Abstract
In schizophrenia there is a consistent epidemiological finding of a birth excess in winter and spring. Season of birth is thought to act as a proxy indicator for harmful environmental factors during foetal maturation. There is evidence that prenatal exposure to harmful environmental factors may trigger pathologic processes in the neurodevelopment, which subsequently increase the risk of schizophrenia. Since brain white matter alterations have repeatedly been found in schizophrenia, the objective of this study was to investigate whether white matter integrity was related to the season of birth in patients with schizophrenia. Thirty-four patients with schizophrenia and 33 healthy controls underwent diffusion tensor imaging. Differences in the fractional anisotropy maps of schizophrenia patients and healthy controls born in different seasons were analysed with tract-based spatial statistics. A significant main effect of season of birth and an interaction of group and season of birth showed that patients born in summer had significantly lower fractional anisotropy in widespread white matter regions than those born in the remainder of the year. Additionally, later age of schizophrenia onset was found in patients born in winter months. The current findings indicate a relationship of season of birth and white matter alterations in schizophrenia and consequently support the neurodevelopmental hypothesis of early pathological mechanisms in schizophrenia.
Introduction
Schizophrenia is a psychiatric disorder with a plethora of mysteries that prevents the assessment of an exact aetiology [1]. Although genetic factors are involved substantially in the risk of developing schizophrenia, environmental factors have recently received much attention [2]. Notably, there is a consistent phenomenon of a 5–8% excess of winter births in patients with schizophrenia, which is replicated throughout the world. The exact mechanisms causing a season of birth effect have not yet been identified, since season of birth merely acts as a proxy indicator for prenatal exposure to seasonally varying harmful environmental factors [3]. Epidemiological, clinical and imaging findings provide evidence for the neurodevelopmental hypothesis of schizophrenia, which suggests that the exposure to harmful factors during foetal maturation may detrimentally affect neurodevelopmental processes resulting in continuous brain and behavioural changes throughout childhood and adolescence, which eventually lead to a higher risk of schizophrenia [4].
Schizophrenia is accompanied by significant, progressive anatomical brain changes, appearing in early adulthood [5]. A finding of interest has been the demonstration of microstructural white matter (WM) alterations in patients with schizophrenia using diffusion tensor imaging (DTI) [6]–[8]. Reduced white matter integrity has been observed as early as first-episode schizophrenia in widespread brain regions [9]. It has been proposed that the downregulation of oligodendrocyte and myelin-related genes may underlie WM alterations in schizophrenia [10]. Typically, brain WM starts to develop during the late first trimester of foetal maturation and is completed by the age of 2 years [11]–[13], followed by a myelination process lasting until early adulthood [14], [15].
Previous studies have already probed for a relationship between season of birth and brain anatomical properties in schizophrenia indicating a ventricular enlargement in winter and spring births, but no other anatomical associations [16]–[21]. Notably, these studies used either computerized tomography or magnetic resonance imaging. However, DTI has not yet been implemented to test the effect of season of birth on WM properties in schizophrenia. Since diffusion is highly anisotropic in brain white matter, reduced anisotropy of water diffusion has been proposed to reflect compromised white matter integrity [22]. Interestingly, after prenatal exposure to viral infections in rodents, WM integrity abnormalities and myelin-related gene alterations were detected [23]. Therefore, according to the neurodevelopmental hypothesis of schizophrenia, it is conceivable that white matter properties in humans may be affected by harmful environmental factors during foetal maturation, which are related to the season of birth. Consequently, in the present study an association between WM integrity and season of birth is hypothesized in patients with schizophrenia. According to previous anatomical findings, we expected to find WM integrity reductions in patients born in winter relative to those born in summer [16]. However, WM integrity may also be compromised during other birth seasons, since the timing of the seasonal factor is unknown and its effects may differ depending on developmental processes affected. For healthy control subjects such an association is not expected, since the lack of psychopathology suggests normal neurodevelopment. To test the current hypotheses, WM integrity of schizophrenia patients and healthy controls was measured with DTI and analysed with tract-based spatial statistics (TBSS).
The purpose of this study was to clarify a possible association between season of birth and WM integrity. Specifically, we hypothesized a relationship between WM integrity and season of birth in patients with schizophrenia and that this association would not be observed in healthy controls.
Materials and Methods
Ethics Statement
Procedures were approved by the local ethics committee (of the Kanton Bern, Switzerland: KEK Bern, 192-05, 064-05, 196-09) and are in accordance with the Declaration of Helsinki. First we explained in detail to all patients and healthy controls the planed study. This was done orally and on the basis of the written description of the study and included the aims and procedures of the study. All subjects were explicitly informed that participation was voluntary and could be declined at any time without any reason and without being subjected to any disadvantage. The patients’ capacity to provide informed consent was assessed and confirmed by their treating psychiatrist who was independent from the study. All participants provided written informed consent prior to the beginning of the examination. The participants received a copy of their signed consent and were not paid for their participation.
Participants
Thirty-four patients with schizophrenia who were admitted to the University Hospital of Psychiatry in Bern and 33 healthy controls who did not differ in age and gender participated in this study. The healthy controls were recruited among the hospital employees of various professions. Inclusion criteria for the patients were Diagnostic and Statistical Manual of Mental Disorders, Fourth edition diagnoses of schizophrenia, schizoaffective disorder or schizophreniform disorder [24]. Out of the 34 patients, 28 were diagnosed with schizophrenia, 4 were diagnosed with schizophreniform disorder, and 2 were diagnosed with schizoaffective disorder. The exclusion criteria for both groups were any serious medical or neurological condition, drug abuse other than nicotine, history of electroconvulsive treatment or head injury with a subsequent loss of consciousness. Additional exclusion criteria of controls were history of any psychiatric disorder and first degree relatives suffering from schizophrenia spectrum disorders. Symptom severity was assessed by the Positive and Negative Symptom Scale (PANSS) [25]. The estimated current equivalent dosage of antipsychotics was calculated according to Woods [26]. Other environmental factors which may play a role in the development of schizophrenia such as migration, cannabis use and minority status were extracted from the medical history of each patient. Minority status was based on the patient’s ethnicity. In order to probe for the seasonal effects on brain white matter, the current study adopted the seasonal differentiation found in literature for the group assignment, which showed significant birth excesses during November and May [3]. Consequently, subjects born from November to May were assigned to the winter half year group (winter-born subjects), whereas the summer half year group (summer-born subjects) comprised subjects born from June to October. In addition, a systematic comparison of different half-year classifications that comprised all 6 possible combinations of equally classifying the year into 2 halves were conducted in order to verify the specificity of the season-of-birth effect within the DTI analysis.
