INTRODUCTION
An aging population has increased demand for orthopaedic implants to restore function. Lumbar and cervical interbody fusion surgery is a commonly used procedure for many types of spine pathology. Advantages to fusing the disc space anteriorly include the fact that the graft has compression loads applied to it (Wolff’s Law), it has excellent vascularity, and it can hold large quantities of bone graft. Another advantage is that there is ready access to mesenchymal stem cells and osteoprogenitor cells, which help in the healing and osseointegration of the implant. While many factors contribute to the success of a spinal fusion procedure, including surgical technique, biologics or bone grafting materials, and the mechanical and structural properties of an interbody device, contributions of the implant material to inter-vertebral bone formation are not well known.
Currently, there are multiple material choices for an interbody implant. Of these, two of the most popular synthetic implant materials are titanium (typically titanium-aluminum-vanadium alloy [Ti6Al4V]) and poly-ether-ether-ketone (PEEK) (1–3). In addition to acting as a spacer between vertebrae, interbody implants provide surfaces that may have impacts on peri-implant bone formation. Studies examining bone formation adjacent to dental and total joint implant surfaces indicate that lack of bone apposition may lead to implant micromotion and loosening with clinical failure (6, 7). Whereas implants fabricated from Ti6Al4V result in good bone-to-implant contact and are osseointegrated into the surrounding bone (8–10), PEEK does not integrate well with the surrounding bone, and instead may form a fibrous connective interface (3–5).
Development of a fusion mass is required for spine fusion and one role of an interbody device is to support osteogenesis across the interbody space. Bone graft materials and biologics facilitate this process by providing a surface and bioactive factors that promote migration of osteoblast progenitor cells and osteoblast differentiation. Macroscale properties, such as implant geometry are important with respect to vascular ingrowth but implant topography at the microscale is important for osteoblastic differentiation, osteoid synthesis and mineralization. In vivo success of titanium alloy implants may be due in part to a stimulatory effect of the device surface on osteoblastic differentiation. In vitro studies show that this effect is greater in osteoblasts cultured on titanium alloy with a micron-scale rough surface texture in comparison to smooth or machined titanium alloy (11, 12). In vivo observations support these in vitro results. Grit-blasted titanium alloy pedicle screws showed a 100% increase in pull-out force in sheep spines when compared with smooth screws (12).
Surface texture is also an important factor in normal bone formation. During healing and remodeling of bone, osteoblasts mature and mineralize their extracellular matrix in areas of the bone that have been pre-conditioned by osteoclasts. The action of the osteoclasts creates micron- and submicron-scale roughness (13). Most importantly, cells on rough surfaces produce increased levels of factors that increase osteogenesis in comparison to cells on smooth surfaces; these factors include transforming growth factor beta-1 (TGF-β1) and bone morphogenetic proteins (BMPs) (14, 15). This suggests that surface texture is an important factor in bone formation.
Bone formation is a result of several processes that work in concert to achieve net new bone. Osteoclast number and/or activity need to decrease in order to achieve less bone remodeling than new bone formation. When osteoblasts grow on microtextured titanium surfaces, they increase production of local factors that reduce osteoclastic bone remodeling in comparison with osteoblasts grown on smooth surfaces (16). These factors include osteoprotegerin (OPG), a decoy receptor for receptor activator for nuclear factor κB (RANK) ligand, which modulates osteoclast activity. It is not known if either titanium alloy or PEEK elicits a similar outcome.
Angiogenesis, new blood vessel formation stemming from existing vasculature, is important in bone formation, fracture healing, bone regeneration, and osseointegration (17–19). Angiogenic factors must create the vascularity needed to support bone creation. Angiogenesis is promoted by several growth factors including vascular endothelial growth factor-A (VEGF-A), fibroblast growth factor-2 (FGF-2), and angiopoietin-1 (Ang-1) (20–22). Studies examining the role of surface micro-architecture on osteoblast production of these factors showed that cells cultured on rough micro-textured titanium substrates produce higher levels of VEGF-A and FGF-2 (23). The results of these studies demonstrate that chemistry and microtexture of surfaces affect cell response, bringing into question how biomaterials used in interbody fusion, PEEK and titanium alloy, differ.
Osteoblasts interact with proteins adsorbed on implant surfaces through integrins, heterodimeric transmembrane receptors that bind specific extracellular matrix components. As cells adopt a more differentiated phenotype, complex interactions between cells and extracellular matrix occur, strengthening cell adhesion and possibly leading to improved biomaterial osseointegration (24, 25). While less differentiated osteoblasts express the integrin pair α5β1, the more differentiated cells on titanium and titanium alloy express α2β1, which recognizes collagen (26–28). Several studies show that levels of integrin subunits α2 and β1 increase on rough titanium surfaces compared to smooth titanium and are required for enhanced osteoblast maturation on these surfaces (26–28). It is not known if osteoblasts on PEEK behave in a similar manner.
