Abstract
Chondrocalcinosis (CC) is a common cause of joint pain and arthritis that is caused by the deposition of calcium-containing crystals within articular cartilage. Although most cases are sporadic, rare familial forms have been linked to human chromosomes 8 (CCAL1) or 5p (CCAL2) (Baldwin et al. 1995; Hughes et al. 1995; Andrew et al. 1999). Here, we show that two previously described families with CCAL2 have mutations in the human homolog of the mouse progressive ankylosis gene (ANKH). One of the human mutations results in the substitution of a highly conserved amino acid residue within a predicted transmembrane segment. The other creates a new ATG start site that adds four additional residues to the ANKH protein. Both mutations segregate completely with disease status and are not found in control subjects. In addition, 1 of 95 U.K. patients with sporadic CC showed a deletion of a single codon in the ANKH gene. The same change was found in a sister who had bilateral knee replacement for osteoarthritis. Each of the three human mutations was reconstructed in a full-length ANK expression construct previously shown to regulate pyrophosphate levels in cultured cells in vitro. All three of the human mutations showed significantly more activity than a previously described nonsense mutation that causes severe hydroxyapatite mineral deposition and widespread joint ankylosis in mice. These results suggest that small sequence changes in ANKH are one cause of CC and joint disease in humans. Increased ANK activity may explain the different types of crystals commonly deposited in human CCAL2 families and mutant mice and may provide a useful pharmacological target for treating some forms of human CC.
Introduction
Deposition of calcium containing crystals in articular cartilage is a common pathological finding, with a prevalence that rises sharply with age. Up to 40% of the elderly show radiographic evidence of mineral deposition in their joints (Dieppe and Watt 1985; Felson et al. 1989). In severe cases, mineral deposits can trigger acute attacks of joint pain and synovitis (pseudogout), or long-term structural alterations and arthritis (chronic pyrophosphate arthropathy) (Kohn et al. 1962; McCarty 1976; Doherty and Dieppe 1988).
The most common mineral deposits found in articular cartilage are crystals of calcium pyrophosphate dihydrate (CPPD) or basic calcium phosphate, including hydroxyapatite (HA). Metabolic diseases that elevate the concentration of either calcium or pyrophosphate ions can lead to CPPD crystal deposition (Doherty et al. 1991a; Jones et al. 1992). However, the causes of most forms of chondrocalcinosis (CC) are still unknown.
Several kindreds have been described that segregate CC as an autosomal dominant trait (van der Korst et al. 1974; van der Korst and Geerards 1976; Gaucher et al. 1977; Richardson et al. 1983; Doherty et al. 1991b; Baldwin et al. 1995; Andrew et al. 1999). In these families, CPPD crystal deposition typically begins in the 3rd and 4th decades, affects several joints, and leads to arthropathy, pseudogout, or pseudo-osteoarthritis. Genetic linkage studies have mapped these familial forms of CC to two different major chromosome regions, CCAL1 MIM 600668 on chromosome 8 and CCAL2 MIM 118600 on chromosome 5 (Baldwin et al. 1995; Hughes et al. 1995; Andrew et al. 1999).
In mice, a classical mutation called progressive ankylosis is known to cause a severe form of generalized joint calcification and arthritis (Sweet and Green 1981). The mouse mutation behaves as an autosomal recessive trait instead of an autosomal dominant one and leads to accumulation of hydroxyapatite crystals rather than CPPD crystals in articular cartilage and synovial fluid (Hakim et al. 1984). Recent cloning and sequencing studies have shown that progressive ankylosis is caused by a nonsense mutation in a novel multiple-pass transmembrane protein that is highly conserved in vertebrates. This protein alters levels of both intracellular and extracellular pyrophosphate in cultured cells and may act as a pyrophosphate transporter that stimulates the elaboration of extracellular pyrophosphate from intracellular stores (Ho et al. 2000).
