Abstract
Background
Major Depressive Disorder (MDD) afflicts up to 10% of adolescents. However, nearly 50% of those afflicted are considered non-responsive to available treatments. Ketamine, a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist has shown potential as a rapid-acting and long-lasting treatment for MDD in adults. Thus, the effectiveness and functional consequences of ketamine exposure during adolescence were explored.
Methods
Adolescent male rats (postnatal day [PD] 35) received two ketamine (0, 5, 10 or 20 mg/kg) injections, 4 hours apart, after exposure to day 1 of the forced swim test (FST). The next day, rats were re-exposed to the FST to assess ketamine-induced antidepressant-like responses. Separate groups were exposed to chronic unpredictable stress (CUS) to confirm findings from the FST. After these initial experiments, adolescent naïve rats were exposed to either 1 or 15 consecutive days (PD35–49) of ketamine (20 mg/kg) twice/daily. Ketamine's influence on behavioral reactivity to rewarding (i.e., sucrose preference) and aversive (i.e., elevated plus-maze, FST) circumstances was then assessed 2 months after treatment. To control for age-dependent effects, adult rats (PD75–89) were exposed to identical experimental conditions.
Results
Ketamine (20 mg/kg) reversed the CUS-induced depression-like behaviors in the FST. Repeated ketamine exposure resulted in anxiolytic- and antidepressant-like responses 2 months after drug exposure. None of the ketamine doses used were capable of inducing drug-seeking behaviors as measured by place preference conditioning.
Conclusions
Repeated ketamine exposure induces enduring resilient-like responses regardless of age of exposure. These findings point to ketamine, and its repeated exposure, as a potentially useful antidepressant during adolescence.
Keywords: ketamine, stress, adolescence, rats, depression, anxiety, resilience
Introduction
Major Depressive Disorder (MDD) is a leading cause of disability (1–3), afflicting approximately 20% of the world's population (4–6), with annual costs of nearly $100 billion (7). MDD also affects about 10% of children and adolescents (8, 9). Pediatric MDD can be highly debilitating, with negative consequences extending into adulthood: increasing risk for conduct and substance abuse disorders, greater likelihood of relapse, and a disproportionate number of those affected do self-harm and/or attempt suicide (8, 10). Although available treatments are generally effective and safe in adults, they are suboptimal, possessing low remission rates, delayed onset of efficacy, and unwanted side effects (1–5). Treatment options for youths are limited, with the selective serotonin reuptake inhibitor fluoxetine as the only pharmacotherapeutic currently approved for pediatric MDD (11, 12). Despite emergence of studies on the efficacy and safety of treatment for childhood depression (12–14), reliable evidence-based indications for antidepressant use, and potential long-term consequences in pediatric populations, are lacking (12, 15–17). Most troubling is that approximately 50% of adolescents with MDD are unresponsive to available treatments (18–20). Therefore, development of better, more effective treatment modalities for juvenile MDD is needed.
Recently, the non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist, ketamine, was identified as rapid acting, long lasting treatment for adult MDD, including those who are treatment resistant (21–26). Unfortunately, acute ketamine is not sufficient to maintain the antidepressant effects, as patients return to the clinic for repeated treatment when experiencing relapse (25–27). Preclinical studies have paralleled clinical findings (28–30), focusing on acute ketamine exposure in adult rodents. Given the limited treatment options available, likelihood of treatment resistance, and higher risk for comorbidity later in life, ketamine's potential as a novel, efficacious treatment for adolescent MDD warrants assessment.
This study was designed to assess ketamine's antidepressant efficacy in adolescent male rats. We also examined enduring functional consequences of repeated ketamine exposure during adolescence by assessing subsequent behavioral reactivity to emotion-eliciting stimuli in adulthood.
Methods and Materials
Subjects
Male Sprague-Dawley rats obtained from our in-house breeding colony were used for this study. To avoid `oversampling' (31) or `within-litter effects' (32), 1 pup/litter was assigned to a particular condition. The age at the start of experimental manipulations in adolescent rats (postnatal day [PD] 35–49) was selected because it roughly approximates adolescence in humans (33–35). Rats were housed in clear polypropylene boxes containing wood shavings in an animal colony maintained at 23–25°C on a 12 h light/dark cycle in which lights were on between 0700 and 1900 hours. Food and water were provided ad libitum.
Drug Treatment and Experimental Design
Ketamine was obtained from Butler Schein (Dublin, OH) in an injectable solution (100 mg/ml), was diluted (5, 10, and 20 mg/kg) in sterile physiological saline (0.9% sodium chloride), and administered intraperitoneally (IP) at a volume of 1 mL/kg. Rats received ketamine (0, 20 mg/kg) twice-daily (BID) for 1 or 15 consecutive days, and their behavioral reactivity to emotion-eliciting situations were assessed 2 months after treatment. Rats were exposed to only two behavioral assays, and were never tested again after exposure to the forced swim test (FST) or the place preference conditioning (CPP) procedure (see Table 1 for experimental groups/testing sequence). There was a rest period of 48h between behavioral testing. All behaviors, except for sucrose preference and locomotor activity, were recorded with a video camera. Behavioral observations and analyses were done by observers with no knowledge of the treatment conditions of each rat. All procedures were in strict accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003) and approved by the Florida State University Animal Care and Use Committee.
Table 1.
Experimental groups and testing sequence
| Group | n | Treatment (mg/kg) | Age | Interval 1 | Test 1 | Interval 2 | Test 2 |
|---|---|---|---|---|---|---|---|
| 1 (Fig. 1) | 8 | 1 day Ketamine (0, 5, 10, 20) | Adolescent | 24 h | FST | - | - |
| 2 (Fig. 2) | 8–12 | CUS + single injection ketamine (0, 20) | Adolescent | 1 h | FST | - | - |
| 3 (Fig. 3) | 19–20 | Single Injection ketamine (0, 20) | Adolescent | - | Locomotion | - | - |
| 4 (Fig 3) | 19–20 | Single Injection ketamine (0, 20) | Adult | - | Locomotion | - | - |
| 5 (Fig. 4, 5, S2) | 10 | 15 days Ketamine (0, 20; BID) | Adolescent | 2 months | Locomotion | 48 h | EPM |
| 6 (Fig. 4, 5, S2) | 10 | 15 days Ketamine (0, 20; BID) | Adult | 2 months | Locomotion | 48 h | EPM |
| 7 (Fig. 5, S2) | 9–10 | 1 day Ketamine (0, 20; BID) | Adolescent | 2 months | Locomotion | 48 h | EPM |
| 8 (Fig. 5, S2) | 10 | 1 day Ketamine (0, 20 BID) | Adult | 2 months | Locomotion | 48 h | EPM |
| 9 (Fig. S1) | 9–10 | Single Injection ketamine (0, 20) | Adolescent | 1 h | Locomotion | - | - |
| 10 (Fig. S3) | 10 | 1 day Ketamine (0, 20; BID) | Adolescent | 2 months | SP* | 48 h | FST |
| 11 (Fig. S3) | 10 | 1 day Ketamine (0, 20; BID) | Adult | 2 months | SP* | 48 h | FST |
| 12 (Fig. 6, S4) | 12 | 15 days Ketamine (0, 20; BID) | Adolescent | 2 months | SP | 48 h | FST |
| 13 (Fig. 6, S4) | 11–12 | 15 days Ketamine (0, 20; BID) | Adult | 2 months | SP | 48 h | FST |
| 14 (Fig. 6) | 6–10 | Ketamine (0, 5,10, 20) | Adolescent | - | CPP | - | - |
Injections intraperitoneal; BID, twice daily; EPM, elevated plus maze; FST, forced swimming test; SP, sucrose preference; CPP, Conditioned Place Preference; S, Supplemental
Data not shown
Behavioral Assays
All behavioral assays were conducted as described previously (see Supplemental Methods for details).
