Abstract
Here, we introduce a solution to low stability of a two-phase slug flow with a chemical reaction occurring at the phase interface in a microfluidic reactor where substantial merging of individual reacting slugs results in the loss of uniformity of the flow. We create a three-phase slug flow by introducing a third fluid phase into the originally two-phase liquid-liquid slug flow, which generates small two-phase liquid slugs separated by gas phase. Introduction of the third phase into our system efficiently prevents merging of slugs and provides beneficial reaction conditions, such as uniform flow pattern along the whole reaction capillary, interfacial area with good reproducibility, and intensive water-oil interface renewal. We tested the three-phase flow on an enzyme hydrolysis of soybean oil and compared the reaction conversion with those from unstable two-phase slug flows. We experimentally confirmed that the three-phase slug flow arrangement provides conversions and pressure drops comparable or even better with two-phase liquid-liquid arrangements.
INTRODUCTION
The use of microfluidic systems in biochemical or chemical synthesis provides unique reaction conditions that can lead to the production of well-defined products with reproducible composition or to a significant reduction of the reaction time in heterogeneous systems.1 Small characteristic dimensions of microfluidic chips result in the reduction of transport resistances, creation of predictable internal conditions (the Stokes or laminar flow, absence of dead volumes, isothermal conditions), increase of work safety because of small processed volumes, etc.2 One class of microfluidic chips employs slug flow of two immiscible fluids (liquid-liquid, gas-liquid) to carry out various operations such as extractions,3, 4 chemical reactions,5, 6 biologically oriented applications,7, 8 controlled polymer stretching,9 passive particle concentration,10 or controlled crystal formation.11
The slug flow systems usually consist of two liquids. One of them, the continuous phase, wets the microchannel and the other one, the dispersed phase, forms uniform droplets. The main advantages of the slug flow systems are intensive mass transfer inside and between the phases, uniform residence time of all droplets, and possible compartmentalization of chemical processes.12 A crucial step that is necessary for the successful realization of slug flow operations is the formation of regular slugs in slug flow generators. Various T, Y, cross and more complex generators have been reported. Particular choices of generator design and fluid arrangements are dictated by many parameters: physical and chemical properties of liquids (density, viscosity, chemical reactivity), surface tensions among phases, flow rates, slug dimensions, etc.13, 14
Robustness of slug flow can be enhanced by the use of a third phase (liquid or gas) that serves as a spacer between discrete volumes of a liquid-liquid dispersion15, 16 or a reservoir containing a reaction component.17 The third phase can prevent spontaneous coalescence of adjacent slugs in long processing microchannels.18 The three-phase flow arrangements have successfully been used for carbonylation reactions17 or in nanoparticle synthesis.19
In this study, we report on the development of a three-phase slug flow system with nitrogen slugs separating discrete volumes of a water-oil dispersion. The system employs a double T-generator or a multi-splitter to form fine water in oil droplets. The regular three-phase flow is then created in a simple T-microfluidic structure. The fabrication procedures and microchip design are described in detail. The developed systems are then tested for an enzyme hydrolysis of soybean oil with Thermomyces lanuginosus lipase.20 This enzyme reaction occurs at the water-oil interface and, as we have shown recently,21 the interface becomes unstable as the chemical compositions of both immiscible phases change during the reaction. It will be demonstrated that the three-phase (3P) slug flow arrangement provides regular separation of water-oil segments and reduces the problems coming from the interface destabilization. Finally, we compare the performance of the developed system with two-phase water-oil slug flow systems either with hydrophobic (2PO) or hydrophilic (2PI) reaction zones.
MATERIALS AND METHODS
Materials, chemicals, equipment
Lipolase 100L stock solution containing T. lanuginosus lipase (L0777, Sigma-Aldrich), phosphate buffer saline—PBS (P5368, Sigma-Aldrich), and crude soybean oil (Sympo Pardubice, Czech Rep.) were used for the hydrolysis experiments.
We employ the following material in our microfluidic systems: Teflon capillaries of i.d. 0.5 and 0.75 mm (Cole—Parmer), Acrifix 192 (Degussa), flexible fused silica capillary of i.d. 0.45 mm (Polymicro technologies, USA), Plexiglass GS (M.K. Plexi, Czech Rep.), PEEK T-connectors of i.d. 0.5 mm (Upchurch Scientific, USA), and isopropylalcohol (Penta, Czech Rep.).
Analysis of the reaction products was carried out in a HPLC system (Waters). Precise liquid and gas dosing was provided by a syringe pump system Nemesys (Cetoni), borosilicat glass syringes (ILSs), and gas flow controller F-200CV (Bronkhorst). The pressure drop in reaction systems was sensed by a digital manometer LEO (Keller). Video sequences were captured by a camera ProgRes CT1 (Jena Optik) and analyzed by NIS-Elements software (Laboratory Imaging).