The aim of this study was to compare osteogenic and angiogenic factor production by human osteoblast-like cells cultured on smooth or microtextured (rough) titanium alloy substrates with cells cultured on PEEK, factors that regulate the cells via autocrine and paracrine pathways and contribute to peri-implant bone formation (16, 29, 30), and correlate these results to expression of specific integrin extracellular matrix receptors. To determine this, we assessed whether cells on these surfaces presented a mature osteoblast phenotype and whether secretion of local factors and angiogenic factors were affected by the chemistry and topography of the substrate. In addition, we investigated the types of integrins expressed by the cells as a first step in understanding why osteoblasts respond differentially to these two materials used in interbody fusions.
METHODS
Disk Preparation
Surgical grade titanium alloy (Ti6Al4V) and poly-ether-ether-ketone (PEEK) disks were provided by Titan Spine, LLC (Mequon, WI). Titanium alloy disks (15mm diameter) were machined, yielding a smooth surface texture (sTiAlV). Alternatively, the machined titanium alloy disks were etched with a proprietary process to create titanium alloy disks with a rough microtexture (rTiAlV). PEEK substrates were machined. All disks were ultrasonically cleaned, sonicated in ultrapure water (Millipore, Billerica, MA), and sterilized by autoclave (Tuttnauer, Hauppauge, NY) for 20 minutes at 121°C and 15 PSI b efore use in cell culture studies.
Disk Characterization
Scanning electron microscopy (SEM) and laser confocal microscopy (LCM) were used to characterize the surface topographies of the titanium alloy and PEEK disks. In addition, the chemistry of the surface was determined using energy dispersive X-ray spectroscopy (EDX) and sessile-drop contact angle. The detailed description of the methods used and the results have been published previously (31). Briefly, the PEEK disks had a machined surface finish with parallel grooves due to processing and no other distinctive features; sTiAlV disks also had a machined surface finish with shallower grooves than were seen on PEEK surfaces; and rTiAlV disks were characterized by 100–300 μm craters with superimposed micron-scale features. The roughness of each surface was determined by LCM [Sa = 0.09 ± 0.01 μm for sTiAlV; Sa = 0.43 ± 0.07 μm for PEEK; and Sa = 1.81 ± 0.51 μm for rTiAlV]. EDX measurements confirmed that PEEK and the titanium alloy substrates had different chemistries. As expected, PEEK samples were composed of C and O. Both sTiAlV and rTiAlV were composed of Ti, Al, and V with no significant compositional differences between the two. Surface wettability assessed by contact angle measurements showed that all three substrates presented similar contact angles.
Cell Culture
Human MG63 cells (American Type Culture Collection, Manassas, VA) were used as a model for these studies. They have been well studied in cell response to titanium (32) and results correlate well with results obtained from in vitro studies using normal human osteoblasts, fetal and adult rat calvarial osteoblasts, and neonatal mouse calvarial osteoblasts (33–37) as well as with in vivo osseointegration of dental and orthopaedic implants (11, 12, 25). Cells were cultured at an initial density of 10,000 cells/cm2 on tissue culture polystyrene (TCPS, the surface of the cell culture plate wells), PEEK, sTiAlV, and rTiAlV. Medium (Dulbecco’s modification of Eagle’s medium [cellgro®, MediaTech, Manassas, VA] containing 10% fetal bovine serum [Hyclone, Thermo Scientific, Pittsburg, PA], and 1% penicillin-streptomycin [Gibco, Invitrogen, Carlsbad, CA]) was changed 24 hours after plating and then every 48 hours thereafter. When cultures reached confluence on TCPS, the cells on all surfaces were treated for an additional 24 hours with fresh medium. To ensure that cells were removed completely from the surfaces, the cells were released with two sequential 10-minute incubations in 0.25% trypsin-EDTA (Invitrogen) at 37°C and counted (Z2 Counter, Beckm an Coulter, Fullerton, CA).
The cell culture model was validated by assessing cell number, alkaline phosphatase specific activity of isolated cells and levels of osteocalcin in the conditioned medium as reported previously (31). Briefly, in comparison to growth on TCPS, cell number was reduced on the test substrates (TCPS>PEEK>sTiAlV>rTiAlV). Alkaline phosphatase specific activity was increased on the titanium alloy surfaces compared to TCPS and PEEK (TCPS=PEEK<sTiAlV<rTiAlV). Similarly, osteocalcin was elevated on the titanium alloy substrates in comparison to TCPS and PEEK, but there was no additional effect of roughness (TCPS=PEEK<sTiAlV, rTiAlV).