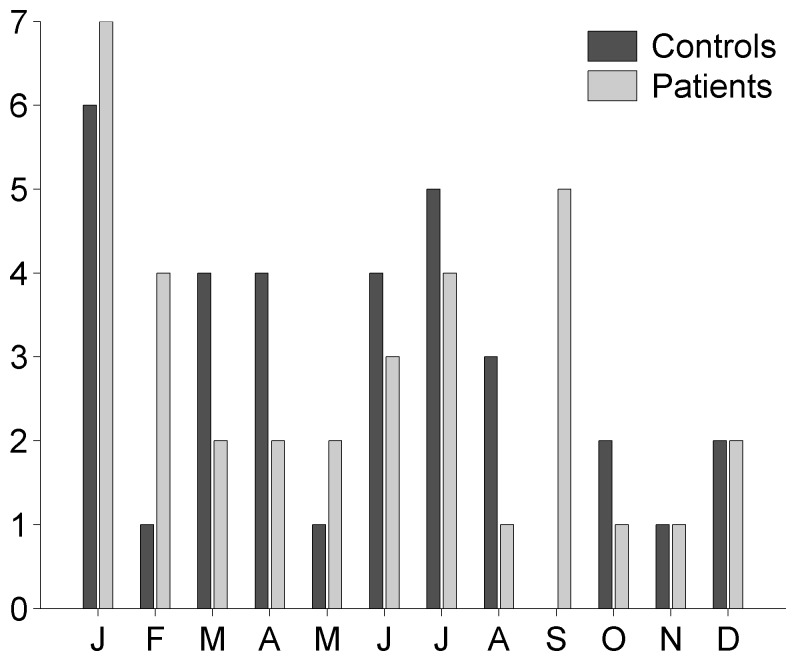
The demographic and clinical characteristics of the participants are shown in Table 1. Patients and healthy controls were not different in age, gender distribution or season of birth. No significant differences were observed for age, gender, migration, cannabis use or minority status ratio among winter- and summer-born patients and winter- and summer-born healthy controls. Because clinical and demographic variables were not normally distributed, non-parametric tests were used to assess differences between groups with significance set at p<0.05 using R software package [27]. Furthermore, a MATLAB Toolbox for Circular Statistics was employed to analyse the birth distribution of patients and healthy controls across the year and their correlates with demographic and clinical variables with significance set at p<0.05 [28]. Circular statistics confirmed the lack of group differences in birth distribution during the year (Figure 1). Additionally, correlation analyses of birth dates with demographic and clinical variables demonstrated an absence of significant relationships, except for the age of schizophrenia onset, which indicated an increase in the age of schizophrenia onset for patients born in January and February (see Supporting Information, Figure S1).
Table 1. Demographic data of patients with schizophrenia and controls, separated for seasons of birth.
| Category | Controls | Patients | ||||
| Total | Winter-born | Summer-born | Total | Winter-born | Summer-born | |
| N | 33 | 19 | 14 | 34 | 20 | 14 |
| Age (years) | 33.2 (±8.9) | 31.15 (±8.17) | 36.07 (±9.41) | 33.65 (±9.89) | 34.00 (±9.45) | 33.14 (±10.83) |
| Age range | 21–53 | 21–52 | 22–53 | 20–66 | 20–55 | 22–66 |
| Gender (M/F) | (16/17) | (11/8) | (5/9) | (18/16) | (12/8) | (6/8) |
| Age at onset (years) | 25.63 (±7.15) | 26.51 (±8.85) | 24.28 (±4.24) | |||
| Duration of illness (years) | 8.22 (±8.79) | 7.75 (±8.11) | 8.86 (±9.91) | |||
| PANSS-P | 14.29 (±6.14) | 14.70 (±7.15) | 13.71 (±4.50) | |||
| PANSS-N | 18.15 (±7.91) | 17.90 (±8.58) | 18.50 (±7.12) | |||
| PANSS-T | 60.91 (±18.96) | 60.10 (±19.18) | 62.07 (±19.30) | |||
| CPZ (mg) | 506.41 (±87.84) | 417.75 (±279.12) | 633.07 (±678.89) | |||
| Migration (yes/no) | (8/26) | (6/14) | (2/12) | |||
| Minority status (yes/no) | (12/22) | (8/12) | (4/10) | |||
| Cannabis use (yes/no) | (17/17) | (10/10) | (7/7) | |||
Except for age range, gender, migration, social adversity and cannabis use, values are represented as mean ± standard deviation. M, male; F, female; PANSS, Positive and Negative Syndrome Scale; PANSS-P, subscale for positive symptoms; PANSS-N, subscale for negative symptoms; PANSS-T, total score of PANSS, CPZ, chlorpromazine equivalents.
Figure 1. Distribution of the months of birth across the year for patients and controls.
The x-axes of the bar plot displays the months from January to December, each abbreviated by the first letter. The y-axis reports the frequency of births.
Imaging Methods
DTI images were acquired for all subjects using a 3-Tesla Magnetom Trio TIM system (Siemens Erlangen, Germany) using a 12-channel array head coil. Spin-echo planar imaging sequence was performed with two 180° pulses and a repetition time/echo time of 6,500/96 ms with a matrix of 128×128, a field of view of 256×256 mm2, 52 slices, a slice thickness of 2 mm, a gap thickness of 0 mm, a pixel bandwidth of 1396 Hz/pixel and N = 2 averages, 4 b-values of 0 s/mm2 and a b-value of 1,300 s/mm2 along 42 non-collinear directions. All images were measured in the axial plane parallel to the anterior/posterior commissure line.