Microfluidic systems
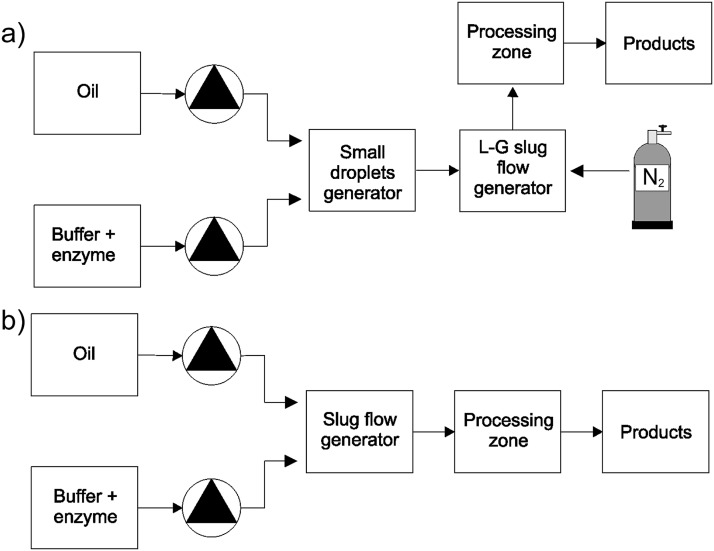
The main reason for generation of a 3P flow system (Figure 1a) is to avoid problems with flow instabilities or irregularities occurring in classical two-phase arrangements (Figure 1b). If there is a low surface tension between two liquid phases, it is difficult to provide regular and stable slug flow in longer capillaries or microchannels. It recently happened in our microreactor with a hydrophobic microcapillary because the surface tension significantly decreased in the course of an enzyme reaction.21 Slug instabilities can lead to an insufficient reproducibility in the composition of reaction products due to irreproducible mass transfer conditions in particular slugs. The main idea of the suggested 3P system is to separate a fine liquid-liquid dispersion into small discrete volumes by means of nitrogen slugs. Each discrete compartment represents a small chemical reactor. Possible liquid-liquid slug flow instabilities are then limited only to the discrete volume between two adjacent gas slugs. Moreover, the presence of the nitrogen slugs prevents formation of large aggregates of one liquid phase in downstream processes.
Figure 1.
Microfluidic systems for the generation of slug flows coupled with the enzyme hydrolysis of soybean oil. (a) Three-phase arrangement and (b) two-phase arrangement.
We suggested and implemented the following design of the 3P flow generator. In the first step, a fine water-oil dispersion is generated in plexiglass microfluidic chips, particularly, either in a double T-generator or in a multi-splitter, see Figure 2. One pair of syringe pumps is used for liquid dosing. The fine dispersion is then led to either a PEEK T-connector or another plexiglass T-generator where it is regularly separated into discrete compartments by means of nitrogen. The formed three-phase dispersion is led to a processing zone represented by a long microcapillary, where the enzyme hydrolysis of soybean oil takes place. After the processing, the three fluid phases are separated by means of the gravity force.
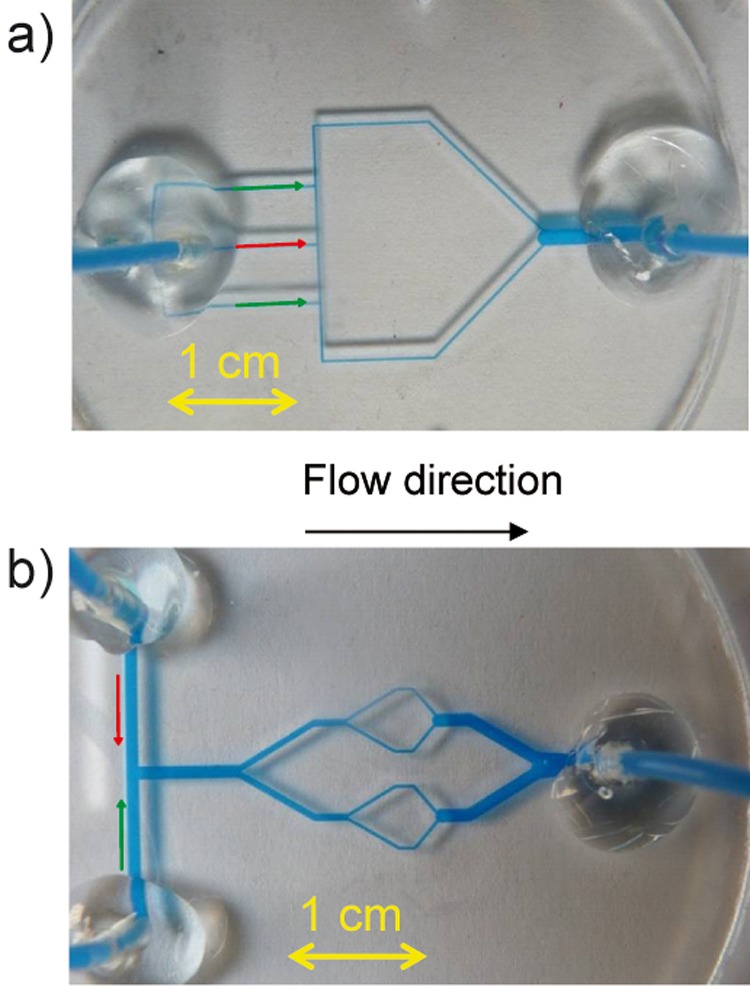
Figure 2.
Design of droplet generators. (a) Double T-generator and (b) multi-splitter. The red and green arrows represent the oil and water phase inlet channels, respectively.
