Abstract
Fine particles were collected in three indoor environments and an outdoor reference site. Samples were acid and aqueous extracted for metal analyses and cytokine expression study using a BEAS-2B line. Results revealed that the average PM2.5 concentration indoors was 5.8 mg/m3 while outside, it was 9.4 mg/m3. The airborne metal concentrations in indoor air ranged from 0.01 ng/m3 (Cd) to 620 ng/m3 (Al). All metals analyzed were higher indoors when compared to outdoor (I/O ratio) indicating a contribution from the workplace. Some metals were more efficiently extracted (e.g., Ni, V, As) in the aqueous phase than others (e.g., Fe and Al). Toxicological assays showed that the aqueous extracts at 20% induced IL-6 and subsequently inhibited it at a higher concentration (50%); both IL-8 and MCP-1 were inhibited at 20 and 50%. As, Ni and V concentrations seem to be the most important metals associated with the cytokine induction/inhibition response probably due to the higher bioavailability.
Keywords: air pollutant concentrations, dust, exposure assessment, heavy metals, indoor
Introduction
Attention has focused on the PM10 in cities because that is where most deaths occur, where pollution is routinely monitored and hence the associations are best seen. Many studies about particulate matter (especially the fine particles) have shown correlations between high concentrations of heavy metals and health effects in humans (Dockery and Pope 1994; Schwartz et al. 1996; Saskia et al. 1998). Epidemiological studies have associated them with increased mortality, morbidity and decreased lung function (Saskia et al. 1998; Samet et al. 2001) and cardiac diseases (Delfino et al. 2005). Indoor PM is affected by the special conditions pertaining to the indoor environment under consideration, e.g., cigarette smoke, hay dust, cooking-derived particles. Local practice, tradition, type of job and behavior could greatly influence the indoor environmental conditions and hence PM exposure profiles.
Due to numerous sources, particulate matter can contain hundreds of different organic and inorganic chemical elements (Gordon et al. 1984; Meszaros et al. 1997). Fine particles can contain sulfates, ammonium, nitrates, elemental carbon and heavy metals such as selenium, cadmium and zinc. Investigators have suggested that trace metals distributed widely throughout the lung could catalyze the formation of oxidants within it. This in turn could induce tissue damage (Ghio et al. 1996; Dreher et al. 1997). In addition, in vitro studies suggest that metal components in air particulate matter are correlated with pulmonary toxicity (Costa and Dreher 1997; Kodavanti et al. 1998; Dye et al. 2001).
Inflammation is a vital response that has evolved to deal with injury and to stimulate the regeneration of healthy tissue, although in an excessive amount, inappropriate or chronic setting inflammation is harmful leading to disease. For some particles it is feasible that the inflammation could result from release of transition metals from the large surface area and in such situations transition metals like iron are considered to play an important role in the oxidative stress process. Iron redox cycles between the II and III forms and in doing so generates hydroxyl radicals that cause severe oxidative damage. It is believed that cytokines play a key role in the development of cardiopulmonary function and diseases (Ulrich et al. 2002; Pope et al. 2004). It is thought that after phagocytosis of inhaled particles, alveolar macrophages release chemo-tactic mediators capable of attracting inflammatory cells into the neighboring lung tissue. In vitro studies have shown that human bronchialepithelial cells can secrete cytokines after exposure to particulate matter (Kennedy et al. 1998; Fujii et al. 2001; Baulig et al. 2003). Few studies however, have looked at the effect of aqueous extracts from PM10 on immune response (Frampton et al. 1999) and to our knowledge few reports have been published concerning the immune response of these cells to aqueous extracts of PM2.5, particularly those from indoor environments (Monn and Becker 1999).
Much of the literature on metals in particulate matter has been conducted in outdoor environments. Consequently, characterization and monitoring of indoor air have been neglected. On the other hand, the bioavailability of metal present in PM2.5 seems to be more important on health effect causes than the total metal content (Graney et al. 2004). This is extremely important if one considers the amount of time spent at the work place. Due to the versatility in workplace environments the specifics and characteristic composition of indoor air will depend on the type of activities performed in that environment.
This study examines the concentrations of heavy metals and arsenic associated with fine particulate matter (PM2.5) from a specialized indoor environment and the effects of their aqueous extracts on immune response by human airway bronchial epithelial cells. Aqueous extracts were used to evaluate the toxicity of PM2.5 since transition metals, endotoxin and proteins present in particles can be water-soluble and hence can be easily absorbed by respiratory airways.
Materials and methods
Sampling sites
Aerosol particles (PM2.5) were collected in three analytical laboratories from a building in the metropolitan area of San Juan, Puerto Rico. To evaluate indoor sources and infiltrations of ambient air, an outdoor site was used as reference and samples simultaneously collected. Samples were taken in a two-storey building located in a relatively calm and non-crowded place. The rooms studied were chemistry laboratories used for specialized analyses and were equipped with distinctive instrumentation. Although these are highly specialized environments the workforce in them was very low. The laboratories were located on the second International Journal of Environmental Health Research floor in the same building. The traffic near the building is due to a busy parking space as well as the proximity to a highway that is more than 20 m from the building.