Analysis of Secreted Factors
Conditioned media were collected and assayed for secreted proteins and factors as described previously (33). OPG, VEGF-A, FGF-2, and Ang-1 were assayed using commercially available enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, MN) following manufacturer’s instructions. Active TGF-β1 was measured prior to acidification of the conditioned media using a commercially available ELISA (R&D Systems). Total TGF-β1 was measured after acidifying the media and latent TGF-β1 was defined as total TGF-β1 minus active TGF-β1. Results of immunoassays were normalized to total cell number.
Integrin Expression
Changes in integrin mRNA expression were measured using real-time PCR. When MG63 cells reached confluence on TCPS, all cultures were incubated for an additional 12 hours with fresh medium. RNA was isolated using TRIzol® (Invitrogen) and quantified using a Nanodrop spectrophotometer (Thermo Scientific, Waltham, MA). 250 ng of RNA was reverse transcribed to cDNA templates using High Capacity Reverse Transcription cDNA kit (Applied Biosystems, Carlsbad, CA). Gene specific primers and Power Sybr® Green Master Mix (Applied Biosystems) were used to quantify mRNA expression using the StepOnePlus Real-time PCR System (Applied Biosystems). Starting mRNA quantities were quantified using a standard curve of mRNA created from known dilutions of MG63 cells cultured on TCPS and related to threshold cycle values. Genes are presented as normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH, F:5′-GCTCTCCAGAACATCATCC-3′; R:5′-TGCTTCACCACCTTCTTG-3′). Primers for integrin α1 (ITGA1, F:5′-CACTCGTAAATGCCAAGAAAAG-3′; R:5′-TAGAACCCAACACAAAGATGC-3′); integrin α2 (ITGA2, F:5′-ACTGTTCAAGGAGGAGAC-3′; R:5′-GGTCAAAGGCTTGTTTAGG-3′); integrin α5 (ITGA5, F:5′-ATCTGTGTGCCTGACCTG-3′; R:5′-AAGTTCCCTGGGTGTCTG-3′); integrin αv (ITGAV, F:5′-GTTGCTACTGGCTGTTTTGG-3′; R:5′-CTGCTCCCTTTCTTGTTCTTC-3′); integrin β1 (ITGB1, F:5′-ATTACTCAGATCCAACCAC-3′; R:5′-TCCTCCTCATTTCATTCATC-3′); and integrin β3 (ITGB3, F:5′-AATGCCACCTGCCTCAAC-3′; R:5′-GCTCACCGTGTCTCCAATC-3′) were designed using Beacon Designer™ (Premier Biosoft, Palo Alto, CA) and synthesized by Eurofins MWG Operon (Huntsville, AL).
Statistical Analysis
For each experiment, there were six independent cultures per type of surface. Experiments were repeated to ensure validity of the results. Data presented are from one representative experiment. Data were analyzed by ANOVA; when statistical differences were detected, Student’s t-test was used with post hoc correction for multiple comparisons using Tukey’s method. P<0.05 was considered significant.
RESULTS
Effects on Factors Modulating Osteoclast Activity
OPG production was sensitive to surface properties. Levels were increased in cultures grown on PEEK and smooth titanium alloy (sTiAlV) compared to TCPS (p<0.05). However, when cells were grown on rough titanium alloy (rTiAlV), production increased by 100% in comparison to TCPS and PEEK and by 30% in comparison to sTiAlV (Fig. 1A, p<0.05). Active TGF-β1 was more than 100% higher on titanium alloy surfaces compared to either TCPS or PEEK (Fig. 1B, p<0.05). Latent TGF-β1 was higher on sTiAlV than PEEK and further increased in cells on rTiAlV (Fig. 1C, p<0.05).
Figure 1.
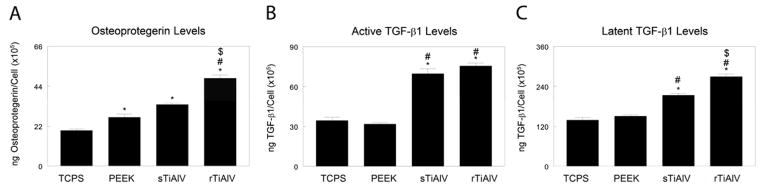
Secreted osteoprotegerin (A), active TGF-β1 (B), and latent TGF-β1 (C) were measured in the conditioned media of cells cultured on TCPS, PEEK, smooth titanium alloy (sTiAlV), or rough titanium alloy (rTiAlV). Levels were normalized to total cell number. *p<0.05, versus TCPS; #p<0.05, versus PEEK; $p<0.05, versus sTiAlV. TCPS, tissue culture polystyrene; PEEK, poly-ether-ether-ketone; sTiAlV, smooth Ti6Al4V; rTiAlV, roughened Ti6Al4V.