It was important to include a gas flow controller in the front of the T-connector. Without precise flow control, it is difficult to form a stable three-phase flow in the processing capillary (Teflon, i.d. 0.75 mm) due to pressure fluctuations in the microfluidic system. When we used a reaction capillary with a smaller diameter (Teflon, i.d. 0.5 mm), the nitrogen flow rate was very low so that no available controller was able to provide a constant flow rate. In such a case, a peristaltic pump was used for the nitrogen dosing. We lost the precise information on the mass flow rate of nitrogen; however, the generated 3P flow was regular.
To compare performance of the 3P arrangement with conventional two-phase slug flow arrangements, another microfluidic system was used (Figure 1b), where regular oil in water or water in oil dispersions was generated in a plexiglass T-structure. Both liquids were dosed by means of a pair of syringe pumps. Teflon (i.d. 0.5 mm) and glass (i.d. 0.45 mm) processing capillaries were used in the hydrophobic and hydrophilic arrangements, respectively. After processing, the biphasic reaction product was separated using the gravity force.
Microchip fabrication
Plexiglass microfluidic chips were fabricated by means of a solvent assisted bonding method developed in our laboratory.22 Briefly, the microfluidic structures were milled into one plexiglass plate. The plate was provided with holes for tubing connections. The plate was then aligned with another blank plexiglass plate. This sandwich was treated with isopropylalcohol in a press (2500 N) under elevated temperature (70 °C for 15 min). UV curable adhesive Acrifix192 was used for the attachment of inlet and outlet tubing to the fabricated microchip.
The design of the double T-generator is shown in Figure 2a. Three microchannels with the cross-section of 300 μm × 300 μm at the left part of the chip serve as inlets. The oil phase is dosed into the central channel; the water phase is introduced into the two side channels. Water droplets in the continuous oil phase are generated in the top and bottom T-structures and then led to a collecting channel in the right part of the microchip. The collecting channel is 1.5 mm wide and 500 μm deep. The water droplet diameter is about 400 μm.
The multi-splitter chip is depicted in Figure 2b. The water and oil phases are introduced in simple T-junction with the channel width of 1.2 mm in the left part of the chip. The dispersed water slugs are split sequentially in two Y-splitters. The microchannel widths after the first and second splitting are 600 μm and 300 μm, respectively. The generated water droplets are collected in one collecting channel with the channel width of 1 mm. The depth of all channels is set to 500 μm.
Simple T-junction microfluidic chips were used as generators of two-phase flow in comparative two-phase experiments. Approximate dimensions of all fluidic channels were 400 μm × 500 μm.
Enzyme hydrolysis of soybean oil
Lipases are enzymes widely used in industry for the production of monoglycerides, free fatty acids, organic esters, production of enantiopure pharmaceuticals, and other products.20 The synthesis of biodiesel by means of lipase has also been tested.23 Because lipases usually catalyze biotransformations at the water-oil interface, the use of well-defined slug flow systems brings a promising alternative to classical batch or packed-bed systems. Especially for small-volume production of fine and rare chemicals and pharmaceuticals, microfluidic platforms seem to be a good solution. The lipase catalyzed hydrolysis of soybean oil is selected to evaluate the performance (triglyceride conversion and pressure drop) of the developed three-phase slug flow microfluidic system. In this reaction, the enzyme catalyzes the hydrolysis of various triglycerides contained in soybean oil to di- and mono-glycerides and free fatty acids.
Crude (without any additives) soybean oil is used as the oil phase. The water phase contains a solution of the enzyme in PBS buffer. The buffer to enzyme volume ratio is set to 9/1. The oil to water phase volume ratio is set to 2/1 in all experiments. Particular flow rates and characteristics of the used capillaries are summarized in Table TABLE I.. In 3P flow experiments, the nitrogen flow rates are only approximate due to a gradual gas expansion in the reaction capillary. The flow rates and capillary lengths provide 20 min residence time of the reaction dispersion in the reaction zone. In the 3P arrangement, the residence time was checked by direct observation of liquid slugs. The enzyme hydrolysis is carried out in a tempered box at 35 °C.
TABLE I.
Reaction capillary characteristics in different slug flow arrangements. (Vw, Vo, and VN are the water, oil, and nitrogen flow rates, respectively.)
| Arrangement | Capillary | i.d. [mm] | Length [m] | Vw [nl s−1] | Vo [nl s−1] | VN [nl s−1] |
|---|---|---|---|---|---|---|
| 3P | Teflon | 0.75 | 3 | 150 | 300 | ∼654 |
| 3P | Teflon | 0.5 | 4.8 | 70 | 140 | ∼575 |
| 2PO | Teflon | 0.5 | 6 | 327 | 654 | … |
| 2PI | Glass | 0.45 | 6 | 265 | 530 | … |
Under a constant residence time, the flow rate of the reaction mixture (Vw + Vo) is always smaller in the three phase arrangement because the gas phase occupies a part of the reaction capillary. If we compare our 3P and 2PO experiments with 0.5 mm hydrophobic capillaries, the flow rate of the reaction mixture is by 78.5% lower in the 3P system. To attain the same flow rate in the 3P arrangement, either a longer capillary or capillary parallelization has to be used.
After leaving the processing capillaries, the reaction products are immediately collected and cooled to 0 °C in a vial that is placed in an ice bath to suppress the enzyme reaction. Such small droplets are cooled within a few seconds. After cooling, the reaction mixture is centrifuged and finally analyzed. HPLC analysis of the oil phase is carried out in order to evaluate the performance of the microfluidic system. Details on the HPLC analysis are specified in Ref. 21. Here, we define the soybean oil conversion as the ratio of the triglyceride concentration decrease in the reaction capillary to the inlet triglyceride concentration. We did not monitor the overall content of free fatty acids, mono- and di-glycerides in the products.
