Abstract
Fibrocalculous pancreatic diabetes (FCPD) is a secondary cause of diabetes due to chronic pancreatitis. Since the N34S variant of the SPINK1 trypsin inhibitor gene has been found to partially account for genetic susceptibility to chronic pancreatitis, we used a family-based and case-control approach in two separate ethnic groups from the Indian subcontinent, to determine whether N34S was associated with susceptibility to FCPD. Clear excess transmission of SPINK1 N34S to the probands with FCPD in 69 Bangladeshi families was observed (P<.0001; 20 transmissions and 2 nontransmissions). In the total study group (Bangladeshi and southern Indian) the N34S variant was present in 33% of 180 subjects with FCPD, 4.4% of 861 nondiabetic subjects (odds ratio 10.8; P<.0001 compared with FCPD), 3.7% of 219 subjects with type 2 diabetes, and 10.6% of 354 subjects with early-onset diabetes (aged <30 years) (P=.02 compared with the ethnically matched control group). These results suggest that the N34S variant of SPINK1 is a susceptibility gene for FCPD in the Indian subcontinent, although, by itself, it is not sufficient to cause disease.
Fibrocalculous pancreatic diabetes (FCPD) has been classified by the World Health Organization as a secondary cause of diabetes due to disease of the exocrine pancreas (Alberti and Zimmet 1998). It is a condition in which, in addition to diabetes being present, there is also evidence of chronic pancreatitis of unknown origin with large intraductal pancreatic stones (Mohan et al. 1998). Patients frequently have a low body mass and a history of chronic abdominal pain and require insulin treatment, although, unlike in type 1 diabetes (T1D), they are not prone to ketosis. We have hypothesized that FCPD is likely to be a multifactorial disease, with genetic and environmental components to both the diabetes and chronic pancreatitis. In support of a genetic background to FCPD, we have demonstrated familial clustering of the disease (Mohan et al. 1989). More specifically, we have demonstrated associations between FCPD and human leukocyte antigen (HLA) genotype in subjects from southern India and Bangladesh, which revealed both similarities to and differences from T1D (Kambo et al. 1989; Chowdhury et al. 2002). Other groups have also demonstrated HLA associations with FCPD, although the disease-associated alleles are not always consistent between studies (Sanjeevi et al. 1999). We have also found an association between the insulin gene hypervariable region and FCPD in southern Indian but not in Bangladeshi subjects (Kambo et al. 1989; Chowdhury et al. 2002).
Recently, there has been rapid progress in understanding the genetic basis of hereditary and idiopathic pancreatitis (Whitcomb 1999, 2000, 2002; Truninger et al. 2001) with the identification of mutations in the cationic trypsinogen gene (Whitcomb et al. 1996; Gorry et al. 1997; Teich et al. 2002), the cystic fibrosis transmembrane conductance regulator (CFTR) (Cohn et al. 1998; Sharer et al. 1998; Ockenga et al. 2000; Noone et al. 2001), and the serine protease inhibitor, Kazal type 1 (SPINK1 [MIM 167790]) genes (Chen et al. 2000; Pfützer et al. 2000, 2002; Witt et al. 2000; Threadgold et al. 2002), all associated with disease. We have already excluded common mutations of the cationic trypsinogen gene in FCPD (Rossi et al. 1998; Hassan et al. 2000). In contrast, recently published data have suggested that the SPINK1 N34S variant may be a cause of FCPD (Rossi et al. 2001; Chandak et al. 2002). The N34S variant was found in 7 of 24 patients with FCPD and in 16 of 44 patients with tropical pancreatitis without diabetes (TCP) living in India (Chandak et al. 2002). In a pilot study of Bangladeshi subjects, the N34S variant was present in five of eight patients with FCPD but was absent in four patients with TCP and in four control individuals (Rossi et al. 2001). More recent data analyzing a further 140 Bangladeshi subjects (14 patients with FCPD, 11 patients with TCP, 43 young patients with T2D, and 72 control individuals) support the pilot data for FCPD (Schneider et al., in press).
