Abstract
The academic setting provides an environment that may foster success in the discovery of certain types of small molecule tools, while proving less suitable in others. For example, small molecule probes for poorly understood systems, those that exploit a specific resident expertise, and those whose commercial return is not apparent are ideally suited to be pursued in a university setting. In this perspective, we highlight five projects that emanated from academic research groups and generated valuable tool compounds that have been used to interrogate biological phenomena: Reactive oxygen species (ROS) sensors, GPR30 agonists and antagonists, selective CB2 agonists, Hsp70 modulators and beta-amyloid PET imaging agents. By continuing to take advantage of the unique expertise resident in university settings, and the ability to pursue novel projects that may have great scientific value, but limited or no immediate commercial value, probes from academic research groups continue to provide useful tools and generate a long-term resource for biomedical researchers.
Introduction
Over the last two decades, two significant changes in the practice of medicinal chemistry have occurred: a greater focus on and an appreciation for the value of small molecules to interrogate biological systems, and greater contributions of academic research to the identification and characterization of chemical tools and probes, as well as to drug discovery.1,2 (For the purpose of this perspective, we broadly use the terms “tool” and “probe” to describe any small molecule that has been used to interrogate biological phenomena. This definition includes not only “chemical probes” that can be used understand the actions of a specific protein target, but also molecules that detect other biological occurrences such as specific macromolecular structures or transient reactive species). Several factors have conspired to affect these changes. One contributor was the passage of the Bayh-Dole Act in 1980 that allows a university to retain patents and commercialize inventions that emanate from federally funded research. Equally important were initiatives by US funding agencies to support “chemical probe” development and drug discovery, for example through the National Institutes of Health (NIH) Molecular Libraries Initiative.3,4 While some of these initiatives are being phased out, the establishment of a new NIH Institute, the National Center for Advancing Translational Sciences (NCATS), focusing on translational research, might continue to inspire academic probe development.5 These initiatives, as well as more direct drug development efforts at the NIH (e.g., the National Cancer Institute's Chemical Biology Consortium and the NIH Blueprint Neurotherapeutics Network), define a new paradigm for collaborative academic research beyond basic science. Further encouragement for small molecule tool and drug development efforts at universities originates in the massive changes in pharmaceutical industry's business plans, which include large scale outsourcing, expanded research collaborations6 and a greater willingness to consider externally initiated projects.7,8 Finally, recent examples of highly successful (and lucrative) drugs invented in academic laboratories9 have also increased awareness of this change among university researchers and administrators.
The academic setting provides a unique environment, distinct from traditional pharmaceutical or biotech companies, which may foster success and long-term value of certain types of probe discovery projects while proving unsuitable in others.10 The ability to launch exploratory high risk - high novelty projects from both chemistry and biology perspectives, for example testing the potential of unconventional chemotypes such as organometallic complexes,11 is one such distinction. Other advantages include the ability to work without overly constrained deadlines, and pursue projects that are not expected to reap commercial rewards - criteria and constraints that are common in “big Pharma.” Furthermore, projects to identify tool molecules in an academic setting often benefit from access to unique and highly specialized biological assays and/or synthetic chemistry expertise12,13 that emerge from innovative basic science discoveries. Indeed, recent data show that the portfolios of academic drug discovery centers contain a larger percentage of long term, high-risk projects compared to the pharmaceutical industry. In addition, many centers focus more strongly on orphan diseases and disorders of third world countries than commercial research organizations.2 In contrast, programs that might be less successful in an academic setting are those that require significant resources – personnel, equipment and funding – that may be difficult to sustain in a university setting.2 Projects whose goals are not consistent with the educational mission of the university and cannot provide appropriate training and / or content for publications or theses would also be better suited for a commercial enterprise.
There are clear challenges and disadvantages in carrying out projects to identify and characterize tools, probes and drugs in an academic setting. Challenges may include a lack of resources14 and expertise in certain disciplines that would expedite progress, such as know-how in certain areas of medicinal chemistry, pharmacokinetics, drug formulation, and animal toxicology.2,15,16 The Bayh-Dole Act opened the door to establishing university-based intellectual property (IP), but it did not resolve complex inventorship, licensing and conflict of interest quandaries. Furthermore, the value of early patenting of university-generated IP is still being debated, with some organizations now moving toward revealing “pre-competitive” matter, leaving to others any optimization or commercialization.17 Finally, the multi-disciplinary nature of research that is essential to probe and drug discovery projects is not a typical research structure in academic science departments and hard to factor into the tenure decision process.