Pressure drop
Pressure drop in the reaction capillaries was monitored as this parameter can strongly affect the economy of the entire process. In the two-phase arrangements (2PO and 2PI), the pressure drop was evaluated directly as a pressure difference between the capillary inlet and outlet. For that reason, the manometer was connected to T-microfluidic generators of the liquid-liquid slug flow. In the three-phase arrangement, the pressure drop was determined indirectly from lengths of the nitrogen slugs at the capillary inlet and outlet. Considering an ideal behavior of nitrogen and the atmospheric pressure at the capillary outlet, the pressure drop can simply be estimated with a precision that corresponds to the optical method used for the slug size determination (digital image analysis with NIS-Elements software).
Interfacial area
We define the liquid-liquid interfacial area density a as the ratio of the water-oil interfacial area to the volume of a representative part of the slug flow. In the case of two-phase flow, the representative volume is the sum of volumes of two adjacent water and oil droplets. The volume delimited by two adjacent nitrogen slugs is the representative volume in three-phase flow systems. The size of interfacial area is given by the surface of the dispersed liquid phase (water phase in Teflon capillaries, oil phase in glass capillaries). All droplets were considered to be axially symmetric spheres or ellipsoids or more complex objects composed from (i) two spherical cups or (ii) a cylinder and two spherical cups added or subtracted. Dimensions of particular droplets were determined by means of NIS Elements software. Due to irregularity of two-phase flow in hydrophobic capillaries, the corresponding interfacial areas are only approximate.
RESULTS AND DISCUSSION
Three-phase slug flow generation
The formation of regular 3P slug flow consists of two steps. First, we need to create water-oil dispersion with small water droplets. The double T-generator provides the formation of the water in oil slug flow in two parallel T-junctions, see Figure 3a. The water droplets are then collected in a central channel in two rows. The droplets are spherical and do not exhibit any merging tendency on short distances. The multi-splitter microchip guarantees the formation of small water droplets by a different mechanism (Figure 3b). Large water droplets previously formed in simple T-generator are mechanically split in two Y-junctions. The splitting process is not fully symmetric and the daughter slugs differ a bit in size. The symmetry is broken due to the limited precision of the fabrication process, i.e., limited precision of the milling process, possible channel deformation during the bonding process, etc. However, the symmetry breaking is beneficial for the droplet formation because the droplets remain separated even if they are finally collected in a central channel. It can be summarized that the developed microchips are appropriate for the formation of small water droplets dispersed in oil. We note that the large channel-like structures in Fig. 3 are not parts of the microchips. They are milled in a chip holder and are visible due to microchip transparency.
Figure 3.
Droplet generator operation. (a) Double T-generator and (b) multi-splitter (enhanced online).
Operation of the plexiglass microchip for the generation of 3P slug flow generator is shown in Figure 4. Nitrogen is introduced through a T-junction into the previously formed water in oil dispersion. It can be seen that the nitrogen slugs uniformly separate the water-oil dispersion. Adjacent nitrogen slugs trap both liquid phases whereby allow for the enzyme reaction and intensive mass transfer. Even if the water-oil interface is unstable, all discrete volumes of the water-oil dispersion have the same residence time in the downstream part of the reaction capillary.
Figure 4.
Three phase flow generator (enhanced online).
Three-phase system coupled with enzyme hydrolysis of soybean oil
The character of three-phase flow at the inlet and outlet of the reaction capillary is shown in Figure 5. Regular segments of nitrogen separating the oil-water reaction mixture are observed at both ends of the capillary. At the inlet, relatively large amount of water droplets is dispersed in the continuous oil phase, which guarantees large interfacial/reaction area. The water droplets undergo partial coalescence as flowing through the capillary where the enzyme hydrolysis takes place. We observe larger water droplets at the capillary outlet, however, the interfacial area is still quite large.
Figure 5.
Character of the 3P slug flow coupled with enzyme reaction in a Teflon capillary with i.d. of 0.75 mm (enhanced online).