DTI Processing
For the pre-processing of DTI images we implemented the Centre for Functional Magnetic Resonance Imaging of the Brain’s diffusion toolbox, part of the Centre for Functional Magnetic Resonance Imaging of the Brain software library (FSL, http://www.fmrib.ox.ac.uk/fsl) [29]. The original data was corrected for the effects of head movement and eddy currents. Subsequently, a brain extraction tool was used to remove all non-brain parts of the image [30]. From these images, fractional anisotropy (FA) values were calculated by fitting a tensor model to the data at each voxel. Tract-Based Spatial Statistics (TBSS, part of FSL) pipeline was used for further pre-processing of FA images [29] and included the following steps; All subjects’ FA data were aligned to a 1 mm×1 mm×1 mm Montreal Neurological Institute (MNI) standard space using as a target the FMRIB58 FA standard-space image. The alignment was performed by the non-linear registration tool FMRIB’s Non-Linear Image Registration Tool [31], [32], which uses a B-spline representation of the registration warp field [33]. Next, a mean FA image was created and thinned in order to create a mean FA skeleton that represented the centres of all tracts common to the group. A lower FA threshold of 0.2 was used to prevent the inclusion of non-skeletal voxels [34]. Each subject’s aligned FA data was then projected onto this skeleton, and the resulting data was subjected to voxel-wise cross-subject statistics.
Statistical Data Analysis
Statistical analysis was carried out with TBSS, which is based on a non-parametric approach using permutation test theory with a standard general linear model (GLM) design matrix [34]. Age and gender as well as PANSS total scores, CPZ and age at onset were entered as covariates of no interest into the analyses. The GLM examined the main effect of season and group, as well as a season by group interaction. Pair-wise differences between groups, and also between subgroups (e.g. summer-born controls versus summer-born patients) were calculated in accordance to a study with a similar design [35]. The permutation testing was performed by the FSL Randomise program using 5,000 Monte Carlo random permutations to generate random permutation maps. This approach allows for inference on the statistical maps when the null distribution is unknown and offers a solution to the problem of multiple testing [36]. Using this setup voxel-wise differences between groups were then assessed, testing the significance corrected for multiple comparisons at p<0.05. Results which were uncorrected for multiple comparisons are reported in the Supporting Information (see Table S1 and Figure S2). A threshold-free cluster enhancement option was employed in order to enhance the cluster-like structures in images without having to define an initial cluster-forming threshold. From the results of the voxel-wise analyses, the skeletal regions showing significant effects were located and labelled anatomically by mapping the FWE-corrected statistical map to the Johns Hopkins University (JHU)-ICBM-DTI-81 WM labels atlas and the JHU-WM tractography atlas in MNI space [37], [38]. For the comparison of FA differences between groups, mean FA values were extracted from the significant clusters within the different WM regions for each subject. Only clusters greater than 100 voxels per WM region were displayed.
Results
Significant Main Effect of Season of Birth
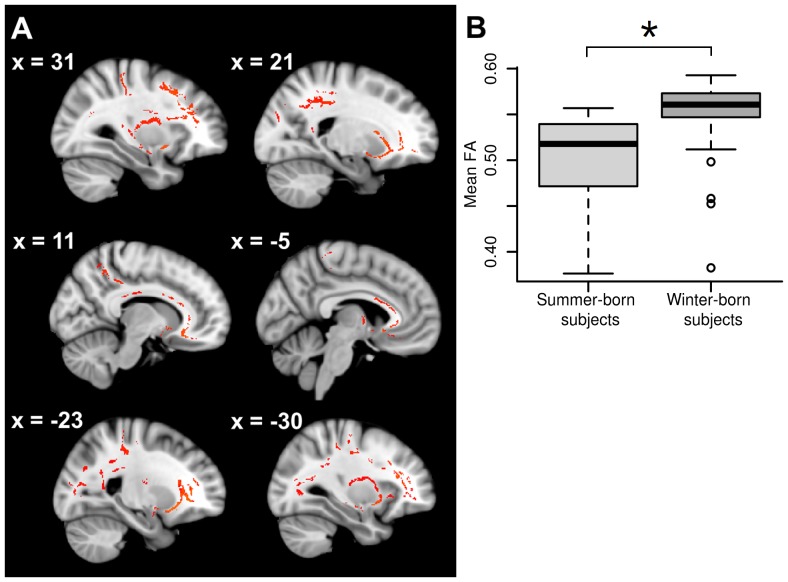
A significant main effect of season was reported by the GLM applied in TBSS in widespread white matter regions (see Figure 2). Significantly decreased FA values were found in summer-born subjects (including healthy controls and patients) compared to winter-born subjects (p<0.05). The anatomical regions comprised sections of the corpus callosum, the internal capsule, the corona radiata, the posterior thalamic radiation, the sagittal striatum, the external capsule and the superior longitudinal fasciculus. For an overview of the size and mean FA values of the significant clusters, see Table 2.
Figure 2. Significant main effect of season of birth.
(A) Summer-born subjects have lower FA values than winter-born subjects in the regions indicated in red. (B) The plot shows the mean FA values in summer and winter-born subjects within the significant TBSS clusters of the main effect of season highlighted in red. The TBSS images show results at p<0.05 corrected for multiple comparisons.
Table 2. Main effect of season of birth for FA values.
| Location | Centre of gravity (MNI coordinates) | Cluster size | Mean FA (±SD) of all subjects | |||
| x | y | z | Summer-born | Winter-born | ||
| Genu of corpus callosum | 3 | −4 | 25 | 920 | 0.74 (±0.07) | 0.78 (±0.06) |
| Body of corpus callosum | 3 | −4 | 25 | 760 | 0.74 (±0.08) | 0.78 (±0.06) |
| Splenium of corpus callosum | −3 | −43 | 20 | 585 | 0.78 (±0.06) | 0.82 (±0.04) |
| Anterior limb of internal capsule | 19 | 13 | 6 | 275 | 0.58 (±0.06) | 0.62 (±0.05) |
| Anterior limb of internal capsule | −16 | 10 | 5 | 492 | 0.53 (±0.04) | 0.58 (±0.05) |
| Retrolenticular part of internal capsule | 32 | −27 | 5 | 203 | 0.60 (±0.07) | 0.65 (±0.06) |
| Anterior corona radiate | 21 | 30 | 0 | 699 | 0.53 (±0.05) | 0.57 (±0.05) |
| Anterior corona radiate | −20 | 31 | 2 | 889 | 0.51 (±0.06) | 0.56 (±0.06) |
| Superior corona radiate | 27 | −10 | 28 | 318 | 0.53 (±0.05) | 0.57 (±0.05) |
| Superior corona radiate | −24 | −12 | 29 | 133 | 0.60 (±0.06) | 0.64 (±0.06) |
| Posterior corona radiate | 22 | −37 | 34 | 193 | 0.50 (±0.06) | 0.54 (±0.05) |
| Posterior corona radiate | −25 | −41 | 28 | 275 | 0.51 (±0.05) | 0.56 (±0.05) |
| Posterior thalamic radiation | −32 | −59 | 7 | 547 | 0.61 (±0.07) | 0.67 (±0.05) |
| Sagittal stratum | 41 | −31 | −9 | 364 | 0.58 (±0.08) | 0.63 (±0.06) |
| Sagittal stratum | −40 | −29 | −9 | 233 | 0.59 (±0.04) | 0.64 (±0.06) |
| External capsule | 30 | 1 | −2 | 714 | 0.46 (±0.05) | 0.51 (±0.04) |
| External capsule | −29 | 4 | 0 | 738 | 0.52 (±0.05) | 0.57 (±0.05) |
| Superior longitudinal fasciculus | 38 | −21 | 29 | 557 | 0.54 (±0.05) | 0.58 (±0.05) |
| Superior longitudinal fasciculus | −36 | −18 | 29 | 662 | 0.55 (±0.05) | 0.59 (±0.05) |
MNI, Montreal Neurological Institute; FA, fractional anisotropy; SD, standard deviation.