Laboratory 1 (approx. 20 m2) is designed to develop water analysis by High Performance Liquid Chromatography (HPLC) and also used for sample preparation. Several reagents are stored and handled in this lab. There are two airconditioner devices that are turned on constantly. Laboratory 2, the smallest of them all, measures approximately 9 m2, and is used for metal analyses by Atomic Absorption (AAS) which also contains an air-conditioned device. Laboratory 3 (approx. 36 m2) is used for water quality analysis and houses a Gas Chromatography (GC) instrument. This lab also contains an air-conditioning unit supplied by a central cooling system. The laboratory also serves as a storage place for reagents used in the analytical process and others.
These specialized environments were selected due to personnel complaints and reported symptoms that could possibly be related to indoor air quality. Consequently, this type of indoor environment is not a typical representation of most workplaces due to the nature of the specialized equipments and activities performed there. An effort to characterize the various indoor air qualities and establish relative comparisons among them was carried out in order to identify possible causes of illness.
Sampling procedure (PM2.5)
An Andersen air sampler (RAAS 2.5-400) (Franklin, MA, USA) which is a filterbased gravimetric instrument with a six-filter module for the capture of specific PM2.5 particles was used. The flow meters (FM) were set to intake air at flows of 17 l/min (FM 1 and 4), 4.1 l/min (FM 2 and 5) and 3.5 l/min (FM 3 and 6). The RAAS 2.5–400 monitors and records the ambient manifold and enclosed temperatures, barometric and pump vacuum pressures, humidity, flow rates and volumes of each individual channel of the instrument. Teflon filters were used for particle collection. The PM2.5 air sampler was placed in the middle of each laboratory, at 1.5 m of height from the floor, in order to obtain a representative sample of room particles. All laboratories were approximately 4 m in height. Each filter represents the material collected in a 129 h period. Blanks were taken during the sampling. Filters were transported in cassettes placed in a stainless steal sealed protective containers. Teflon filters for PM2.5 were weighed (balance model M- 220D, Denver Instrument, Denver, CO, USA) and pre-conditioned at a temperature of 20– 23 + 28C and a humidity of 40–45 + 5%. Filters were stored in Petri slides and kept in dry keepers (Samplatec, Corp., Osaka, Japan) until the conditioning process was achieved. Once the weight of the PM2.5 was stable, filters were stored at −20°C until further analyses. The PM2.5 mass was determined by gravimetric analyses and the concentration obtained dividing the mass by air volume.
Metal analyses
The Teflon filters containing particulate matter (PM2.5) from each laboratory and outdoor site were used to obtain the acid and aqueous extracts. For acid extraction, filters were digested by adding nitric acid and ultra pure water. It was conducted on a hot plate, heated and refluxed at 908C + 58C for 2 h (Gioda et al. 2006).
For obtaining the aqueous extracts, the filters were minced and extracted by shaking for 13 h in a 1.5 ml volume of double-processed tissue culture water (Sigma-Aldrich Corp., St Louis, MO, USA). The soluble fraction was then used for cell treatment and trace elements analyses.
Six blank filters were digested in a similar manner and analyzed for trace elements accordingly as done for samples. Metals were analyzed using Atomic Absorption Spectroscopy technique (Perkin Elmer Atomic Absorption Spectrophotometer Model AAnalyst 800 with a Graphite Furnace). US EPA methods 202.2, 7060A, 7131A, 7381, 7421, 7521 and 7911 were used for the particular analyses of Al, As, Cd, Fe, Pb, Ni and V, respectively (US EPA 1986–1996). Detailed metal analyses were described elsewhere (Gioda et al. 2006).
Culture and extract exposure to bronchial epithelial cells
Bronchial epithelial cells (BEAS-2B) were cultured in 75 cm2 Flask (Corning Inc., Corning, NY, USA) using a Keratinocyte Basal Medium (KGM1 – Keratinocyte Medium, Cambrex, Walkersville, MD, USA, 2.0 ml of BPE, hEGF 0.5 ml, Insulin 0.5 ml, Hydrocortisone 0.5 ml, GA-1000 0.5 ml) and maintained at 378C in a humidified atmosphere containing 5% of CO2. Media was changed every 48 h until the cells reached 90–100% of confluence. Cells were then removed by brief trypsinization and sub-cultured in a Tissue Cultured Plate (Corning Inc., Corning, NY, USA), 24- or 96-wells. At 90–100% confluence cells were treated with aqueous extracts at three different concentrations: (a) 6 ml of aqueous extract per ml of KGM bullet kit media (0.6%), using 0.5 ml of treatment solution per well; each well containing 100,000 cells-p. 46; in triplicate; 24-wells; (b) 200 ml of aqueous extract per ml of KGM bullet kit media (20%), using 0.5 ml of treatment solution per well; each well containing 100,000 cells – p47; in triplicate; 24-wells; and (c) 200 ml of aqueous extract per 200 ml of KGM bullet kit media (50%), using 200 ml of treatment solution per well; each well containing 70,000 cells – p56; in duplicate; 96-wells. The control is 0.5 ml of KGM bullet kit media. BEAS-2B cells were incubated for 24 h.
Cytokine quantifications
After 24 h treatment and cell incubation, the resultant supernatant was transferred to a 1.5 ml vial and kept in ice. A volume of 50 ml from each supernatant was transferred to a 96-well micro plate and analyzed for cytokine proteins using the Bioplex system. Seventeen human cytokines were measured using the Bio-Plex Cytokine Assay (Bio-Rad Lab. Inc.). Bio-Plex cytokine assays are multiplex beads based assays designed to quantify multiple cytokines in diverse matrices including serum samples, plasma, and tissue culture supernatants. It permits the simultaneous detection of up to 100 cytokines in a single well. In this research the following cytokines were analyzed: interleukin-1 beta (IL-1b), IL-2, IL-4, IL-6, IL-8, IL-10, IL-5, IL-7, IL-12, IL-13, IL-17, granulocyte-monocyte colony stimulating factor (GM-CSF), interferon-gamma (IFN-g), tumor necrosis factor-alpha (TNF-a), G-CSF, macrophage chemotactic protein-1 (MCP-1; MCAF) and MIP-1β.