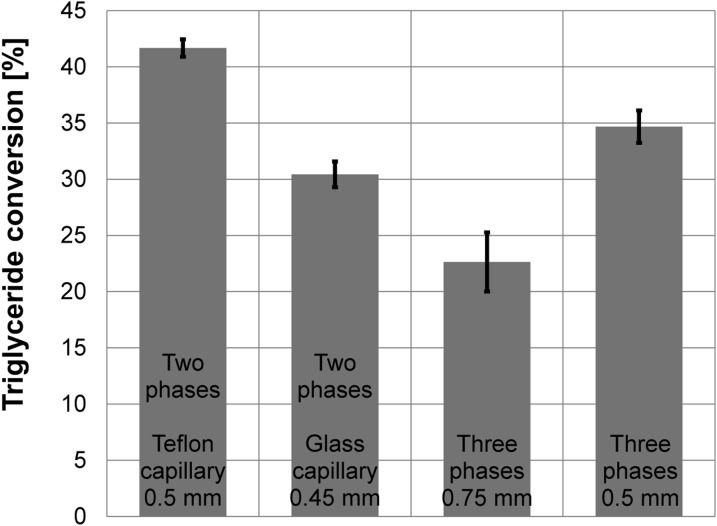
The reaction conversion of soybean oil hydrolysis is limited by the thermodynamic equilibrium.24 However, the conversion values about 20% obtained for the capillary with i.d. of 0.75 mm are quite low, see Figure 6. As we have found in our previous work,21 an important factor that affects the reaction rate is the surface density a of the water-oil interface. The surface density is often called the surface-to-volume ratio. The surface density typically grows with a decreasing characteristic dimension of particles or droplets. If an ideal sphere with the diameter d fills a cylindrical capillary of the same diameter, the surface density is inversely proportional to the capillary diameter in geometrically similar systems. We can hypothesize that the surface density of the water-oil interface will be higher in the capillary with i.d. of 0.5 mm. However, Figure 5 shows that the water phase forms a couple of small non-spherical droplets in the water phase. These droplets undergo a partial coalescence along the capillary. Then, the dependence of the surface density on the capillary diameter is not fully recognizable. When i.d. decreases from 0.75 mm to 0.5 mm in our experiments, the conversion increases from 22.6% to 34.6%, which corresponds to the relative increase about 50%. Hypothetically in geometrically similar systems, the surface density increase is equal to the same value. This result indicates that the surface density can be an important parameter that strongly affects the conversion values. Nevertheless, phenomena such as the internal circulation or reduction of transport distances can also contribute to the conversion increase in the thinner capillary. By analyzing our experimental data, we will show in Sec. 3D that (i) the surface density increase is slightly more than 50% and (ii) not only the surface density contributes to the conversion results.
Figure 6.
The arithmetic means and 90% confidence intervals for the soybean oil conversion data. Ten independent experiments were carried out with the 2PI, 2PO, and 3P (i.d. 0.5 mm) arrangements; five experiments with the 3P arrangement (0.75 mm).
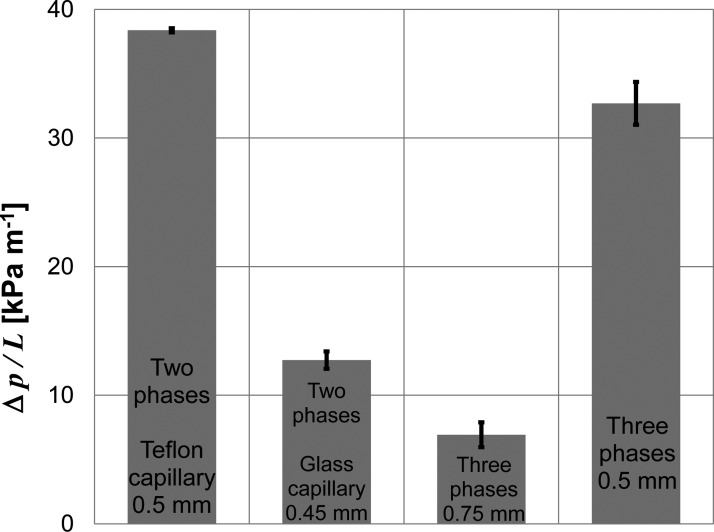
Smaller i.d. of the reaction capillary certainly results in higher pressure drop, see Figure 7. In the 3P experiments, we observed that the pressure drops per unit length of the reaction capillary () were 6.9 kPa m−1 and 32.7 kPa m−1 for capillaries with i.d. = 0.75 mm, L = 3 m and i.d. = 0.5 mm, L = 4.8 m, respectively. The Hagen–Poiseuille equation25 derived for laminar one-phase flow predicts . We note that the Hagen–Poiseuille equation gives us only a very rough estimate of the pressure drop in multi-phase systems. For better estimates, the surface tension among phases, effects of a thin film of the continuous phase, and physical properties of all phases have to be taken into account.26 However, these more precise relations also contain terms with the confirming the strong dependence of the pressure drop on the diameter. Because the residence time is constant in our experiments, the flow velocity is directly proportional to the capillary length. Thus, we can write a simple proportionality relation . This relation underestimates the experimentally observed pressures drop. For example, considering the experimental pressure drop 6.9 kPa m−1 for the capillary with i.d. of 0.75 mm, the relation predicts only 25 kPa m−1 drop for the capillary with i.d. of 0.5 mm. In summary, our results show that the soybean conversion significantly increases with decreasing capillary diameter; however, the use of capillaries with small diameters is accompanied by undesirable pressure increase.
Figure 7.
The arithmetic means and 90% confidence intervals for the pressure drop. Ten independent experiments were carried out with the 2PI and 2PO arrangements; four experiments with the 3P arrangements.
Two-phase systems coupled with enzyme hydrolysis of soybean oil
To discuss possible benefits or disadvantages of the 3P slug flow, we studied the enzyme hydrolysis of soybean oil in two phase arrangements either with a Teflon hydrophobic capillary (2PO) or with a hydrophilic glass capillary (2PI). In agreement with the results of our previous study, the enzyme reaction destabilizes slug flow in the 2PO arrangement (Figure 8) due to the variations of surface tension.21 We observe large slugs of different sizes of one or the other phase at the capillary outlet as the slugs tend to merge by irregular manner. These irregularities work against the purpose of the microchip use, e.g., mass transfer intensity is no longer uniform or the Taylor dispersion is not efficiently suppressed.