The human homolog (ANKH [MIM 605145]) of the mouse progressive ankylosis gene maps to chromosome 5, closely linked to D5S1954 (Ho et al. 2000). This location places the gene within the critical region previously defined for both a British family and a French family with CCAL2 (Hughes et al. 1995; Andrew et al. 1999). On the basis of the map location of ANKH and its previously established role in pyrophosphate regulation and joint calcification, we have tested whether human patients with CC harbor sequence changes in ANKH. The previously described British and French families have small amino acid alterations in ANKH that segregate completely with disease status. An additional 1 of 95 sporadic patients shows a single amino acid deletion in the ANKH protein. Reconstruction of each mutation in a full-length ANK expression construct followed by in vitro tissue culture assays showed that the altered human proteins have significantly more activity than the truncated protein of mice with progressive ankylosis. These results show that some forms of human CC are caused by mutations in the ANKH gene and that these mutations may lead to increased rather than decreased ANKH function.
Material and Methods
Mutation Screening
ANKH gene sequences were amplified from blood cell genomic DNA prepared from two previously described families with CCAL2, from 95 patients with sporadic CC diagnosed by x-ray examination and from 200 unaffected control subjects. Forward and reverse primer pairs were designed to amplify all 12 exons and flanking splice sites of the ANKH gene (primers and amplification conditions available upon request). Amplified products were purified from agarose gels using Wizard columns (Promega) and were sequenced using the BigDye terminator cycle protocol (ABI). All heterozygous base pair changes were confirmed by independent amplifications, sequencing reads on both strands, or by cloning and resequencing of individual subclones from the bulk amplification reactions.
The sequence changes found in exon 1 and exon 12 were reanalyzed in 190 control chromosomes by amplification and direct sequencing. For the –11CT change in exon 1, we used flanking primers 5′-gatctttgttgtgtgggagg-3′ and 5′-cacccgaccaaatgtttcagg-3′. For the change in exon 12, we used flanking primers 5′-gaaggtttaagcctacagtga-3′ and 5′-tgatgccgaagtgtcatcct-3′. The sequence change in the French family creates a new Hga1 restriction site in exon 2. A 389-bp PCR product spanning this mutation was amplified using an initial round of PCR with forward primer 5′-catgagatgcaccctataagctac-3′ and reverse primer 5′-gaaattgccaaagctagattcgtc-3′, followed by a second round of amplification with the same forward primer and a nested reverse primer, 5′-ttgccaaagctagattcgtca-3′. Hga1 digestion of these products resulted in the predicted band of 389 bp in unaffected family members and 107 unrelated control subjects and resulted in the expected additional smaller fragments of 213 bp and 176 bp in all affected patients carrying the missense mutation.
In Vitro Translation and Mass Determination
The effect of the –11CT mutation was assessed by first subcloning the wild type and mutant exon 1 sequences in the TA cloning vector (Invitrogen), amplifying with primers that contained an upstream T7 RNA polymerase promoter (5′-taatacgactcactatagggagcggcagca-3′) and a downstream termination codon (5′-acacccgaccaaatgtttaagg-3′), and then testing the size of the encoded proteins using a TNT-coupled in vitro transcription and rabbit reticulocyte translation system (Promega). We concentrated, desalted, and removed detergents from the peptide product with 10 ml C18 ZipTips (Millipore), and we analyzed the final eluted peptide using a Termofinnigan LCQ deca Electrospray Ionization Mass Spectrometer (ESI). The wild-type sequence should generate a polypeptide of 67 amino acids in this system (estimated Mw: 7303). If the novel upstream ATG sequence is recognized as a new translational start site, the mutant sequence should generate a larger polypeptide with four additional residues added to the amino terminus (estimated Mw: 7664).
Cell Culture and Pyrophosphate Determination
Each of the human sequence changes was recreated in the previously described full-length ANK expression construct (pCMV-ANK) (Ho et al. 2000). The French M48T mutation was generated using the Stratagene QuickChange site-directed mutagenesis protocol. Briefly, 25–45 bp forward and reverse primers were designed that completely overlap and incorporate the desired sequence change (forward: 5′-gatgcagtagaaaCgctggccagctac-3′; reverse: 5′-gtagctggccagcGtttctactgcatc-3′). The original template was digested with DpnI and the circular PCR product was transformed into electrocompetent DH10B cells. Finally, the modified ANK cDNA insert was removed by restriction digest and was ligated into a freshly cut vector to ensure that experimental results were not influenced by base-pair changes incorporated into the vector during PCR.