Statistical analysis
Unpaired t-test or Student-Newman-Keuls Multiple Comparisons Test and analysis of variance were used to evaluate statistical differences between sample International Journal of Environmental Health Research concentrations. The amount of cytokine secreted per treatment was analyzed in relation to the concentrations of PM2.5, each individual metal, and total metal content. A Chisquare test was also employed to compare the effects of concentrations. Differences between sampling sites were also evaluated. All statistical analyses were performed using the GraphPad InStat version 3.0 computer software package (GraphPad Software Inc., San Diego, CA, USA). Tests were considered significant at 0.05 level of significance.
Results and discussion
Particulate matter (PM2.5)
Average concentrations of heavy metals in PM2.5 for all chemistry laboratories are shown in Table 1. The average concentrations of PM2.5 in the indoor environments ranged from 3–8.5 mg/m3 versus 9.2 mg/m3 at the outdoor location. Results for Laboratory 1 ranging from 4–11.5 mg/m3; for Laboratory 2 from 6.4–6.6 mg/m3, for Laboratory 3 from 2.4–3.2 mg/m3, and outdoor from 9.2–9.6 mg/m3 were obtained.
Table 1.
Average concentrations for PM2.5 (standard deviation (±); n = 6) and metal (indoor: n = 3; outdoor: n = 1) at chemical laboratories.
| Site | PM2.5 (μg/m3) | Al (μg/m3) | As (ng/m3) | Cd (ng/m3) | Fe (μg/m3) | Pb (ng/m3) | Ni (ng/m3) | V (ng/m3) |
|---|---|---|---|---|---|---|---|---|
| Laboratory 1 | 8.5 (±3.9) | 0.62 (±0.2) | 1.4 (±0.4) | 0.23 (±0.01) | 0.54 (±0.1) | 15.0 (±5.9) | 10.0 (±5.1) | 1.9 (±0.7) |
| Laboratory 2 | 6.5 (±2.5) | 0.13 (±0.1) | 0.7 (±0.3) | 0.02 (±0.01) | 0.12 (±0.03) | 1.0 (±0.5) | 1.0 (±0.5) | 0.5 (±0.3) |
| Laboratory 3 | 3.0 (±0.9) | 0.24 (±0.01) | 0.95 (±0.05) | 0.01 (±0.001) | 0.23 (±0.1) | 1.7 (±0.5) | 4.70 (±0.8) | 0.3 (±0.1) |
| Outdoor | 9.4 (±6.1) | 0.21 | 1.7 | 0.2 | 0.8 | 3.0 | 1.0 | 0.8 |
The low levels of particulate matter in indoor environments are probably due to reduced level of human activity (1–3 employees) in each of the laboratories. The differences among laboratory levels could be also related to the type of human activity and movement in this laboratory at the time of sampling. The average indoor PM2.5 concentrations were lower than outdoor. This suggests that few outdoor particulate matter finds its way into the indoor environments. This is also supported by the differences in chemical composition of PM2.5 collected outside and inside the building, suggesting that most of the particles are generated from indoor sources.
In a previous study in the city of Guaynabo, the outdoor concentrations of PM2.5 averaged 11.6 mg/m3 (Gioda et al. 2007). These findings are similar to the concentration obtained outdoors during this research. The literature for indoor PM2.5 concentrations shows data that greatly varies between environments. PM2.5 concentrations from households from different cities ranged from 8 to as much as 530 mg/m3; in offices from 67 to as much as 111 mg/m3; and hospitals from 40– 215 mg/m3 (Gemenetzis et al. 2006). The indoor PM2.5 concentrations found in this study were far below what has been described elsewhere for indoor environments. There are no PM2.5 guideline values available for indoor air; however, total dust should not exceed 100 mg/m3 (Indoor Air Quality 2003). The United States Environmental Protection Agency (US EPA), recommends a 24 h PM2.5 value of 35 mg/m3 and an annual average of 15 mg/m3 for outdoor environments. The average indoor and outdoor levels obtained in this study were below the suggested EPA annual mean value (15 mg/m3), and the individual daily standard was never exceded (35 mg/m3) during the sampling period. The concentrations of PM2.5 in the building were maintained at levels suggested for low health risk. However, it is of utmost importance to know the relative composition and constituents of indoor dust in order to fully evaluate its toxic potential.
Trace elements
One of the chemical constituents of indoor dust were evaluated as heavy metals and arsenic concentrations and compared to outdoor airborne PM2.5. The aqueous and acid extracts from PM2.5 were measured for comparison. The total content in indoor air ranged from 0.01 ng/m3 for Cd and as much as 620 ng/m3 for Al (Table 1). These components were also measured in outdoor air with values ranging from 0.2 ng/m3 for Cd and as much as 800 ng/m3 for Fe (Table 1). The acid/aqueous percentiles show that the most efficiently extracted metals in the aqueous phase were V, Ni, As (25% or greater) and; on the other hand, there was less Al and Fe, as expected. The solubility of individual metal ranged from low such as for Al (0.8%) as high as to V (46%). Previous studies for PM2.5 from indoor environment showed higher percentages in water-soluble form for some metals such Al (83%), Cd (95%), Ni (89%) and Pb (58%) than we found (Graney et al. 2004). Maybe the differences in aerosol pH could be responsible for more or less dissolution of metals. Also, the extraction methodologies employed were different.