Angiogenic Factor Production
All experimental surfaces supported higher levels of VEGF than cells cultured on TCPS (Fig. 2A). However, cells on sTiAlV produced higher levels of VEGF than cells on PEEK, and rTiAlV enhanced this effect (p<0.05). Culture on TCPS and PEEK produced similar levels of FGF-2, but levels were 75% higher on sTiAlV and 100% higher on rTiAlV than on PEEK (Fig. 2B, p<0.05). Levels of Ang-1 decreased on PEEK in comparison to TCPS, but culture on titanium alloy, both smooth and rough, increased Ang-1 50% over cells on TCPS (Fig. 2C, p<0.05). The results show that cells cultured on titanium alloy produce higher levels of angiogenic factors than cells on PEEK, but the effect on VEGF and FGF-2 was enhanced on rough titanium alloy substrates.
Figure 2.
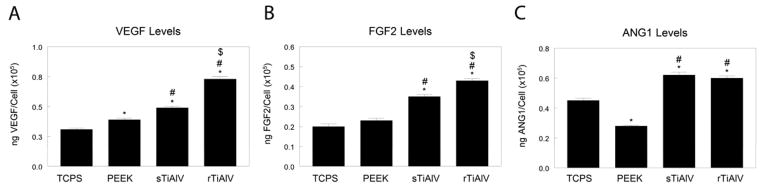
Secreted VEGF-A (A), FGF-2 (B), and angiopoietin-1 (ANG1, C) were measured in the conditioned media of cells cultured on TCPS, PEEK, smooth titanium alloy (sTiAlV), or rough titanium alloy (rTiAlV). Levels were normalized to total cell number. *p<0.05, versus TCPS; #p<0.05, versus PEEK; $p<0.05, versus sTiAlV. TCPS, tissue culture polystyrene; PEEK, poly-ether-ether-ketone; sTiAlV, smooth Ti6Al4V; rTiAlV, roughened Ti6Al4V.
Integrin Expression
Culture on sTiAlV and rTiAlV substrates stimulated higher expression of ITGA1 mRNA (Table 1), ITGA2 (Fig. 3B), ITGAV (Table 1), and ITGB1 (Fig. 3D) than on TCPS or PEEK (p<0.05). Moreover, ITGA2 expression was greater on rTiAlV than on sTiAlV (Fig. 3B, p<0.05). Expression of ITGA5 was higher on PEEK than on TCPS, reduced on titanium alloy surfaces in comparison to TCPS, and further reduced on rTiAlV was further reduced in comparison to sTiAlV (Table 1, p<0.05). Expression of ITGB3 was lower on PEEK than on TCPS, sTiAlV, or rTiAlV (Table 1, p<0.05).
Table 1.
Expression of mRNA for ITGA1, ITGA5, ITGAV, and ITGB3. Human MG63 osteoblast-like cells were harvested 12 hours after confluence on TCPS. Expression of mRNA for ITGA1, ITGA5, ITGAV, and ITGB3 were measured by real-time quantitative PCR of cells cultured on TCPS, PEEK, smooth titanium alloy (sTiAlV), or rough titanium alloy (rTiAlV). Expression is normalized to GAPDH.
| Surface | Gene Expression (Mean ± SEM) | |||
|---|---|---|---|---|
| ITGA1 | ITGA5 | ITGAV | ITGB3 | |
| TCPS | 0.935±0.057 | 1.403±0.026 | 1.008±0.030 | 1.211±0.040 |
| PEEK | 0.875±0.128 | 1.686±0.022* | 0.829±0.020 | 0.862±0.102* |
| sTiAlV | 1.407±0.114*,# | 1.115±0.023*,# | 1.402±0.079*,# | 1.301±0.091# |
| rTiAlV | 1.577±0.108*,# | 0.892±0.023*,#,$ | 1.569±0.037*,# | 1.161±0.059 |
p<0.05, v. TCPS;
p<0.05, v. PEEK;
p<0.05, v. sTiAlV.
Figure 3.
