Abstract
Deterministic lateral displacement (DLD) is a microfluidic size-based particle separation or filter technology with applications in cell separation and enrichment. Currently, there are no cost-effective manufacturing methods for this promising microfluidic technology. In this fabrication paper, however, we develop a simple, yet robust protocol for thermoplastic DLD devices using regulatory-approved materials and biocompatible methods. The final standalone device allowed for volumetric flow rates of 660 μl min−1 while reducing the manufacturing time to <1 h. Optical profilometry and image analysis were employed to assess manufacturing accuracy and precision; the average replicated post height was 0.48% less than the average post height on the master mold and the average replicated array pitch was 1.1% less than the original design with replicated posts heights of 62.1 ± 5.1 μm (mean ± 6 standard deviations) and replicated array pitches of 35.6 ± 0.31 μm.
INTRODUCTION
Particle separation is a critical process in medicine and biology allowing clinicians to detect—and researchers to study—rare cell populations. Microfluidic methods provide continuous cell and particle separation and improved size resolution over conventional methods. Passive hydrodynamic separation may be achieved by deterministic lateral displacement (DLD).1 DLD is a central research focus because it is capable of enriching rare cell populations1, 2, 3, 4 with high-throughput2 and promises to improve clinical point-of-care diagnostics. These advantages can only be achieved if the relevant engineering challenges can be overcome.
Engineering microfluidic devices for cell-based applications requires careful consideration of the microenvironment surrounding the cells. A critical step in the commercial development of microfluidics is to identify the ideal set of materials and methods for manufacturing the device.5 The materials commonly used by biologists and engineers are polystyrene (PS) and polydimethylsiloxane (PDMS), respectively.6 While these materials are popular, PS is not ideal for biological microfluidic devices7 and may not satisfy clinical regulatory requirements;8 PDMS has negative biological implications.9 Additionally, devices manufactured with regulatory-approved materials may be more rapidly approved for use.8 The commercial development of clinical microfluidic devices requires low-cost materials from regulatory-approved suppliers.
Medical-grade microfluidic substrates include polyolefin (COP)8 and select thermoplastic elastomers (TPEs). COPs are optically transparent and cyto-compatible thermoplastics exhibiting limited hydrophobic recovery after plasma treatment and low small molecule absorbance.7 The high Young's modulus of COP may be used to reduce feature deformation during operation, when compared to PDMS. TPEs offer improved gas-permeability over thermoplastics, which is ideal for long-term cell culture and wetting complex microfluidic devices when vacuum immersion10 is used. Vacuum immersion pulls trapped air through the gas permeable to thermoplastic elastomer creating a vacuum where the trapped air bubbles previously existing. The fluid surrounding the vacuum then fills the vacuum, completely wetting the device. Thermoplastic materials, including thermoplastic elastomers are 2–100 times less expensive than PDMS.11 Additionally, TPEs allow for integrated microfluidic devices with valves and pumps.11 COP and TPE are the ideal materials for low-cost, biomicrofluidic devices requiring rigid features and gas permeability. Once the ideal materials are selected, a manufacturing method for replicating the devices is required.
Common methods for replicating thermoplastic microfluidic devices include: injection molding,12 casting,13 and hot embossing.14, 15, 16, 17, 18 The overhead costs of injection molding are prohibitively expensive except at economies of scale. Feature aspect ratios are limited to 2 and draft angles are required to facilitate demolding.19 Casting allows for high aspect ratio features with vertical sidewalls,13 but cycle times are prohibitive when it comes to medium- and large-scale production. Soft embossing, hot embossing with an elastomeric mold, is a robust process that allows for dense arrays of high aspect ratio features with vertical sidewalls,17, 18 such as those required by DLD devices. Recent advances in hot embossing equipment have reduced cycle times down to 2 min with low-cost, bench top equipment16 while PDMS curing requires 10 or more minutes.20 Once replicated, microfluidic parts need to be bonded or sealed prior to operation.
The bonding step—required to close the channels—follows microfluidic part replication. There are numerous methods for bonding homogenous microfluidic devices (devices manufactured with one material).21 However, these methods are not adequate for manufacturing complex microfluidic devices, such as those requiring the physical properties of different materials (i.e., high Young's modulus and gas permeability). A simple, robust method for bonding different thermoplastic substrates is required. The bonding method presented here uses the existing replication equipment to minimize costs, requires no additional consumables and results in a standalone device with negligible channel deformation.