Forced Swimming
Latency to immobility, total immobility, swimming, floating, and climbing counts were recorded (36).
Chronic Unpredictable Stress
Adolescent rats were subjected to a 15-day (PD31–46) chronic unpredictable stress (CUS) schedule with slight modifications (30).
Corticosterone Enzyme Immunoassay
Subgroups of control and CUS-exposed adolescent rats were subsequently used 72h after an injection of saline or ketamine to assess corticosterone levels. Half of the control group received an acute stressor (5 minutes of swimming stress) immediately before blood collection. A corticosterone enzyme immunoassay (Assay Designs, Ann Arbor, Michigan) was performed as previously described (37). See Supplemental Methods for details.
Sucrose Preference
The sucrose preference test consisted of a two-bottle choice paradigm (38). Rats were exposed to either 1% under the CUS paradigm (Figure 2C–D), or to ascending concentrations of sucrose (0.125–1%; wt/vol) for 2-days/concentration after ketamine (Supplement: Figure S4A–D). The preference for sucrose over water was used as a measure for sensitivity to reward.
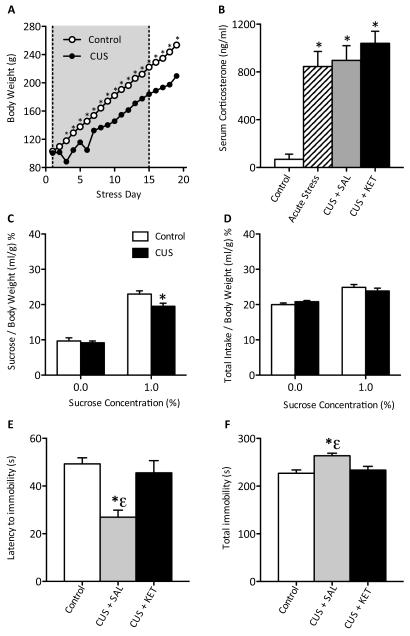
Figure 2.
Effects of chronic unpredictable stress (CUS) and ketamine's (20 mg/kg) antidepressant efficacy in adolescent rats (n=8–12/group). (A) Exposure to CUS reduced weight gain across days. (B) Levels of circulating serum corticosterone (CORT) were assessed in control (i.e., no stress) and CUS-exposed adolescent rats 72 hours after receiving saline or ketamine (20 mg/kg), and in controls exposed to stress for 5 minutes immediately before blood collection. Serum CORT levels were significantly elevated by acute as well as CUS exposure, and were not affected by ketamine treatment (p<0.05). (C) Exposure to CUS significantly reduced adolescent rats' preference for sucrose (p<0.05). (D) No changes in total liquid intake were observed. (E) CUS significantly reduced rats' latency to become immobile (p<0.05), whereas a single ketamine injection (20 mg/kg) reversed the CUS-induced deficit back to control levels (p>0.05). (F) Exposure to CUS significantly increased total immobility (p<0.05) while a single ketamine injection (20 mg/kg) reduced total immobility back to control levels (p>0.05). Data are presented as latencies to become immobile and total immobility (in seconds; mean ± SEM). CUS + SAL, chronic unpredictable stress and saline; CUS + KET, chronic unpredictable stress and ketamine. *Significantly different from saline-treated controls (p<0.05). εSignificantly different from CUS + ketamine-treated group (p<0.05).
Locomotor Activity
Ketamine-induced locomotor activity was indexed as the distance traveled (cm) in an open-field apparatus immediately (Figure 3A–B), 1h after (Supplement: Figure S1) a single injection, or 60 days after repeated (1 or 15 days, BID) ketamine (0, 20 mg/kg) exposure (Supplement: Figure S2A and C for adolescents; B, and D for adults).
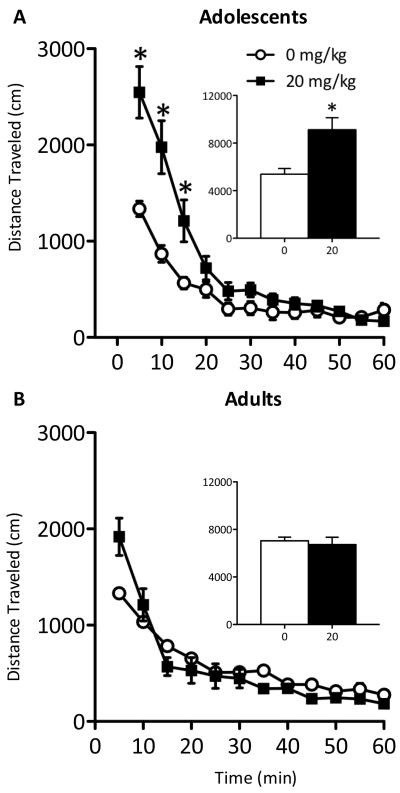
Figure 3.
Immediate effects of a single injection of ketamine (20 mg/kg) on distance traveled in adolescent and adult rats. (A) Adolescent rats treated with ketamine (n=19) had significantly increased locomotor activity during the first 15 minutes immediately after a single ketamine exposure when compared to saline-treated adolescent controls (n=20; p<0.05). Their total distance traveled was also significantly higher than controls (p<0.05). (B) Ketamine treated adults (n=19) exhibit no differences in distance traveled across time or total distance traveled compared to their respective saline-treated controls (n=20). Data are represented as mean distance traveled (mean ± SEM, in cm). *Significantly different from saline-treated controls (p<0.05).
Elevated Plus-Maze
Time spent in the open and closed arms of an elevated plus-maze (EPM) was assessed over 5 minutes (38).
Place Preference Conditioning
Conditioning trials occurred over 4 days. During conditioning, rats received saline (1.0 mL/kg, IP) and were confined to one of the side compartments of the apparatus for 30 minutes. After 3h, rats received ketamine (5, 10, or 20 mg/kg, IP) and were confined to the opposite side compartment for 30 minutes. On the test day (day 5), rats received saline (IP) and were allowed to explore the entire apparatus freely for 30 minutes (39, 40).
Statistical Analyses
Behavioral data were analyzed using mixed-design ANOVAs followed by Fisher Least Significant Difference (LSD) post hoc tests. The Nyholt correction was used to control for multiple comparisons (41). When appropriate, Student's t tests were used to determine statistical significance of planned comparisons. Data are expressed as the mean ± SEM. Statistical significance was set at p<0.05.