The British –11CT sequence change was recreated by inserting 18 bp of the mutant 5′ UTR flanking human sequence into the pCMV-ANK vector (forward primer: 5′-cgcggatccggtcagcccATGgcggggactATGgtgaaattcccggcgc-3′; reverse primer: 5′-cgcgaattcttactcattttcttctctcatct-3′) via PCR. A control construct carrying the wild-type human 5′ UTR was generated in parallel (forward: 5′-cgcggatccggtcagcccacggcggggactATGgtgaaattcccggcgc-3′; reverse: 5′-cgcgaattcttactcattttcttctctcatct-3′). The control construct was called “pCMV-hum5ANK.” Finally, the E490del mutation was recreated in the pCMV-hum5ANK control vector via site-directed mutagenesis, as described for the French M48T mutation (forward primer: 5′-cagacatcgtagagatgagagaaaatgagtaagaattctgcag-3′; reverse primer: 5′-ctgcagaattcttactcattttctctcatctctacgatgtctg-3′). All constructs were sequenced across the entire open reading frame to confirm that the predicted sequence alterations had been made without introducing undesired mutations.
COS-7 cells were transfected with 7 μg of DNA using fugene 6 reagent, and cells from three 10 cm plates were scraped and pooled 28–34 h later. PPi was extracted from cells in percholoric acid, as described elsewhere (Ho et al. 2000), and PPi concentrations were measured using a coupled enzymatic assay (Doherty et al. 1991a; Terkeltaub et al. 1994) containing 57 mM Tris, 5.2 mM magnesium acetate, 4 mM NADP, 18.6 mM glucose 1,6-diphosphate, 7.5 mM UDPG, 0.4 units glucose-6-phosphate dehydrogenase, 0.057 units phosphoglucomutase, 0.136 units UDPG pyrophosphorylase, and tritiated UDPG (0.04 mCi: specific activity 25 Ci/mmol), in 100 μl and 40 μl of sample or PPi standard (0–400 pmoles). After 1 h at 37°C, 0.2 ml of 4% charcoal suspended in 50 mM Tris acetate was added to each sample to separate unreacted substrate from labeled 6-phosphogluconate. Samples were vortexed and incubated at 4°C for 10 min before centrifugation at 2,000g for 10 min at 4°C. Supernatants (0.2 ml) were placed in 5 ml of scintillation fluid and were counted. The effects of each sequence variant were compared to matched wild-type internal controls performed in parallel in each experiment, and the results of multiple experiments were analyzed by paired t-tests.
Results
Sequencing Analysis
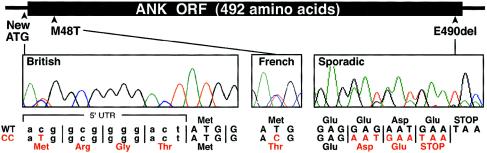
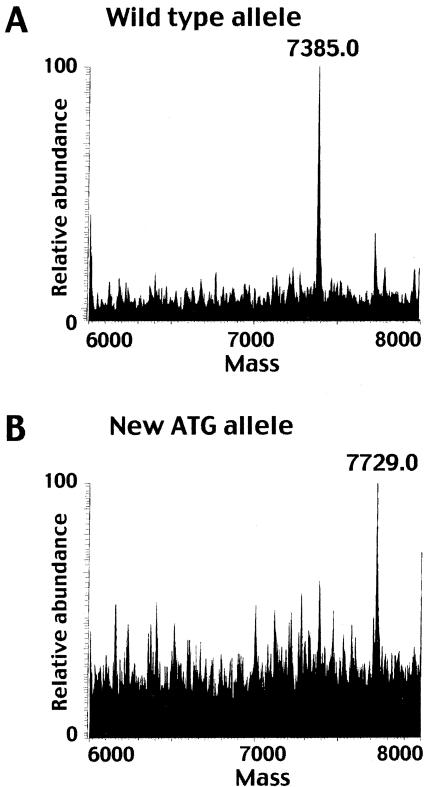
ANKH maps within the overlapping 5p candidate region previously defined for two CCAL2 families (Hughes et al. 1995; Andrew et al. 1999; Ho et al. 2000). To test for sequence changes in human CC, we amplified each of the 12 ANKH coding exons from family members in the French (Andrew et al. 1999) and British (Hughes et al. 1995) kindreds with CCAL2 (fig. 1). All affected individuals of the French family were heterozygous for a thymine-to-cytosine base change (143T→C) in exon 2 (fig. 2). This missense mutation substitutes threonine for methionine in a predicted transmembrane domain at a position that is absolutely conserved in the ANKH protein over 400 million years of evolution, from fish to mammals (Ho et al. 2000; Nurnberg et al. 2001). In contrast, all of the affected members of the U.K. kindred were heterozygous for a cytosine-to-thymine base change (–11C→T) located 11 bp upstream of the normal ATG initiation codon (fig. 2). This change generates an alternative ATG initiation codon and is predicted to add four amino acids to the highly conserved N-terminus of the ANKH protein. Reconstruction of the –11CT mutation showed that the new upstream ATG sequence is recognized as a translational start in a rabbit reticulocyte translation system. ESI mass spectrometry confirmed that the ANKH polypeptide translated from the mutant expression construct is 344 Da larger than the polypeptide from the wild-type construct (fig. 3). In both the French and British kindreds, the mutation resides on the disease allele and segregates completely with CC (fig. 1). Neither of the mutations was found in DNA samples from unaffected family members or in 200 chromosomes from unaffected control individuals.
