Abstract
HPC1/RNASEL was recently identified as a candidate gene for hereditary prostate cancer. We identified a novel founder frameshift mutation in RNASEL, 471delAAAG, in Ashkenazi Jews. The mutation frequency in the Ashkenazi population, estimated on the basis of the frequency in 150 healthy young women, was 4% (95% confidence interval [CI] 1.9%–8.4%). Among Ashkenazi Jews, the mutation frequency was higher in patients with prostate cancer (PRCA) than in elderly male control individuals (6.9% vs. 2.4%; odds ratio = 3.0; 95% CI 0.6–15.3; P=.17). 471delAAAG was not detected in the 134 non-Ashkenazi patients with PRCA and control individuals tested. The median age at PRCA diagnosis did not differ significantly between the Ashkenazi carriers and noncarriers included in our study. However, carriers received diagnoses at a significantly earlier age, compared with patients with PRCA who were registered in the Israeli National Cancer Registry (65 vs. 74.4 years, respectively; P<.001). When we examined two brothers with PRCA, we found a heterozygous 471delAAAG mutation in one and a homozygous mutation in the other. Loss of heterozygosity was demonstrated in the tumor of the heterozygous sib. Taken together, these data suggest that the 471delAAAG null mutation is associated with PRCA in Ashkenazi men. However, additional studies are required to determine whether this mutation confers increased risk for PRCA in this population.
Prostate cancer (PRCA), like other common forms of cancer, has a hereditary component. Linkage analysis identified several chromosomal loci that may harbor PRCA-susceptibility genes (Smith et al. 1996; Berthon et al. 1998; Xu et al. 1998; Berry et al. 2000; Ostrander and Stanford 2000; Tavtigian et al. 2001). Mutations in these genes are estimated to cause ∼9% of all PRCA cases (Carter et al. 1992). The HPC1/RNASEL gene (MIM 180435) on chromosome 1q25 was recently identified, and germline mutations in this gene cosegregated with the disease in two families showing linkage with HPC1 (Carpten et al. 2002). One of the RNASEL mutations, E265X, was also associated with hereditary prostate cancer (HPC) in Finnish patients (Rokman et al. 2002). RNASEL encodes the 2′,5′-oligoisoadenylate-synthetase–dependent ribonuclease L protein (RNASEL), which regulates cell proliferation and apoptosis through the interferon-regulated 2-5A pathway (Zhou et al. 1993). It has been suggested to be a tumor suppressor gene (Carpten et al. 2002).
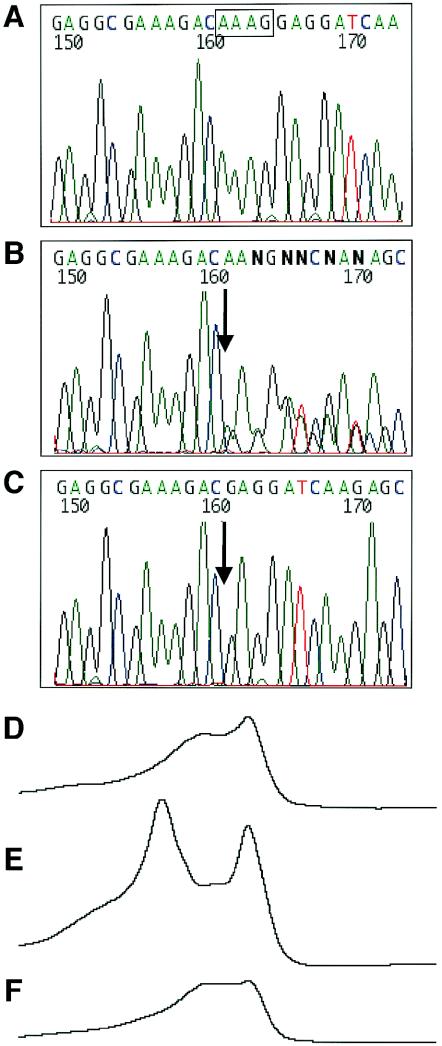
Unlike breast, ovarian, and colon cancers (Struewing et al. 1995; Laken et al. 1997), very little is known about susceptibility genes for prostate cancer in Jewish men. To examine the role of RNASEL in PRCA among Jews, we first analyzed the entire coding sequence of the gene through use of RNA extracted from blood leukocytes of two Ashkenazi sib pairs affected with PRCA. This was performed using RT-PCR followed by denaturing high-performance liquid chromatography (DHPLC) and sequencing analysis. We identified a novel, 4-bp deletion mutation, 471delAAAG, in one sib pair (fig. 1). The mutation, at codon 157 in exon 1, results in a premature truncation at codon 164. In this sib pair, one brother, who received a diagnosis at age 65 years, was heterozygous for the mutation (fig. 1B and 1E), whereas the other, who received a diagnosis at age 57 years, was homozygous for this mutation (fig. 1C and 1F).
