Abstract
Measures of brain and hippocampal volume in 40 healthy young (aged 18–30 years) and 36 healthy elderly (aged 60–83 years) subjects were compared with composite cognitive function scores in three conceptual domains: memory ability, processing speed, and general fluid intelligence. Through a series of general linear models testing the relationship between these brain measures and cognitive performance scores, a significant positive relationship between hippocampal volume and fluid intelligence ability was found in elderly subjects but not in young. No relationship between the other cognitive domains and brain or hippocampal volume was found. The findings suggest a role of hippocampal atrophy in the decline in fluid intelligence in the elderly.
Keywords: Aging, Hippocampus, Brain atrophy, Cognitive decline, Fluid intelligence, Memory
INTRODUCTION
As individuals age, their total brain size will grow, peaking in their mid-twenties, and then decline, dropping sharply by their mid-sixties (Pfefferbaum et al., 1994). Rates of total brain atrophy vary among individuals but generally increase with age and disease and, importantly, have been associated with age-related losses in cognitive function. Because the brain does not atrophy uniformly (Sowell et al., 2003), different brain regions will lose volume at different rates; atrophy-related loss of cognitive ability may represent the influence of atrophy in specific brain regions (Sowell et al., 2003). Atrophy of the hippocampus (HC), a region generally associated with memory processing, has been identified as a measure related to distinct declines in cognitive performance in the elderly.
Investigations of direct correlations between age-related HC atrophy and specific declines in cognitive function have produced mixed findings (Raz & Rodrigue, 2006). Early magnetic resonance imaging (MRI) studies reported declines in memory ability with HC atrophy (de Leon et al., 1997; Golomb et al., 1993), but a more recent review of MRI studies reported only weak correlations between HC size and memory ability in the elderly when considering the extant literature collectively (Van Petten, 2004). Raz and colleagues (2007) found an association between HC volume at follow-up over 5 years and lower fluid intelligence ability across a range of ages, pointing to a possible relationship between age-related HC atrophy and age-related fluid intelligence declines in the elderly. But no study, to our knowledge, has investigated this relationship as a function of age-group, leaving an open question of whether hippocampal associations with cognition are due to normal inter-individual differences or due to age-related atrophy.
The aim of the current study was to investigate the association of brain and hippocampal volume with cognitive function in the elderly with the goal of resolving the issue of mixed findings in memory decline correlations as well as the issue of the relative impact of individual variations in HC volume and HC volume atrophy on fluid intelligence ability. We predicted that there would be a positive relationship between brain and HC volumes and cognitive abilities and that, importantly, the manner of these relationships would differ as a function of age group, with low cognitive function only being associated with low brain and HC volume in the elderly. Cognitive function was summarized in three ability domains based on performance on a series of neuropsychological tests. These domains, memory, speed processing, and general fluid intelligence ability, represent distinct cognitive functions that all have been reported to decline with age (Siedlecki et al., 2009). Based on findings in healthy young and older individuals, we predicted that HC volume would relate to memory and fluid intelligence ability but not speed processing.
METHODS
Participants
The study included 40 healthy young and 36 healthy older adults. Young participants were recruited through fliers, internet advertisement, and market mailings and older adults were recruited through market mailings and from senior day centers located in Manhattan, New York. Participant demographics are displayed in Table 1. To be eligible for participation, all subjects had to speak English, be right-handed, and have normal or corrected-to-normal vision. All subjects were screened to ensure the absence of any past or current medical, neurological, or psychiatric disorders, including dementia, or treatment with psychoactive drugs. In particular, all subjects had to score 133 or higher on the Mattis Dementia Rating Scale (Mattis, 1998) to be included in the study (Lucas et al., 1998). Informed consent was obtained from all participants in accordance with the procedures of Columbia University Medical Center.
Table 1.