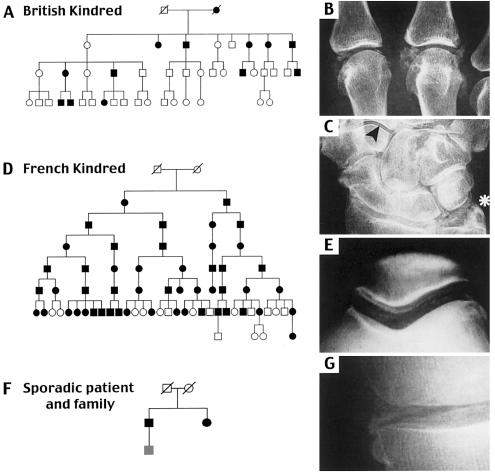
Figure 1.
Families with CC mutations in ANKH. A, Affected family members of the British family with CCAL2 have one or more benign febrile fits as children and develop florid polyarticular CC in their 3rd or 4th decade (Doherty et al. 1991b). B, and C, Radiographs of the hand of a female family member show gross calcification of the metacarpophalangeal joints and extensive CC in both the triangular ligament fibrocartilage (asterisk) and articular cartilage of nearly all wrist joints (arrowhead). D, Members of the previously described French family with CCAL2 develop CC in many joints of the legs, arms, and spine by age 35 (Gaucher et al. 1977). E, Radiographs of the knee of a 56-year-old man show pronounced calcific deposits of the femoropatellar articular cartilage. F, and G, Wider screening of sporadic cases identified a 79-year-old man with CC affecting his medial tibiofemoral compartment (G), wrist triangular ligament, symphysis pubis, and hips. His sister has had bilateral knee replacements for “osteoarthritis.” In all three pedigrees, black shading represents affected status. All affected family members were heterozygous for different ANKH mutations (see text), and all unaffected family members were homozygous for wild-type ANKH alleles with the exception of the youngest member of the third pedigree, who was heterozygous for ANKH mutations but not yet old enough to reliably determine CC status (gray box, panel F).
Figure 2.
Identification and location of ANKH mutations. Three different mutations were identified in DNA sequence traces of the families with CC. The –11CT change creates a novel ATG initiation codon in the 5′ untranslated region of ANKH. A thymine-to-cytosine transition at position 143 leads to a substitution of threonine for methionine. Deletion of three base pairs removes a glutamate codon near the carboxy terminus of the ANK protein.
Figure 3.
Mass spray analysis of ANKH polypeptides generated from wild-type and mutant constructs. A, and B, The presence of the –11CT mutation in the 5′ UTR of ANKH creates a new in-frame ATG and directs translation of a predominantly 7,729 Da ANK polypeptide compared to a smaller, 7,385 Da form translated from the wild-type allele.
To test whether ANKH sequence changes may also be involved in sporadic forms of CC, we sequenced ANKH coding exons from 95 British patients with CC. One patient who presented with late-onset polyarticular CC with structural arthropathy of both knees had a 3-bp deletion, in exon 12, that deletes a glutamate residue (E490del) three amino acids from the highly conserved carboxyl terminus of the ANKH protein (fig. 2). This deletion was not seen in any of the control chromosomes. However, analysis of DNA from two other available family members showed that a sister and son of the index case were also heterozygous for the same 3-bp deletion (fig. 1). Although neither had demonstrable CC, the sister had undergone bilateral total knee replacement surgery for “osteoarthritis.” Her preoperative radiographs were unavailable, but there was no CC on her pelvis or hand radiographs. Knee radiographs of the son were normal, but at the age of 42 years, he may not yet display CC. No further mutations were identified in 95 patients with sporadic CC.