Main Effect of Group
The GLM indicated a significant main effect of group exclusively when no correction for multiple comparisons was applied. Patients with schizophrenia showed a reduction in FA values compared to healthy controls (p uncorrected<0.05) in the bilateral superior longitudinal fasciculus, bilateral inferior-frontal fasciculus, in the bilateral forceps major and in the bilateral cingulum (see Figure S2 and Table S1).
Interaction of Group and Season of Birth
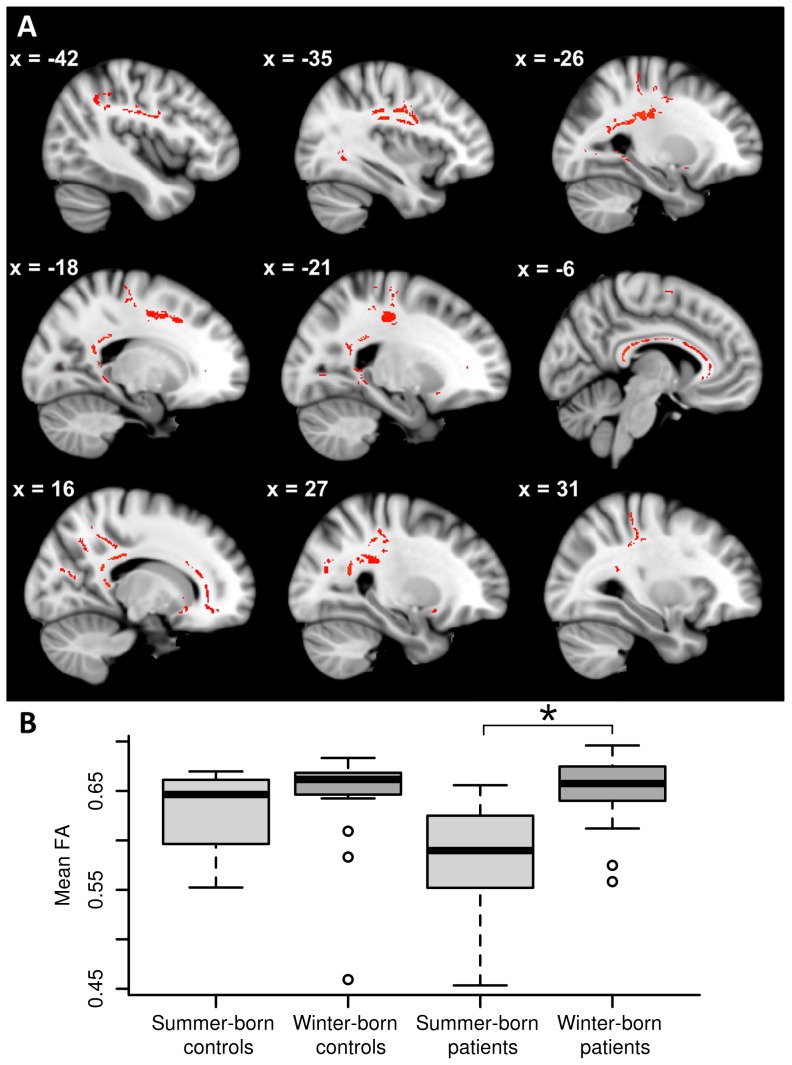
The GLM revealed that summer-born patients with schizophrenia had significantly lower FA values than winter-born patients (p<0.05). As can be seen in Figure 3, the regions of decreased FA in summer-born patients relative to winter-born patients are predominantly located within the genu, body, and splenium of the corpus callosum. Furthermore, there were significant differences in parts of the inferior fronto-occipital fasciculi on both sides, the uncinate fasciculi on both sides, the right anterior corona radiata, the left posterior cingulum near the hippocampus, the posterior corona radiata in both hemispheres, the left posterior thalamic radiation, parts of the corticospinal tracts on both sides, the superior longitudinal fasciculi on both sides, and the forceps major. For an overview of the size and mean FA values of the significant results see Table 3. Systematically comparing different half-year classifications demonstrated significant results within patients but not within healthy controls. In particular, lower FA values were found for patients born between June and November compared to those born between December and May (p<0.05), as well as significantly lower FA for patients born between May and October compared to those born between November and April (p<0.05). No other significant interactions were found between group and season of birth when correcting for multiple comparisons. Notably, healthy controls displayed no areas of significantly altered FA values between different seasons of birth. For significant results which are uncorrected for multiple comparisons see the Supporting Information (see Table S1 and Figure S2). Except for age being negatively correlated with FA values, the voxel-wise analysis detected no significant associations between the duration of illness, the age at schizophrenia onset, CPZ or the PANSS total scores and FA values.
Figure 3. Interaction of group and season of birth.
(A) Summer-born patients with schizophrenia showed reduced FA values compared to winter-born patients with schizophrenia within the areas indicated in red. (B) The plot shows the mean FA values within the significant TBSS clusters highlighted in red in summer and winter-born patients. For the purpose of comparison, mean FA values extracted from the same areas indicated in red were additionally displayed for summer and winter-born controls. The TBSS images show results at p<0.03 corrected for multiple comparisons.