RESULTS
Establishing Ketamine's Antidepressant Efficacy
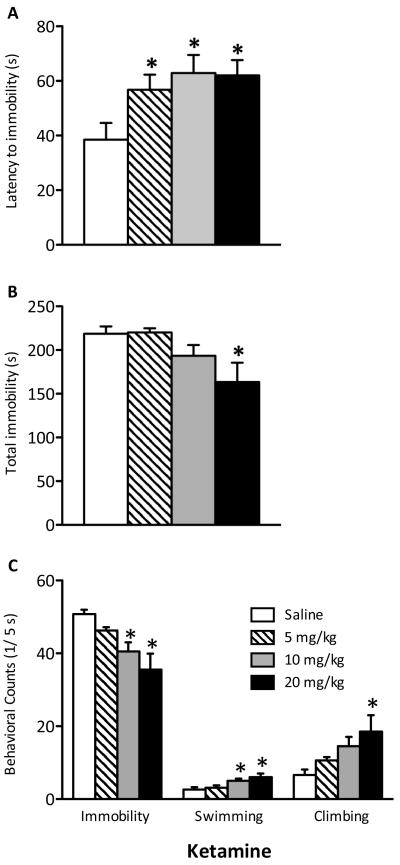
An initial experiment was conducted to determine the antidepressant efficacy of ketamine in adolescent rats using the FST. Rats received a single injection of ketamine (0, 5, 10, or 20 mg/kg) 24h before FST day 2, but this treatment failed to reliably induce escape-like behaviors (data not shown). Therefore, in a subsequent experiment, PD35 rats received two injections of ketamine (0, 5, 10, and 20 mg/kg), 4h apart, after day 1 of FST. Twenty-four hours later (PD36), their behavioral reactivity to swimming stress was assessed. Ketamine increased latency to immobility in adolescent rats (F(3,28)=3.568, p<0.05; Figure 1A; n=32). Analyses revealed that all three doses significantly increased latency to immobility when compared to controls (p<0.05). Ketamine treatment reduced total immobility (F(3,28)=3.873, p<0.05; Figure 1B), but only reliably at 20 mg/kg when compared to controls (p<0.05). Treatment also influenced immobility (F(3,28)=6.275, p<0.005), swimming (F(3,28)=4.572, p<0.05), and climbing (F(3,28)=3.411, p<0.05) counts (Figure 1C). Specifically, 10 and 20 mg/kg decreased immobility and increased swimming (p<0.05), while only 20 mg/kg increased climbing counts (p<0.05).
Figure 1.
Effects of ketamine (0, 5, 10, or 20 mg/kg) on behavioral despair as measured in the forced swim test (FST) in adolescent (PD35) male rats 24 hours after 1-day (i.e., two injections, 4 hours apart) of ketamine (n=8/dose). (A) Ketamine significantly increased latency to become immobile regardless of dose (p<0.05, respectively). (B) Only the 20 mg/kg ketamine dose significantly reduced total immobility (p<0.05). (C) Ketamine (10 and 20 mg/kg) significantly reduced immobility and increased swimming counts, whereas only 20 mg/kg significantly increased climbing counts when compared to controls. Data are presented as latencies to become immobile and total immobility (in seconds) and as cumulative 5-second intervals of immobility, swimming, and climbing counts (mean ± SEM). *Significantly different from saline-treated controls (p<0.05).
Ketamine's Antidepressant Efficacy Following CUS
Although ketamine increased escape-like behavioral reactivity, only the highest dose did so reliably on all measures (Figure 1). To confirm this finding, we exposed rats to a 15-day (PD31–46) CUS regimen to induce a depression-like phenotype to further assess ketamine's (20 mg/kg) antidepressant efficacy. Exposure to CUS affected weight gain in adolescent rats (Figure 2A; n=31). Repeated measures ANOVA revealed that while rats gained weight as they matured (within-subject main effect: F(18,522)=2707.444, p<0.001), CUS significantly reduced bodyweight (between-subject main effect: F(1,522)=57.081, p<0.001) when compared to controls. There was a significant interaction between days and stress (F(18,522)=50.181, p<0.001). CUS-exposed rats displayed lower bodyweights beginning on day 3, never returning to control levels (p<0.05).
We also determined the effect of CUS and ketamine treatment on serum corticosterone (CORT) levels (Figure 2B). Serum CORT concentrations varied as a function of CUS (F(3,27)=8.039, p<0.001). Acute stress, CUS + Saline, and the CUS + Ketamine groups had significantly elevated CORT levels when compared to No Stress controls (p<0.05, respectively).
To verify that CUS produced a depressive-like phenotype in adolescents, we assessed sucrose preference. Exposure to CUS significantly reduced preference for sucrose (t(29)=2.161, p<0.05) compared to controls (Figure 2C), indicating a decreased sensitivity to natural reward (i.e., anhedonia). No differences in total liquid intake were detected between groups in either condition (Figure 2D).
We also assessed ketamine's (20 mg/kg) ability to influence behavioral despair after CUS (Figure 2E–F). In addition to controls, rats were divided into groups receiving a single saline (CUS + SAL) or ketamine (CUS + KET) injection 60 minutes before FST (day 2). CUS + SAL-treated rats displayed pro-depressive behaviors manifested in significantly reduced latencies to immobility (F(2,28)=8.542, p<0.005), and increased total immobility (F(2,28)=7.647, p<0.005), when compared to No Stress and the CUS + KET-treated groups. Ketamine reversed the effects of CUS (i.e., anti-depressant effect), as escape-like behaviors of these rats did not differ from the No Stress controls (p>0.05).
Based on these results (Figures 1 and 2E–F), we chose the 20 mg/kg dose to assess the long-term functional consequences of repeated ketamine in adolescent and adult (PD75) rats.
Ketamine-induced Locomotor Activity
Because forced swimming can be influenced by motor activity, we assessed the immediate effects of acute ketamine (20 mg/kg) on locomotor activity in adolescent and adult rats (Figure 3A–B). Repeated measures ANOVA revealed drug-induced changes in locomotor activity across time (F(11,407)=65.944, p<0.001) and as a function of time by drug (F(11,407)=10.45, p<0.001). Post-hoc analyses revealed adolescents with higher activity for the first 15 minutes following ketamine (p<0.05) before returning to control levels. Ketamine-exposed adolescents traveled greater cumulative distance overall (t(37)=3.303, p<0.005; Figure 3A inset).
Ketamine did not influence adult rats' locomotor activity when compared to controls (Figure 3B).
To control for novelty influencing ketamine-induced locomotion, distance traveled was assessed 1h after acute ketamine (20 mg/kg) in adolescent rats (Supplement: Figure S1). Repeated measures ANOVA revealed main effects indicating reduced activity across time (F(11,187)=55.075, p<0.001) and drug treatment (F(1,187)=6.896, p<0.05), without interaction between variables. These results indicate that novelty did not contribute to the ketamine-induced locomotion observed in Figure 3A.