To take advantage of the unique academic environment and have a significant long-term impact on the scientific community, ideal projects might include those that generate tools to detect important biological phenomena or characterize poorly understood disease targets and biological systems. In addition, probe projects that exploit a specific, unique expertise (or a combination of tools and skills) that is resident at a particular university are also likely to be successful and have high impact.
Notable examples of successful biological probes and drugs have emanated from academic laboratories. The contributions of academic and research institute laboratories to the characterization and development of taxol are an excellent historic example of exploiting highly specialized skills (e.g. natural products structure elucidation, cancer and tubulin pharmacology, and synthetic organic chemistry) that are typically resident in university settings. Taxol also highlights the crucial role of the government (e.g., NCI) in supporting and facilitating such work, as well as developing a probe molecule into a viable drug candidate.9,18,19,20 It is worth noting that the time from the isolation of taxol (1967) to its approval as a therapeutic agent (1992) may also be characteristic of academic probe and drug discovery programs.
In this perspective, we highlight five examples of small molecule probes and tools that have found use in interrogating biological systems. A “chemical probe” is often thought of as a molecule that interrogates a specific biological target, with ideal characteristics involving high potency, adequate water solubility, and other appropriate physical properties. Although distinct from a drug, these chemical probes can often be starting points for drug discovery efforts.21,22 While several of the examples included in this perspective fit this definition (GPR30 agonists and antagonists, Hsp70 modulators, CB2 agonists), we chose to include others (ROS sensors, beta-amyloid imaging agents) that fall outside this classification in an effort to highlight a broad range of tool development efforts in academic labs. The selection of molecules to highlight was neither exhaustive nor completely objective; however, several criteria were applied. First, each featured molecule was discovered in an academic laboratory. While it is often the case that multiple groups from multiple departments and locations contribute to the development of specific probes or a family of probes, we based our criteria on the dates of the first publications found. A second criterion was a judgment of the utility and impact of the probe. Most examples have already been used as tool compounds in studies external to the originating laboratory or have been the basis for further investigations by others. All have opened up new areas of research. Additional measures for utility and impact included commercial availability and reports of in vivo activity. Third, the highlighted probes were novel at their report, and similar tool compounds were not previously known. Among the many probe molecules that met these criteria, we selected to showcase different approaches, different therapeutic areas, and different “forms” of probes. Accordingly, we aim to highlight the specific and unique aspects of the academic setting that contributed to these probe molecules gaining widespread utility and serving as catalysts for further scientific study.
Chemical Probes for the Detection of Reactive Oxygen Species
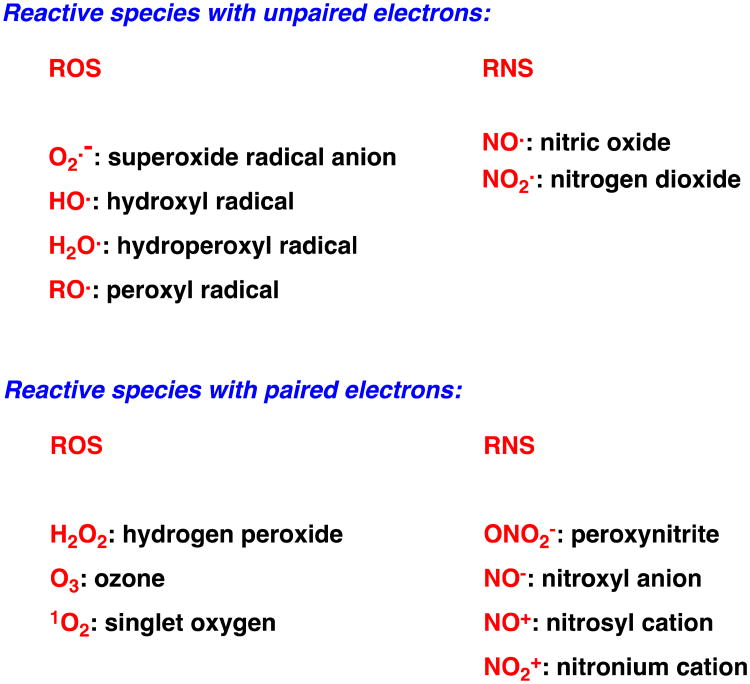
The presence of reactive oxygen species (ROS) and reactive nitrogen species (RNS) in intracellular compartments reflects a balance between the need for energy (ATP) generation through the oxidative phosphorylation (OxPhos) pathway, regulatory feedback mechanisms for oxygen metabolism, and the biochemical functions of transition metal cofactors. The most pertinent ROS are radicals such as superoxide radical anion, O2.-, and hydroxyl radical, OH., as well as reactive species with paired electrons, such as hydrogen peroxide, H2O2 (Scheme 1).23 Among RNS, nitric oxide, NO., is a well-known secondary messenger, but this free radical can also cause severe intracellular damage and induce programmed cell death (apoptosis).24
Scheme 1.