The purpose of the present study was multifold. First, we used a family-based study of subjects from Bangladesh to test for an association between the SPINK1 N34S variant and either FCPD or early-onset diabetes. Second, we tested for an interaction between SPINK1 and either HLA-DQB genotype or the insulin-gene hypervariable region, in the family resources. Since we found an association between FCPD susceptibility and the SPINK1 N34S variant, we then proceeded to determine the frequency of this variant in two further ethnic groups from the Indian subcontinent, in order to replicate the original study and to investigate a possible association with type 2 diabetes (T2D). No patients are duplicated between this study and the other pilot data (Rossi et al. 1998; Schneider et al., in press).
We used a PCR-RFLP assay (endonuclease TaaI) to identify SPINK1 N34S (Plendl et al. 2001) in 69 families from Bangladesh, which were included in the study if they met the following two criteria: (1) an index case individual with FCPD was present and (2) both parents of the index case individual were available for study. Twenty-six percent of the probands with FCPD (mean ± SD age at onset 18.8 ± 4.7 years; mean ± SD BMI 15.9 ± 2.83 kg/m2) possessed the variant. There was clear excess transmission of the variant from the parents to the index case individual in the trios (P<.0001); 20 transmissions and 2 nontransmissions. The multifactorial nature of FCPD is supported by the observation that, although parents carried the SPINK1 N34S variant, none had FCPD on the basis of clinical criteria.
In our previous study of the same families (Chowdhury et al. 2002), there was a significantly decreased transmission of HLA-DQB1*0202 from the parents to the index case individual. It could therefore be postulated that HLA-DQB1*0202 might protect a subject from FCPD in the presence of the disease-associated N34S variant. To address this question, we compared phenotype frequencies in the parents according to the presence or absence of N34S and DQB1*0202; no difference in distribution was found (P=.86). We have also observed increased transmission of the HLA marker TNFc and HLA-DQB1*0302 (Chowdhury et al. 2002) to the probands with FCPD. No gene-to-gene interaction in the index case individuals with FCPD was found between SPINK1 and either of two major histocompatibility complex (MHC) markers on chromosome 6 (TNFc, P=.70; and HLA-DQB1*0302, P=1.0). Furthermore, no interaction between SPINK1 and alleles defined by HphI of the insulin gene (P=.68) was found. This would suggest that, in an individual with FCPD, the known genetic factors predisposing to either chronic pancreatitis or diabetes are acting independently of each other, rather than in a synergistic manner.
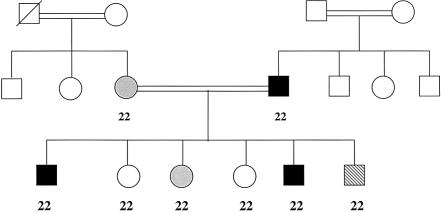
The frequencies of the N34S variant in the other study groups are presented in table 1. This variant was present in 41.9% of the additional unrelated Bangladeshi subjects with FCPD (mean age at onset of diabetes 20.4±5.7 years; mean BMI 17.0±3.33 kg/m2), compared with 5.6% of control individuals (mean age 22.8±4.8 years; mean BMI 20.0±3.4 kg/m2) (P<.0001 for genotype differences). In southern Indian subjects with FCPD (mean age at onset of diabetes 28.0±7.9 years; mean BMI 19.4±3.7 kg/m2), the variant was present in 35.3% of patients, compared with 3.5% of control individuals (mean age 42.8±12.7 years; mean BMI 22.0±4.3 kg/m2) (P<.0001), suggesting the SPINK1 N34S predisposes to FCPD in subjects from both southern India and Bangladesh. Five southern Indian families were also screened (three families had more than one member with FCPD, and two had one member with FCPD and at least one other with T2D). The variant was present in only one family. In this consanguineous family (fig. 1) the father had FCPD and the mother had idiopathic TCP; both were homozygous for the N34S variant, as were all six children (two with FCPD, one with TCP, one with impaired glucose tolerance [IGT], and two without diabetes).