Figure 8.
Character of the two-phase slug flow in a hydrophobic capillary (enhanced online).
In the 2PI arrangement (Figure 9), surface properties of the glass hydrophilic capillary result in the formation of regular slug flow with continuous water phase and dispersed oil phase. Slug flow remains stable along the entire reaction capillary. It is a bit surprising as the surface tension varies similarly in the both two-phase arrangements due to the same enzyme reaction. The different flow behavior can be explained as follows. The viscous stress τ in a Newtonian fluid is directly proportional to the velocity gradient with the viscosity η as a proportionality constant25
| (1) |
where the upper index t represents the velocity gradient transposition. The largest velocity gradients are localized at walls in the continuous phase.27 In both the two-phase arrangements, the velocity gradients are similar because of the same mean velocity (the residence times and capillary lengths are the same). However, the oil phase viscosity is much higher than that of the water phase (the mean water and oil phase viscosities were about 0.94 mPa s and 33.2 mPa s, respectively21). It results in much higher viscous stress in the 2PO system with the continuous oil phase. Higher viscous stress then destabilizes the oil-water interface, which leads to droplet merging, splitting, and other instabilities.
Figure 9.
Character of the two-phase slug flow in a hydrophilic capillary (enhanced online).
The triglyceride conversion attained in the 2PO and 2PI arrangements are depicted in Figure 6. Despite of the flow irregularities, the 2PO system gives the highest conversion (41.7%) in our study. Conversion in the 2PI system with regular slug flow slightly exceeds 30%. We suggest the following reason for such behavior. Jurado et al.28 showed that the rate of the enzyme hydrolysis of triglycerides is limited by the total interfacial area and by the transport of diglycerides and triglycerides at the water-oil interface. By other words, to attain high conversion, a large amount of the enzyme binding sites has to be available at the interface for the substrate binding and the reaction products have to be intensively released from the interface to a bulk. Irregularities of the two-phase flow (slug splitting and merging) occurring along the hydrophobic capillary favor the renewal of the water-oil interface, which helps to an intensification of the substrate transport to the interfacially localized catalyst as well as to the product stripping from the interface. The interface irregularities are supported by high shear stress. According to Eq. 1, the shear stress is directly proportional to the viscosity. Hence, the shear stress destabilizing the interface is much higher in the hydrophobic capillary with the continuous oil phase. Further, the internal circulation in the dispersed phase29, 30 should be more intensive in the system with the continuous oil phase due to the tangential stress balance at the interface.25
The pressure drops per unit length are 38.4 kPa m−1 and 12.7 kPa m−1 in the 2PO and 2PI arrangements, respectively, see Figure 7. This finding confirms that the viscous dissipation of energy in the continuous phase is a critical factor affecting the pressure drop. While the observed pressure drop in the 2PO system is higher than in the 3P arrangement (i.d. = 0.5 mm), the 2PI system exhibits pressure drop significantly smaller.
In summary, two of the tested microfluidic arrangements are favorable to carry out the lipase catalyzed hydrolysis of soybean oil: (i) 3P system with i.d. = 0.5 mm and (ii) 2PI system. Both systems offer stable and regular slug flow, which is the most valuable feature of the suggested microfluidic platform. While the former one allows for a higher conversion, the other one for a smaller pressure drop.
Discussion on size and reproducibility of interfacial area
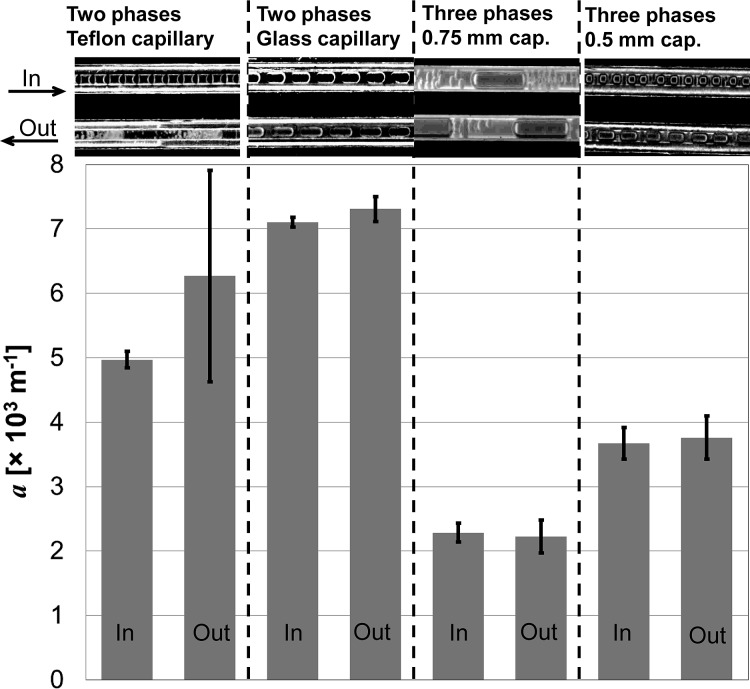
As we have discussed in Sec. 3B and in our previous work,21 large interfacial area favors high triglyceride conversion. Hence, we determined the surface density of the water-oil interface, see Fig. 10. The character of slug flow can differ at the capillary inlet and outlet due to the enzyme reaction that changes properties of the liquids. For that reason, we determined the interfacial areas at both sides of the reaction capillaries.