Categories of reactive species.
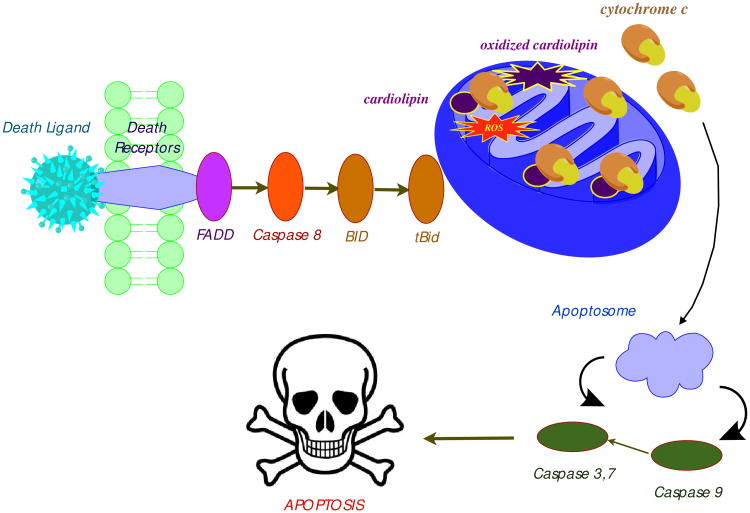
The majority of the intracellular ROS is generated in mitochondria and can be triggered by radiation, hemorrhagic shock, aging, exposure to microbial toxins, or activation of membrane-associated death receptors. For example, the FAS-associated protein with death domain (FADD) activates caspase 8 and the BH3-interacting-domain death agonist (BID). Cleaved BID (tBid) mediates translocation of cytochrome c into the cytosol, formation of the apoptosome, and irreversible commitment to apoptosis (Scheme 2).25 ROS oxidize cytochrome c-associated cardiolipin, which amplifies the release of cytochrome c from the inner mitochondrial membrane.26
Scheme 2.
ROS trigger apoptosis through the release of cytochrome c from mitochondria.
In addition to genetically encoded redox sensors,27 the detection of ROS with small molecule probes represents an active field of academic research focused on improving our understanding of cellular energy conversion and signaling pathways.28,29 Moreover, the opportunities to correlate the redox environment to the regulation of cell division, circadian rhythms, immune response, and apoptosis has inspired commercial developments in ROS detection and regulation.
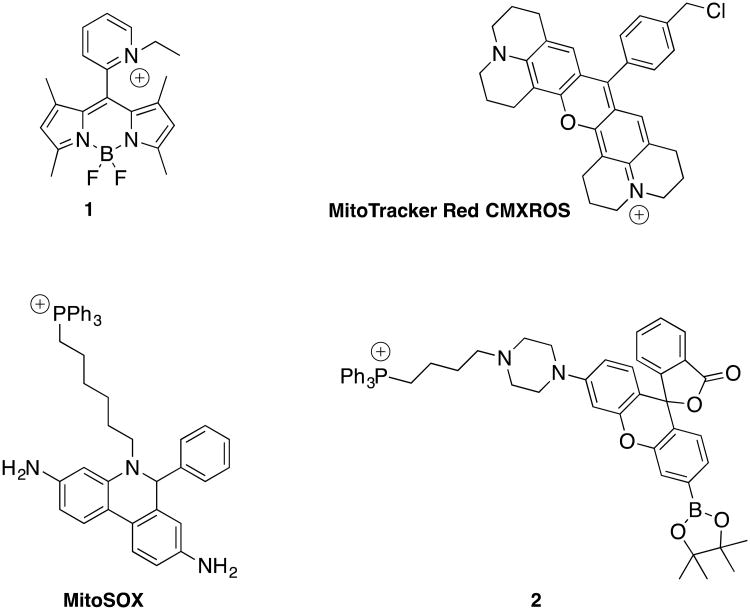
Most sensors are based on fluorescent signaling, and some of them have additional intracellular targeting capabilities. It is often sufficient to add a positive charge to a fluorescent probe to develop a mitochondria-specific ROS sensor that can provide information about morphology, number, and membrane potential of these energy-producing organelles. For example, quaternization of the pyridyl group in the BODIPY dye OBP provided the photostable mitochondrial redox probe 1 (OBEP), which has similar staining properties but much lower cytotoxicity compared to MitoTracker Red CXMRos (Figure 1).30
Figure 1.