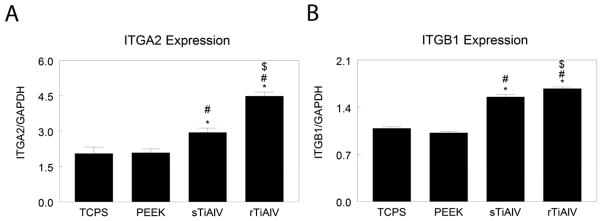
Expression of messenger RNA for ITGA2 (A) and ITGB1 (B) were measured by real-time qPCR of cells cultured on TCPS, PEEK, smooth titanium alloy (sTiAlV), or rough titanium alloy (rTiAlV). Expression is normalized to GAPDH. *p<0.05, versus TCPS; #p<0.05, versus PEEK; $p<0.05, versus sTiAlV. TCPS, tissue culture polystyrene; BMP, bone morphogenetic protein; qPCR, quantitative polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PEEK, poly-ether-ether-ketone; sTiAlV, smooth Ti6Al4V; rTiAlV, roughened Ti6Al4V.
DISCUSSION
Studies using both commercially pure titanium and titanium alloys (i.e., Ti6Al4V) have demonstrated in vitro that increased surface roughness enhances osteoblast maturation and production of local factors associated with osteogenesis and in vivo that the same topographies increase bone-to-implant contact and torque removal forces (12, 23, 38). We previously showed that osteoblasts on rough titanium substrates produce angiogenic factors (23). The present study indicates that osteoblasts also produced significantly higher VEGF-A and FGF-2 levels on smooth and roughened titanium alloy than on PEEK, an effect significantly more robust on rough titanium alloy. These results suggest that peri-implant osteoblasts may create an environment that modulates angiogenesis around the implant and in the adjacent tissue, indicating that the chemistry of the implant plays an important role in determining the nature of the angiogenic milieu. Interestingly, cells grown on PEEK surfaces did not stimulate production of angiogenic factors.
The importance of angiogenesis in bone homeostasis is well appreciated. Vasculature is required for delivery of nutrients and removal of wastes, and provides a source of multipotent cells for tissue regeneration and remodeling (39). The factors measured in this study play distinct but cooperative roles in the process. VEGF-A is produced by diverse cells, including osteoblasts, and is one of the most important initiators of the signaling cascade during neovascularization in endothelial cells (40). FGF-2, a soluble factor with autocrine and paracrine functions, induces proliferation and migration of endothelial cells and is considered a key factor in angiogenesis (41). Ang-1 is known to control late stages of blood vessel formation, such as stabilization of the endothelial sprout and endothelial interaction with pericytes (42). Our results suggest that failure of osseointegration observed with PEEK implants is associated with reduced ability of cells on the implant surface to generate an environment rich in these factors.
Our results suggest that angiogenic factor production is associated with osteoblast maturation state. As we have noted previously, MG63 cells exhibit a more differentiated phenotype on rough titanium alloy, characterized by reduced cell number and increased osteocalcin production (31). This suggests that osteoblast differentiation is sensitive to general micron-scale elements. PEEK surfaces differ both chemically and physically from titanium alloy, so it is difficult to ascribe a specific parameter or feature of the surface to the lack of an angiogenic response. Cellular responses studies of PEEK have been limited to cell attachment and proliferation, but we previously showed that MG63 cells and normal human osteoblasts on PEEK do not exhibit increased alkaline phosphatase or osteocalcin production typical of differentiated osteoblast (31). Moreover, studies have attempted to modify PEEK using coatings of hydroxyapatite (43), titanium (44), or diamond-like carbon (45) to improve cellular response, supporting our findings that PEEK does not induce an osteogenic response.
In this experimental in vitro study, MG63 cells grown on roughened titanium alloy increased levels of active and latent TGF-β1 and OPG in their media, both of which are associated with bone formation. Osteoblasts produce TGF-β1 in latent form and store it in the extracellular matrix. In its active form, TGF-β1 stimulates osteoblast differentiation and matrix synthesis (46) while inhibiting osteoclast activity (47). OPG is produced by osteoblasts as a decoy receptor for receptor activator of nuclear factor κB (RANK) ligand, thereby reducing osteoblast-dependent osteoclast activation (48). Together these factors result in net new bone formation. This microenvironment may enhance bone formation while regulating bone remodeling in areas adjacent to the implant.
We previously showed that osteoblast differentiation and production of VEGF-A and FGF-2 on microtextured titanium are mediated by α2β1 integrin signaling (23). Here we show that mRNAs for integrins α1, α2, αv, and β1 were upregulated in cells grown on titanium alloy surfaces. Interestingly, ITGA2 and ITGB1 expression was higher on roughened titanium alloy surfaces than smooth surfaces, as was noted in cells grown on titanium (26). MG63 cells were grown on PEEK express similar integrin subunits as seen on TCPS, specifically α5, which is associated with cell attachment and proliferation but not with differentiation (27). These results may explain why PEEK failed to induce osteoblast maturation or yield an osteogenic environment.