Participant demographics and neuropsychological test results
| Parameter | Elder | Young |
|---|---|---|
| Age range | 60–83 | 18–30 |
| Age* | 70.2 ± 6.5 | 24.0 ± 3.2 |
| Education | 16.1 ± 2.5 | 15.5 ± 2.5 |
| DRS | 140.1 ± 3.7 | 141.9 ± 2.0 |
| SRT_tot* | 50.08 ± 9.06 | 60.20 ± 5.48 |
| SRT_delayed recall* | 8.50 ± 2.27 | 11.0 ± 1.24 |
| SRT_delayed recog | 11.25 ± 2.22 | 11.73 ± 1.74 |
| Digit Symbol* | 49.36 ± 11.71 | 65.53 ± 11.32 |
| Letter Number Sequencing* | 10.97 ± 3.16 | 14.40 ± 3.20 |
| Matrix Reasoning* | 13.61 ± 5.58 | 21.05 ± 4.06 |
| Z_Memory* | −0.58 ± 0.68 | 0.22 ± 0.41 |
| Z_Processing Speed* | −0.91 ± 0.80 | 0.24 ± 0.80 |
| Z_gF* | 0.66 ± 0.87 | 0.47 ± 0.66 |
Note. Values for age, education, neuropsych tests, Z_Memory, Z_Processing Speed, Z_Gf, and DRS are the mean ± standard deviation. Education is measured in years.
p <.05 for t test comparing aged and young.
Domain Scores
Scores for the three observed domains were generated for each participant by taking an average of construct-relevant z-transformed neuropsychological test scores. Test selection for each construct was based on the results of structural equation modeling as previously reported (Siedlecki et al., 2009). To avoid removing possible age effects on performance, only raw test scores were used to generate composite domain scores; however, because analyses were conducted to explore the relationships between cognitive performance and brain volume measures within a group, the use of raw versus age-scaled scores would not necessarily affect the results of the analyses.
Memory (Z_mem)
We assessed memory with three subscores of the Selective Reminding Test (SRT; Buschke & Fuld, 1974), which required participants to recall words over six trials from a list of 12 words read aloud. After each recall attempt, participants were reminded of the words they failed to recall. SRT-total refers to the total number of words out of a possible 72 that the participant recalled across all six trials. SRT-delayed refers to the correct number of words from the 12 word list that the participant recalled after a 15-min delay. Finally, SRT-recognition refers to the total number of words correctly recognized by the participant in a recognition test, which followed the delayed recall trial, where each of the 12 memorized words is presented, one by one, alongside three distraction words.
Processing Speed (Z_speed)
Processing Speed was assessed with the Wechsler Adult Intelligence Scale-Revised (WAIS-R; Wechsler, 1981) Digit Symbol subtest. The Digit Symbol subtest involves writing symbols that correspond with single-digit numbers, as provided by a key at the top of the test form, as quickly as possible.
General fluid intelligence (Z_gF)
General fluid intelligence was assessed with the Wechsler Adult Intelligence Scale-III (WAIS-III; Wechsler, 1997) Letter Number Sequencing subtest and the Matrix Reasoning Test (Raven, 1962). In the Letter Number Sequencing subtest, intermixed letters and numbers are read aloud to participants who must then repeat the presented items back in a specific sequence of alphabetically increasing letters followed by numerically increasing numbers. The length of presented letter-number strings increases with each trial. The Matrix Reasoning task involves determining which pattern out of a set of eight possible patterns best completes the missing cell of a matrix.
Brain Volume Measures
All brain volume measures were generated for each participant from T1-weighted structural images acquired on a Philips Electronics 1.5 Tesla (T) Intera MRI scanner (http://www.medical.philips.com/main/products/mri/systems/index.wpd) on the day of neuropsychological testing. Whole brain volume calculation and segmentation was conducted with SPM5 (Wellcome Department of Cognitive Neurology) software. Each T1-weighted image was segmented into three tissue classes (i.e., gray matter, white matter, and cerebrospinal fluid) using standardized procedures in SPM 5. Total intracranial volume was defined as the sum of all three tissue classes and normalized, or relative, whole brain volume (nWBV) was defined as the ratio of the sum of gray matter and white matter to total intracranial volume (Fotenos, Snyder, Girton, Morris, & Buckner, 2005). Hippocampal identification and segmentation was conducted via automated processing described and validated by Firbank, Barber, Burton, and O’Brien (2008). Hippocampus volumes were summed and divided by total intracranial volume to generate a normalized hippocampus volume (nHippV).