These results presented here indicate that the association of specific metals (V and Ni) to indoor particulate matter appear to be different than the association for the same metals to outdoor particles since the average percentiles are extremely different indoor (V . 39% indoor vs. 26 outdoor and N . 26% vs. 44) to outdoor. Consequently, the more water-soluble metals (As, Ni and V) will also be more bioavailable for uptake by the cells in the lung. Metals, independent of their sources, are mainly in insoluble oxide form, being most of them insoluble in aqueous solution (Pritchard et al. 1996). Low recovery was expected since most metals are in an insoluble form or complexed to other constituents. Therefore, it is also of importance the possible form of these metals associated with organic constituents of PM which need to be evaluated separately and are being reported elsewhere (Fuentes-Mattei et al. 2010). Solubility of metal is dependent on the ligand to which it is coupled and to its environmental pH. Acid medium helps the solubilization of metals. This research shows low concentrations of metals solubilized in aqueous extract of neutral pH, but it should be taken into consideration that the respiratory system has areas of lower pH, therefore, greater solubilization of metals is expected (Ghio et al. 1999).
To have an idea of the amount of metal incorporated in the working environment, we estimated the absorbed mass of metal during a year of exposure. We based this exposure on an 8 h working day, a 152 days/year, 20 m3/day as the inhalation rate constant, which has been previously used by the United States Environmental Protection Agency (US EPA 1991) and a 50% absorption rate per metal. The estimated annual absorbed mass for the elements ranged from 0.3– 0.56 mg for As among laboratories; Cd from 0.02–0.12 mg; Fe from 45.6–324 mg; Ni from 0.5–5.1 mg; Pb from 0.4–7.6 mg and V from 0.3–1.0 mg. However, our results cannot be directly compared to the literature estimates since different assumptions were adopted. These estimates are conservative since they only consider exposure of 8 h at the laboratories and need to include the exposure and uptake of metal during the other 17 h of the day.
According to Graney et al. (2004), low concentrations of trace metals are typical for PM collected in indoor air. In our study the heavy metal concentrations were higher than those reported in other indoor environments. The differences may be attributed to the type of indoor environment activities being tested, laboratory vs. household or other. According to several studies, metal content of particles has been suggested as causative agents associated with adverse health effects, such as mutagenic and carcinogenic (Brewer and Yuzbasiyan-Gurkan 1992), airways inflammation (Schaumann et al. 2004), pulmonary toxicity (Costa and Dreher 1997; Kodavanti et al. 1998; Dye et al. 2001). This ultrafine size particle (PM2.5) has greater possibilities to reach the deep respiratory tract and lung alveoli than larger ones exerting higher health problems due to its greater surface area and trace metal content throughout the particle.
Cytokines response
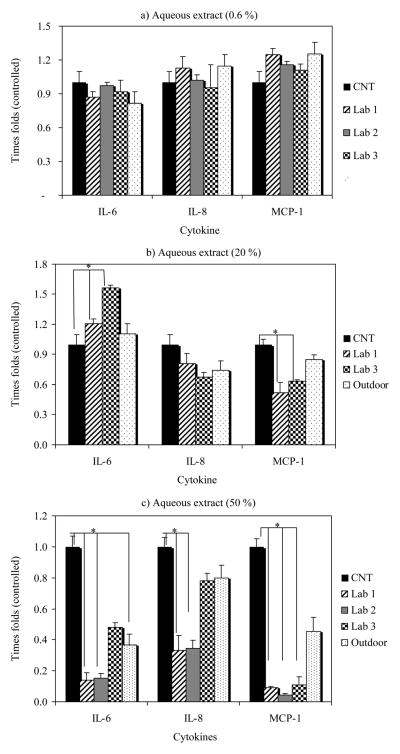
In order to evaluate the inflammatory potential of indoor PM2.5, its aqueous extracts were used to expose human lung epithelial cells (BEAS-2B) and the media analyzed to determine cytokines secretions. The results from aqueous extract for the indoor and outdoor samples on human airways epithelial cells are illustrated in Figure 1 and Table 2. The greatest effect of PM2.5 aqueous extracts on immune response of 17 human cytokines was observed for IL-6 and IL-8 and MCP-1. The effect of aqueous extracts at three different concentrations exerted different responses on cytokine release dependent on the concentration tested. No significant differences (P 5 0.05) were observed between controls and aqueous extract treatments at 0.6% for any of the cytokine tested (Figure 1a). Probably this low concentration does not cause significant effects on cells although trends on IL-8 and MCP-1 increases were seen for Laboratory 1 and outdoor aqueous extracts. The greatest immune response was the induction of IL-6 (labs 1 and 3) and inhibitory effects on IL-8 and MCP-1 for PM2.5 aqueous extracts exposure at 20% concentration (Figure 1b). Similar results, but more markedly (IL-6 increased secretion; IL-8 and MCP-1 repressed secretion), were observed after exposure with outdoor polar organic extract from urban and rural sites from Puerto Rico (Fuentes-Mattei et al. 2010). The interleukin IL-6 is known to play an important role in acute inflammation and to be induced in response to environmental insults to the lung (Baulig et al. 2003; Thacker 2006).