CONCLUSIONS
This experimental study demonstrates that roughened titanium alloy stimulates an angiogenic and osteogenic environment with factors important in bone formation and remodeling. This osteogenic environment may enhance bone formation, implant stability, and fusion. Clinically, these findings point to the possibility that surface texture and material composition of spinal interbody implants can be manipulated to maximize the endogenous production of bone growth and angiogenic factors.
Acknowledgments
Sources of Funding:
This research was supported by US PHS grant NIH AR052102. Titan Spine, LLC provided the Ti6Al4V and PEEK substrates as a gift.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Toth JM, Wang M, Estes BT, Scifert JL, Seim HB, 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324–34.16115677. doi: 10.1016/j.biomaterials.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Bruner HJ, Guan Y, Yoganandan N, Pintar FA, Maiman DJ, Slivka MA. Biomechanics of polyaryletherketone rod composites and titanium rods for posterior lumbosacral instrumentation. Presented at the 2010 Joint Spine Section Meeting. Laboratory investigation. J Neurosurg Spine. 2010;13(6):766–72.21121756. doi: 10.3171/2010.5.SPINE09948. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28(32):4845–69.17686513. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos ER, Goss DG, Morcom RK, Fraser RD. Radiologic assessment of interbody fusion using carbon fiber cages. Spine (Phila Pa 1976) 2003;28(10):997–1001.12768137. doi: 10.1097/01.BRS.0000061988.93175.74. [DOI] [PubMed] [Google Scholar]
- 5.Anjarwalla NK, Morcom RK, Fraser RD. Supplementary stabilization with anterior lumbar intervertebral fusion--a radiologic review. Spine (Phila Pa 1976) 2006;31(11):1281–7.16688045. doi: 10.1097/01.brs.0000217692.90624.ab. [DOI] [PubMed] [Google Scholar]
- 6.Bauer TW, Schils J. The pathology of total joint arthroplasty. II. Mechanisms of implant failure. Skeletal Radiol. 1999;28(9):483–97.10525792. doi: 10.1007/s002560050552. [DOI] [PubMed] [Google Scholar]
- 7.Brunski JB. In vivo bone response to biomechanical loading at the bone/dental-implant interface. Adv Dent Res. 1999;13:99–119.11276755. doi: 10.1177/08959374990130012301. [DOI] [PubMed] [Google Scholar]
- 8.Stenport VF, Johansson CB. Evaluations of bone tissue integration to pure and alloyed titanium implants. Clin Implant Dent Relat Res. 2008;10(3):191–9.18241219. doi: 10.1111/j.1708-8208.2007.00077.x. [DOI] [PubMed] [Google Scholar]
- 9.De Leonardis D, Garg AK, Pecora GE. Osseointegration of rough acid-etched titanium implants: 5-year follow-up of 100 minimatic implants. Int J Oral Maxillofac Implants. 1999;14(3):384–91.10379112. [PubMed] [Google Scholar]
- 10.Linder L. Osseointegration of metallic implants. I. Light microscopy in the rabbit. Acta Orthop Scand. 1989;60(2):129–34.2658464. doi: 10.3109/17453678909149239. [DOI] [PubMed] [Google Scholar]
- 11.Lincks J, Boyan BD, Blanchard CR, Lohmann CH, Liu Y, Cochran DL, et al. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials. 1998;19(23):2219–32.9884063. doi: 10.1016/s0142-9612(98)00144-6. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz Z, Raz P, Zhao G, Barak Y, Tauber M, Yao H, et al. Effect of micrometer-scale roughness of the surface of Ti6Al4V pedicle screws in vitro and in vivo. J Bone Joint Surg Am. 2008;90(11):2485–98.18978418. doi: 10.2106/JBJS.G.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyan BD, Schwartz Z, Lohmann CH, Sylvia VL, Cochran DL, Dean DD, et al. Pretreatment of bone with osteoclasts affects phenotypic expression of osteoblast-like cells. J Orthop Res. 2003;21(4):638–47.12798063. doi: 10.1016/S0736-0266(02)00261-9. [DOI] [PubMed] [Google Scholar]