Figure 10.
Surface density of the water-oil interface a in different slug flow arrangements. The arithmetic means and 90% confidence intervals are plotted. The surface density was evaluated ten times for each arrangement at the capillary inlet and ten times at the capillary outlet. Snapshots at the top part represent typical flow pattern at the capillary inlets and outlets.
Except the 2PO arrangement with irregular slug flow, the surface density does not change remarkably along the capillary. Flow in the other three arrangements remains regular. One can clearly see the expansion of the nitrogen bubbles in the 3P system with 0.5 mm capillary due to the high pressure drop between the capillary ends (Fig. 7).
Important finding is that two-phase arrangements provide about two times larger interfacial area than those three-phases. However, the triglyceride conversion values are comparable or even better than in the two-phase systems. In the two-phase arrangements, a thin film of a continuous phase wets the capillary surface. Thus, a big part of the water-oil interface is localized at this thin film. The character of three-phase flow is completely different. Small water droplets are dispersed in the oil phase and the entire water-oil interface is localized between two adjacent nitrogen bubbles.
This fact indicates that not only interfacial area determines the conversion degree. The intensity of mass transport at the interface also significantly affects the conversion values. The work by Jurado et al.28 indicates that the enzyme hydrolysis of triglycerides is limited by the transport of diglycerides and triglycerides to and out of the water-oil interface. In the two-phase systems, the thin film region at the capillary wall is characterized by nearly zero velocity (the velocity gradients can be large). Hence, no intensive convection, which provides intensive reactant and product transport to and out of the enzyme binding sites, is expected in the film.27, 31 In the three-phase arrangement conversely, small droplets of water are not in a tight contact with the walls. Then, the entire water-oil interfacial area can be intensively renewed, which is favorable for the enzyme reaction. From this point of view, the three-phase flow provides beneficial reaction-transport conditions in the microfluidic system.
CONCLUSIONS
In this study, we bring an idea to use regular three-phase slug flow to carry out multiphase enzyme reactions. Such a system overcomes problems that arise from the liquid-liquid interface instabilities and keeps the most valuable characteristics related to slug flow microsystems, i.e., intensive water-oil interface renewal, suppression of the Taylor dispersion, and regularity and reproducibility of flow. Enzyme hydrolysis of soybean oil by the enzyme lipase was chosen to test our hypothesis.
First, we develop plexiglass microfluidic chips called a double T-generator and multi-splitter that allows for the formation of fine water droplets dispersed in a continuous oil phase. Regular three-phase slug flow is then formed in simple T-generator where nitrogen slugs uniformly separate small volumes of the liquid-liquid reaction mixture. We showed that the three-phase flow is stable along a several meters long reaction capillary. Water droplet coalescence is limited to the space between two adjacent nitrogen slugs, which guarantees reproducible interfacial area.
To evaluate performance of the model enzyme reaction in the 3P arrangement, we compared it with classical two-phase arrangements either with a hydrophobic or hydrophilic reaction capillary. We found that the 2PO arrangement provides the highest conversion values; however, the slug flow becomes irregular, which is against to our demand on the uniform mass transfer intensity in the reactor. The other two systems (3P and 2PI) guarantee the regularity of slug flow along the entire reaction capillary as well as reasonable conversion values and pressure drops.
We can conclude that our concept of three-phase flow systems is a possible way how to provide uniform reaction-transport properties in heterogeneous reaction systems with unstable interface in long microchannels or reaction capillaries. We believe that the suggested concept is not limited only to enzyme synthesis but can be useful in many chemical, biochemical, and biological applications such as cell cultivation, production of fine chemicals, synthesis of micro and nanoparticles, etc.32
In feature, we are going to carry out theoretical and experimental studies that confirm and refine our ideas about reaction-transport processes in three-phase slug flow microsystems.
ACKNOWLEDGMENTS
The authors extend thanks for the support by the specific university research program MŠMT ČR (MSM No. 20/2013). The result was developed within the CENTEM Project, Reg. No. CZ.1.05/2.1.00/03.0088, co-funded by the ERDF as part of the Ministry of Education, Youth and Sports' OP RDI Program.