Effects of Repeated Ketamine Exposure on Weight Gain and Food Intake
Adolescent and adult rats received ketamine (20 mg/kg, BID) for 1 or 15 consecutive days. One day of ketamine exposure had no effect on weight gain or food intake (data not show).
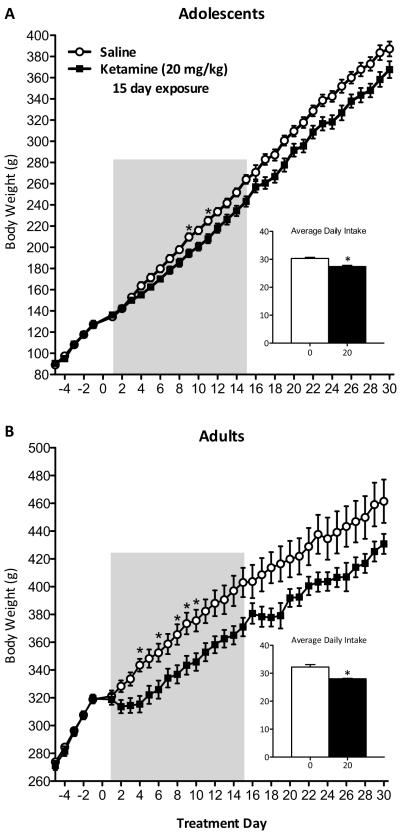
Fifteen days of ketamine treatment reduced weight gain in both adolescent (Figure 4A) and adult rats (Figure 4B). Repeated measures ANOVA revealed changes in bodyweight across days (F(34,612)=2642.339, p<0.001), drug (F(1,612)=5.932, p<0.05), and as a function of day by drug (F(34,612)=6.907, p<0.001) in adolescents. Although ketamine-treated rats gained weight at a lower rate than controls, Noyholt corrections for multiple comparisons indicated significantly reduced bodyweights only on days 9 and 11 of treatment (p<0.05). Nevertheless, adolescents' bodyweight remained lower, and was accompanied by reduced daily food intake (t(18)=4.933, p<0.001; Fig. 4A inset) throughout the experiment.
Figure 4.
Repeated exposure to ketamine (20 mg/kg, twice daily) disrupts normal weight gain and average daily food intake of adolescent- and adult-treated rats (n=10/group). (A) Repeated ketamine from postnatal days (PD) 35–49 reduced weight gain on days 9 and 11 of treatment (p<0.05) in adolescent rats. Average daily chow intake during and 15 days after treatment was significantly reduced in ketamine-treated adolescents. (B) Adults (PD75+) repeatedly treated with ketamine also show reduced weight gain on days 4, 6, and 8–10 (p<0.05). Ketamine-treated adults daily intake was also significantly reduced when compared to saline-treated controls (p<0.05). Data are represented as body weight in grams and average daily intake across days in grams (mean ± SEM). Shaded area indicates ketamine treatment days. *Significantly different from saline-treated controls (p<0.05).
Ketamine reduced adult weight gain in a similar fashion as the adolescents (Fig. 4B). The ANOVA revealed changes in weight across days (F(34,612)=45887.454, p<0.001), drug (F(1,612)=5.269, p<0.05), and as a function of day by drug (F(34,612)=3.83, p<0.001). Nyholt corrections revealed lower bodyweights on days 4, 6, and 8–10 of treatment (p<0.05); but similarly to adolescents, adult rats displayed lower bodyweights and reduced daily food intake (t(18)=4.476, p<0.001; Fig. 4B inset) throughout the experiment.
Long-term Effects of Repeated Ketamine Exposure on Basal Locomotor Activity
Repeated ketamine had not effect on adolescent or adult rats' basal locomotor activity 2 months after drug exposure (Supplement: Figure S2A–D).
Long-term Effects of Repeated Ketamine Exposure on Anxiety-like Behavior
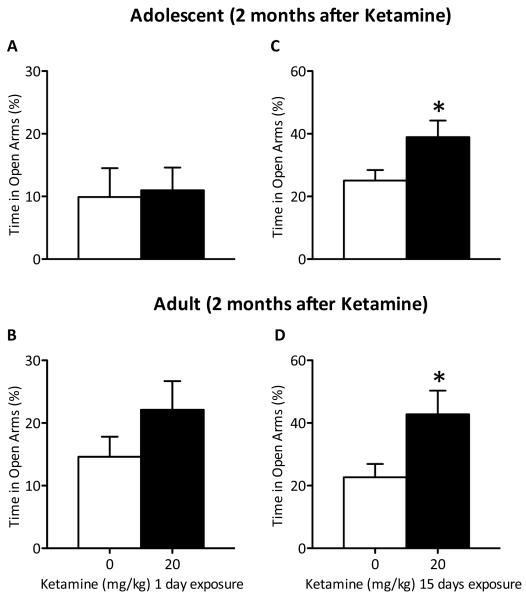
One-day of ketamine exposure did not affect time spent in the open arms of the EPM, regardless of age at time of treatment, 2 months after exposure (Figure 5A–B; n=19–20/group). Conversely, 15 days of ketamine significantly increased time spent in the open arms of the EPM of both adolescent- (t(18)=2.205, p<0.05) and adult-treated (t(18)=2.314, p<0.05) rats when compared to controls (Figure 5C–D; n=20/group) 2 months after drug exposure.
Figure 5.
Effects of 1 and 15 days of ketamine exposure (20 mg/kg; twice daily) on anxiety-like behavior. (A–B) One day of ketamine treatment did not produce changes in anxiety-like behaviors as measured in the elevated-plus maze (EPM) regardless of age of exposure (n=10/group). (C–D) Conversely, repeated exposure to ketamine (20 mg/kg), twice daily, induced significant increases in time spent in the open arms of the EPM in both adolescent-and adult-treated rats (p<0.05; n=10/group). Data are presented as percent time spent (mean ±DSEM) in the open arms of the EPM. *Significantly different from saline-treated controls (p<0.05).
Long-Term Effects of Ketamine Exposure on Behavioral Despair
We used the FST to assess rats' responsiveness to stress 2 months after 1 day of ketamine exposure. No differences on any measures of the FST regardless of age at time of treatment were observed (adolescents: Supplement: Figure S3A–C; adults: Supplement: Figure S3D–F; n=20/group).
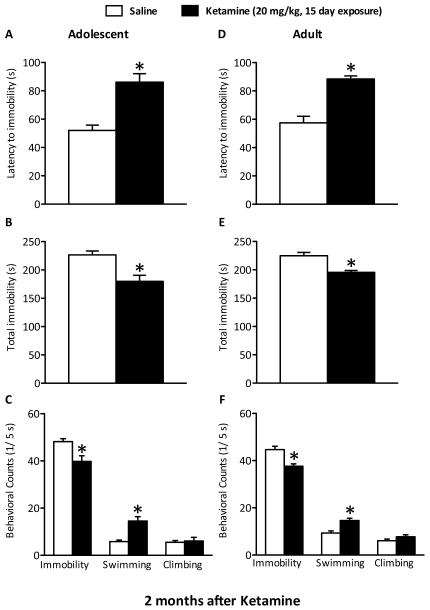
Behavioral despair was also assessed 2 months after 15 days of treatment in adolescent (n=24) and adult (n=20) rats (Figure 6A–F). Ketamine exposure during adolescence significantly increased latency to immobility (t(22)=4.743, p<0.005) and decreased total immobility (t(22)=3.684, p<0.005) when compared to controls (Figure 6A–B). These rats also displayed less immobility (t(22)=3.092, p<0.05) and higher swimming (t(22)=4.364, p<0.005) counts than controls (Figure 6C).