- 14.Vlacic-Zischke J, Hamlet SM, Friis T, Tonetti MS, Ivanovski S. The influence of surface microroughness and hydrophilicity of titanium on the up-regulation of TGFbeta/BMP signalling in osteoblasts. Biomaterials. 2011;32(3):665–71.20933273. doi: 10.1016/j.biomaterials.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Kieswetter K, Schwartz Z, Hummert TW, Cochran DL, Simpson J, Dean DD, et al. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like MG-63 cells. J Biomed Mater Res. 1996;32(1):55–63.8864873. doi: 10.1002/(SICI)1097-4636(199609)32:1<55::AID-JBM7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz Z, Olivares-Navarrete R, Wieland M, Cochran DL, Boyan BD. Mechanisms regulating increased production of osteoprotegerin by osteoblasts cultured on microstructured titanium surfaces. Biomaterials. 2009;30(20):3390–6.19395022. doi: 10.1016/j.biomaterials.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geris L, Gerisch A, Sloten JV, Weiner R, Oosterwyck HV. Angiogenesis in bone fracture healing: a bioregulatory model. J Theor Biol. 2008;251(1):137–58.18155732. doi: 10.1016/j.jtbi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Duvall CL, Taylor WR, Weiss D, Wojtowicz AM, Guldberg RE. Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin-deficient mice. J Bone Miner Res. 2007;22(2):286–97.17087627. doi: 10.1359/jbmr.061103. [DOI] [PubMed] [Google Scholar]
- 19.Abshagen K, Schrodi I, Gerber T, Vollmar B. In vivo analysis of biocompatibility and vascularization of the synthetic bone grafting substitute NanoBone. J Biomed Mater Res A. 2009;91(2):557–66.18985779. doi: 10.1002/jbm.a.32237. [DOI] [PubMed] [Google Scholar]
- 20.Schweigerer L, Neufeld G, Friedman J, Abraham JA, Fiddes JC, Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987;325(6101):257–9.2433585. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- 21.Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84(5):1470–8.2478587. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta VB, Besner GE. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors. 2007;25(4):253–63.18092233. doi: 10.1080/08977190701773070. [DOI] [PubMed] [Google Scholar]
- 23.Raines AL, Olivares-Navarrete R, Wieland M, Cochran DL, Schwartz Z, Boyan BD. Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy. Biomaterials. 2010;31(18):4909–17.20356623. doi: 10.1016/j.biomaterials.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anselme K, Bigerelle M. Topography effects of pure titanium substrates on human osteoblast long-term adhesion. Acta Biomater. 2005;1(2):211–22.16701798. doi: 10.1016/j.actbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Anselme K, Bigerelle M, Noel B, Dufresne E, Judas D, Iost A, et al. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J Biomed Mater Res. 2000;49(2):155–66.10571901. doi: 10.1002/(sici)1097-4636(200002)49:2<155::aid-jbm2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Olivares-Navarrete R, Raz P, Zhao G, Chen J, Wieland M, Cochran DL, et al. Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proc Natl Acad Sci U S A. 2008;105(41):15767–72.18843104. doi: 10.1073/pnas.0805420105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raz P, Lohmann CH, Turner J, Wang L, Poythress N, Blanchard C, et al. 1alpha,25(OH)2D3 regulation of integrin expression is substrate dependent. J Biomed Mater Res A. 2004;71(2):217–25.15386491. doi: 10.1002/jbm.a.30134. [DOI] [PubMed] [Google Scholar]
- 28.Siebers MC, ter Brugge PJ, Walboomers XF, Jansen JA. Integrins as linker proteins between osteoblasts and bone replacing materials. A critical review. Biomaterials. 2005;26(2):137–46.15207460. doi: 10.1016/j.biomaterials.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Boyan BD, Lohmann CH, Sisk M, Liu Y, Sylvia VL, Cochran DL, et al. Both cyclooxygenase-1 and cyclooxygenase-2 mediate osteoblast response to titanium surface roughness. J Biomed Mater Res. 2001;55(3):350–9.11255188. doi: 10.1002/1097-4636(20010605)55:3<350::aid-jbm1023>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 30.Bessho K, Carnes DL, Cavin R, Chen HY, Ong JL. BMP stimulation of bone response adjacent to titanium implants in vivo. Clin Oral Implants Res. 1999;10(3):212–8.10522181. doi: 10.1034/j.1600-0501.1999.100304.x. [DOI] [PubMed] [Google Scholar]
- 31.Olivares-Navarrete R, Gittens IRA, Schneider JM, Hyzy SL, Haithcock DA, Ullrich P, et al. Osteoblasts Exhibit a More Differentiated Phenotype and Increased BMP Production on Titanium Alloy Substrates than on PEEK. The Spine Journal: official journal of the North American Spine Society. 2012 doi: 10.1016/j.spinee.2012.02.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz Z, Nasazky E, Boyan BD. Surface microtopography regulates osteointegration: the role of implant surface microtopography in osteointegration. Alpha Omegan. 2005;98(2):9–19.16122142. [PubMed] [Google Scholar]