In Vitro Functional Assays
Previous studies have shown that overexpression of wild-type ANK in COS cells decreases intracellular levels of pyrophosphate (PPi) and increases extracellular pyrophosphate, most likely by transport via the multiple-pass transmembrane ANK protein (Ho et al. 2000). This is biologically relevant, because PPi can both promote deposition of CPPD crystals (as a constituent ion) and inhibit deposition of hydroxyapatite crystals (by binding to the surface of calcium phosphate crystals and blocking further crystal growth) (Kohn et al. 1962; Fleisch 1981; Rachow and Ryan 1988). The nonsense mutation in progressive ankylosis mice truncates the ANK protein and greatly reduces its ability to alter pyrophosphate levels in transfected tissue culture cells (Ho et al. 2000). To compare the effect of the mouse and human mutations, we reconstructed the –11CT, M48T, and E490del mutations and tested their effect on pyrophosphate levels in transfected COS cells. As expected, full-length wild-type ANK caused a significant reduction in intracellular pyrophosphate (58 ± 5% of levels seen in cells transfected with vector only control, P<.0001 in n=23 experiments). The activity of the mouse ank mutant allele was sharply reduced (24% ± 14% of the decrease seen with wild type ANK, P<.0001; 23 experiments). In contrast, −11CT, M48T, and E490del mutations had mean activities that were 148% ± 33%, 101% ± 25%, and 109% ± 12% of matched wild-type ANK values (mean ± SEM percent reduction of intracellular PPi concentration compared to cells transfected with wild type ANK, P values of .18, .97, and .47 compared to wild type ANK; and P values of .016, .018, .003 compared to the mouse mutant form of ANK in n=13, 18, and 13 experiments, respectively).
Discussion
None of the CC mutations presented here appear to be null mutations. Their differing location in the ANKH gene may help explain important phenotypic differences between the human CCAL2 families and the mouse progressive ankylosis mutant. For example, the U.K. family is unique in that adult-onset CC is preceded by repeated benign fits during childhood. The extra 4-aa extension found in the U.K. family may produce a new activity or interaction in neural cells, a known site of ANK expression in mice and humans (Ho et al. 2000).
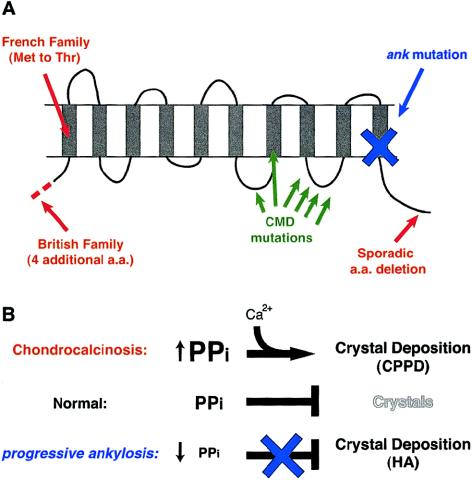
Although the recessive mouse mutation causes early and widespread deposition of hydroxyapatite mineral in articular cartilage and synovial fluid (Hakim et al. 1984), the dominant human mutations described here lead to slow adult-onset deposition of calcium pyrophosphate dihydrate (CPPD) crystals (Gaucher et al. 1977; Doherty et al. 1991b). The different crystal types formed in the mouse and human diseases and the slower time course of the human disease may result from differing effects of the mutant alleles on pyrophosphate levels (fig. 4). The mouse nonsense mutation truncates the ANK protein, sharply reduces protein activity in vitro, and causes a decrease in pyrophosphate levels outside cells. This drop in PPi levels would eliminate a normal physiological block to hydroxyapatite formation (Fleisch 1981) and would lead to the widespread deposition of hydroxyapatite mineral seen in mutant mice (Ho et al. 2000). In contrast, the human alleles cause small amino acid changes instead of truncations, and each of these alleles shows a high level of activity compared to the mouse mutation in vitro. We have only measured intracellular pyrophosphate levels in the transfected COS cells. However, given the substantial activity of the CC mutations in the COS cell experiments and the known ability of wild-type ANK to increase extracellular pyrophosphate levels (Ho et al. 2000), we think it likely that the human CC mutations will also lead to reciprocal small increases in extracellular pyrophosphate. Although the total difference in activity between wild-type ANK and the CC alleles appears to be minor, even a small increase in total ANK activity may be sufficient to lead to ectopic crystal formation over the course of many years. Elevated pyrophosphate concentrations can trigger direct precipitation of calcium and pyrophosphate ions, leading to deposition of CPPD crystals instead of hydroxyapatite. This effect is also seen in several other metabolic diseases that raise pyrophosphate levels including hypomagnesaemia, haemochromatosis, and hypophosphatasia (Doherty et al. 1991a; Jones et al. 1992). The combined data and the dominant inheritance of CC suggest that the human mutations might be gain-of-function alleles that lead to slow accumulation of pyrophosphate in vivo.