In addition to the technical and regulatory hurdles, moving a microfluidic technology to market requires a cost-effective,22 verifiable23 manufacturing process. In this paper, we compare the cytotoxicity of medical-grade materials to the industry standard materials (PS, PDMS), develop a robust, cost-effective manufacturing and wetting method for high aspect ratio DLD devices and rapidly verify the manufacturing method using optical profilometry and in-house image analysis software.
METHOD
Cytotoxicity
Cryopreserved KG1A cells, a human leukemic cell line, were used in this study. Cells were thawed and suspended in Iscove's Modified Dulbecco's Medium (IMDM) (I7633, Sigma-Aldrich, MO, USA) supplemented with 10% fetal bovine serum (7c0030, JRH Biosciences, KS, USA) and 1% penicillin/streptomycin (Sigma-Aldrich, MO, USA) in T25 flasks (Grenier bio-one, Germany). Cultures were split at 1.5 × 106 cells ml−1 then diluted to 1.0 × 105 cells ml−1 a total of 6 times prior to the study.
Identical 20 mm × 20 mm sheets of COP, TPE and PDMS were prepared in triplicate. Surface modification of the COP sheets was achieved by Corona treatment for 1 min. For comparison, 5 mm diameter sheets of latex (CL), which is known to be cytotoxic, were also prepared in triplicate. All sheets were sterilized in 80% ethanol for 1 h, rinsed in 0.9% sodium chloride (NaCl), immersed in NaCl overnight, dried by compressed nitrogen and placed in the centre of 35-mm diameter PS cell culture dishes then placed in an 80 °C oven for 1 hto promote adhesion to the bottom of the dish. An additional 3 cell culture dishes were used for comparing growth and viability to PS. All dishes were sterilized by UV irradiation for 30 min then inoculated at a concentration of 1.1 × 105 cells ml−1. The growth and viability counts were taken by a ViCell XR (BeckmanCoulter Australia, NSW, Australia) every 24 h for a period of 5 days.
Soft embossing and bonding
DLD device master molds, designed for enriching white blood cells, were fabricated by deep reactive ion etching (DRIE). An elastomeric mold was cast in PDMS by standard soft lithography techniques. The mold was backed with a glass microscope slide, cured at 100 °C for 1 h, then annealed at 150 °C for 1 day. COP sheets were dehydrated at 60 °C overnight to prevent bubble formation during embossing. The mold was used to soft emboss 12 parts in COP with a heated and air-cooled press (4386, Carver, IN, USA) at 200 kPa and 150 °C for 2 min. The pressure was reduced to 20 kPa prior to cooling then manually demolded at 80 °C. Scanning electron microscopy (SEM) was used to image the embossed COP structures. Each mold and all parts were characterized by optical profilometry (Bruker Biosciences, VIC, Australia) and in-house image analysis software was used to investigate manufacturing tolerances. Surface modification of the embossed COP parts was achieved by corona treatment for 1 min. 600-μm thick TPE lids, with bored inlet and outlet holes, were placed on top of the soft embossed parts, weighted with a glass microscope slide, then thermally bonded at 80 °C for 45 min.
Device wetting and operation
A total of 3 devices were wetted by vacuum immersion, filled with deionized water at 19.5 °C and operated at a pressure of 35 kPa for 4 min. Water was collected at the outlet in a pre-weighed micro-centrifuge tubes. The mass of each sample and respective tube was weighed for volumetric flow rate calculations. The operating pressure of each device was ramped up at a rate of 20 kPa min−1 until a leak was detected—the peak pressure recorded was used as the burst pressure. A bonded, dry DLD device was fitted with tubing at the inlets and wetted by pressure driven flow at 314-μl min−1 (∼95 kPa) for 90 s. An additional device was immersed in blue dye diluted with deionized water then placed under vacuum with a minimum pressure of −95 kPa for 90 s. Both devices were imaged. After burst pressure testing, the TPE lids were manually removed and inspected by microscopy and optical profilometry.