Statistical Analysis
To test the relationship between brain and hippocampal volumes and cognitive ability in old and young we built a series of general linear models (GLM) that proceeded in stages. In the first stage (“full-model”), two models were constructed using brain volume measures, nWBV and nHippV, respectively, as outcome measures. The three composite cognitive function scores (Z_mem, Z_speed, Z_gF) were used as predictors, along with age-group, in each model. We also added interaction terms in each model by multiplying the group-membership predictor with each of the subject-specific predictors. Thus, the two full models comprised 7 total predictors: group, Z_mem, Z_speed, Z_gF, group*Z_mem, group*Z_speed, and group*Z_gF. Inclusion of these interaction terms allowed for formal tests of heterogeneity of the slope of the relation between the brain volume measures and cognition as a function of age group. After performing the full-model analysis, we retained only those covariate main effects and interaction terms that yielded statistically significant regression weights. The results of the general linear model with the reduced set of predictors are reported as the “reduced model.”
RESULTS
Neuropsychological Measures
Participant demographics and neuropsychological test results are presented in Table 1. All participants had comparable levels of education and performed comparably on the DRS. On the average, the aged performed significantly worse than the young participants on all other measures.
Analysis of Brain Measures and Cognition
Bivariate correlations between all tested measures are presented in Table 2. Age showed strong negative correlation with both normalized brain measures, as well as all of the tested composite cognition scores.
Table 2.
Correlations between brain volume measures, group, and cognitive performance scores
| Parameter | Age | nWBV | nHippV | Z_Memory | Z_Speed | Z_gF |
|---|---|---|---|---|---|---|
| Age | — | −.835** | −.535** | −.615** | −.626** | −.627** |
| nWBV | — | .655** | .560** | .512** | .591** | |
| nHippV | — | .383** | .379** | .474** | ||
| Z_Memory | — | .539** | .580** | |||
| Z_Speed | — | .591** | ||||
| Z_gF | — |
Note. Values are standardized regression coefficients (i.e., β).
p <0.05;
p <0.01.
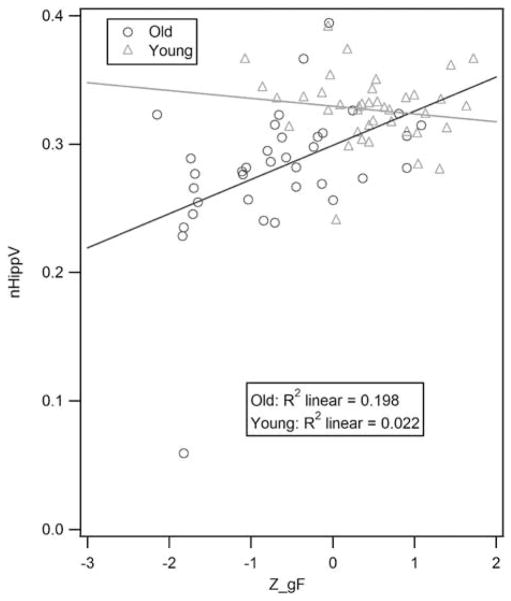
Analysis of the two full GLM models constructed (one with nWBV and one with nHippV) yielded two statistically significant regression weights for the nHippV predicted model (group and Z_gF by group) and one statistically significant regression weight for the nWBV predicted model (Z_gF). Consequently, we generated two reduced models (one with each brain measure as a predicted outcome) retaining only the significant effects from the full model (namely group, group by Z_gF, and Z_gF). The results of all four models are presented in Table 3. The final, reduced model predicting nHippV found significant positive relationship between group and nHippV as well as Z_gF by group with nHippV. A graph of the interaction between nHippV and Z_gF by group is presented in Figure 1. The final, reduced model predicting nWBV found no significant interactions.
Table 3.