Table 1.
Frequency of the SPINK1 N34S Variant in the Various Study Groups
|
SPINK1 Genotype Frequency (n) |
||||
| Group (n) | HomozygoteWild-Type | HeterozygoteN34S | HomozygoteN34S | P Valuea |
| Bangladeshi: | ||||
| Probands with FCPD (69) | .739 (51) | .217 (15) | .043 (3) | <.0001 |
| Other FCPD (43) | .581 (25) | .279 (12) | .140 (6) | <.0001 |
| Nondiabeticb (393) | .944 (371) | .053 (21) | .003 (1) | |
| Under-30 diabetes (354) | .893 (316) | .099 (35) | .008 (3) | .02 |
| Sylheti Nondiabeticc (156) | .968 (151) | .032 (5) | 0 | |
| Sylheti T2D (142) | .965 (137) | .035 (5) | 0 | NS |
| South Indian: | ||||
| FCPD (68) | .647 (44) | .265 (18) | .088 (6) | <.0001 |
| Nondiabetic (312)b | .965 (301) | .032 (10) | .003 (1) | |
| Impaired fasting glucose/IGT (56) | .964 (54) | .036 (2) | 0 | NS |
| T2D (77) | .961 (74) | .039 (3) | 0 | NS |
For comparison with nondiabetic group. NS = not significant.
Defined by fasting blood glucose <6 mmol/liter.
Defined by either a random or fasting blood glucose <6 mmol/liter.
Figure 1.
Southern Indian family in which each individual genotyped is homozygous for the SPINK1 N34S variant. A symbol with a line through it denotes a deceased individual, an unshaded symbol denotes an unaffected individual, a black symbol denotes an individual with FCPD, a gray symbol denotes an individual with TCP, and a diagonally striped symbol denotes an individual with IGT. SPINK1 genotypes are given under the symbols; 2 = variant. The wild-type allele was not detected in genotyped individuals from this family.
To further investigate a possible association between N34S and diabetes, we investigated several additional resources (table 1). The first group (n=354) consisted of Bangladeshi subjects presenting with diabetes before the age of 30 years (subsequently referred to as “under-30 diabetes” [mean age at onset 18.7±6.2 years; mean BMI 18.3±5.1 kg/m2]) at the Bangladesh Institute of Research and Rehabilitation in Diabetes, Endocrine and Metabolic Disorders (BIRDEM) clinic, none of whom had either FCPD (normal abdominal x-ray and no history of severe abdominal pain) or T1D (defined by an acute onset of disease with ketosis or insulin dependency). The N34S variant was present in 10.9% of subjects in the under-30 diabetes group (P=.02 compared with the control group). The increased frequency of the variant in this group is likely to reflect the presence of subjects with diabetes and subclinical chronic pancreatitis. A different study design and further genetic and immunological investigations are required to investigate this further.
The second cohort we studied were Bangladeshi subjects from Sylhet, ascertained either from a diabetes clinic at the Royal London Hospital, London, or from a coronary heart disease study in East London. In subjects from Sylhet with T2D (mean age at onset of diabetes 44.7±10.0 years; mean BMI 26.5±3.4 kg/m2), the frequency of N34S (3.5%) was no different than in ethnically matched control individuals (mean age 41.5±10.4 years; mean BMI 26.9±9.7 kg/m2) (P=1). Similarly, in the third cohort of southern Indian subjects (Ramachandran et al. 1992), the frequencies of either IGT (mean age 47.9±12.6 years; mean BMI 23.4±4.2 kg/m2) (3.6%) or T2D (mean age 53.6±10.7 years; BMI 23.8±3.2 kg/m2) (3.9%) was no different than that in control individuals (mean age 42.8±12.7 years; mean BMI 23.4±4.1 kg/m2) (3.5%) (P=.94). It is, therefore, highly unlikely that an association exists between the SPINK1 N34S variant and the more common forms of T2D or IGT.