Table 3. Interaction of group and season of birth shows reduced FA values in summer-born patients compared to winter-born patients with schizophrenia.
| Centre of gravity (MNI coordinates) | Cluster Size | Mean FA (±SD) of patients | ||||
| Location | x | y | z | Summer-born | Winter-born | |
| Genu corpus callosum | 2 | 26 | 8 | 808 | 0.73 (±0.07) | 0.81 (±0.04) |
| Body of corpus callosum | 0 | −4 | 25 | 1149 | 0.74 (±0.08) | 0.81 (±0.04) |
| Splenium of corpus callosum | 0 | −40 | 19 | 1318 | 0.79 (±0.06) | 0.85 (±0.03) |
| Anterior corona radiata | 17 | 33 | −1 | 227 | 0.55 (±0.06) | 0.62 (±0.04) |
| Superior corona radiata | −23 | −18 | 36 | 155 | 0.53 (±0.06) | 0.59 (±0.05) |
| Posterior corona radiata | 25 | −39 | 28 | 422 | 0.50 (±0.05) | 0.56 (±0.05) |
| Posterior corona radiata | −26 | −39 | 25 | 293 | 0.53 (±0.06) | 0.58 (±0.05) |
| Posterior thalamic radiation | −30 | 59 | 7 | 195 | 0.60 (±0.06) | 0.66 (±0.04) |
| Inferior fronto-occipital fasciculus | −29 | −59 | 5 | 197 | 0.54 (±0.09) | 0.61 (±0.08) |
| Inferior fronto-occipital fasciculus | 25 | −39 | 19 | 396 | 0.52 (±0.06) | 0.58 (±0.06) |
| Corticospinal tract | −22 | −23 | 45 | 614 | 0.51 (±0.05) | 0.57 (±0.04) |
| Corticospinal tract | 23 | −30 | 39 | 209 | 0.49 (±0.05) | 0.55 (±0.05) |
| Cingulum near hippocampus | −20 | −45 | 0 | 191 | 0.49 (±0.06) | 0.56 (±0.03) |
| Superior longitudinal fasciculus | −36 | −19 | 29 | 584 | 0.47 (±0.05) | 0.54 (±0.04) |
| Forceps major | 1 | −50 | 15 | 859 | 0.71 (±0.05) | 0.77 (±0.04) |
| Uncinate fasciculus | 19 | 21 | −10 | 185 | 0.44 (±0.04) | 0.50 (±0.04) |
MNI, Montreal Neurological Institute; FA, fractional anisotropy; SD, standard deviation.
Discussion
We report a significant main effect of season of birth on white matter integrity which has not been previously noted. This significant main effect of season demonstrated that summer-born subjects had significantly lower FA values relative to winter-born subjects. The interaction of group by season of birth indicated that WM integrity impairments were higher within summer-born patients than within summer-born healthy controls relative to their winter-born counterparts. Furthermore, our findings indicated impaired WM integrity in schizophrenia patients relative to healthy controls. Although these findings are uncorrected for multiple comparisons they are broadly consistent with previous DTI studies [39]–[42]. In addition, a correlation of birth date and illness onset was demonstrated, indicating a later age of disease onset for patients born in winter months (see Supporting Information, Figure S1).
In previous studies, a birth excess in winter-born patients has repeatedly been found [3]. Harmful environmental factors are thought to affect neurodevelopment in perinatal stages and thus increase the risk of schizophrenia for winter-born subjects [43]. Furthermore, some studies have observed a ventricular enlargement in winter-born patients with schizophrenia relative to patients born in the remainder of the year suggesting detrimental effects by perinatal seasonal factors [19]. According to these observations and the neurodevelopmental hypothesis of schizophrenia, we expected WM integrity impairments in winter-born schizophrenia patients relative to the patients born in the reminder of the year. However, the current DTI study displayed structural white matter impairments in patients born in summer relative to patients born in winter. Consequently, this finding suggests that the seasonal birth excess in winter and WM integrity impairments in schizophrenia are unrelated.
The exact mechanisms that account for the observation of reduced WM integrity in patients born in summer are beyond the scope of this study. Since the season of birth is regarded as a proxy indicator for prenatal exposure to harmful factors, the current findings are still in line with the neurodevelopmental hypothesis of schizophrenia [4]. According to this hypothesis, the prenatal exposure to harmful factors may affect subsequent neurodevelopment resulting in an increased risk of schizophrenia [44]. It is suggested that abnormal brain development may start as early as late first or early second trimester and trigger further pathologic mechanisms [4]. This is evidenced by neuroanatomical irregularities already present at disease onset such as ventricular enlargement, grey and white matter changes in patients with schizophrenia [45], [46]. Consequently, the current findings of a relationship between season of birth and WM properties support the hypothesis of perinatal events involving a seasonal factor and subsequent pathologic brain development in schizophrenia [47].
As mentioned in the introduction, previous neuroimaging findings have already revealed a relationship between ventricular enlargement and season of birth, but not for other morphological measures [16]–[21]. Particularly, patients with schizophrenia born in winter-spring months had an increased risk of ventricular enlargement compared to patients born in the rest of the year. An animal study using immune activation in different gestational stages corroborated this finding showing a marked enlargement of lateral ventricles at adult stage, without affecting total white and grey matter volumes [48]. These findings contributed to the notion of an anatomical specificity of seasonally harmful environmental factors affecting neurodevelopment [19]. However, the current findings may add to the previous relationship between season of birth and ventricular enlargement because it is suggested that using DTI, more subtle changes of WM properties can be detected [22]. As mentioned in the introduction, animal models have already shown that the exposure to viral infections during foetal maturation resulted in subsequent white matter alterations, such as glial fibrillary acidic protein immunoreactivity [49] and abnormal expression of myelination gens which were detected using DTI [23]. As in rodents, human WM development starts during early foetal maturation and may thus be comparably affected by prenatal exposure to harmful factors [12].
The interaction of group by season of birth indicated that FA values in summer-born subjects relative to winter-born subjects were more reduced within patients than in healthy controls. As already mentioned, the current findings are unexpected regarding the previous findings of a ventricular enlargement in winter-born schizophrenia patients [20]. Nevertheless, this reported dissociation of season of birth and related anatomical alterations may indicate the importance of the timing of exposure to harmful factors during foetal maturation. Animal studies have demonstrated that middle or late prenatal exposure to infections were dissociable regarding brain and behavioural pathology [50]–[52]. Since the exact timing of action of the seasonal factor is unknown, the current results of reduced WM integrity in summer-born patients may merely indicate that WM properties and ventricular enlargement may be the result of exposure to harmful factors during different stages of perinatal development. Furthermore, the current observation of a later age at schizophrenia onset in winter-born patients together with previous findings of enduring negative symptoms for schizophrenia patients born in summer months contribute to the notion of differential effects of a seasonal factor in the aetiology of schizophrenia [53]. Interestingly, negative symptoms have been found to correlate with WM integrity in schizophrenia patients [54]. Consequently, the current results might indicate a differential effect of the timing of exposure to prenatal harmful factors during foetal maturation.