- 33.Olivares-Navarrete R, Hyzy SL, Chaudhri RA, Zhao G, Boyan BD, Schwartz Z. Sex dependent regulation of osteoblast response to implant surface properties by systemic hormones. Biol Sex Differ. 2010;1(1):4.21208469. doi: 10.1186/2042-6410-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lohmann CH, Tandy EM, Sylvia VL, Hell-Vocke AK, Cochran DL, Dean DD, et al. Response of normal female human osteoblasts (NHOst) to 17beta-estradiol is modulated by implant surface morphology. J Biomed Mater Res. 2002;62(2):204–13.12209940. doi: 10.1002/jbm.10290. [DOI] [PubMed] [Google Scholar]
- 35.Schneider GB, Perinpanayagam H, Clegg M, Zaharias R, Seabold D, Keller J, et al. Implant surface roughness affects osteoblast gene expression. J Dent Res. 2003;82(5):372–6.12709504. doi: 10.1177/154405910308200509. [DOI] [PubMed] [Google Scholar]
- 36.Dekker RJ, van Blitterswijk CA, Hofland I, Engelberts PJ, Li J, de Bruijn JD. Studying the effect of different macrostructures on in vitro cell behaviour and in vivo bone formation using a tissue engineering approach. Novartis Found Symp. 2003;249:148–67. doi: 10.1002/0470867973.ch11. discussion 67–9, 70–4, 239–41.12708655. [DOI] [PubMed] [Google Scholar]
- 37.Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, Lankford J, Jr, et al. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63) J Biomed Mater Res. 1995;29(3):389–401.7542245. doi: 10.1002/jbm.820290314. [DOI] [PubMed] [Google Scholar]
- 38.Cochran DL, Nummikoski PV, Higginbottom FL, Hermann JS, Makins SR, Buser D. Evaluation of an endosseous titanium implant with a sandblasted and acid-etched surface in the canine mandible: Radiographic results. Clin Oral Implants Res. 1996;7(3):240–52. doi: 10.1034/j.1600-0501.1996.070306.x. ISI:A1996VU66800007. [DOI] [PubMed] [Google Scholar]
- 39.Heino TJ, Hentunen TA. Differentiation of osteoblasts and osteocytes from mesenchymal stem cells. Curr Stem Cell Res Ther. 2008;3(2):131–45.18473879. doi: 10.2174/157488808784223032. [DOI] [PubMed] [Google Scholar]
- 40.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22(5):617–25.20817428. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Chen CH, Poucher SM, Lu J, Henry PD. Fibroblast growth factor 2: from laboratory evidence to clinical application. Curr Vasc Pharmacol. 2004;2(1):33–43.15320831. doi: 10.2174/1570161043476500. [DOI] [PubMed] [Google Scholar]
- 42.Saharinen P, Bry M, Alitalo K. How do angiopoietins Tie in with vascular endothelial growth factors? Curr Opin Hematol. 2010;17(3):198–205.20375888. doi: 10.1097/MOH.0b013e3283386673. [DOI] [PubMed] [Google Scholar]
- 43.Tan KH, Chua CK, Leong KF, Naing MW, Cheah CM. Fabrication and characterization of three-dimensional poly(ether- ether- ketone)/-hydroxyapatite biocomposite scaffolds using laser sintering. Proc Inst Mech Eng H. 2005;219(3):183–94.15934394. doi: 10.1243/095441105X9345. [DOI] [PubMed] [Google Scholar]
- 44.Han CM, Lee EJ, Kim HE, Koh YH, Kim KN, Ha Y, et al. The electron beam deposition of titanium on polyetheretherketone (PEEK) and the resulting enhanced biological properties. Biomaterials. 2010;31(13):3465–70.20153890. doi: 10.1016/j.biomaterials.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Xu M, Zhang W, Kwok DT, Jiang J, Wu Z, et al. Mechanical and biological characteristics of diamond-like carbon coated poly aryl-ether-ether-ketone. Biomaterials. 2010;31(32):8181–7.20692699. doi: 10.1016/j.biomaterials.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 46.Bonewald LF, Dallas SL. Role of active and latent transforming growth factor beta in bone formation. J Cell Biochem. 1994;55(3):350–7.7962167. doi: 10.1002/jcb.240550312. [DOI] [PubMed] [Google Scholar]
- 47.Janssens K, ten Dijke P, Janssens S, Van Hul W. Transforming growth factor-beta1 to the bone. Endocrine Reviews. 2005;26(6):743–74.15901668. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi Y, Udagawa N, Takahashi N. Action of RANKL and OPG for osteoclastogenesis. Crit Rev Eukaryot Gene Expr. 2009;19(1):61–72.19191757. doi: 10.1615/critreveukargeneexpr.v19.i1.30. [DOI] [PubMed] [Google Scholar]