Figure 1.
Detection of the RNASEL 471delAAAG frameshift mutation by sequencing (A–C) and DHPLC (D–F) analyses. A–C, The AAAG deleted sequence is marked by a box; the deletion site is indicated by an arrow. A and D, unaffected control individual. B and E, PRCA-affected individual heterozygous for the 471delAAAG mutation. C and F, PRCA-affected individual homozygous for the 471delAAAG mutation.
We further analyzed DNA samples from a microdissected tumor and a benign prostatic hyperplasia (BPH) of the brother who had the heterozygous 471delAAAG mutation. DHPLC analysis demonstrated loss of heterozygosity (LOH) in the tumor DNA, whereas heterozygosity was maintained in the BPH sample. Sequencing confirmed the sole presence of the 471delAAAG allele in the tumor DNA. This finding is in agreement with the results of Carpten et al. (2002), who showed LOH of the wild-type allele and absence of RNASEL protein in tumor cells from a patient with HPC carrying the E265X mutation. Taken together, these observations further support the important role of RNASEL in prostate cancer pathogenesis.
To assess the frequency of 471delAAAG among Jewish patients with PRCA and the control population, we analyzed DNA from an additional 119 unselected patients (85 Ashkenazi and 34 non-Ashkenazi) and from 333 control individuals (233 Ashkenazi and 100 non-Ashkenazi). These DNA samples were obtained from patients who received diagnoses during the years 1991–1997 in two different medical centers in Israel (Hubert et al. 1999). The median age at the time of diagnosis, for all patients and for the subgroup of Ashkenazi patients, was 68 years (range 48–80 years). Two control groups were studied: the first consisted of elderly Ashkenazi Jewish men with no personal history of cancer (n=83; median age 74 years, range 59–92 years). These were recruited on the basis of a self-administrated questionnaire in which they indicated no history of cancer, including prostate cancer. They were not screened for PRCA by either a prostate-specific antigen blood test or a medical examination. The second control group included healthy young Jewish women, aged 20–45 years (150 Ashkenazi and 100 non-Ashkenazi). The non-Ashkenazi female control group consisted of four distinct ethnic groups, originating from northern Africa, Iran/Iraq, the Balkans, and Yemen (25 individuals from each group). All participating subjects signed written informed consent and identified themselves as either Ashkenazi or non-Ashkenazi Jews of a particular ethnic origin. DNA samples were blinded and tested anonymously.
The exon 1 fragment containing the 471delAAAG mutation (bases 286–667 of RNASEL) was amplified, and the 382-bp PCR products were analyzed by the WAVE DHPLC apparatus (Transgenomics), under conditions described elsewhere (Gavert et al. 2002). The primer sequences were as follows: forward primer, 5′ TTT ATC CTC GCA GCG ATT G 3′; and reverse primer, 5′ GCG TAA TAG CCT CCA CAT CAC 3′. Because the DHPLC profiles of the homozygous wild-type and mutant alleles were similar (fig. 1D and 1F), mixing studies were performed to identify 471delAAAG homozygotes. All abnormal DHPLC profiles were confirmed by sequence analysis on the initial PCR product and on an independent PCR, using an automated ABI Prism 310 Genetic Analyzer (Perkin Elmer Applied Biosystems). No discrepancies between DHPLC results and sequencing were detected. Statistical analyses were performed using SPSS Base 11.0 and EpiInfo 2000 software. The mutation frequency in the Ashkenazi population was estimated using the young Ashkenazi female control group as a point in a binomial distribution. Upper and lower percentage points were then calculated as the limits of the CI around it. The odds ratio (OR) and CI were calculated as an estimation of risk among mutation carriers. χ2 and Fisher exact tests were used, when appropriate, to determine significant differences in mutation and marker frequencies. Statistical differences in age at diagnosis of PRCA were calculated using the median test.