General linear models predicting the two brain volume measures, nWBV and nHippV
| Parameter | Full | Reduced |
|---|---|---|
| nWBV | ||
| Group | .114** | .160** |
| Z_Memory | .002 | — |
| Z_Speed | .001 | — |
| Z_gF | .009* | .008 |
| Group* Z_Memory | .008 | — |
| Group* Z_Speed | .001 | — |
| Group* Z_gF | .005 | — |
| nHippV | ||
| Group | .011** | 0.11** |
| Z_Memory | .000 | — |
| Z_Speed | .000 | — |
| Z_gF | .005 | — |
| Group* Z_Memory | .001 | — |
| Group* Z_Speed | .000 | — |
| Group* Z_gF | .011** | .019** |
Note. Values are standardized regression coefficients (i.e., β). The “full” model includes all covariates and interactions. The “reduced” model drops all nonsignificant interactions and covariates. All probabilities determined nonparametrically via the method of permutations (see Methods section for details).
p <.05;
p <.01.
Fig. 1.
Scatter plot of the interaction between nHippV and Z_gF as a function of age group, including fitted regression lines. Elders in our study group (darker line) evidenced increased gF performance with greater hippocampal volume. Young participants evidenced no similar associations.
Note. The removal of the outlier does not significantly alter the slope of the fitted line in the elder group.
DISCUSSION
Our study found an association between greater HC atrophy and lower general fluid intelligence (gF) ability in the elderly, but not in younger adults, suggesting a specific influence of HC atrophy on the decline of gF functioning that is associated with advanced aging. Although memory and speed processing ability decreased with increasing age in our study group, their relationship with normalized hippocampal volume was not significant. There was also no significant direct association between rates of total brain atrophy and reduced cognitive abilities in any of the tested domains.
General fluid intelligence refers to logical reasoning ability: it is the mental process underlying novel problem solving and the ability to understand complex relationships between items or events (Gary, Chabris, & Braver, 2003; Yuan, Steedle, Shavelson, Alonzo, & Oppezo, 2006). gF is partly heritable and decreases with age, and as a multifaceted mental process it is likely mediated by multiple brain regions (Gong et al., 2005). Functional neuroimaging studies in healthy young individuals have implicated brain regions associated with working memory, processing speed, and executive control functions in gF processing. A majority of imaging studies point to activity in the medial and lateral prefrontal cortex (PFC) as being related to gF processing (Gary et al., 2003; Gong et al., 2005; Kane & Engle, 2002; Thompson et al., 2001).
That HC atrophy should relate to losses in gF ability in the elderly specifically may seem unexpected, given the HC’s accepted role as a memory processing hub (and the lack of associations found between HC volume and gF ability in the young). However, Raz and colleagues (2007) found a correlation between gF ability and HC size in healthy adults, and the HC in general has been implicated in some of the processes underlying logical reasoning. Specifically, select areas of the HC are used in the spatial organization of memory events and, therefore, in the formation of relationships between memorized items (Goel, Makale, & Grafman, 2004; Preston, Shrager, Dudukovic, & Gabrieli, 2004; Rapp, 2004). The organization of events and items is a fundamental step in logical reasoning, as the perception of patterns and relationships can only occur within an understood context of order. Should the spatial organizing capabilities of the HC decline as a result of atrophy, it holds that overall gF functioning will similarly decline, as was witnessed in the elder age group. Indeed, in a study examining the information processing capabilities of the HC, Buckmaster, Eichenbaum, Amaral, Suzuki, and Rapp (2004) found that monkeys with entorinal cortex (i.e., HC) lesions had greatly reduced capacity, compared to normal monkeys, for relational information processing, losing their ability, in this case, for making judgments about the relationships between familiar items.
Another possible explanation for the impact of HC atrophy on gF ability in the elder group involves the role that the HC may play in supporting executive control (EC) processes in the elderly. Areas related to EC processes (notably in the PFC) have been implicated in gF ability in the young (Gary et al., 2003), but in a test of age-related differences in gF ability in healthy older adults, Schretlen et al. (2000) found that EC ability accounted for some of the variance in adult gF ability independently of PFC volume or of ability in shared processes that underlie both gF and EC (such as speed processing). This finding could indicate that either age-related decreases in EC processing contribute directly to decreases in gF processing in the elderly or that decreases in EC and gF ability are the result of decreases in a shared third factor, such as another mental process or a non-PFC brain region. Schretlen and colleagues did not implicate any specific brain regions in this PFC-independent EC processing but our current findings, and studies that have found HC atrophy to be associated with executive function loss in the elderly and ill, indicate that the HC could potentially represent this non-PFC brain region (Frodl et al., 2006; Van Petten, Plante, Davidson, Bajuscak, & Glisky, 2004). Further studies exploring the relationship between HC atrophy and EC ability on gF ability in the elderly will be necessary to identity the exact impacts of these different measures.