Figure 4.
Summary of known ANKH mutations and model of different effects on hydroxyapatite and CPPD mineral deposition. A, The locations of the new CC mutations (red) are compared to previously identified mutations responsible for mouse progressive ankylosis (blue) and human craniometaphyseal dysplasia (green). B, Previous studies suggest that the ANK multiple-pass transmembrane protein may transport pyrophosphate across the membrane (Ho et al. 2000). The wild-type protein promotes accumulation of extracellular pyrophosphate from intracellular stores. Extracellular pyrophosphate acts as an inhibitor of hydroxyapatite deposition, normally preventing mineralization of articular cartilage (Fleisch 1981). Loss of ANK function in progressive ankylosis mice leads to a decrease in extracellular pyrophosphate and results in deposition of hydroxyapatite in joints. In contrast, the dominant human CC mutations lead to excess extracellular pyrophosphate, eventually stimulating precipitation of calcium and pyrophosphate as CPPD crystals.
Previous studies have shown that extracellular pyrophosphate levels are elevated in synovial fluid of the British family, a result fully consistent with our working model of increased ANK activity caused by the CC mutations (Doherty 1991b). In contrast, previous studies have shown that intracellular pyrophosphate levels are increased in cultured fibroblasts and lymophocytes of patients from the French family, a result that differs from the effect seen when the French mutant form of ANK is transfected into cultured COS cells in the current studies. We note, however, that the cell types, growth conditions, and serum supplementation levels are markedly different in the two types of experiments. Pyrophosphate levels are known to respond to changes in culture conditions (Rosenthal et al. 1991), and recent studies suggest that ANK expression is also markedly sensitive to changes in cytokines and serum levels (Guo et al. 2001). An important goal for the future will be a more detailed characterization of the effects of the mouse and human mutations on both intracellular and extracellular pyrophosphate levels in stable cell lines, in articular cartilage cells, and in synovial fluids. We are in the process of recreating the human mutations in mouse animal models to facilitate a more detailed comparison of their effects on different tissues under uniform conditions.
The sequence changes reported here provide the first evidence that some forms of human CC are due to mutations in ANKH. Our studies have only examined coding region changes in the gene. Changes in cis-acting regulatory sequences might also lead to increased ANKH activity. It will be important to test in the future whether additional cases of CC may be due to mutations in regulatory regions of ANKH or to induction of ANKH activity by cytokines or growth factors (Guo et al. 2001) in joint regions. Other mutations in ANKH have recently been reported in patients with craniometaphyseal dysplasia (CMD [MIM 123000]), a dominant skeletal disease characterized by hyperostosis and sclerosis of the skull and long bones (Nurnberg et al. 2001; Reichenberger et al. 2001). The clustering of CMD mutations in a single predicted loop of the ANKH protein suggests that they are specialized alleles that alter transport specificity or activity (fig. 4). The current data indicate that distinct mutations in the ANKH protein can lead to CPPD deposition. The dual role of pyrophosphate as both an inhibitor of hydroxyapatite deposition and a promoter of CPPD deposition may help explain how different mutations in the same gene can lead to formation of different types of crystal.