The mitochondrially-targeted fluorescent dyes 1, MitoTracker Red CXMRos, and the ROS detecting MitoSOX and 2.
The most widely used intracellular probe for O2.- is hydroethidium, which relays oxidative stress and can be targeted to mitochondria by attachment to a triphenylphosphonium (TPP) group, thus yielding MitoSOX.31,32 Similarly, the attachment of a TPP group onto a boronate-masked hybrid fluorescein/rhodamine scaffold provides a probe molecule, 2 (MitoPY1), that can detect increases in mitochondrial H2O2 levels in mammalian cells.33 Furthermore, addition of the mitochondrial electron transport chain uncoupler and H2O2 inducer paraquat to HeLa cells leads to a robust fluorescence enhancement in mitochondria observed in confocal fluorescence imaging.
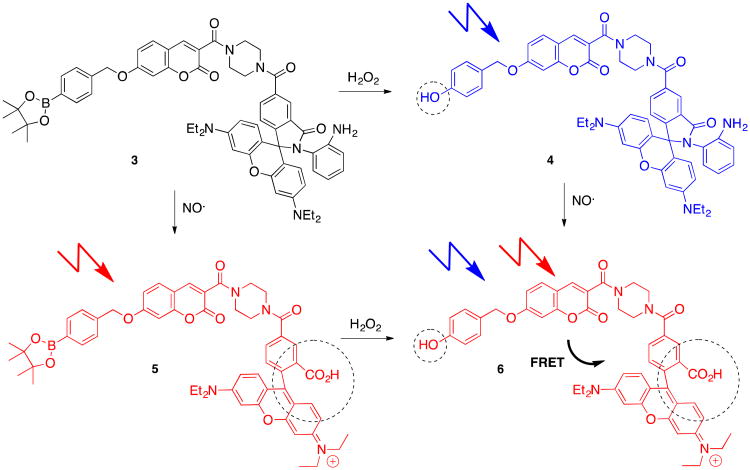
Prior to the development of 2, among nine commercial H2O2 fluorescent molecular probes and thirty mitochondrial fluorescent probes, no H2O2 probe specifically targeted to mitochondria was available.34 Compound 2 filled this gap and quickly inspired other subcellular targeting fluorescent devices. For example, in a recent study that also nicely illustrates the opportunities for ROS-selective chemical sensing, the fluorescent probe 3 responding to H2O2, NO. , and the combination of the two reactive species was developed and tested in HeLa cells (Scheme 3).35 The reaction of H2O2 with the pinacol boronate moiety of 3 generates a phenol function in 4 and leads to a blue fluorescence emission at 460 nm upon excitation at 400 nm. Alternatively, exposure of the aniline moiety to NO. leads to hydrolysis of the lactam to give 5 and triggers a red fluorescence emission at 580 nm upon excitation at 550 nm. Finally, if both ROS and RNS are present, Förster resonance energy transfer (FRET) in the 7-hydroxycoumarin-rhodamine scaffold 6 causes a red fluorescence emission at 580 nm upon excitation at either 400 or 550 nm.
Scheme 3.
Triple sensing fluorescent probe design.
These versatile dual sensors of NO. and H2O2 can serve as high-content molecular tools for the investigation of intracellular networks, in particular signal transduction pathways and redox regulation of protein activation. In this context, having a single fluorescent probe available that is capable of monitoring multiple species is particularly valuable, since the biological system is less disturbed, and mutual interference by sensors is less likely than upon administration of multiple, more specialized probes. Strategically, this research also illustrates the advantage of the academic environment to anticipate the demand for higher content diagnostic agents that can offer new opportunities for the SAR analysis and potential clinical development of dual ROS/RNS modulators and theranostics.36
Identifying Selective Ligands for Orphan GPCR's: GPR-30 Modulators
Orphan G-protein Coupled Receptors (GPCR's) are receptors, often identified via sequence analysis, whose natural ligand is unknown. “De-orphaning” - that is, identifying the endogenous ligand - provides further insight into the physiological and potential pathophysiological role of the receptor; however such work is often hampered by the lack of selective tools to probe the biology of the novel GPCR The identification of selective ligands (both agonists and antagonists) for orphan and de-orphaned GPCR's provides a valuable biological tool, and is an area where academic research centers can have a significant impact. The fundamental biology involved and the high-risk nature of the work make these projects ideal for an academic setting.