Table 1 presents the frequencies of the 471delAAAG mutation in the different study groups. The estimation of the mutation frequency in the Ashkenazi Jewish population was calculated on the basis of the frequency among 150 young female control individuals, because women are not at risk for PRCA. In this group, the mutation frequency was 4% (95% CI 1.9%–8.4%), compared with 0% in the non-Ashkenazi female control group (P<.05). To determine the mutation frequency among Ashkenazi patients with PRCA, we included only one member affected with prostate cancer per family, on the basis of “first sample obtained.” Among Ashkenazi patients with PRCA, 471delAAAG was found in 6.9% (6/87), compared with 2.4% (2/83) among elderly Ashkenazi male control individuals (OR=3.0; 95% CI 0.6–15.3; P=.17). Of the 87 Ashkenazi patients with PRCA, 7 had a first-degree relative affected with PRCA, 2 (28.6%) of whom carried the mutation (OR=16.2; 95% CI 1.9–140.2; P=.029). Finally, the mutation was not detected in any of the 34 non-Ashkenazi patients with PRCA.
Table 1.
Frequency of the RNASEL 471delAAAG Mutation in Unselected Patients with PRCA and Control Individuals, Stratified by Ethnic Origin
| Study Group | No.Tested | No. ofCarriers (%)a |
| Ashkenazi Jews: | ||
| Patients with PRCA | 87 | 6 (6.9)b |
| Elderly male control individuals | 83 | 2 (2.4) |
| Young female control individuals | 150 |
6 (4.0) |
| Total | 320 | 14 |
| Non-Ashkenazi Jews: | ||
| Patients with PRCA | 34 | 0 |
| Young female control individuals | 100 |
0 |
| Total | 134 | 0 |
Ashkenazi patients with PRCA versus elderly male control individuals: OR=3.0 (95% CI 0.6–15.3; P=.17). Young Ashkenazi female control individuals (95% CI 1.9%–8.4%) versus non-Ashkenazi young female control individuals: P<.05.
A homozygous sib was not included.
Rokman et al. (2002) noted that an earlier age of PRCA diagnosis (∼11 years) was associated with the RNASEL E265X mutation in patients from Finnish families with HPC. In our group of Ashkenazi patients with PRCA, there was a small, nonsignificant difference in the median age at diagnosis between 471delAAAG carriers and noncarriers (65 vs. 68.5 years, respectively). However, the median ages at diagnosis for carrier and noncarrier patients with PRCA in the study were 9.4 and 5.9 years earlier, respectively, compared with all 4,866 Ashkenazi patients with PRCA registered in the Israel National Cancer Registry (INCR) in the years 1991–1997 (74.4 years; P<.001) (INCR Web site).
To determine whether 471delAAAG is a founder mutation, we performed genotyping using two closely linked markers, D1S2818 and D1S158 (NCBI Web site), flanking the RNASEL gene (∼2 Mb apart). This analysis revealed that the patient homozygous for 471delAAAG was also homozygous for D1S2818 and D1S158 alleles, containing 22- and 15-dinucleotide repeats, respectively. Moreover, this D1S281822/D1S15815 haplotype was present in 100% (15/15) of 471delAAAG carriers, compared with only 32.5% (13/43) of noncarrier Ashkenazi patients with PRCA (P<.001). These data suggest that 471delAAAG is a founder mutation in the Ashkenazi Jewish population.
Interestingly, we found the 471delAAAG mutation in LNCaP cells, one of the most commonly used human PRCA cell lines, but not in two other PRCA cell lines, PC3 and DU145. LNCaP cells originated from a lymphatic metastasis of a prostatic adenocarcinoma in a 50-year-old white male (Horoszewicz et al. 1980). The LNCaP cells, however, did not carry the commonly linked D1S15822 allele. This finding could be explained by the many rearrangements that occurred in these cells during repeated passages, or by de novo occurrence of 471delAAAG in either the LNCaP cells or in other non-Jewish white populations.
The 471delAAAG is the first founder null mutation in a known HPC gene that is potentially associated with an increased risk of PRCA in Ashkenazi Jewish men. Since our preliminary results regarding this risk are not statistically significant, additional population-based studies are required to determine whether there is an age-specific PRCA risk conferred by heterozygous or homozygous 471delAAAG mutations. Further studies are also needed to determine whether this relatively common mutation in Ashkenazi Jews is also associated with familial clustering of PRCA or with other cancer types, as well as to verify the clinical value of genetic screening for this mutation.