There is overwhelming evidence that the SPINK1 N34S variant predisposes to chronic pancreatitis, since it is present in 13%–40% of patients with idiopathic chronic pancreatitis (Truninger et al. 2001; Witt 2002). SPINK1 is a pancreatic secretory trypsin inhibitor, secreted from the pancreatic acinar cells into the pancreatic juice, that prevents premature activation of zymogens within the pancreas and pancreatic duct. Functional studies on the N34S variant have not yet been published, but it is likely to be of functional significance because of its location near the reactive lysine-isoleucine site of SPINK1 (Threadgold et al. 2002); furthermore, structural modeling has revealed several possible pathological mechanisms for the N34S mutation (Pfützer et al. 2000). In one of the earliest publications to indicate the importance of SPINK1 in chronic pancreatitis, a clear excess transmission of SPINK1 variants to affected subjects (P<.0001 in 29 informative transmissions), similar to what we have observed for FCPD (Witt et al. 2000), was found.
In many studies of non-Asian subjects, the frequency of N34S has been ⩽1% in control subjects (Chen et al. 2000; Pfützer et al. 2000; Witt et al. 2000; Threadgold et al. 2002). In people without FCPD from the Indian subcontinent, we have found the variant in 38 of 861 (4.4%; range 3.2%–5.6%) nondiabetic subjects, with no heterogeneity between ethnic groups (P=.54). Furthermore, a prevalence of 3% has been reported in a control group from Hyderabad, India (Chandak et al. 2002). Although the frequency appears higher than in non-Asian subjects, in a recently reported study in subjects from Leeds, United Kingdom, the frequency was 4% (4/100) (Threadgold et al. 2002). However, these control subjects were ascertained as healthy blood donors and the ethnic group was not reported, and it is known that Leeds has a large South Asian community. It therefore remains to be determined whether the frequency of N34S is higher in South Asians and whether this may, in part, account for the comparatively higher frequency of FCPD in this ethnic group.
In conclusion, we found a striking association, using family-based and case-control methods, between N34S and FCPD, with the variant being present in 33% of 180 subjects with FCPD, compared with 4.4% of 861 subjects without diabetes (odds ratio 10.8; 95% CI 6.9–17.0). These studies demonstrate that SPINK1 is an important gene for FCPD susceptibility, although, by itself, it is not sufficient for full expression of the disease. This finding is very similar to that observed in idiopathic pancreatitis. Our data also emphasize the importance of other environmental and genetic factors that are required for the full expression of FCPD.
Acknowledgments
We gratefully acknowledge the subjects and members of the families, for their participation in the study; and Diabetes U.K., Juvenile Diabetes Research Foundation International, and The International Program in the Chemical Sciences (Uppsala, Sweden), for grant support involved in this work. Dr. A. Timmis (Barts and the Royal London National Health Service Trust, London, United Kingdom), as well as Dr. A. Ramachandran and C. Snehalatha (M.V. Hospital for Diabetes, Chennai, India), contributed to subject collection and characterization.