RESULTS AND DISCUSSION
Cytotoxicity
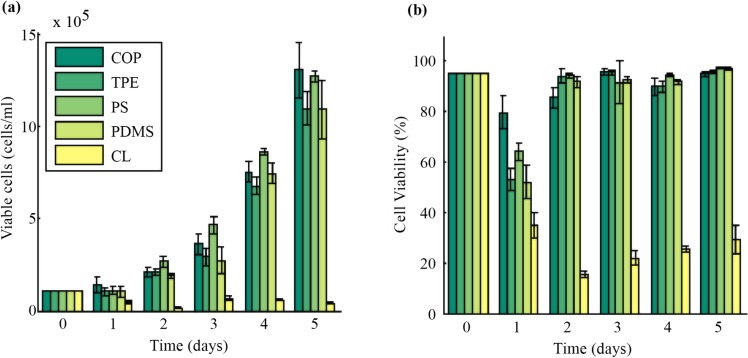
The purpose of the cytotoxicity assay is to determine if the materials and methods used to manufacture the DLD device will influence the viability of cells after separation. COP and TPE were selected as substitutes for PS and PDMS due to their physical properties and compliance with the parts of the International Organization for Standardization (ISO) standards for the biological evaluation of medical devices (10993) regarding hemotoxicity (Part 4) and cytotoxicity (Part 5). As shown in Figure 1, the average cell growth and viability of KG1A cells was approximately the same when in contact with corona-treated COP and TPE relative to PS and PDMS. After 5 days, the COP samples contained approximately the same concentration of viable cells as PS samples and the TPE samples contained about the same viable cell concentration as PDMS. On average, the elastomeric materials (TPE, PDMS) had 17% less viable cells than the rigid thermoplastics (COP, PS). For PDMS, this is attributed to the ethanol sterilization method; the absorption24 subsequent leaching of trace amounts of ethanol may have reduced cell growth and proliferation. Additionally, the estimated cell residence time in this device is 350 ms when operating at half the burst pressure or more than 6 orders of magnitude less than the length of the cytotoxicity assay; any cytotoxic effects observed after separating cells in this thermoplastic DLD device will be due to other aspects of the microenvironment such as chemical leaching or shear stress. Finally, it should be noted that the use of regulatory-approved materials or modified regulatory-approved materials does not mean the device is regulatory-approved, but that it may encounter less regulatory resistance.8
Figure 1.
Cytotoxicity assay comparing KG1A cell growth when in contact with surface modified COP, TPE, PS, PDMS, and CL. (a) Growth and viability plot of novel medical-grade thermoplastics (COP, TPE), industry-standard materials (PS, PDMS) and negative control (CL). (b) Cell viability plot of the aforementioned materials. Error bars represent 1 standard deviation.
Soft embossing and bonding
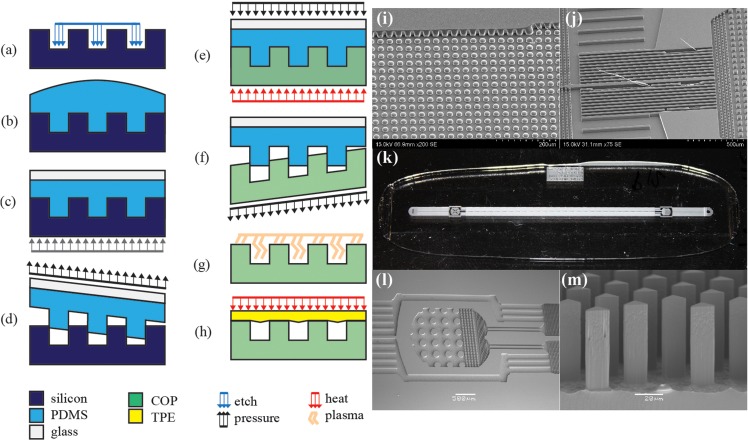
Preliminary experiments showed that previously reported methods17, 18 are not suitable for rapidly manufacturing DLD devices or similar devices with dense arrays of high aspect ratio features over large surface area (Figures 2i, 2j). These previously reported methods resulted in incomplete mold filling and feature failure during demolding as shown in Figure 2. A high melt flow index COP resolved the mold filling challenge, while high-temperature demolding15 reduced feature failure.
Figure 2.