Figure 6.
Lasting effects of repeated (15 days) exposure to ketamine (20 mg/kg, twice daily) on behavioral despair using the forced swim test (FST) paradigm, 2 months after drug exposure, in adolescent (A–C) and adult (D–F) rats. Adolescent (PD35–49; n=12/group) rats show significantly increased latencies to immobility (A), lower total immobility (B), decreased immobility as well as higher swimming counts (C) when compared with saline-treated rats 2 months after drug exposure (p<0.05). Similarly treated adult rats (PD 75–89; n=11–12/group) also exhibited significantly increased latencies to immobility (D), lower total immobility (E), decreased immobility and increased swimming counts (F) 2 months after drug treatment (p<0.05). Data are presented as latencies to become immobile and total immobility (in seconds) and as cumulative 5-second intervals of swimming, climbing, and immobile counts (mean ± SEM). *Significantly different from saline-treated rats (p<0.05).
Separate groups of adult rats were tested on the FST 2 months after 15 days of ketamine (matched treatment and testing schedule). Ketamine-treated adult rats displayed longer latencies to immobility (t(21)=5.748, p<0.005) and decreased total immobility (t(21)=4.247, p<0.005) when compared to controls (Figure 6D–E). They also displayed lower immobility (t(21)=3.995, p<0.005) and higher swimming (t(21)=4.125, p<0.005) counts than controls (Figure 6F).
Effects of Ketamine on Reward-related Behavior
Because stress can induce anhedonia and ketamine is a drug of abuse that interacts with brain reward circuits, we determined the effects of prior exposure to ketamine on sucrose preference in adolescent and adult rats. We also assessed whether ketamine would induce place preference using the CPP paradigm in drug naïve adolescent rats. Adolescent and adult rats receiving 1 (data not shown) or 15 days of ketamine (Supplement: Figure S4A–D) did not show differences in sucrose preference 2 months after drug exposure.
Ketamine (0, 5, 10, and 20 mg/kg) failed to induce place conditioning in adolescent rats when compared to controls (Supplement: Figure S4E).
Discussion
This study was designed to examine the effectiveness of ketamine's antidepressant properties in adolescent male rats, and assess the consequences of its repeated exposure during adolescence on functional reactivity to emotion-eliciting situations in adulthood. Here we report that ketamine yields rapid antidepressant-like effects in adolescent rats exposed to control and CUS conditions. Additionally, 15 days, but not 1 day, of twice-daily ketamine treatment results in an enduring stress-resistant phenotype, regardless of age at time of exposure.
Initially, adolescent rats received a single injection of ketamine (5, 10, or 20 mg/kg) 24h before re-exposure to the FST (day 2) without yielding reliable results. Subsequently, the same experiment was conducted in separate groups, but this time receiving two injections of ketamine, 4h apart. Exposure to ketamine induced antidepressant-like responses manifested in higher latencies to immobility and increased total immobility on day 2 of the FST (42–44). Interestingly, only the highest dose of ketamine (20 mg/kg) produced reliable antidepressant-like effects in adolescents, a dose higher than reported in adult rats (45). Because these findings were derived from naïve rats, it was important to validate ketamine's antidepressant effects after exposure to CUS, a commonly used model of depression and antidepressant efficacy (46–48). Our data shows that 15 days of CUS produces a robust depressive-like phenotype in adolescent rats, as evidenced by reduced weight gain, elevated serum CORT levels, shorter latencies to immobility and greater total immobility in the FST, and anhedonia (i.e., reduced sucrose preference). Although reduced body weight in CUS-exposed rats was expected to influence sucrose preference, this was not the case because there were no significant changes in total liquid intake (sucrose+water) between the groups, and decreased preference for sucrose remained after controlling for body weight (48–50). Importantly, a single ketamine exposure (20 mg/kg) 1h before forced swimming successfully reversed the effects of CUS as indicated by coping patterns categorized as antidepressant-like behaviors (42–44). These effects were not due to ketamine-induced changes in locomotor activity because these rats were forced to swim long after ketamine's influence on motor behavior had dissipated (see Supplement: Figure S1 and Figure S3A). These findings parallel those demonstrating acute ketamine's antidepressant effects in adults (51–53), and we now expand ketamine's antidepressant effects to adolescent rats.
Given ketamine's ability to ameliorate the effects of stress on CORT levels in adults (53), we assessed for similar effects in adolescent rats. Acute ketamine failed to influence serum CORT levels of CUS-exposed adolescents, yet it was capable of inducing antidepressant-like responses in the FST. The mechanism(s) underlying these effects are unknown, but suggests that ketamine's antidepressant-like effects may be independent of hypothalamic-pituitary-adrenal (HPA) axis modulation in adolescents. The effects of NMDA antagonists on HPA axis activity are equivocal, increasing or decreasing its functioning depending on the species and drug dose utilized (54–56), method, and duration of drug administration (57). Within the CUS context, it is conceivable that discrepancies of our findings and those by Garcia et al. (53) may be explained by differences in experimental design and methods (e.g., stress schedule), and/or by ontogenetic differences (i.e., age of exposure) that often emerge when manipulating the nervous system during maturational stages before adulthood (35, 36, 38), and more detailed studies are needed to assess these phenomena.
Based on these results, we assessed for enduring consequences after 1 or 15 days of twice-daily ketamine (20 mg/kg) exposure during adolescence on behavioral reactivity in adulthood. In the clinic, acute ketamine yields very rapid and robust antidepressant and anxiolytic effects (21–26, 58), but patients experience relapse within days (25–27). Preclinical studies also report antidepressant- and anxiolytic-like responses lasting several days (28–30, 52, 59), but none have looked beyond two weeks. We report here that adolescent rats and their adult controls tested 2 months after 1 day of ketamine exposure show no significant changes in responsivity to anxiety- and stress-eliciting situations, as well as no changes in hedonic responses as measured by the sucrose preference paradigm. These results are in agreement with clinical and preclinical findings demonstrating that acute ketamine produces rapid antidepressant responses, but that it is insufficient to sustain long-lasting antidepressant or anxiolytic effects.