Our measure of HC atrophy (total HC volume at time of testing normalized to total intracranial volume) is cross-sectional and derived from the strong relationship found between increasing age and decreasing HC volume in our elderly participants. Because HC volume decreases with increasing age in our older group but not our younger group, we can conclude that these volumetric differences are age-related. Furthermore, we can be confident that the association between HC volume and gF ability was the result of age-related atrophy, and not simply individual variation in HC size, because the same association was absent in the younger group, who, although lacking age-related atrophy, nevertheless evidenced considerable but normal variation in HC volume. Although cross-sectional brain atrophy measures cannot match the power of longitudinal study designs, recent studies have found good correlation between cross-sectional and longitudinal measures of brain atrophy, with cross-sectional measures, if anything, generally underestimating total atrophy (Raz et al., 2005).
A surprising finding in our analysis was the lack of a relationship between HC atrophy and memory function in the elderly. We predicted that age-related declines in memory ability would be associated with age-related atrophy, either of the total brain or the HC, such as we witnessed with gF ability. Although memory ability declined with age, there was no evidence for HC atrophy influencing age-related memory decline. There may be many explanations for this finding, but perhaps the best explanation lies in a consideration of our participant-eligibility screening methods. Our study subjects were heavily screened for disorders of cognitive impairment (e.g., Alzheimer’s disease; AD), which are diagnosed primarily through tests of memory ability. Subjects with low memory ability were, by nature of this exclusion, necessarily exempted from the study, effectively truncating the range of possible HC atrophy effect witnessed in the study population. Because there is a strong relationship between HC atrophy and the severity of degenerative disorders (Chetelat & Baron, 2003; de Leon et al., 1997), we might have seen a significant relationship between HC atrophy and memory decline were individuals meeting formal criteria for mild cognitive impairment or AD patients included in the study. Conversely, we implemented no screening process that would have selected specifically against low gF ability in our study population. Of additional importance, because the SRT-recognition test may consider a different aspect of memory function than the SRT-total or the SRT-recall test, we reran the main analysis with SRT-recognition dropped from the memory domain construct. Results of the analysis run with this reduced memory construct were not significantly different from those generated in the initial analysis with the full memory construct.
Given that HC atrophy appears to be related to gF ability in the elderly, a reasonable course for future studies would involve further exploration of the mechanisms of gF decline through advanced structural and functional neuroimaging of the HC using techniques not used in this current study. Diverse imaging techniques, such as arterial spin labeling and diffusion tensor imaging would indicate the manner of structural changes that underpin gF loss with HC atrophy. Additionally, comparative functional imaging of young and elderly patients performing logical reasoning tasks could reveal possible age or atrophy-related functional network changes; inclusion of executive function tasks in these studies could also identify the individual impacts of EC ability and HC atrophy on gF decline in the elderly. In this study, we only considered individuals in two discrete age strata. Future studies should address the impact of atrophy on cognition across a more diverse age-range, including middle-aged (40–60 years) individuals. Understanding the neural mechanisms of fluid intelligence decline in the elderly is a crucial first step in the eventual development of effective cognitive decline interventions.
Acknowledgments
The present study was supported by NIA R01 AG26158.