The main effect of season of birth showed that WM integrity was reduced in summer-born subjects relative to winter-born subjects. This effect was driven by the patients group showing significantly decreased WM integrity in summer-born patients relative to winter-born patients. However, this effect was also observed in healthy controls when correction for multiple comparisons was omitted. Since WM integrity changes are unspecific for schizophrenia and have been found in different neurologic and psychiatric diseases as well as in healthy aging [55]–[57], it is conceivable that it may vary in a small degree in healthy controls as well. Interestingly, the season of birth has already been related to cognitive and physical characteristics in healthy controls. Notably, a study which analysed the cognitive and physical outcome of healthy children depending on their season of birth revealed a superior cognitive and physical development in winter and spring-born children compared to summer and autumn-born children [58]. Moreover, higher schizotypal traits and a tendency of lower agreeableness were found in healthy controls born during winter months compared to those born in the remainder of the year [59], [60]. These findings suggest that the season of birth may not only affect patients with schizophrenia but as well –in a lesser degree- also healthy controls. Therefore, it would be interesting in future research to probe for an association between the season of birth, WM integrity and different psychometric measures in healthy controls and schizophrenia.
The reported WM integrity impairments in patients born in summer were in line with general findings of WM abnormalities in schizophrenia, which have predominantly been observed in left frontal and left temporal parts of the brain [61]. WM integrity alterations have also been linked to specific symptoms in schizophrenia [62]. Especially, the corpus callosum, which connects both hemispheres, has repeatedly been found to be affected in schizophrenia and was related to “hypofrontality” as well as to the dysfunctions of the heteromodal association cortex and social cognition deficits [63]–[65]. Further, decreased white matter integrity in the corpus callosum, the corona radiata and other long association fibers was found to be related to poor outcome in schizophrenia as well as to cognitive impairment [66], [67]. Another study showed that posterior regions near the right supplemental motor area were associated with motor activity in schizophrenia [8]. Moreover, white matter impairments in the uncinate fasciculus were associated with increased severity of negative symptoms and impaired memory functioning [68]. The cingulum bundles, part of the limbic system, were associated with positive symptoms in schizophrenia [69]. Additionally, the superior longitudinal fasciculus, which connects frontal and parietal areas, was reported to be related to working memory as well as to auditory hallucinations in schizophrenia [70], [71].
A number of confounding factors, including age, age of illness onset, chronicity, and medication use, may affect WM integrity [72], [73]. Apart from age correlating negatively with WM integrity in widespread regions within subjects, no other confounding variables, such as PANSS scores, CPZ, age of onset or illness duration, were found to be related to WM integrity. Moreover, a later age of schizophrenia onset was found for patients born during January and February. Nevertheless, all above mentioned variables were included as covariates in the statistical analyses in TBSS. While WM integrity alterations have consistently been reported in patients with schizophrenia, the detected differences in FA values between patients and healthy controls disappeared after correction for multiple comparisons [6]. This might be due to the inclusion of a broader schizophrenia spectrum instead of core schizophrenia or differences in the implemented methods [74]. Furthermore, urban birth which was not assessed in the current study may interact with the season of birth and thus represent a potential confounding factor [3], [75]. However, migration, minority status and drug abuse which may as well contribute to the aetiology of schizophrenia did not differ between summer and winter-born patients.
In summary, the present DTI study observed a clear and novel relationship between FA values and season of birth in patients with schizophrenia, but not in healthy controls. The alterations were located in distinct WM fibre bundles. The findings are in line with the neurodevelopmental hypothesis of schizophrenia, which suggests an early contribution to abnormal brain development.
Supporting Information
Relationship of birth date and age of schizophrenia onset. Polar plot of birth distribution in relation to age at schizophrenia onset (circular-linear correlation, r = 0.42, p = 0.049). In particular, patients born in January and February showed a later age of schizophrenia onset compared to patients born in the remainder of the year.
(DOCX)
Location of significant TBSS results of pair-wise differences between groups and between subgroups uncorrected for multiple comparisons. The locations of significant FA value reductions are shown for patients relative to controls (red), for summer-born controls relative to winter-born controls (green), summer-born patients relative to summer-born controls (cyan), summer-born patients relative to winter-born controls (blue), winter-born patients relative to winter-born controls (yellow).
(DOCX)
Significant TBSS results of pair-wise differences between groups and between subgroups uncorrected for multiple comparisons and corrected for multiple comparisons.
(DOCX)
Acknowledgments
We thank Othmar Würmle for his support in the statistical analysis related to the manuscript.
Funding Statement
This work was supported by The Swiss National Science Foundation (http://www.snf.ch/D/Seiten/default.aspx) (SNSF: 32003B-112578 and SNSF: 2003B-124947). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tandon R, Keshavan MS, Nasrallah HA (2008) Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr Res 102: 1–18. [DOI] [PubMed] [Google Scholar]
- 2. Brown AS (2011) The environment and susceptibility to schizophrenia. Prog Neurobiol 93: 23–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torrey EF, Miller J, Rawlings R, Yolken RH (1997) Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophr Res 28: 1–38. [DOI] [PubMed] [Google Scholar]
- 4. Fatemi SH, Folsom TD (2009) The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull 35: 528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Peri L, Crescini A, Deste G, Fusar-Poli P, Sacchetti E, et al. (2012) Brain structural abnormalities at the onset of schizophrenia and bipolar disorder: a meta-analysis of controlled magnetic resonance imaging studies. Curr Pharm Des 18: 486–494. [DOI] [PubMed] [Google Scholar]
- 6. Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, et al. (2007) A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res 41: 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Federspiel A, Begre S, Kiefer C, Schroth G, Strik WK, et al. (2006) Alterations of white matter connectivity in first episode schizophrenia. Neurobiol Dis 22: 702–709. [DOI] [PubMed] [Google Scholar]
- 8. Walther S, Federspiel A, Horn H, Razavi N, Wiest R, et al. (2011) Alterations of white matter integrity related to motor activity in schizophrenia. Neurobiol Dis 42: 276–283. [DOI] [PubMed] [Google Scholar]
- 9. Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, et al. (2008) A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol Med 38: 877–885. [DOI] [PubMed] [Google Scholar]
- 10. Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, et al. (2003) Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 362: 798–805. [DOI] [PubMed] [Google Scholar]
- 11. Walterfang M, Velakoulis D, Whitford TJ, Pantelis C (2011) Understanding aberrant white matter development in schizophrenia: an avenue for therapy? Expert Rev Neurother 11: 971–987. [DOI] [PubMed] [Google Scholar]
- 12.Nolte N (1999) The Human Brain. Mosby, MO, USA.