Chronic ketamine exposure disrupts appetite and weight gain in adult humans and rats (60, 61). Although 15 days of repeated ketamine significantly reduced food intake and weight gain in both adolescent and adult rats, it took longer for adolescents to show deficits than their adult counterparts (Figure 4), and both groups displayed lower bodyweights than controls throughout the experiment. Ketamine also induced age-dependent effects on locomotor activity as adolescents showed initial hyper-locomotion lasting 15 minutes, while adult-treated rats were unaffected. These results demonstrate that adolescents are more sensitive to the locomotor effects of ketamine, yet required higher doses than adults to elicit antidepressant-like responses. The underpinnings underlying these effects are unknown. NMDA receptor expression peaks during adolescence and steadily drops-off thereafter (35, 62, 63). That adolescents have higher concentrations of NMDA receptors and typically metabolize drugs faster than adults (64) may explain why they require higher doses of ketamine to produce reliable antidepressant-like responses. Sub-anesthetic doses of ketamine are thought to enhance glutamatergic signaling and dopamine release within the prefrontal cortex (PFC) by reducing excitatory input into GABAergic neurons that subsequently leads to hyperactivity of corticolimbic pathways (59, 65, 66). Thus, it is conceivable that there is more GABAergic inhibition of this pathway at rest during adolescence, and that ketamine treatment results in increased hyperactivity as compared to adults.
Repeated ketamine is being explored as a treatment modality for the long lasting maintenance of antidepressant response (67–69). Repeated ketamine exposure in adult rats is effective (70), but the longevity of these effects in adult or adolescent rats is unknown. It is well documented that drug exposure during development can yield mood-related perturbations later in life (36, 38, 39, 71–73), therefore we expected repeated ketamine to induce deficits in coping reactivity to anxiety- and stress-eliciting challenges. Surprisingly, repeated ketamine exposure yields long-lasting (i.e., at least 2 months) anxiolytic- and antidepressant-like responses in both adolescent and adult rats. These effects cannot be explained by ketamine-induced changes in basal locomotor activity since no differences were detected 2 months after drug exposure (Supplement: Figure S2). Our results provide support for repeated ketamine's improved efficacy in maintaining antidepressant-like effects and demonstrate its effectiveness in adolescent rats.
Despite ketamine's therapeutic potential, this drug possess abuse liability (74–76), and its repeated exposure has the capacity to induce behavioral sensitization in rodents (77, 78), a known mechanism implicated in drug addiction (79, 80). Therefore, we further evaluated whether the doses utilized in these studies could produce place preference conditioning, as measured by the CPP paradigm, in adolescent rats. CPP is a well-established behavioral assay used to assess drug reward (40, 81–84). Ketamine failed to produce place preference at any of the doses tested (Supplement: Figure S4E). These results suggest that the range of ketamine doses, specifically the 20 mg/kg, do not induce rewarding effects in adolescent rats. Few studies have successfully reported ketamine-induced CPP in adult rats (85–87). Though unlikely, given our experience with this behavioral assay (40, 73, 88, 89), it is possible that our drug conditioning procedure was insufficient to induce CPP. However, to our knowledge, there is no literature assessing ketamine-induced rewarding effects in juvenile rodents. It is therefore conceivable that low doses of ketamine in adolescents could serve as a relatively safe antidepressant with low abuse potential. This assumption is supported, at least partially, by findings demonstrating lack of behavioral sensitization in healthy human subjects after repeated ketamine (90). Furthermore, no long-term changes in hedonic responses were observed, as measured by sucrose preference, following repeated exposure to ketamine in adolescent- or adult-treated rats (Supplement: Figure S4A–D). Nevertheless, the adolescent rats utilized here were ketamine-naïve, and different results could be observed in rats previously treated with ketamine during adolescence, and future studies should assess this possibility.
The mechanism(s) underlying ketamine's antidepressant effects have only recently begun to be elucidated, with most research centering on understanding its rapid actions. Current evidence suggests that ketamine rapidly enhances the structure and function of cortical synapses known to play a role in mood (45, 65). Studies demonstrate that ketamine's antidepressant effects may depend on rapid activation of the mammalian target of rapamycin (mTOR) pathway, including increases in extracellular signal-regulated kinase (ERK), protein kinase B (PKB/Akt), and brain-derived neurotrophic factor (BDNF) in the hippocampus, and number of new spines in the PFC (28, 65). The mechanism(s) involved in the sustained effects observed with ketamine are unclear, although mounting evidence points to changes in AMPA receptor density and their intracellular signaling cascades (52, 91). Although speculative, our data suggests that repeated ketamine might lead to robust, perhaps permanent, changes to cortical synapses thus maintaining longer-lasting antidepressant effects. Nevertheless, ketamine's actions on the nervous system are complex, and much more detailed assessments of its effects on other behavioral outputs, signaling pathways, and brain areas are clearly needed to understand the mechanism(s) involved in its rapid and sustained antidepressant effects.
While this study provides evidence that repeated ketamine induces long-lasting antidepressant effects, there are some limitations. Our experiments were conducted on males, and given higher prevalence of MDD in women (92), it will be important to replicate these findings using female rats. NMDA receptors play a role in learning and memory (93, 94), and ketamine can affect memory (95, 96). Therefore the effects of repeated sub-anesthetic exposure to ketamine on learning and memory must be determined, as findings in the FST could have been influenced by disruption in memory retention in ketamine-treated rats. In addition, the role of ketamine-induced impulsivity within the context of these findings must be explored.