Summary of fabrication and manufacturing process: (a) master mold fabrication, in this case, DRIE, (b)–(d) soft lithography, (e) soft embossing, (f) demolding, (g) surface modification, and (h) thermal bonding. SEMs of embossing failure modes: (i) incomplete mold filling and (j) feature failure during demolding, (k) image of 25 mm × 75 mm DLD chip, (l) SEM of pristine inlet features and (m) high-fidelity, high aspect ratio DLD array with vertical sidewalls.
The manufacturing process, shown in Figure 2, reduced post height from 63.2 ± 2.5 μm (mean ± 6 standard deviations) on the master mold to 62.9 ± 5.1 μm for all 12 parts and reduced the pitch of the COP array to 35.6 ± 0.31 μm from 36 μm in the original design with a final post aspect ratio of 3.6. There was significant variability in part quality due to the manual embossing and manual demolding processes. However, the soft embossing process resulted in parts that typically showed no defects. Parts that were immediately demolded after cooling to 80 °C (<10 s) with minimal substrate bending during demolding showed no defects, while parts demolded after exposure to ambient temperatures for a significant period of time required additional demolding force resulting in significant substrate bending. Bent posts were observed in an area of significant substrate bending during demolding. For example, parts 10 and 11 required additional time between cooling and demolding; 84 bent posts were observed in part 10 and 12 bent posts were observed in part 11 for defect rates of 0.19% and 0.03%, respectively. For this specific design, this suggests temperature-controlled demolding15 may be used to eliminate part defects observed during manual demolding. The vertical sidewalls of the triangular posts were maintained throughout the soft embossing and surface modification processes as shown in Figure 2m. The use of air plasma is a significant advantage over other plasma since no vacuum or additional gases are required. This significantly reduced the surface modification process time and costs. The DLD array contained over 43 904 posts with a feature density of 1080 features mm−2. COP feature geometry was maintained throughout the bonding process. The sharp feature edges shown in Figures 2l, 2m were maintained by bonding 20 °C below the glass transition of COP. Optical profilometry of the lid and part after manual lid removal indicated that channel deformation was negligible—i.e., lid sag only reduced channel height by 1.1%.
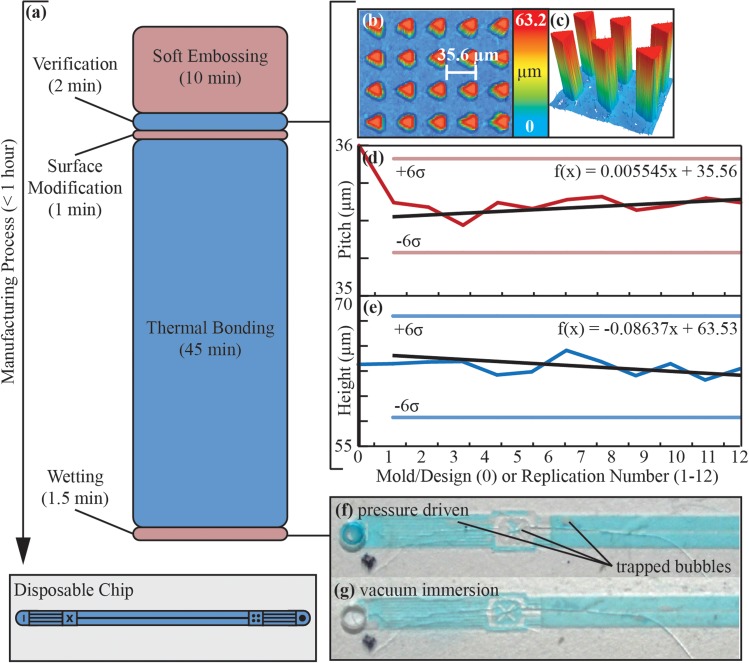
It is essential to monitor trends when replicating part geometry to keep track of mold and part quality. Simply stating the average and standard deviation may be acceptable if the slope of the trend line is known to be 0. In this case, the data suggest there are subtle, but relevant changes in the mold. This trend line may be used to approximate the lifetime of the mold. For example, if we only want parts that are within the six standard deviation boundaries shown in Figs. 3d, 3e, then we select the upper bounds for pitch calculations (f(x) = 35.9 μm) and lower bounds for height calculations (f(x) = 57.9 μm) and use the equations shown in the Figs. 3d, 3e to calculate x. For the pitch, x is 61 replications and for the height x is 65 replications. This suggests the lifetime of the mold, within the six standard deviation boundaries, is approximately 60–65 replications. This mold lifetime estimation requires significant extrapolation and does not account for structural and chemical changes that may occur after 12 replications but is a useful indicator.