- 13. Kasprian G, Brugger PC, Weber M, Krssak M, Krampl E, et al. (2008) In utero tractography of fetal white matter development. Neuroimage 43: 213–224. [DOI] [PubMed] [Google Scholar]
- 14. Paus T (2010) Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn 72: 26–35. [DOI] [PubMed] [Google Scholar]
- 15. Barkovich AJ (2000) Concepts of myelin and myelination in neuroradiology. AJNR Am J Neuroradiol 21: 1099–1109. [PMC free article] [PubMed] [Google Scholar]
- 16.Sacchetti E, Vita A, Battaglia M, Calzeroni A, Conte G, et al.. (1987) Season of birth and cerebral ventricular enlargement in schizophrenia. In: Cazullo GL, Invernizzi G, Sacchetti E, Vita A, editors. Etiopathogenetic Hypotheses of Schizophrenia. Boston, MA: MTP Press. 93–98.
- 17. Zipursky RB, Schulz SC (1987) Seasonality of birth and CT findings in schizophrenia. Biol Psychiatry 22: 1288–1292. [DOI] [PubMed] [Google Scholar]
- 18. Degreef G, Mukherjee S, Bilder R, Schnur D (1988) Season of birth and CT scan findings in schizophrenic patients. Biol Psychiatry 24: 461–464. [DOI] [PubMed] [Google Scholar]
- 19. Sacchetti E, Calzeroni A, Vita A, Terzi A, Pollastro F, et al. (1992) The brain damage hypothesis of the seasonality of births in schizophrenia and major affective disorders: evidence from computerised tomography. Br J Psychiatry 160: 390–397. [DOI] [PubMed] [Google Scholar]
- 20. d’Amato T, Rochet T, Dalery J, Chauchat JH, Martin JP, et al. (1994) Seasonality of birth and ventricular enlargement in chronic schizophrenia. Psychiatry Res 55: 65–73. [DOI] [PubMed] [Google Scholar]
- 21. DeQuardo JR, Goldman M, Tandon R (1996) VBR in schizophrenia: relationship to family history of psychosis and season of birth. Schizophr Res 20: 275–285. [DOI] [PubMed] [Google Scholar]
- 22. Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111: 209–219. [DOI] [PubMed] [Google Scholar]
- 23. Fatemi SH, Folsom TD, Reutiman TJ, Abu-Odeh D, Mori S, et al. (2009) Abnormal expression of myelination genes and alterations in white matter fractional anisotropy following prenatal viral influenza infection at E16 in mice. Schizophr Res 112: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.APA (1994) American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association.
- 25. Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276. [DOI] [PubMed] [Google Scholar]
- 26. Woods SW (2003) Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 64: 663–667. [DOI] [PubMed] [Google Scholar]
- 27.R Development Core Team (2010) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna Austria.
- 28. Berens P (2009) CircStat: a MATLAB toolbox for circular statistics. Journal of Statistical Software 31: 1–21. [Google Scholar]
- 29. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, et al. (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1S208–219. [DOI] [PubMed] [Google Scholar]
- 30. Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersson JLR, Jenkinson M, Smith S (2007) Non-linear optimisation FMRIB technical report TR07JA1.
- 32.Andersson JLR, Jenkinson M, Smith S (2007) Non-linear registration, aka Spatial normalisation.
- 33. Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, et al. (1999) Non-rigid registration using free-form deformations: Application to breast MR images. IEEE Transactions on Medical Imaging 18: 712–721. [DOI] [PubMed] [Google Scholar]
- 34. Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, et al. (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- 35. O’Dwyer L, Lamberton F, Bokde AL, Ewers M, Faluyi YO, et al. (2012) Sexual dimorphism in healthy aging and mild cognitive impairment: a DTI study. PLoS One 7: e37021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, et al. (2001) A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans R Soc Lond B Biol Sci 356: 1293–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori S, Wakana S, Zijl PCM (2005) MRI atlas of human white matter. Amsterdam, The Netherlands: Elsevier.