Figure 1.
Comparison between control (CNT) and cytokines excretion after treatment with aqueous extracts of PM2.5 (0.6%, 20% and 50%) on human airway epithelial cells (BEAS-2B). Representation in time folds regarding control concentrations (*P < 0.05).
Table 2.
Total metal concentrations (ng/well) present in each aqueous extracts and cytokine secretions (pg/ml) (standard deviation; n = 3) produced by BEAS cells.
| Location | Treatment (0.60%)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trace element (ng/well)
|
Cytokines (pg/ml)
|
ng/well Total metal | |||||||||
| Al | As | Cd | Fe | Ni | Pb | V | IL-8 | IL-6 | MCP-1 | ||
| Control | – | – | – | – | – | – | – | 108.6 (±13.7) | 73.0 (±4.2) | 55.2 (±10.9) | – |
| Lab 1 | 4.26 | 0.10 | 0.00 | 0.74 | 0.26 | 0.27 | 0.10 | 122.8 (±8.6) | 63.4 (±2.5) | 69.0 (±4.2) | 5.73 |
| Lab 2 | 0.12 | 0.07 | 0.00 | 0.13 | 0.23 | 0.02 | 0.12 | 110.5 (±4.5) | 71.0 (±6.6) | 63.8 (±1.8) | 0.69 |
| Lab 3 | 0.49 | 0.05 | 0.00 | 0.74 | 0.22 | 0.06 | 0.05 | 103.7 (±12.8) | 67.1 (±8.9) | 61.3 (±0.9) | 1.61 |
| Outdoor | 2.15 | 0.03 | 0.02 | 7.09 | 0.89 | 0.18 | 3.85 | 124.4 (±8.7) | 59.7 (±8.5) | 69.3 (±1.0) | 14.20 |
| Treatment (20%) | |||||||||||
| Control | – | – | – | – | – | – | – | 306.9 (±23.3) | 129.2 (±4.9) | 47.1 (±2.0) | – |
| Lab 1 | 142.1 | 3.3 | 0.2 | 24.7 | 8.7 | 8.9 | 3.2 | 248.5 (±4.2) | 155.6 (±10.9) | 24.6 (±1.5) | 191.2 |
| Lab 2 | 2.6 | 2.5 | 0.1 | 4.3 | 7.7 | 0.8 | 3.9 | – | – | – | 21.8 |
| Lab 3 | 16.4 | 1.7 | 0.0 | 24.8 | 7.2 | 2.0 | 1.5 | 206.3 (±6.3) | 201.6 (±5.7) | 30.0 (±3.0) | 53.7 |
| Outdoor | 71.6 | 1.0 | 0.8 | 236.4 | 29.5 | 5.9 | 128.2 | 226.7 (±34.4) | 143.0 (±34.2) | 39.9 (±5.2) | 473.5 |
| Treatment (50%) | |||||||||||
| Control | 511.3 (±116.4) | 165.7 (±43.2) | 80.6 (±7.6) | ||||||||
| Lab 1 | 355.3 | 8.2 | 0.4 | 61.8 | 21.7 | 22.4 | 8.1 | 168.5 (±0.7) | 22.9 (±3.0) | 6.3 (±3.4) | 477.9 |
| Lab 2 | 9.9 | 6.2 | 0.1 | 10.7 | 19.2 | 1.9 | 9.7 | 177.0 (±29.7) | 25.6 (±0.8) | 3.0 (±0.1) | 57.7 |
| Lab 3 | 41.0 | 4.2 | 0.1 | 62.0 | 18.1 | 5.0 | 3.8 | 390.4 (±60.7) | 79.3 (±5.2) | 7.6 (±2.5) | 134.1 |
| Outdoor | 178.9 | 2.6 | 1.9 | 591.0 | 73.8 | 14.8 | 320.6 | 409.0 (±53.4) | 61.1 (±1.8) | 31.3 (±9.3) | 1183.6 |
Interesting, ICAM-1 has been suggested to have a significant role in inflammatory airway disease (Kose et al. 2007), and IL-6 is also known to have the capacity to induce ICAM-1 expression (Zambon et al. 2006). At relative low concentration (20%) of indoor PM2.5 aqueous extract exerts an increase in IL-6 secretion. Inhibitions of IL-8 and MPC-1 secretions suggest that no neutrophils or monocytes recruitment is triggered, in response to PM2.5 exposure. Further increase to 50% of PM2.5 aqueous extract resulted in inhibition of all three pro inflammatory cytokines, IL-6, IL-8 and MCP-1 (Figure 1c). The metal content available in these extracts was higher and a more interaction among them could increase the effects on the cytokine response. Overall, the difference in chemical composition of PM2.5 extracts from each indoor environment is probably the cause of different responses to cytokine productions.