Figure 3.
Summary of manufacturing process: (a) graphical summary of manufacturing time; (b) surface profile of DLD array from part 10 with array pitch measured; (c) 3D profile of DLD posts from part 10; (d) plot of COP array pitch where 0 represents the original designed pitch (36 μm), error bars represent 6 standard deviations and the black trend line and first-order polynomial indicate that pitch is slightly increasing with each replication, (e) plot of COP post height where 0 represents the silicon mold, error bars represent 6 standard deviations and the black trendline and first-order polynomical indicate the post height is slightly decreasing with each replication, (f) image of thermoplastic device wetted with DI water and blue dye with pressure driven (95 kPa) flow for 90 s (g) image of thermoplastic device wetted by vacuum immersion (−95 kPa) for 90 s—vacuum immersion results in no trapped bubbles after 90 s.
Based on this work, there are a number of potential limitations for this manufacturing technology, including: master fabrication, demolding during soft lithography, buckling during embossing, and post failure during demolding. First, the design of the replicated parts is limited by the master fabrication method. For DRIE, 40 nm wide with aspect ratios of 37.5 are reported.25 Elastomeric mold fabrication is limited by the spacing between features18 and PDMS is known to shrink when cured at high temperatures26—thermal stress may damage the master mold. The next potential limitation for high aspect ratio replication is during the soft lithography demolding step Fig. 2d where the friction forces acting on the structures during demolding may exceeds the fracture toughness of silicon. A successfully casted and demolded elastomeric mold is also prone to buckling during embossing.27 Additionally, a replicated plastic part is prone to failure during cooling and demolding from the elastomeric mold, where forces caused by thermal stress and adhesion may exceed the yield strength of the plastic. Ultimately, this fabrication technology is limited by the fracture toughness of the silicon mold, material properties of the uncured elastomer, buckling of the elastomeric mold and yield strength of the plastic.
Device wetting and operation
Figures 3f, 3g show the conventional wetting approach of flowing water through the device results in stochastic trapping of air. These air pockets can take many minutes to remove by continued flowing of degassed liquid. In a commercial use environment, setup time must be minimized. Therefore, we demonstrate complete wetting by vacuum immersion in 90 s. Devices were submerged in water, placed under vacuum (−95 kPa) for 90 s then the chamber was vented to atmospheric pressure. Thermally bonded devices burst at 200 ± 7 kPa (29 psi) with flow rates of 3.3 μl min−1 kPa−1 and a peak flow rate of 660 μl min−1. Also, these devices are expected to have a significant shelf life as they were found to rapidly wet under vacuum immersion after 9 months of dry storage.
