakura et al., 2005; Yarilina et al., 2007). These are acute induced models of the inflammatory effector phase of disease that occurs mostly independently of autoimmunity. In these models type I IFNs likely target myeloid lineage cells and possibly non-immune tissue cells to suppress production and function of inflammatory mediators such as IL-1 (Katakura et al., 2005; Prinz et al., 2008; Yarilina et al., 2007). Thus, this work suggests that loss of function of PTPN22 in myeloid cells can result in an augmented inflammatory effector phase of autoimmune diseases. Although suppression of inflammation by type I IFNs, and thus the role of PTPN22, is strikingin these models, therapy with type I IFNs exhibits limited efficacy in human rheumatoid arthritis and inflammatory bowel disease (IBD) (reviewed in (Kalliolias and Ivashkiv, 2010)). This raises questions about the importance of this IFN-mediated suppressive mechanism in human diseases. An alternative, albeit at this point speculative, pathogenic mechanism is that diminished IFN production can compromise host defense and lead to uncontrolled infections that trigger autoimmunity. Another question is why diseases characterized by a strong innate immune component, such as IBD and ankylosing spondylitis, are not associated with PTPN22W, which instead is associated increased susceptibility to diseases characterized by dysregulation of adaptive immunity and autoantibody production, such as type I diabetes, RA, SLE, Graves disease and ANCA-associated vasculitis (Burn et al., 2011). As type I IFNs generally promote adaptive immunity and antibody production, decreased IFN production associated with the loss-of-function PTPN22W allele described Wang at al. would be expected to have the opposite effect, namely decreased autoimmunity.
Furthermore, if the findings of Wang at al. reflect the major contribution of PTPN22W to disease susceptibility, one would predict that PTPN22W (and thus low IFN production) would be associated with diseases such as multiple sclerosis where IFNs are protective, while PTPN22R (high IFN production) would be associated with diseases where IFNs are likely pathogenic, such as systemic lupus erythematosus (Kalliolias and Ivashkiv, 2010). Contrary to these predictions, PTPN22W is not associated with MS, while it is apparently paradoxically associated with SLE (Burn et al., 2011; Zheng et al., 2012). The dominant paradigm of SLE pathogenesis posits that SLE is driven by excessive signaling by TLRs leading to increased type I IFN production, with downstream increased autoimmunity and autoantibody production (Kalliolias and Ivashkiv, 2010). Possible explanations for the association of PTPN22W with SLE are: 1. PTPN22W plays a pathogenic role in a non-myeloid cell type, for example by altering TCR or BCR signaling, and relatively diminished TLR-induced IFN production in patients harboring PTPN22W is still sufficient to promote disease. 2. A relative deficit in IFN production facilitates subclinical or chronic infections that trigger autoimmunity. 3. PTPN22W expression in myeloid cells triggers a pathogenic that remains to be discovered. In this scenario, the true pathogenic consequences of decreased PTPN22W-TRAF3 interactions would not be related to IFN production, but to other TRAF3-mediated signaling events, for example increased activation of MAPKs or noncanonical NF-κB signaling (Figure 1B) (Hacker et al., 2011). A more extensive investigation of this possibility than performed in Wang et al., for example by gene expression profiling, could be instructive. Equally importantly, TRAF3 is also a key mediator of signaling by TNF family cytokines (Figure 1B), including TNFα, a key pathogenic factor in several PTPN22W-associated diseases such as RA, psoriatic arthritis, ANCA-associated vasculitis and inflammatory myopathies. Thus, investigation of the role of PTPN22 in TNFR responses, including the recently described crosstalk between TNFα and IFN responses (Gordon et al., 2012 and refs. therein), may provide additional insights about pathogenic mechanisms related to PTPN22W.
In summary, the study by Wang at al. identifies a new function for PTPN22 – regulation of IFN production in innate immune cells – and a new molecular mechanism of PTPN22 action via regulation of TRAF3 ubiquitination rather than dephosphorylation of signaling intermediates. This work also establishes that the autoimmune disease-associated PTPN22W is a hypomorphic allele in the context of TLR-stimulated IFN production in innate immune cells. These findings highlight the importance of studying function of disease-associated variants in different cell types, and open new lines of investigation of PTPN22 function and mechanisms of action. The study by Wang at al. also raises thought-provoking questions about how PTPN22W increases susceptibility to autoimmune diseases.
This is a commentary on article Wang Y, Shaked I, Stanford SM, Zhou W, Curtsinger JM, Mikulski Z, Shaheen ZR, Cheng G, Sawatzke K, Campbell AM, Auger JL, Bilgic H, Shoyama FM, Schmeling DO, Balfour HH Jr, Hasegawa K, Chan AC, Corbett JA, Binstadt BA, Mescher MF, Ley K, Bottini N, Peterson EJ. The autoimmunity-associated gene PTPN22 potentiates toll-like receptor-driven, type 1 interferon-dependent immunity. Immunity. 2013 Jul 25;39(1):111-22.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burn GL, Svensson L, Sanchez-Blanco C, Saini M, Cope AP. Why is PTPN22 a good candidate susceptibility gene for autoimmune disease? FEBS Lett. 2011;585:3689–3698. doi: 10.1016/j.febslet.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Fiorillo E, Orru V, Stanford SM, Liu Y, Salek M, Rapini N, Schenone AD, Saccucci P, Delogu LG, Angelini F, et al. Autoimmune-associated PTPN22 R620W variation reduces phosphorylation of lymphoid phosphatase on an inhibitory tyrosine residue. J Biol Chem. 2010;285:26506–26518. doi: 10.1074/jbc.M110.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon RA, Grigoriev G, Lee A, Kalliolias GD, Ivashkiv LB. The interferon signature and STAT1 expression in rheumatoid arthritis synovial fluid macrophages are induced by tumor necrosis factor alpha and counter-regulated by the synovial fluid microenvironment. Arthritis Rheum. 2012;64:3119–3128. doi: 10.1002/art.34544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker H, Tseng PH, Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat Rev Immunol. 2011;11:457–468. doi: 10.1038/nri2998. [DOI] [PubMed] [Google Scholar]
- Kalliolias GD, Ivashkiv LB. Overview of the biology of type I interferons. Arthritis Res Ther. 2010;12(Suppl 1):S1. doi: 10.1186/ar2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Schmidt H, Mildner A, Knobeloch KP, Hanisch UK, Raasch J, Merkler D, Detje C, Gutcher I, Mages J, et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity. 2008;28:675–686. doi: 10.1016/j.immuni.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shaked I, Stanford SM, Zhou W, Curtsinger JM, Mikulski ZRSZ, Cheng G, Sawatzke K, Campbell AM, et al. Ptpn22 potentiates Toll-like receptor-driven, type I interferon-dependent immunity. Immunity. 2013 doi: 10.1016/j.immuni.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarilina A, DiCarlo E, Ivashkiv LB. Suppression of the effector phase of inflammatory arthritis by double-stranded RNA is mediated by type I IFNs. J Immunol. 2007;178:2204–2211. doi: 10.4049/jimmunol.178.4.2204. [DOI] [PubMed] [Google Scholar]
- Zheng J, Ibrahim S, Petersen F, Yu X. Meta-analysis reveals an association of PTPN22 C1858T with autoimmune diseases, which depends on the localization of the affected tissue. Genes Immun. 2012;13:641–652. doi: 10.1038/gene.2012.46. [DOI] [PubMed] [Google Scholar]