- 39. Seal ML, Yucel M, Fornito A, Wood SJ, Harrison BJ, et al. (2008) Abnormal white matter microstructure in schizophrenia: a voxelwise analysis of axial and radial diffusivity. Schizophr Res 101: 106–110. [DOI] [PubMed] [Google Scholar]
- 40. Mori T, Ohnishi T, Hashimoto R, Nemoto K, Moriguchi Y, et al. (2007) Progressive changes of white matter integrity in schizophrenia revealed by diffusion tensor imaging. Psychiatry Res 154: 133–145. [DOI] [PubMed] [Google Scholar]
- 41. Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, et al. (2005) DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage 26: 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO (2003) MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport 14: 2025–2029. [DOI] [PubMed] [Google Scholar]
- 43. Tochigi M, Okazaki Y, Kato N, Sasaki T (2004) What causes seasonality of birth in schizophrenia? Neurosci Res 48: 1–11. [DOI] [PubMed] [Google Scholar]
- 44. Rapoport JL, Addington AM, Frangou S, Psych MR (2005) The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry 10: 434–449. [DOI] [PubMed] [Google Scholar]
- 45. Frangou S, Murray RM (1996) Imaging as a tool in exploring the neurodevelopment and genetics of schizophrenia. Br Med Bull 52: 587–596. [DOI] [PubMed] [Google Scholar]
- 46. Rapoport JL, Gogtay N (2011) Childhood onset schizophrenia: support for a progressive neurodevelopmental disorder. Int J Dev Neurosci 29: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boyd JH, Pulver AE, Stewart W (1986) Season of birth: schizophrenia and bipolar disorder. Schizophr Bull 12: 173–186. [DOI] [PubMed] [Google Scholar]
- 48. Li Q, Cheung C, Wei R, Hui ES, Feldon J, et al. (2009) Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PLoS One 4: e6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fatemi SH, Emamian ES, Sidwell RW, Kist DA, Stary JM, et al. (2002) Human influenza viral infection in utero alters glial fibrillary acidic protein immunoreactivity in the developing brains of neonatal mice. Mol Psychiatry 7: 633–640. [DOI] [PubMed] [Google Scholar]
- 50. Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, et al. (2006) The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci 26: 4752–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J (2008) Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun 22: 469–486. [DOI] [PubMed] [Google Scholar]
- 52. Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, et al. (2008) Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr Res 99: 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Messias E, Kirkpatrick B, Bromet E, Ross D, Buchanan RW, et al. (2004) Summer birth and deficit schizophrenia: a pooled analysis from 6 countries. Arch Gen Psychiatry 61: 985–989. [DOI] [PubMed] [Google Scholar]
- 54. Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, et al. (2001) Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry 158: 234–243. [DOI] [PubMed] [Google Scholar]
- 55. Gunning-Dixon FM, Raz N (2000) The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology 14: 224–232. [DOI] [PubMed] [Google Scholar]
- 56. Werring DJ, Brassat D, Droogan AG, Clark CA, Symms MR, et al. (2000) The pathogenesis of lesions and normal-appearing white matter changes in multiple sclerosis: a serial diffusion MRI study. Brain 123 (Pt 8): 1667–1676. [DOI] [PubMed] [Google Scholar]
- 57. Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, et al. (2007) White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 130: 2508–2519. [DOI] [PubMed] [Google Scholar]
- 58. McGrath JJ, Saha S, Lieberman DE, Buka S (2006) Season of birth is associated with anthropometric and neurocognitive outcomes during infancy and childhood in a general population birth cohort. Schizophr Res 81: 91–100. [DOI] [PubMed] [Google Scholar]
- 59. Hori H, Teraishi T, Sasayama D, Matsuo J, Kawamoto Y, et al. (2012) Relationships between season of birth, schizotypy, temperament, character and neurocognition in a non-clinical population. Psychiatry Res 195: 69–75. [DOI] [PubMed] [Google Scholar]
- 60. Tochigi M, Marumo K, Hibino H, Otowa T, Kato C, et al. (2004) Season of birth effect on personality in a general population. Neurosci Lett 365: 120–123. [DOI] [PubMed] [Google Scholar]
- 61. Ellison-Wright I, Bullmore E (2009) Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res 108: 3–10. [DOI] [PubMed] [Google Scholar]
- 62. Knochel C, O’Dwyer L, Alves G, Reinke B, Magerkurth J, et al. (2012) Association between white matter fiber integrity and subclinical psychotic symptoms in schizophrenia patients and unaffected relatives. Schizophr Res 140: 129–135. [DOI] [PubMed] [Google Scholar]
- 63. Henze R, Brunner R, Thiemann U, Parzer P, Klein J, et al. (2012) White matter alterations in the corpus callosum of adolescents with first-admission schizophrenia. Neurosci Lett 513: 178–182. [DOI] [PubMed] [Google Scholar]
- 64. Patel S, Mahon K, Wellington R, Zhang J, Chaplin W, et al. (2011) A meta-analysis of diffusion tensor imaging studies of the corpus callosum in schizophrenia. Schizophr Res 129: 149–155. [DOI] [PubMed] [Google Scholar]
- 65. Miyata J, Yamada M, Namiki C, Hirao K, Saze T, et al. (2010) Reduced white matter integrity as a neural correlate of social cognition deficits in schizophrenia. Schizophr Res 119: 232–239. [DOI] [PubMed] [Google Scholar]
- 66. Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, et al. (2007) Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophr Res 92: 211–224. [DOI] [PubMed] [Google Scholar]
- 67. Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, et al. (2010) Anterior thalamic radiation integrity in schizophrenia: a diffusion-tensor imaging study. Psychiatry Res 183: 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Szeszko P, Robinson DG, Ashtari M, Vogel J, Betensky J, et al. (2008) Clinical and Neuropsychological Correlates of White Matter Abnormalities in Recent Onset Schizophrenia. Neuropsychopharmacology 33: 976–984. [DOI] [PubMed] [Google Scholar]
- 69. Fujiwara H, Namiki C, Hirao K, Miyata J, Shimizu M, et al. (2007) Anterior and posterior cingulum abnormalities and their association with psychopathology in schizophrenia: a diffusion tensor imaging study. Schizophr Res 95: 215–222. [DOI] [PubMed] [Google Scholar]
- 70. Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, et al. (2008) Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry 63: 512–518. [DOI] [PubMed] [Google Scholar]
- 71. Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, et al. (2004) Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry 61: 658–668. [DOI] [PubMed] [Google Scholar]
- 72. Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, et al. (2011) Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry 70: 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kanaan R, Barker G, Brammer M, Giampietro V, Shergill S, et al. (2009) White matter microstructure in schizophrenia: effects of disorder, duration and medication. Br J Psychiatry 194: 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Peters BD, de Haan L, Dekker N, Blaas J, Becker HE, et al. (2008) White matter fibertracking in first-episode schizophrenia, schizoaffective patients and subjects at ultra-high risk of psychosis. Neuropsychobiology 58: 19–28. [DOI] [PubMed] [Google Scholar]
- 75. Krabbendam L, van Os J (2005) Schizophrenia and urbanicity: a major environmental influence–conditional on genetic risk. Schizophr Bull 31: 795–799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relationship of birth date and age of schizophrenia onset. Polar plot of birth distribution in relation to age at schizophrenia onset (circular-linear correlation, r = 0.42, p = 0.049). In particular, patients born in January and February showed a later age of schizophrenia onset compared to patients born in the remainder of the year.
(DOCX)
Location of significant TBSS results of pair-wise differences between groups and between subgroups uncorrected for multiple comparisons. The locations of significant FA value reductions are shown for patients relative to controls (red), for summer-born controls relative to winter-born controls (green), summer-born patients relative to summer-born controls (cyan), summer-born patients relative to winter-born controls (blue), winter-born patients relative to winter-born controls (yellow).
(DOCX)
Significant TBSS results of pair-wise differences between groups and between subgroups uncorrected for multiple comparisons and corrected for multiple comparisons.
(DOCX)