Agents that inhibit ANKH transport activity, such as the anion transport inhibitor probenecid (Ho et al. 2000; Rosenthal and Ryan 1994), might provide a useful therapeutic approach for lowering pyrophosphate levels and reducing CPPD deposition in a subset of patients with CC. However, any effective therapy is likely to depend upon maintaining extracellular PPi within a narrow physiological range, given that we now know that an abnormal increase in extracellular PPi/ANKH function leads to pathological CPPD formation, whereas an excessive decrease in extracellular PPi/ANKH function may lead to pathological hydroxyapatite formation.
Acknowledgments
We thank Prof. A. Gaucher, Dr. J. Peterschmitt, and the group of Rheumatologists of Colmar for helpful scientific discussion. This work was supported in part by grants from the Projet Hospitalier de Recherche Clinique du CHU de NANCY (1997) (P.N.), NIH/NIAMS R01 AR44360 (C.W.), the Arthritis Research Campaign UK ICAC D0541 (M.D.), the Arthritis Foundation A21320 (R.S.), by a Northern Ireland Research and Development fellowship to A.P., a Howard Hughes predoctoral fellowship to K.A.G., and a Medical Science Training Program fellowship to A.M.H. D.M.K. is an associate investigator of the Howard Hughes Medical Institute.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CCAL1 [MIM 600668], CCAL2 [MIM 118600], CMD [MIM 123000], and ANKH [MIM 605145])
References
- Andrew LJ, Brancolini V, de la Pena LS, Devoto M, Caeiro F, Marchegiani R, Reginato A, Gaucher A, Netter P, Gillet P, Loeuille D, Prockop DJ, Carr A, Wordsworth BF, Lathrop M, Butcher S, Considine E, Everts K, Nicod A, Walsh S, Williams CJ (1999) Refinement of the chromosome 5p locus for familial calcium pyrophosphate dihydrate deposition disease. Am J Hum Genet 64:136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin CT, Farrer LA, Adair R, Dharmavaram R, Jimenez S, Anderson L (1995) Linkage of early-onset osteoarthritis and chondrocalcinosis to human chromosome 8q. Am J Hum Genet 56:692–697 [PMC free article] [PubMed] [Google Scholar]
- Dieppe P, Watt I (1985) Crystal deposition in osteoarthritis: an opportunistic event? Clinics Rheum Dis 11:367–392 [PubMed] [Google Scholar]
- Doherty M, Chuck A, Hosking D, Hamilton E (1991a) Inorganic pyrophosphate in metabolic diseases predisposing to calcium pyrophosphate dihydrate crystal deposition. Arthritis Rheum 34:1297–1303 [DOI] [PubMed] [Google Scholar]
- Doherty M, Dieppe P (1988) Clinical aspects of calcium pyrophosphate dihydrate crystal deposition. Rheum Dis Clin North Am 14:395–414 [PubMed] [Google Scholar]
- Doherty M, Hamilton E, Henderson J, Misra H, Dixey J (1991b) Familial chondrocalcinosis due to calcium pyrophosphate dihydrate crystal deposition in English families. Br J Rheumatol 30:10–15 [DOI] [PubMed] [Google Scholar]
- Felson DT, Anderson JJ, Naimark A, Kannel W, Meenan RF (1989) The prevalence of chondrocalcinosis in the elderly and its association with knee osteoarthritis: the Framingham Study. J Rheumatol 16:1241–1245 [PubMed] [Google Scholar]
- Fleisch H (1981) Diphosphonates: history and mechanisms of action. Metab Bone Dis Relat Res 3:279–287 [DOI] [PubMed] [Google Scholar]
- Gaucher A, Faure G, Netter P, Pourel J, Raffoux C, Streiff F, Tongio MM, Mayer S (1977) Hereditary diffuse articular chondrocalcinosis. Dominant manifestation without close linkage with the HLA system in a large pedigree. Scand J Rheumatol 6:217–221 [DOI] [PubMed] [Google Scholar]
- Guo Y, Hsu DK, Feng SL, Richards CM, Winkles JA (2001) Polypeptide growth factors and phorbol ester induce progressive ankylosis (Ank) gene expression in murine and human fibroblasts. J Cell Biochem 84:27–38 [DOI] [PubMed] [Google Scholar]