The overall results from our study demonstrate that repeated exposure to ketamine results in an enduring resilient phenotype. To our knowledge, we show for the first time that ketamine is capable of inducing antidepressant-like responses in adolescent rats. Furthermore, we show that repeated, but not acute, ketamine produces lasting anxiolytic- and antidepressant-like responses independent of age at time of exposure. This supports the view that repeated ketamine is more efficacious, and that it could serve as a novel antidepressant for pediatric MDD.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants R21DA022351 and R01DA026854 from the National Institute on Drug Abuse (NIDA), and the Developing Scholar Award from Florida State University to CABG. BL Warren was supported by a Neuroscience Fellowship from Florida State University, and by training grant T32MH093311 from the National Institute of Mental Health. SD Iñiguez was supported by a McKnight Fellowship from the Florida Education Fund, a Neuroscience Fellowship from Florida State University, and NRSA (F31DA027300) from NIDA. We are grateful to Dr. Jon K. Maner and Dr. Stephen J. Glatt for their advice and statistical expertise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE/CONFLICT OF INTEREST The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Hyman S, Chisholm D, Kessler R, Patel V, Whiteford H. Mental Disorders. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al., editors. Disease Control Priorities in Developing Countries. 2nd ed. Washington (DC): 2006. [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Evidence-based health policy--lessons from the Global Burden of Disease Study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan PF, Daly MJ, O'Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nature reviews Genetics. 2012;13:537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 5.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 6.Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, et al. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 8.Richmond TK, Rosen DS. The treatment of adolescent depression in the era of the black box warning. Curr Opin Pediatr. 2005;17:466–472. doi: 10.1097/01.mop.0000166347.53102.e7. [DOI] [PubMed] [Google Scholar]
- 9.Slomski A. Chronic mental health issues in children now loom larger than physical problems. Jama. 2012;308:223–225. doi: 10.1001/jama.2012.6951. [DOI] [PubMed] [Google Scholar]
- 10.Hawton K, Saunders KE, O'Connor RC. Self-harm and suicide in adolescents. Lancet. 2012;379:2373–2382. doi: 10.1016/S0140-6736(12)60322-5. [DOI] [PubMed] [Google Scholar]
- 11.Safer DJ. Should Selective Serotonin Reuptake Inhibitors Be Prescribed for Children With Major Depressive and Anxiety Disorders? Pediatrics. 2006;118:1248–1251. doi: 10.1542/peds.2006-0215. [DOI] [PubMed] [Google Scholar]
- 12.Birmaher B. Should we use antidepressant medications for children and adolescents with depressive disorders? Psychopharmacol Bull. 1998;34:35–39. [PubMed] [Google Scholar]
- 13.Jureidini JN, Doecke CJ, Mansfield PR, Haby MM, Menkes DB, Tonkin AL. Efficacy and safety of antidepressants for children and adolescents. BMJ. 2004;328:879–883. doi: 10.1136/bmj.328.7444.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kratochvil CJ, Vitiello B, Walkup J, Emslie G, Waslick BD, Weller EB, et al. Selective serotonin reuptake inhibitors in pediatric depression: is the balance between benefits and risks favorable? J Child Adolesc Psychopharmacol. 2006;16:11–24. doi: 10.1089/cap.2006.16.11. [DOI] [PubMed] [Google Scholar]
- 15.Emslie GJ, Mayes TL. Mood disorders in children and adolescents: psychopharmacological treatment. Biol Psychiatry. 2001;49:1082–1090. doi: 10.1016/s0006-3223(01)01149-0. [DOI] [PubMed] [Google Scholar]
- 16.Zito JM, Safer DJ, DosReis S, Gardner JF, Soeken K, Boles M, et al. Rising prevalence of antidepressants among US youths. Pediatrics. 2002;109:721–727. doi: 10.1542/peds.109.5.721. [DOI] [PubMed] [Google Scholar]
- 17.Coyle JT, Pine DS, Charney DS, Lewis L, Nemeroff CB, Carlson GA, et al. Depression and bipolar support alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2003;42:1494–1503. doi: 10.1097/00004583-200312000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Maalouf FT, Atwi M, Brent DA. Treatment-resistant depression in adolescents: review and updates on clinical management. Depress Anxiety. 2011;28:946–954. doi: 10.1002/da.20884. [DOI] [PubMed] [Google Scholar]
- 19.Hazell P. Depression in children and adolescents. Clinical evidence. 2011;2011 [PubMed] [Google Scholar]
- 20.Emslie GJ, Mayes T, Porta G, Vitiello B, Clarke G, Wagner KD, et al. Treatment of Resistant Depression in Adolescents (TORDIA): week 24 outcomes. Am J Psychiatry. 2010;167:782–791. doi: 10.1176/appi.ajp.2010.09040552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 22.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Archives of general psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14:1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 24.Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of general psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 26.Aan Het Rot M, Zarate CA, Jr., Charney DS, Mathew SJ. Ketamine for Depression: Where Do We Go from Here? Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, Aan Het Rot M, et al. Rapid and Longer-Term Antidepressant Effects of Repeated Ketamine Infusions in Treatment-Resistant Major Depression. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engin E, Treit D, Dickson CT. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience. 2009;161:359–369. doi: 10.1016/j.neuroscience.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 30.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- 32.Hughes CW. Outcome of early experience studies as affected by between-litter variance. J Nutr. 1979;109:642–645. doi: 10.1093/jn/109.4.642. [DOI] [PubMed] [Google Scholar]
- 33.Andersen SL, Navalta CP. Altering the course of neurodevelopment: a framework for understanding the enduring effects of psychotropic drugs. Int J Dev Neurosci. 2004;22:423–440. doi: 10.1016/j.ijdevneu.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 35.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 36.Iñiguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, et al. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology. 2009;34:1609–1624. doi: 10.1038/npp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, et al. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73:7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolaños CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- 39.Bolaños CA, Willey MD, Maffeo ML, Powers KD, Kinka DW, Grausam KB, et al. Antidepressant treatment can normalize adult behavioral deficits induced by early-life exposure to methylphenidate. Biol Psychiatry. 2008;63:309–316. doi: 10.1016/j.biopsych.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Iñiguez SD, Warren BL, Neve RL, Nestler EJ, Russo SJ, Bolaños Guzmán CA. Insulin receptor substrate-2 in the ventral tegmental area regulates behavioral responses to cocaine. Behavioral neuroscience. 2008;122:1172–1177. doi: 10.1037/a0012893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iñiguez SD, Warren BL, Bolaños-Guzmán CA. Short- and long-term functional consequences of fluoxetine exposure during adolescence in male rats. Biol Psychiatry. 2010;67:1057–1066. doi: 10.1016/j.biopsych.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 44.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends in pharmacological sciences. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 45.Murrough JW. Ketamine as a novel antidepressant: from synapse to behavior. Clinical pharmacology and therapeutics. 2012;91:303–309. doi: 10.1038/clpt.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: Parallels to major depression. Neurosci Biobehav Rev. 2012;36:2085–2117. doi: 10.1016/j.neubiorev.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 48.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 49.Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- 50.Willner P, Moreau JL, Nielsen CK, Papp M, Sluzewska A. Decreased hedonic responsiveness following chronic mild stress is not secondary to loss of body weight. Physiol Behav. 1996;60:129–134. doi: 10.1016/0031-9384(95)02256-2. [DOI] [PubMed] [Google Scholar]
- 51.Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 52.Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behavioural brain research. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 53.Garcia LS, Comim CM, Valvassori SS, Reus GZ, Stertz L, Kapczinski F, et al. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:450–455. doi: 10.1016/j.pnpbp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Farah JM, Jr., Rao TS, Mick SJ, Coyne KE, Iyengar S. N-methyl-D-aspartate treatment increases circulating adrenocorticotropin and luteinizing hormone in the rat. Endocrinology. 1991;128:1875–1880. doi: 10.1210/endo-128-4-1875. [DOI] [PubMed] [Google Scholar]