Electronic-Database Information
The accession number and URL for data presented herein are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for SPINK1 [MIM 167790])
References
- Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15:539–553 [DOI] [PubMed] [Google Scholar]
- Chandak GR, Idris MM, Reddy DN, Bhaskar S, Sriram PV, Singh L (2002) Mutations in the pancreatic secretory trypsin inhibitor gene (PSTI/SPINK1) rather than the cationic trypsinogen gene (PRSS1) are significantly associated with tropical calcific pancreatitis. J Med Genet 39:347–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JM, Mercier B, Audrezet MP, Ferec C (2000) Mutational analysis of the human pancreatic secretory trypsin inhibitor (PSTI) gene in hereditary and sporadic chronic pancreatitis. J Med Genet 37:67–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury ZM, McDermott MF, Davey S, Hassan Z, Sinnott PJ, Hemmatpour SK, Sherwin S, Ali L, Aganna E, Allotey RA, North BV, Cassell PG, Azad Khan AK, Hitman GA (2002) Genetic susceptibility to fibrocalculous pancreatic diabetes in Bangladeshi subjects: a family study. Genes Immun 3:5–8 [DOI] [PubMed] [Google Scholar]
- Cohn JA, Friedman KJ, Noone PG, Knowles MR, Silverman LM, Jowell PS (1998) Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med 339:653–658 [DOI] [PubMed] [Google Scholar]
- Gorry MC, Gabbaizedeh D, Furey W, Gates LK Jr, Preston RA, Aston CE, Zhang Y, Ulrich C, Ehrlich GD, Whitcomb DC (1997) Mutations in the cationic trypsinogen gene are associated with recurrent acute and chronic pancreatitis. Gastroenterology 113:1063–1068 [DOI] [PubMed] [Google Scholar]
- Hassan Z, Mohan V, McDermott MF, Ali L, Ogunkolade WB, Aganna E, Cassell PG, Deepa R, Khan AK, Hitman GA (2000) Pancreatitis in fibrocalculous pancreatic diabetes mellitus is not associated with common mutations in the trypsinogen gene. Diabetes Metab Res Rev 16:454–457 [DOI] [PubMed] [Google Scholar]
- Kambo PK, Hitman GA, Mohan V, Ramachandran A, Snehalatha C, Suresh S, Metcalfe K, Ryait BK, Viswanathan M (1989) The genetic predisposition to fibrocalculous pancreatic diabetes. Diabetologia 32:45–51 [DOI] [PubMed] [Google Scholar]
- Mohan V, Chari ST, Hitman GA, Suresh S, Madanagopalan N, Ramachandran A, Viswanathan M (1989) Familial aggregation in tropical fibrocalculous pancreatic diabetes. Pancreas 4:690–693 [DOI] [PubMed] [Google Scholar]
- Mohan V, Nagalotimath SJ, Yajnik CS, Tripathy BB (1998) Fibrocalculous pancreatic diabetes. Diabetes Metab Rev 14:153–170 [DOI] [PubMed] [Google Scholar]
- Noone PG, Zhou Z, Silverman LM, Jowell PS, Knowles MR, Cohn JA (2001) Cystic fibrosis gene mutations and pancreatitis risk: relation to epithelial ion transport and trypsin inhibitor gene mutations. Gastroenterology 121:1310–1319 [DOI] [PubMed] [Google Scholar]
- Ockenga J, Stuhrmann M, Ballmann M, Teich N, Keim V, Dork T, Manns MP (2000) Mutations of the cystic fibrosis gene, but not cationic trypsinogen gene, are associated with recurrent or chronic idiopathic pancreatitis. Am J Gastroenterol 95:2061–2067 [DOI] [PubMed] [Google Scholar]
- Pfützer RH, Barmada MM, Brunskill AP, Finch R, Hart PS, Neoptolemos J, Furey WF, Whitcomb DC (2000) SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterology 119:615–623 [DOI] [PubMed] [Google Scholar]