While examining the aqueous metal concentration and correlating it with cytokine response the main effect appears to be associated with concentrations of As, Ni and V. At 20%, IL-6 showed strong negative correlations for Ni and V (r . *71.0) while at 50% for As and V (r . *70.7). For IL-8, the strongest positive relationship at 20% for Pb (r . 0.97) and V (r . 0.8) and negatively for As and V (r . 70.7) at 50% was observed. For MCP-1, at 20 and 50% an inhibition occurred and negative correlations were obtained for As (r . 70.9) and positive correlations for Al (r . 0.95). The positive correlations are interpreted as an increase in the specific metal and cytokine production; on the other hand, a negative correlation means that an increase in metal concentrations inhibits the cytokine production. Both V and As seem to inhibit the release of IL-6 and IL-8. Arsenic increased the inhibition of MCP-1 secretion but V did not influence this response. These results indicate that the secretion of specific cytokines could be related to a specific metal exposure and differ from cytokine to cytokine. However, these analyses were performed just to provide an idea of possible associations between metals and immune responses and were not designed to determine specific associations. These analyses provide the bases for future metal evaluation on immune responses in human lung cells. A detailed study on dose response and speciation of these metals needs to be conducted in order to validate and confirm these results. However our results are consistent with those found in the literature. High vanadium content in oil fly ash has been implicated in the increased production of IL-6 in NHBE cells (Baeza-Squiban et al. 1999), while transition metals could mediate a dose-dependent manner the release of IL-6 and TNF-a in monocytic cells after PM exposure (Osornio-Vargas et al. 2003). V and Ni seem to play a substantial role in toxic effects on alveolar epithelial cells (Dye et al. 1999). IL-6 is recognized by causing lung inflammation and injury due to air pollutants (Riley et al. 2003).
Other studies showed that low levels of IL-6 production in rat lung epithelial cells are related to high concentrations of metals (Riley et al. 2003). Studies of impact of metals on rat lung epithelial cells showed that some metals are more toxic based on TC50 values than others (V 4 Zn 4 Cu 4 Ni 4 Fe). However, interactions among these metals showed that Zn decreased the negative effect of V and Cu; and additive for Ni; but minimal for Fe (Riley et al. 2003). Therefore, the different effects caused by PM2.5 in each experiment could be due to variation in the chemical composition since interactions among metals could increase or decrease toxicological effects. A study performed in a smelter area, with high metal content (Fe, Cu, Zn, Pb, Ni and V) in an aqueous extract of PM10 showed an increase in IL-8 and IL-6 production by BEAS cells (Ghio 2004).
Conclusions
The results from this research shows that the concentration of PM2.5 found in the indoor and outdoor environments are not directly responsible for the immunological response caused on human lung epithelial cells. However, the real concern and significant variable is the heavy metal content associated with the PM2.5 from each particular location, which is relatively high in the indoor environment. Although no indoor guidelines or standards exist for metals in these indoor environments it is important to note that metal concentrations found in Lab 1 and 3, are relatively high. The source of heavy metals is mainly due to the type of activity and frequencies of these in each of the indoor environments. An accumulation of heavy metals indoor PM2.5 may result from a series of different factors: (1) Specific indoor sources; (2) settlement of infiltrated outdoor airborne particles, and (3) adsorption differencesof metals due to specific indoor environmental conditions such as humidity, available surfaces and air circulation patterns.
Exposure to aqueous extract of PM2.5 to human airway bronchial epithelial cells tends to inhibit proinflammatory cytokines IL-6 IL-8 and MCP-1 at high concentrations of 50% of extract. Intermediate concentrations (20%) also showed an inhibition of IL-8 and MCP-1, but a clear induction of IL-6. Interleukin IL-6 is a multifunctional cytokine that influences the expression of cell adhesion molecules and which can mediate the movement of inflammatory cells from the blood circulation to the airway epithelium or lumen. In this study, As and V seem to exhibit the highest influence on cytokine secretion or its inhibition on human epithelium cells.
This study was designed to gather chemical and biological data in order to provide irrefutable evidence between PM2.5 differences among various indoor environments within the same building. Although specific cytotoxicity analyses were not performed on the PM2.5 aqueous extracts obtained in this study, other studies have reported as much as 50 mg/ml in PM2.5 polar organic extracts from a rural outdoor (Fuentes-Mattei et al. 2010) and in aqueous extracts from PM10 from both rural and urban sites with no evident cytotoxicity (Ortiz-Martínez et al. 2010).
In addition, this study was not designed to determine specific associations of heavy metals nor biological constituents encountered in PM2.5 with cytokine secretion in human lung cells. However, the study provides the fundamental data needed for future metal evaluation on immune responses using human lung cells in indoor environments. We believe that the data obtained in this study is relevant and adds to the amount of existing knowledge in the field of indoor air.
Acknowledgments
This work was supported in part by the grants: Minority Biomedical Research Support –Support of Continuous Research Excellence (MBRS-SCORE), National Institutes of Heath [grant number 5S06-GM008224]; Research Center in Minority Institutions (RCMI) Program at the University of Puerto Rico Medical Sciences Campus from the National Center for Research Resources, National Institutes of Heath [grant number G12RR03051]; Minority Biomedical Research Support – Research Initiative for Scientific Enhancement (MBRSRISE), National Institutes of Heath [grant numbers 5R25GM061838-08, 2R25GM061838-09]; and the Center for Environmental and Toxicological Research at the University of Puerto Rico Medical Sciences Campus.