- 55.Elvidge H, Challis JR, Robinson JS, Roper C, Thorburn GD. Influence of handling and sedation on plasma cortisol in rhesus monkeys (Macaca mulatta) The Journal of endocrinology. 1976;70:325–326. doi: 10.1677/joe.0.0700325. [DOI] [PubMed] [Google Scholar]
- 56.Adams HA, Thiel A, Jung A, Fengler G, Hempelmann G. Studies using S-(+)-ketamine on probands. Endocrine and circulatory reactions, recovery and dream experiences. Anaesthesist. 1992;41:588–596. [PubMed] [Google Scholar]
- 57.Broadbear JH, Winger G, Woods JH. Self-administration of fentanyl, cocaine and ketamine: effects on the pituitary-adrenal axis in rhesus monkeys. Psychopharmacology (Berl) 2004;176:398–406. doi: 10.1007/s00213-004-1891-x. [DOI] [PubMed] [Google Scholar]
- 58.Sappington AA, Corssen G, Becker AT, Tavakoli M. Effects of ketamine on patient responsiveness during the various phases of a single induced anxiety session. The Alabama journal of medical sciences. 1977;14:121–124. [PubMed] [Google Scholar]
- 59.Maeng S, Zarate CA, Jr., Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 60.Cvrcek P. Side effects of ketamine in the long-term treatment of neuropathic pain. Pain Med. 2008;9:253–257. doi: 10.1111/j.1526-4637.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- 61.Venancio C, Magalhaes A, Antunes L, Summavielle T. Impaired spatial memory after ketamine administration in chronic low doses. Current neuropharmacology. 2011;9:251–255. doi: 10.2174/157015911795016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacology, biochemistry, and behavior. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Wenk GL, Barnes CA. Regional changes in the hippocampal density of AMPA and NMDA receptors across the lifespan of the rat. Brain research. 2000;885:1–5. doi: 10.1016/s0006-8993(00)02792-x. [DOI] [PubMed] [Google Scholar]
- 64.Hein K. The use of therapeutics in adolescence. Journal of adolescent health care : official publication of the Society for Adolescent Medicine. 1987;8:8–35. doi: 10.1016/0197-0070(87)90244-0. [DOI] [PubMed] [Google Scholar]
- 65.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 68.Liebrenz M, Stohler R, Borgeat A. Repeated intravenous ketamine therapy in a patient with treatment-resistant major depression. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2009;10:640–643. doi: 10.1080/15622970701420481. [DOI] [PubMed] [Google Scholar]
- 69.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, Aan Het Rot M, et al. Rapid and Longer-Term Antidepressant Effects of Repeated Ketamine Infusions in Treatment-Resistant Major Depression. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia LS, Comim CM, Valvassori SS, Reus GZ, Andreazza AC, Stertz L, et al. Chronic administration of ketamine elicits antidepressant-like effects in rats without affecting hippocampal brain-derived neurotrophic factor protein levels. Basic & clinical pharmacology & toxicology. 2008;103:502–506. doi: 10.1111/j.1742-7843.2008.00210.x. [DOI] [PubMed] [Google Scholar]
- 71.Andersen SL. Stimulants and the developing brain. Trends in pharmacological sciences. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 72.Carlezon WA, Jr., Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry. 2003;54:1330–1337. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 73.Warren BL, Iñiguez SD, Alcantara LF, Wright KN, Parise EM, Weakley SK, et al. Juvenile administration of concomitant methylphenidate and fluoxetine alters behavioral reactivity to reward-and mood-related stimuli and disrupts ventral tegmental area gene expression in adulthood. J Neurosci. 2011;31:10347–10358. doi: 10.1523/JNEUROSCI.1470-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morgan CJ, Curran HV. Ketamine use: a review. Addiction. 2012;107:27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- 75.Coppola M, Mondola R. Methoxetamine: From drug of abuse to rapid-acting antidepressant. Medical hypotheses. 2012 doi: 10.1016/j.mehy.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 76.Wood D, Cottrell A, Baker SC, Southgate J, Harris M, Fulford S, et al. Recreational ketamine: from pleasure to pain. BJU international. 2011;107:1881–1884. doi: 10.1111/j.1464-410X.2010.10031.x. [DOI] [PubMed] [Google Scholar]
- 77.Trujillo KA, Zamora JJ, Warmoth KP. Increased response to ketamine following treatment at long intervals: implications for intermittent use. Biol Psychiatry. 2008;63:178–183. doi: 10.1016/j.biopsych.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 78.Uchihashi Y, Kuribara H, Morita T, Fujita T. The repeated administration of ketamine induces an enhancement of its stimulant action in mice. Japanese journal of pharmacology. 1993;61:149–151. doi: 10.1254/jjp.61.149. [DOI] [PubMed] [Google Scholar]
- 79.Kalivas PW. Recent understanding in the mechanisms of addiction. Curr Psychiatry Rep. 2004;6:347–351. doi: 10.1007/s11920-004-0021-0. [DOI] [PubMed] [Google Scholar]
- 80.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 81.Carlezon WA., Jr. Place conditioning to study drug reward and aversion. Methods Mol Med. 2003;84:243–249. doi: 10.1385/1-59259-379-8:243. [DOI] [PubMed] [Google Scholar]
- 82.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Progress in neurobiology. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 83.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 84.Bolaños CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki T, Aoki T, Kato H, Yamazaki M, Misawa M. Effects of the 5-HT(3) receptor antagonist ondansetron on the ketamine- and dizocilpine-induced place preferences in mice. European journal of pharmacology. 1999;385:99–102. doi: 10.1016/s0014-2999(99)00762-1. [DOI] [PubMed] [Google Scholar]
- 86.van der Kam EL, De Vry J, Tzschentke TM. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates ketamine and heroin reward as assessed by acquisition, extinction, and reinstatement of conditioned place preference in the rat. European journal of pharmacology. 2009;606:94–101. doi: 10.1016/j.ejphar.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 87.Li F, Fang Q, Liu Y, Zhao M, Li D, Wang J, et al. Cannabinoid CB(1) receptor antagonist rimonabant attenuates reinstatement of ketamine conditioned place preference in rats. European journal of pharmacology. 2008;589:122–126. doi: 10.1016/j.ejphar.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 88.Iñiguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, et al. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:1609–1624. doi: 10.1038/npp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iñiguez SD, Warren BL, Neve RL, Russo SJ, Nestler EJ, Bolaños Guzmán CA. Viral-mediated expression of extracellular signal-regulated kinase-2 in the ventral tegmental area modulates behavioral responses to cocaine. Behavioural brain research. 2010;214:460–464. doi: 10.1016/j.bbr.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cho HS, D'Souza DC, Gueorguieva R, Perry EB, Madonick S, Karper LP, et al. Absence of behavioral sensitization in healthy human subjects following repeated exposure to ketamine. Psychopharmacology (Berl) 2005;179:136–143. doi: 10.1007/s00213-004-2066-5. [DOI] [PubMed] [Google Scholar]
- 91.Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA., Jr. Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacology & therapeutics. 2009;123:143–150. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. Journal of affective disorders. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 93.Cotman CW, Monaghan DT, Ganong AH. Excitatory amino acid neurotransmission: NMDA receptors and Hebb-type synaptic plasticity. Annual review of neuroscience. 1988;11:61–80. doi: 10.1146/annurev.ne.11.030188.000425. [DOI] [PubMed] [Google Scholar]
- 94.Rezvani AH. Involvement of the NMDA System in Learning and Memory. In: Levin ED, Buccafusco JJ, editors. Animal Models of Cognitive Impairment. Boca Raton (FL): 2006. [PubMed] [Google Scholar]
- 95.Moosavi M, Yadollahi Khales G, Rastegar K, Zarifkar A. The effect of sub-anesthetic and anesthetic ketamine on water maze memory acquisition, consolidation and retrieval. European journal of pharmacology. 2012;677:107–110. doi: 10.1016/j.ejphar.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 96.Morgan CJ, Muetzelfeldt L, Curran HV. Ketamine use, cognition and psychological wellbeing: a comparison of frequent, infrequent and ex-users with polydrug and non-using controls. Addiction. 2009;104:77–87. doi: 10.1111/j.1360-0443.2008.02394.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.