References
- Burns J. R. and Ramshaw C., Lab Chip 1(1), 10–15 (2001). 10.1039/b102818a [DOI] [PubMed] [Google Scholar]
- Brand O., Fedder G. K., Hierold C., Korvink J. G., and Tabata O., Micro Process Engineering (Wiley-VCH, Weinheim, 2006). [Google Scholar]
- Assmann N. and von Rohr P. R., Chem. Eng. Process. 50(8), 822–827 (2011) 10.1016/j.cep.2011.05.009. [DOI] [Google Scholar]
- Jovanovic J., Rebrov E. V., Nijhuis T. A., Kreutzer M. T., Hessel V., and Schouten J. C., Ind. Eng. Chem. Res. 51(2), 1020–1031 (2012) 10.1021/ie200715m. [DOI] [Google Scholar]
- Ahmed B., Barrow D., and Wirth T., Adv. Synth. Catal. 348(9), 1043–1048 (2006). 10.1002/adsc.200505480 [DOI] [Google Scholar]
- Jovanovic J., Rebrov E. V., Nijhuis T. A., Hessel V., and Schouten J. C., Ind. Eng. Chem. Res. 49(6), 2681–2687 (2010). 10.1021/ie9017918 [DOI] [Google Scholar]
- Martin K., Henkel T., Baier V., Grodrian A., Schon T., Roth M., Kohler J. M., and Metze J., Lab Chip 3(3), 202–207 (2003). 10.1039/b301258c [DOI] [PubMed] [Google Scholar]
- Reddy V. and Zahn J. D., J. Colloid Interface Sci. 286(1), 158–165 (2005). 10.1016/j.jcis.2004.12.052 [DOI] [PubMed] [Google Scholar]
- Hu S. W., Sheng Y. J., and Tsao H. K., Biomicrofluidics 6(2), 024130 (2012). 10.1063/1.4729129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup G. K. and Basu A. S., Biomicrofluidics 6(2), 022008 (2012). 10.1063/1.3700120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashina A., Meldrum F., and deMello A., Biomicrofluidics 6(2), 022001 (2012). 10.1063/1.3683162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashid M. N., Renken A., and Kiwi-Minsker L., Chem. Eng. Sci. 66(17), 3876–3897 (2011) 10.1016/j.ces.2011.05.015. [DOI] [Google Scholar]
- Sobieszuk P., Aubin J., and Pohorecki R., Chem. Eng. Technol. 35(8), 1346–1358 (2012). 10.1002/ceat.201100643 [DOI] [Google Scholar]
- Dessimoz A. L., Cavin L., Renken A., and Kiwi-Minsker L., Chem. Eng. Sci. 63(16), 4035–4044 (2008) 10.1016/j.ces.2008.05.005. [DOI] [Google Scholar]
- Zheng B. and Ismagilov R. F., Angew. Chem. Int. Edit. 44(17), 2520–2523 (2005) 10.1002/anie.200462857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. A. and Duraiswamy S., Lab Chip 9(13), 1840–1842 (2009). 10.1039/b904119b [DOI] [PubMed] [Google Scholar]
- Rahman M. T., Fukuyama T., Kamata N., Sato M., and Ryu I., Chem. Commun. 2006, 2236–2238 10.1039/b600970k. [DOI] [PubMed] [Google Scholar]
- Chen D. L. L., Li L., Reyes S., Adamson D. N., and Ismagilov R. F., Langmuir 23(4), 2255–2260 (2007). 10.1021/la062152z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraiswamy S. and Khan S. A., Nano Lett. 10(9), 3757–3763 (2010). 10.1021/nl102478q [DOI] [PubMed] [Google Scholar]
- Jaeger K. E. and Eggert T., Curr. Opin. Biotechnol. 13(4), 390–397 (2002). 10.1016/S0958-1669(02)00341-5 [DOI] [PubMed] [Google Scholar]
- Cech J., Schrott W., Slouka Z., Pribyl M., Broz M., Kuncova G., and Snita D., Biochem. Eng. J. 67, 194–202 (2012). 10.1016/j.bej.2012.06.015 [DOI] [Google Scholar]
- Svoboda M., Slouka Z., Schrott W., Cervenka P., Pribyl M., and Snita D., Microelectron. Eng. 87(5–8), 1590–1593 (2010). 10.1016/j.mee.2009.11.010 [DOI] [Google Scholar]
- Guan G., Kusakabe K., Moriyama K., and Sakurai N., Ind. Eng. Chem. Res. 48(3), 1357–1363 (2009). 10.1021/ie800852x [DOI] [Google Scholar]
- Molinari R., Santoro M. E., and Drioli E., Ind. Eng. Chem. Res. 33(11), 2591–2599 (1994). 10.1021/ie00035a010 [DOI] [Google Scholar]
- Deen W. M., Analysis of Transport Phenomena (Oxford University Press, New York, 1998). [Google Scholar]
- Jovanovic J., Zhou W. Y., Rebrov E. V., Nijhuis T. A., Hessel V., and Schouten J. C., Chem. Eng. Sci. 66(1), 42–54 (2011) 10.1016/j.ces.2010.09.040. [DOI] [Google Scholar]
- Kashid M. N., Platte F., Agar D. W., and Turek S., J. Comput. Appl. Math. 203(2), 487–497 (2007). 10.1016/j.cam.2006.04.010 [DOI] [Google Scholar]
- Jurado E., Carnacho F., Luzon G., Fernandez-Serrano M., and Garcia-Roman M., Biochem. Eng. J. 40(3), 473–484 (2008). 10.1016/j.bej.2008.02.002 [DOI] [Google Scholar]
- Kashid M. N., Agar D. W., and Turek S., Chem. Eng. Sci. 62(18–20), 5102–5109 (2007) 10.1016/j.ces.2007.01.068. [DOI] [Google Scholar]
- Kashid M. N., Gupta A., Renken A., and Kiwi-Minsker L., Chem. Eng. J. 158, 233–240 (2010). 10.1016/j.cej.2010.01.020 [DOI] [Google Scholar]
- Kashid M. N., Gerlach I., Goetz S., Franzke J., Acker J. F., Platte F., Agar D. W., and Turek S., Ind. Eng. Chem. Res. 44(14), 5003–5010 (2005). 10.1021/ie0490536 [DOI] [Google Scholar]
- Salic A., Tusek A., and Zelic B., J. Appl. Biomed. 10(3), 137–153 (2012) 10.2478/v10136-012-0011-1. [DOI] [Google Scholar]