References
- Baeza-Squiban A, Bonvallot V, Boland S, Marano F. Airborne particles evoke an inflammatory response in human airway epithelium: Activation of transcription factors. Cell Biol Toxicol. 1999;15:375–380. doi: 10.1023/a:1007653900063. [DOI] [PubMed] [Google Scholar]
- Baulig A, Sourdeval M, Meyer M, Marano F, Baeza-Squiban A. Biological effects of atmospheric particles on human bronchial epithelial cells. Comparison with diesel exhaust particles. Toxicol In Vitro. 2003;17:567–573. doi: 10.1016/s0887-2333(03)00115-2. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Yuzbasiyan-Gurkan V. Wilson disease. Medicine. 1992;71:139–164. doi: 10.1097/00005792-199205000-00004. [DOI] [PubMed] [Google Scholar]
- Costa DL, Dreher KL. Bioavailable transition metals in particulate matter mediate cardiopulmonary injury in healthy and compromised animal models. Environ Health Perspect. 1997;105:1053–1060. doi: 10.1289/ehp.97105s51053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ Health Perspect. 2005;113:934–946. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery DW, Pope CA., III Acute respiratory effects of particulate air pollution. Ann Rev Pub Health. 1994;15:107–132. doi: 10.1146/annurev.pu.15.050194.000543. [DOI] [PubMed] [Google Scholar]
- Dreher KL, Jaskot RH, Lehmann JR, Richards JH, McGee JK, Ghio AJ, Costa DLJ. Soluble transition metals mediate residual oil fly ash induced acute lung injury. J Toxicol Environ Health. 1997;50:285–305. [PubMed] [Google Scholar]
- Dye JA, Adler KB, Richards JH, Dreher KL. Role of soluble metals in oil fly ash-induced airway epithelial injury and cytokine gene expression. Am J Physiol Lung Cel Mol Physiol. 1999;277:L498–L510. doi: 10.1152/ajplung.1999.277.3.L498. [DOI] [PubMed] [Google Scholar]
- Dye JA, Lehman JR, McGee JK, Winset DW, Ledbetter AD, Everitt JI, Ghio AJ, Costa DL. Acute pulmonary toxicity of particle matter filter extracts in rats coherence with epidemiologic studies in Utah Valley residents. Environ Health Perspect. 2001;109:395–403. doi: 10.1289/ehp.01109s3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton MW, Ghio AJ, Samet JM, Carson JL, Carter JD, Devlin RB. Effects of aqueous extracts of PM(10) filters from the Utah valley on human airway epithelial cells. Am J Physiol. 1999;277:L960–967. doi: 10.1152/ajplung.1999.277.5.L960. [DOI] [PubMed] [Google Scholar]
- Fuentes-Mattei E, Rivera E, Gioda A, Jimenez-Velez J. Immunological markers resulting from organic extract exposure of airborne fine particulate matter (PM2.5) in Puerto Rico, using human bronchial epithelial cells (BEAS-2B) Toxic Appl Pharm. 2010;243:381–389. doi: 10.1016/j.taap.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Hogg JC, Vincent R, Van Eeden S. Particulate matter induces cytokine expression in human bronchial epithelial cells. Am J Resp Cell and Mol Biol. 2001;25:265– 271. doi: 10.1165/ajrcmb.25.3.4445. [DOI] [PubMed] [Google Scholar]
- Gemenetzis P, Moussas P, Arditsoglou A, Samara C. Mass concentration and elemental composition of indoor PM2.5 and PM10 in University rooms in Thessaloniki, northern Greece. Atmos Environ. 2006;17:3195–3206. [Google Scholar]
- Ghio AJ, Stonehuerner J, Pritchard RJ, Piantadosi CA, Quigley DR, Dreher KL, Costa DL. Humic-like substances in air pollution particulates correlate with concentrations of transition metals and oxidant generation. Inhal Toxicol. 1996;8:479– 494. [Google Scholar]
- Ghio AJ, Stonehuerner J, Dailey LA, Carter JD. Metals associated with both the wáter soluble and insoluble fractions of an ambient air pollution particle catalyze an oxidative stress. Inhal Toxicol. 1999;11:37–49. doi: 10.1080/089583799197258. [DOI] [PubMed] [Google Scholar]
- Ghio AJ. Biological effects of Utah Valley ambient air particles in humans: A review. J Aerosol Med. 2004;17:157–164. doi: 10.1089/0894268041457200. [DOI] [PubMed] [Google Scholar]
- Gioda A, Perez U, Rosa Z, Jimenez-Velez B. Particulate matter (PM10 and PM2.5) from different areas of Puerto Rico. Fres Environ Bull. 2007;16:8761–8767. [Google Scholar]
- Gioda A, Perez U, Rosa Z, Jimenez-Velez BD. Concentration of trace elements in airborne PM10 from Jobos Bay National Estuary, Puerto Rico. Water Air Soil Poll. 2006;174:141–159. [Google Scholar]
- Gordon GE, Pierson WR, Daisey JM, Lioy PJ, Cooper JA, Watson JG, Cass GR. Considerations for design of source apportionment studies. Atmos Environ. 1984;18:1567– 1582. [Google Scholar]
- Graney JR, Landis MS, Norris GA. Concentrations and solubility of metals from indoor and personal exposure PM2.5 samples. Atmos Environ. 2004;38:237–247. [Google Scholar]
- Indoor Air Quality and Comfort Parameters. Appendix 3, August 2003. Alberta, Canada: Technical Services Branch; 2003. [Google Scholar]