Acknowledgments
We thank Dr. Art Beaudet of the Baylor College of Medicine in Houston, TX, for critical review of the manuscript. This work was supported by the M.K. Humanitarian Fund.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- Israel National Cancer Registry (Prostate 1997), http://www.health.gov.il/icr/HTML_97/Prostate97_2.htm
- National Center for Biotechnology Information (NCBI), http://www.ncbi.nlm.nih.gov/entrez/query.fcgi (for D1S158 [sequence gi: 30403] and D1S2818 [sequence gi: 1235493])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for RNASEL [MIM 180435] and HPC1 [MIM 601518])
References
- Berry R, Schroeder JJ, French AJ, McDonnell SK, Peterson BJ, Cunningham JM, Thibodeau SN, Schaid DJ (2000) Evidence for a prostate cancer-susceptibility locus on chromosome 20. Am J Hum Genet 67:82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthon P, Valeri A, Cohen-Akenine A, Drelon E, Paiss T, Wohr G, Latil A, et al (1998) Predisposing gene for early-onset prostate cancer, localized on chromosome 1q42.2-43. Am J Hum Genet 62:1416–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpten J, Nupponen N, Isaacs S, Sood R, Robbins C, Xu J, Faruque M, et al (2002) Germline mutations in the ribonuclease L gene in families showing linkage with HPC1. Nat Genet 30:181–184 [DOI] [PubMed] [Google Scholar]
- Carter BS, Beaty TH, Steinberg GD, Childs B, Walsh PC (1992) Mendelian inheritance of familial prostate cancer. Proc Natl Acad Sci USA 89:3367–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavert N, Yaron Y, Naiman T, Bercovich D, Rozen P, Shomrat R, Legum C, Orr-Urtreger A (2002) Molecular analysis of the APC gene in 71 Israeli families: 17 novel mutations. Hum Mutat 19:664 [DOI] [PubMed] [Google Scholar]
- Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, Sandberg AA (1980) The LNCaP cell line: a new model for studies on human prostatic carcinoma. Prog Clin Biol Res 37:115–132 [PubMed] [Google Scholar]
- Hubert A, Peretz T, Manor O, Kaduri L, Wienberg N, Lerer I, Sagi M, Abeliovich D (1999) The Jewish Ashkenazi founder mutations in the BRCA1/BRCA2 genes are not found at an increased frequency in Ashkenazi patients with prostate cancer. Am J Hum Genet 65:921–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laken SJ, Petersen GM, Gruber SB, Oddoux C, Ostrer H, Giardiello FM, Hamilton SR, Hampel H, Markowitz A, Klimstra D, Jhanwar S, Winawer S, Offit K, Luce MC, Kinzler KW, Vogelstein B (1997) Familial colorectal cancer in Ashkenazim due to a hypermutable tract in APC. Nat Genet 17:79–83 [DOI] [PubMed] [Google Scholar]
- Ostrander EA, Stanford JL (2000) Genetics of prostate cancer: too many loci, too few genes. Am J Hum Genet 67:1367–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokman A, Ikonen T, Seppala EH, Nupponen N, Autio V, Mononen N, Bailey-Wilson J, Trent J, Carpten J, Matikainen MP, Koivisto PA, Tammela TL, Kallioniemi OP, Schleutker J (2002) Germline alterations of the RNASEL gene, a candidate HPC1 gene at 1q25, in patients and families with prostate cancer. Am J Hum Genet 70:1299–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Freije D, Carpten JD, Gronberg H, Xu J, Isaacs SD, Brownstein MJ, Bova GS, Guo H, Bujnovszky P, Nusskern DR, Damber JE, Bergh A, Emanuelsson M, Kallioniemi OP, Walker-Daniels J, Bailey-Wilson JE, Beaty TH, Meyers DA, Walsh PC, Collins FS, Trent JM, Isaacs WB (1996) Major susceptibility locus for prostate cancer on chromosome 1 suggested by a genome-wide search. Science 274:1371–1374 [DOI] [PubMed] [Google Scholar]
- Struewing JP, Abeliovich D, Peretz T, Avishai N, Kaback MM, Collins FS, Brody LC (1995) The carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individuals. Nat Genet 11:198–200 [DOI] [PubMed] [Google Scholar]
- Tavtigian SV, Simard J, Teng DH, Abtin V, Baumgard M, Beck A, Camp NJ, et al (2001) A candidate prostate cancer susceptibility gene at chromosome 17p. Nat Genet 27:172–180 [DOI] [PubMed] [Google Scholar]
- Xu J, Meyers D, Freije D, Isaacs S, Wiley K, Nusskern D, Ewing C, et al (1998) Evidence for a prostate cancer susceptibility locus on the X chromosome. Nat Genet 20:175–179 [DOI] [PubMed] [Google Scholar]
- Zhou A, Hassel BA, Silverman RH (1993) Expression cloning of 2-5A-dependent RNAase: a uniquely regulated mediator of interferon action. Cell 72:753–765 [DOI] [PubMed] [Google Scholar]