The vacuum wetting expedited the removal of trapped air and is attributed to the gas permeability of TPEs,11 in a commercial environment this would require either wet device storage or a control system with a vacuum pump and vacuum chamber. Alcohol elution was considered for wetting the devices, but this requires a cytotoxic fluid and significant post-processing to remove alcohol absorbed by the materials. Wet storage may be the most effective option as COPs have low water absorption,21 however, the permeability of TPEs its effect on long-term thermal bond strength is unknown; further investigation into a cyto-compatible wet-storage fluid is required. In addition to testing wet storage lifetime, a cost-benefit analysis comparing pre-wetting and wet device storage to pressure-driven wetting is required. The high bond strength is attributed to the chemical properties of TPE, a hot melt pressure sensitive adhesive. The peak flow rate is a 28.7-fold increase over previous PDMS-glass device.1 The bonding process represents 91% of the manufacturing cycle time and further optimization will significantly increase manufacturing throughput. The longevity of the device is attributed to the limited hydrophobic recovery of corona treated COP when compared to other materials common in microfluidics.7
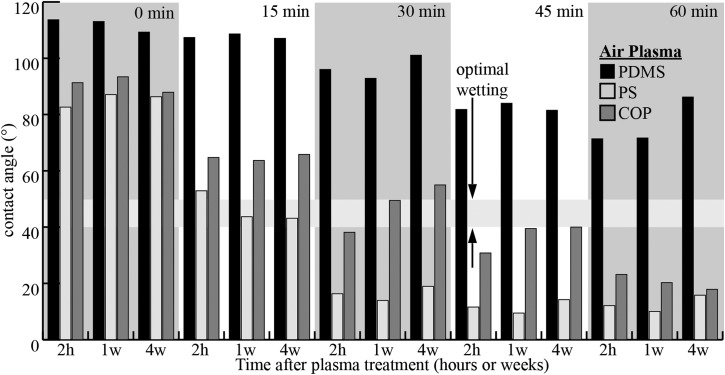
The corona treatment was used to modify the surface of COP for improved wetting, where wetting is defined as the act of completely filling the bonded channels with a fluid. The unmodified, unstructured COP is hydrophobic7 with contact angles of approximately 90° (Figure 4). Surface modification by air plasma or corona treatment creates a hydrophilic surface with contact angles as low as 20° (as shown in Fig. 4). For cell processing, the optimal contact angle is between 40° and 50° as indicated by the contact angle of polystyrene tissue culture-ware; this range of contact angles allows for complete wetting while minimizing surface charges.7 Preliminary experiments indicated the unmodified, micro-structured COP surface was super hydrophobic, exhibiting the Cassie-Baxter wetting state. Preliminary surface modification experiments using corona treatment indicated that briefly modifying the micro-structured COP with the corona treatment induced the Wenzel wetting state. The brief plasma surface treatment period is significantly less than those used by van Midwoud et al. and shown in Figure 4; this is attributed to differences in electrode proximity and plasma energy. During the surface modification process, air plasma is formed using a high frequency power source and electrode. The plasma forms across the air gap between the electrode and COP substrate such that ions bombard the surface of the substrate, creating reactive oxygen species at the surface of the substrate and increasing the surface energy to improve wetting. Additionally, the ion bombardment is thought to induce polymer chain scission,27 which decreases the molecular weight of the surface polymer chains and may reduce the glass transition temperature at the polymer substrate surface. This reduced glass transition temperature may also increase bond strength by increasing the amount of entanglement between the TPE polymer chains and shortened COP polymer chains during thermal bonding. In this fabrication process, the TPE bonding surface was not modified, but modifying both surfaces may improve bond strength by lowering the glass transition temperature at the surface of both polymers to increase polymer chain entanglement.
Figure 4.
Overview of changes in contact angles for PDMS, PS and COP after corona treatment times of 0 min, 15 min, 30 min, 45 min, and 60 min with data taken from Ref. 7. The contact angle of untreated COP and PS are approximately the same while untreated PDMS is significantly more hydrophobic, however, significantly longer treatment times are required for COP when compared to PS. Hydrophobic recovery for COP is observed for corona treatment of 30 min over the course of 4 weeks and over 1 week for a 45 min treatment. The optimal wetting range is defined by contact angles between 40° and 50°.7
CONCLUSIONS AND FUTURE WORK
This paper describes a relatively simple and robust method for manufacturing microfluidic devices containing an array of features using regulatory-approved materials and cyto-compatible methods. We decreased device manufacturing time from day(s) to less than one hour and increased peak DLD flow rates by 28.7-fold with manufacturing tolerances of ±1.1%. This work resulted in a standalone, all-thermoplastic DLD device that reduces the manufacturing times, costs and improved volumetric flow rates. A number of microfluidic manufacturing methods require additional equipment (i.e., plasma etcher, vacuum chamber, and pump) and consumables (i.e., solvents and gases), resulting in complicated and expensive fabrication methods. To improve on these complex methods, we developed a method requiring only an elastomeric mold, thermoplastic materials, heat, pressure, and corona treatment designed to minimize costs. This method moves DLD and similar microfluidic technologies— requiring dense arrays of high aspect ratio features over large surface areas—towards the marketplace.
Future work should focus on maximizing feature aspect ratio and minimizing feature size in the replicated parts. This may include exploring the limitations of the manufacturing method on a nano-scale using a DRIE master mold and determining the ideal demolding temperature for the given materials and methods. Ultimately, this proposed work may improve our understanding of the limitations of the manufacturing technology.