- Kennedy T, Ghio AJ, Reed W, Samet J, Zagorski J, Quay J, Carter J, Dailey L, Hoidal JR, Devlin RB. Copper-dependent inflammation and nuclear factor-kappaB activation by particulate air pollution. Am J Respir Cell Mol Biol. 1998;19:366–378. doi: 10.1165/ajrcmb.19.3.3042. [DOI] [PubMed] [Google Scholar]
- Kodavanti UP, Hauser R, Christiani DC, Meng ZH, McGee J, Ledbetter A, Richards J, Costa DL. Pulmonary responses to oil fly ash particles in the rat differ by virtue of their specific soluble metals. Toxicol Sci. 1998;43:204–212. doi: 10.1006/toxs.1998.2460. [DOI] [PubMed] [Google Scholar]
- Kose S, Karaman O, Islekel H, Uzuner N, Babayigit A, Olmez D, Altun Z, Turgut S, Tezcan D. Circulating adhesion molecules in sera of asthmatic children before and after steroid therapy. Allergy Asthma Proc. 2007;28:199–203. doi: 10.2500/aap.2007.28.2944. [DOI] [PubMed] [Google Scholar]
- Meszaros E, Barcza T, Gelencser A, Hlavay J, Kiss GY, Krivacsy Z, Molnar A, Polyak K. Size distributions of inorganic and organic species in the atmospheric aerosol in Hungary. J Aerosol Sci. 1997;28:1163–1175. [Google Scholar]
- Monn C, Becker S. Cytotoxicity and induction of proinflamatory cytokines from human monocytes exposed to fine (PM2.5) and coarse particles (PM10–2.5) in indoor air. Toxicol Appl Pharmacol. 1999;155:245–252. doi: 10.1006/taap.1998.8591. [DOI] [PubMed] [Google Scholar]
- Ortiz-Martínez M, Rivera-Ramírez E, Méndez-Torres L, Jiménez-Vélez BD. Role of chemical and biological constituents of PM10 from Saharan Dust in the exacerbation of asthma in Puerto Rico. In: Theophanides M, Theophanides T, editors. Biodiversity science for humanity. Athens: Athens Institute for Education and Research (ATINER); 2010. pp. 101–118. [Google Scholar]
- Osornio-Vargas AR, Bonner JC, Alfaro-Moreno E, Martinez L, Garcia-Cuellar C, Poncede-Leon Rosales S, Miranda J, Rosas I. Proinflammatory and cytotoxic effects of Mexico City air pollution particulate matter in vitro are dependent on particle size and composition. Environ Health Perspect. 2003;111:1289–1293. doi: 10.1289/ehp.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Pritchard RJ, Ghio AJ, Lehmann JR, Winsett DW, Tepper JS, Park P, Gilmour MI, Dreher KL, Costa DL. Oxidant generation and lung injury after particulate air pollutant exposure increase with the concentrations of associated metals. Inhal Toxicol. 1996;8:457–477. [Google Scholar]
- Riley MR, Boesewetter DE, Kim AM, Sirvent FP. Effects of metals Cu, Fe, Ni, V, and Zn on rat lung epithelial cells. Toxicology. 2003;190:171–184. doi: 10.1016/s0300-483x(03)00162-8. [DOI] [PubMed] [Google Scholar]
- Samet JM, Zeger SL, Dominici F. Research report 94, part II. Cambridge, MA: Health Effects Institute; 2001. The national morbidity, mortality, and air pollution study. Part II, Morbidity and mortality from air pollution in the United States; pp. 27–29. [PubMed] [Google Scholar]
- Saskia C, Van Der Zee SC, Hoek G, Harrssema H, Brunekreef B. Characterization of particulate air pollution in urban and non-urban areas in the Netherlands. Atmos Environ. 1998;32:3717–3729. [Google Scholar]
- Schaumann F, Borm PJA, Herbrich A, Knoch J. Metal-rich ambient particles cause airway inflamation. Am J Respirat Crit Care Med. 2004;170:898–892. doi: 10.1164/rccm.200403-423OC. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Dockery DW, Neas LM. Is daily mortality associated specifically with fine particles? J Air Waste Manag Assoc. 1996;46:927–939. [PubMed] [Google Scholar]
- Thacker EL. Lung inflammatory responses. Veter Res. 2006;37:469–486. doi: 10.1051/vetres:2006011. [DOI] [PubMed] [Google Scholar]
- Ulrich MM, Alink GM, Kumarathasan P, Vincent R, Boere AJ, Cassee FR. Health effects and time course of particulate matter on the cardiopulmonary system in rats with lung inflammation. J Toxicol Environ Health. 2002;65:1571–1595. doi: 10.1080/00984100290071676. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (US EPA) Human health evaluation manual. I. Washington, DC: Office of Emergency and Remedial Response; 1991. Standard default exposure factors. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA) 1986–1996 Atomic Absorption, Furnace Technique Methods’, 1978: Aluminum (Method 202.2); 1986: Lead (Method 7421), Vanadium (Method 7911); 1992: Copper (Method 7211), Iron (Method 7381); 1994: Arsenic (Method 7060 A), Cadmium (Method 7131 A); 1996: Nickel (Method 7521); Atomic Absorption, Direct Absorption, 1986: Potassium (Method 7610) and Sodium (Method 7770) [Google Scholar]
- Zambon A, Gervois P, Pauletto P, Fruchart JC, Staels B. Modulation of hepatic inflammatory risk markers of cardiovascular diseases by PPAR-alpha activators: Clinical and experimental evidence. Arterioscler Thromb Vasc Biol. 2006;26:977–986. doi: 10.1161/01.ATV.0000204327.96431.9a. [DOI] [PubMed] [Google Scholar]