- Hakim FT, Cranley R, Brown KS, Eanes ED, Harne L, Oppenheim JJ (1984) Hereditary joint disorder in progressive ankylosis (ank/ank) mice. I. Association of calcium hydroxyapatite deposition with inflammatory arthropathy. Arthritis Rheum 27:1411–1420 [DOI] [PubMed] [Google Scholar]
- Ho A, Johnson M, Kingsley DM (2000) Role of the mouse ank gene in tissue calcification and arthritis. Science 289:265–270 [DOI] [PubMed] [Google Scholar]
- Hughes AE, McGibbon D, Woodward E, Dixey J, Doherty M (1995) Localisation of a gene for chondrocalcinosis to chromosome 5p. Hum Mol Genet 4:1225–1228 [DOI] [PubMed] [Google Scholar]
- Jones AC, Chuck AJ, Arie EA, Green DJ, Doherty M (1992) Diseases associated with calcium pyrophosphate deposition disease. Semin Arthritis Rheum 22:188–202 [DOI] [PubMed] [Google Scholar]
- Kohn N, Hughes R, McCarty D, Faires J (1962) The significance of calcium pyrophosphate crystals in the synovial fluid of arthritic patients: the “pseudogout syndrome.” Identification of crystals. Ann Intern Med 56:738–745 [DOI] [PubMed] [Google Scholar]
- McCarty DJ (1976) Calcium pyrophosphate dihydrate crystal deposition disease—1975. Arthritis Rheum 19:275–285 [DOI] [PubMed] [Google Scholar]
- Nurnberg P, Thiele H, Chandler D, Hohne W, Cunningham ML, Ritter H, Leschik G, Uhlmann K, Mischung C, Harrop K, Goldblatt J, Borochowitz ZU, Kotzot D, Westermann F, Mundlos S, Braun HS, Laing N, Tinschert S (2001) Heterozygous mutations in ANKH, the human ortholog of the mouse progressive ankylosis gene, result in craniometaphyseal dysplasia. Nat Genet 28:37–41 [DOI] [PubMed] [Google Scholar]
- Rachow JW, Ryan LM (1988) Inorganic pyrophosphate metabolism in arthritis. Rheum Dis Clin North Am 14:289–302 [PubMed] [Google Scholar]
- Reichenberger E, Tiziani V, Watanabe S, Park L, Ueki Y, Santanna C, Baur ST, Shiang R, Grange DK, Beighton P, Gardner J, Hamersma H, Sellars S, Ramesar R, Lidral AC, Sommer A, Raposo do Amaral CM, Gorlin RJ, Mulliken JB, Olsen BR (2001) Autosomal dominant craniometaphyseal dysplasia is caused by mutations in the transmembrane protein ANK. Am J Hum Genet 68:1321–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BC, Chafetz NI, Ferrell LD, Zulman JI, Genant HK (1983) Hereditary chondrocalcinosis in a Mexican-American family. Arthritis Rheum 26:1387–1396 [DOI] [PubMed] [Google Scholar]
- Rosenthal AK, Cheung HS, Ryan LM (1991) Transforming growth factor beta 1 stimulates inorganic pyrophosphate elaboration by porcine cartilage. Arthritis Rheum 34:904–911 [DOI] [PubMed] [Google Scholar]
- Rosenthal AK, Ryan LM (1994) Probenecid inhibits transforming growth factor-beta 1 induced pyrophosphate elaboration by chondrocytes. J Rheumatol 21:896–900 [PubMed] [Google Scholar]
- Sweet HO, Green MC (1981) Progressive ankylosis, a new skeletal mutation in the mouse. J Hered 72:87–93 [DOI] [PubMed] [Google Scholar]
- Terkeltaub R, Rosenbach M, Fong F, Goding J (1994) Causal link between nucleotide pyrophosphohydrolase overactivity and increased intracellular inorganic pyrophosphate generation demonstrated by transfection of cultured fibroblasts and osteoblasts with plasma cell membrane glycoprotein-1: relevance to calcium pyrophosphate dihydrate deposition disease. Arthritis Rheum 37:934–941 [DOI] [PubMed] [Google Scholar]
- van der Korst JK, Geerards J (1976) Articular chondrocalcinosis in a Dutch pedigree. Arthritis Rheum Suppl 19:405–409 [DOI] [PubMed] [Google Scholar]
- van der Korst JK, Geerards J, Driessens FC (1974) A hereditary type of idiopathic articular chondrocalcinosis: survey of a pedigree. Am J Med 56:307–314 [DOI] [PubMed] [Google Scholar]