References
- Buckmaster AC, Eichenbaum H, Amaral GD, Suzuki AW, Rapp RP. Entorhinal cortex lesions disrupt the relational organization of memory in monkeys. The Journal of Neuroscience. 2004;24(44):9811–9825. doi: 10.1523/JNEUROSCI.1532-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Baron JC. Early diagnosis of Alzheimer’s disease: Contribution of structural neuroimaging. Neuroimage. 2003;18:525–541. doi: 10.1016/s1053-8119(02)00026-5. [DOI] [PubMed] [Google Scholar]
- De Leon M, George AE, Golomb J, Tarshish C, Convit A, Kluger A, Wisniewski HM. Frequency of hippocampal formation atrophy in normal aging and Alzheimer’s disease. Neurobiology of Aging. 1997;18(1):1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- Firbank MJ, Barber R, Burton E, O’Brien JT. Validation of a fully automated hippocampal segmentation method on patients with dementia. Human Brain Mapping. 2008;29:1442–1449. doi: 10.1002/hbm.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64(6):1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Frodl T, Schaub A, Banac S, Charypar M, Jager M, Kummler P, Meisenzahl E. Reduced hippocampal volume correlates with executive dysfunctioning in major depression. Journal of Psychiatry Neuroscience. 2006;31(5):316–325. [PMC free article] [PubMed] [Google Scholar]
- Gary J, Chabris C, Braver T. Neural mechanisms of general fluid intelligence. Nature Neuroscience. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Goel V, Makale M, Grafman J. The hippocampal system mediates logical reasoning about familiar spatial environments. Journal of Cognitive Neuroscience. 2004;16(4):654–664. doi: 10.1162/089892904323057362. [DOI] [PubMed] [Google Scholar]
- Golomb J, de Leon M, Kluger A, Ajax E, Tarshish C, Ferris S. Hippocampal atrophy in normal aging. Archives of Neurology. 1993;50(9):967–973. doi: 10.1001/archneur.1993.00540090066012. [DOI] [PubMed] [Google Scholar]
- Gong Q, Sluming V, Mayes A, Keller S, Barrick T, Cezayirli E, Roberts N. Voxel-based morphometry and stereology provide convergent evidence of the importance of medial prefrontal cortex for fluid intelligence in healthy adults. Neuroimage. 2005;25:1175–1186. doi: 10.1016/j.neuroimage.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review. 2002;9(4):637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Lucas JA, Icnick RJ, Smith GE, Bohac DL, Tangalos EG, Kokmen E, Graff-Radford NR, Petersen RC. Normative data for the Mattis Dementia Rating Scale. Journal of Clinical and Experimental Neuropsychology. 1998;20:536–547. doi: 10.1076/jcen.20.4.536.1469. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;6:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Preston AR, Shrager Y, Dudukovic NM, Gabrieli JDE. Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus. 2004;14(2):148–152. doi: 10.1002/hipo.20009. [DOI] [PubMed] [Google Scholar]
- Rapp P. Who’s the fairest of them all? Role of the human hippocampus in the relational organization of memory. Hippocampus. 2004;14(2):141–142. doi: 10.1002/hipo.20008. [DOI] [PubMed] [Google Scholar]
- Raven J. Advanced progressive matrices, set II. London, UK: H.K. Lewis; 1962. pp. 1–14. [Google Scholar]
- Raz N, Lindenberger U, Ghisletta P, Rodrigue KM, Kennedy KM, Acker JD. Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cerebral Cortex. 2007;18(3):718–726. doi: 10.1093/cercor/bhm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue K. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neuroscience and Behavioral Reviews. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, Barta P. Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age-related differences in fluid intelligence. Journal of the International Neuropsychological Society. 2000;6:25–61. doi: 10.1017/s1355617700611062. [DOI] [PubMed] [Google Scholar]
- Siedlecki KL, Stern Y, Reuben A, Sacco RL, Elkind MSV, Wright CB. Construct validity of cognitive reserve in a multi-ethnic cohort: The Northern Manhattan Study. Journal of the International Neuropsychological Society. 2009;15:558–569. doi: 10.1017/S1355617709090857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Toga AW. Genetic influences on brain structure. Nature Neuroscience. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Plante E, Davidson PSR, Bajuscak L, Glisky EL. Memory and executive function in older adults: relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42(10):1313–1335. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Yuan K, Steedle J, Shavelson R, Alonzo A, Oppezo M. Working memory, fluid intelligence, and science learning. Educational Research Review. 2006;1:83–98. [Google Scholar]