- Pfützer R, Myers E, Applebaum-Shapiro S, Finch R, Ellis I, Neoptolemos J, Kant JA, Whitcomb DC (2002) Novel cationic trypsinogen (PRSS1) N29T and R122C mutations cause autosomal dominant hereditary pancreatitis. Gut 50:271–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plendl H, Siebert R, Steinemann D, Grote W (2001) High frequency of the N34S mutation in the SPINK1 gene in chronic pancreatitis detected by a new PCR-RFLP assay. Am J Med Genet 100:252–253 [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Snehalatha C, Dharmaraj D, Viswanathan M (1992) Prevalence of glucose intolerance in Asian Indians. Urban-rural difference and significance of upper body adiposity. Diabetes Care 15:1348–1355 [DOI] [PubMed] [Google Scholar]
- Rossi L, Pfützer RH, Parvin S, Ali, L, Sattar S, Azad Khan AK, Whitcomb DC (2001) SPINK1/PSTI mutations are associated with tropical pancreatitis in Bangladesh. Pancreatology 1:242–245 [DOI] [PubMed] [Google Scholar]
- Rossi L, Whitcomb DC, Ehrlich GD, Gorry MC, Parvin S, Sattar S, Ali L, Azad Khan AK, Gyr N (1998) Lack of R117H mutation in the cationic trypsinogen gene in patients with tropical pancreatitis from Bangladesh. Pancreas 17:278–280 [DOI] [PubMed] [Google Scholar]
- Sanjeevi CB, Kanungo A, Shtauvere A, Samal KC, Tripathi BB (1999) Association of HLA class II alleles with different subgroups of diabetes mellitus in Eastern India identify different associations with IDDM and malnutrition-related diabetes. Tissue Antigens 54:83–87 [DOI] [PubMed] [Google Scholar]
- Schneider A, Suman A, Rossi L, Barmada MM, Beglinger C, Parvin S, Sattar S, Ali L, Azad Khan AK, Gyr N, Whitcomb DC. SPINK1/PST1 mutations are associated with tropical pancreatitis and type II diabetes in Bangladesh. Gastroenterology (in press) [DOI] [PubMed] [Google Scholar]
- Sharer N, Schwarz M, Malone G, Howarth A, Painter J, Super M, Braganza J (1998) Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N Engl J Med 339:645–652 [DOI] [PubMed] [Google Scholar]
- Teich N, Monssner J, Keim V (2002) Systemic overview of genetic variants of cationic trypsinogen and SPINK1 in pancreatitis patients. In: Durie P, Lerch MM, Lowenfles AB, Masisonneuve P, Ulrich CD, Whitcomb DC (eds) Genetic disorders of exocrine pancreas: an overview and update. Karger, Basel, pp 20–22 [Google Scholar]
- Threadgold J, Greenhalf W, Ellis I, Howes N, Lerch MM, Simon P, Jansen J, Charnley R, Laugier R, Frulloni L, Olah A, Delhaye M, Ihse I, Schaffalitzky de Muckadell OB, Andren-Sandberg A, Imrie CW, Martinek J, Gress TM, Mountford R, Whitcomb D, Neoptolemos JP (2002) The N34S mutation of SPINK1 (PSTI) is associated with a familial pattern of idiopathic chronic pancreatitis but does not cause the disease. Gut 50:675–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truninger K, Ammann RW, Blum HE, Witt H (2001) Genetic aspects of chronic pancreatitis: insights into aetiopathogenesis and clinical implications. Swiss Med Wkly 131:565–574 [PubMed] [Google Scholar]
- Whitcomb DC (1999) Hereditary pancreatitis: new insights into acute and chronic pancreatitis. Gut 45:317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ——— (2000) Genetic predispositions to acute and chronic pancreatitis. Med Clin North Am 84:531–547 [DOI] [PubMed] [Google Scholar]
- ——— (2002) How to think about SPINK and pancreatitis. Am J Gastroenterol 97:1085–1088 [DOI] [PubMed] [Google Scholar]
- Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK Jr, Amann ST, Toskes PP, Liddle R, McGrath K, Uomo G, Post JC, Ehrlich GD (1996) Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet 14:141–145 [DOI] [PubMed] [Google Scholar]
- Witt H (2002) The SPINK in chronic pancreatitis: similar finds, different minds. Gut 50:590–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt H, Luck W, Hennies HC, Classen M, Kage A, Lass U, Landt O, Becker M (2000) Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet 25:213–216 [DOI] [PubMed] [Google Scholar]