ACKNOWLEDGMENTS
This work was partially funded by the Australian Research Council (DP0110102207). We thank Dr. Huaying Chen for the cell culture training and Zeonex for donating the COP.
References
- Huang L. R. et al. , Science 304, 987 (2004). 10.1126/science.1094567 [DOI] [PubMed] [Google Scholar]
- Inglis D. W. et al. , J. Micromech. Microeng. 21, 054024 (2011). 10.1088/0960-1317/21/5/054024 [DOI] [Google Scholar]
- Loutherback K. et al. , AIP Adv. 2, 042107 (2012). 10.1063/1.4758131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. R. et al. , Prenat Diagn. 28, 892 (2008). 10.1002/pd.2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides G. M., Nature 442, 368 (2006). 10.1038/nature05058 [DOI] [PubMed] [Google Scholar]
- Berthier E. et al. , Lab Chip 12, 1224 (2012). 10.1039/c2lc20982a [DOI] [PubMed] [Google Scholar]
- van Midwoud P. M., Anal. Chem. 84, 3938 (2012). 10.1021/ac300771z [DOI] [PubMed] [Google Scholar]
- Kuo J. S. and Chiu D. T., Lab Chip 11, 2656 (2011). 10.1039/c1lc20125e [DOI] [PubMed] [Google Scholar]
- Regehr K. J. et al. , Lab Chip 9, 2132 (2009). 10.1039/b903043c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis D. W. et al. , Lab Chip 8, 925 (2008). 10.1039/b800721g [DOI] [PubMed] [Google Scholar]
- Roy E. et al. , Lab Chip 11, 3193 (2011). 10.1039/c1lc20251k [DOI] [PubMed] [Google Scholar]
- Piotter V. et al. , Micro. Tech. 8, 387 (2002). 10.1007/s00542-002-0178-6 [DOI] [Google Scholar]
- Wang Y. et al. , Lab Chip 11, 3089 (2011). 10.1039/c1lc20281b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worgull M., Hot Embossing: Theory and Technology of Microreplication (Oxford, UK; Burlington, MA: William Andrew, 2009). [Google Scholar]
- Dirckx M. E. and Hardt D. E., J. Micromech. Microeng. 21, 085024 (2011). 10.1088/0960-1317/21/8/085024 [DOI] [Google Scholar]
- Hale M. and Hardt D. E., ASME Conf. Proc. 2009, 43857. [Google Scholar]
- Goral V. N. et al. , J. Micromech. Microeng. 21, 017002 (2011). 10.1088/0960-1317/21/1/017002 [DOI] [Google Scholar]
- Russo A. P. et al. , Biomed. Microdev. 4, 277 (2002). 10.1023/A:1020902106144 [DOI] [Google Scholar]
- Microfluidic Chip Shop, “Lab-on-a-Chip Catalog” (2013), p. 146.
- Dow Corning, “Sylgard 184 Silicone Elastomer” Technical Data Sheet (2008).
- Tsao C. W. and DeVoe D., Microfluid. Nanofluid. 6, 1 (2009). 10.1007/s10404-008-0361-x [DOI] [Google Scholar]
- Whitesides G. M., Lab Chip 13, 11 (2013). 10.1039/c2lc90109a [DOI] [PubMed] [Google Scholar]
- Tantra R. and van Hereen H., Lab Chip 13, 2199 (2013). 10.1039/c3lc50246e [DOI] [PubMed] [Google Scholar]
- Lee J. N. et al. , Anal. Chem. 75, 6544 (2003). 10.1021/ac0346712 [DOI] [PubMed] [Google Scholar]
- Lee S. W. and Lee S. S., Microsyst. Technol. 14, 205 (2008). 10.1007/s00542-007-0417-y [DOI] [Google Scholar]
- Petrzelka J. E. and Hardt D. E., J. Micromech. Microeng. 22, 075015 (2012). 10.1088/0960-1317/22/7/075015 [DOI] [Google Scholar]
- Gerenser L. J. et al. , J. Adhes. Sci. Technol. 7, 1019 (1993). 10.1163/156856193X00556 [DOI] [Google Scholar]