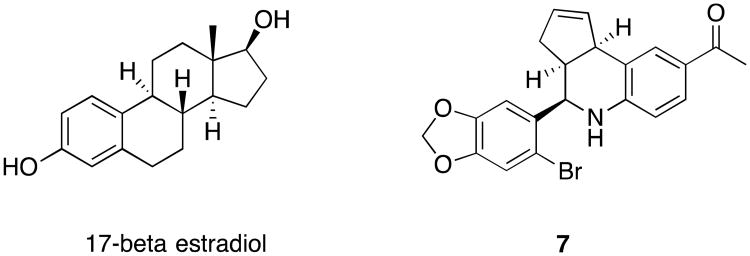
In 1997, the gene, GPR30 (also known as G-protein coupled estrogen receptor, GPER1), and the orphan receptor it codes for, were identified and characterized as a GPCR that was associated with estrogen receptor-expression in breast cancers.37,38,39 Further characterization showed that GPR30's endogenous ligand was estrogen, and suggested this newly characterized GPCR played a role in estrogen-mediated physiology and pathology.40 Estrogen was found to act similarly on GPCR30 and the known nuclear receptors, Estrogen Receptor α (ER-α), Estrogen Receptor β (ER-β); therefore, understanding the specific roles of each receptor in estrogen-mediated physiology was not possible due to the lack of selective tool compounds. Researchers at the University of New Mexico and New Mexico State University, in collaboration with Chemical Diversity Laboratories, initiated an effort to identify highly selective small molecule probes of this poorly understood receptor. A 10,000 compound library that was designed to contained GPCR privileged structures was used in a virtual screen for 2-D and 3-D based similarity to 17-beta estradiol. This effort reduced the number of compounds that were included in the biomolecular screen to 100 compounds. Compound 7 (G-1) was identified as a robust hit that inhibited the binding of estrogen to GPR30, with a binding constant equal to 11 nM, and EC50 for calcium mobilization equal to 2 nM.41 17-β–estradiol exhibited a similar binding constant (Ki=5.7nM) ad EC50 (0.3nM). However, unlike estradiol, 7 exhibited no binding to ER-α or ER-β at concentrations as high at 1μM, nor did it mobilize calcium in functional assays. This tool compound was the first that could differentiate GPR30 from the classical estrogen receptors α and β.
Since estrogens are responsible for a plethora of actions, understanding which receptor contributes to which biological and pharmacological function allows for further understanding of this system as well as for validating these receptors as potential targets for new drugs. Numerous researchers have used this probe to characterize the role of GPR30's in various in vitro and in vivo systems. For example, by using compound 7 as a tool in in vivo models, Lindsey et al. showed that estrogen acting through GPR30 may contribute to the beneficial cardiovascular effects attributed to the hormone.42 This probe molecule was also used in experiments to suggest that the presence of ER-α, but not GPR 30, provides protection against Hepatitis C viral infection in cell culture models.43 Studies to understand the contributions of each estrogen receptor to cognitive performance used compound 7 to show improvements in spatial learning in animal models, compared to ovariectomized animals. This work suggests that GPR30 may be a target for pharmaceutical intervention to treat cognitive defects that offers advantages over targeting the classical estrogen receptors.44 Additional studies suggest a role for GPR 30 in disorders such as obesity, multiple sclerosis45 and cancer.46
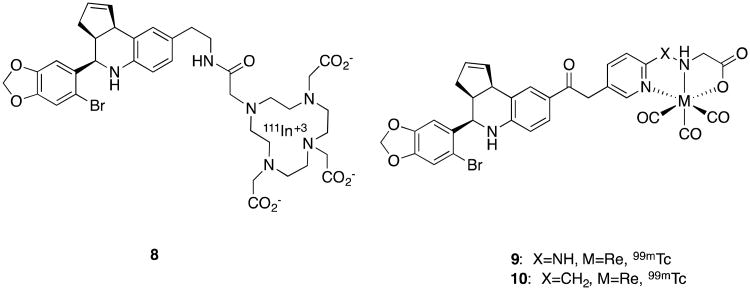
This work and the availability of such a tool have spurred additional studies, including those to develop new tool compounds.47,48 The 1,2,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) derivative of 7, along with others, were synthesized and labeled with 111/113In in order to evaluate their potential as imaging agents (Figure 3). The 111In-labelled derivative 8 exhibited promising in vitro results, but only moderate selectivity between blood and tumor and tumor and muscle, and poor pharmacokinetics.49 Related work reported the tricarbonyl chelate derivatives 9 and 10 as a potential SPECT imaging agent.50
Figure 3.
GPR30 imaging agents.
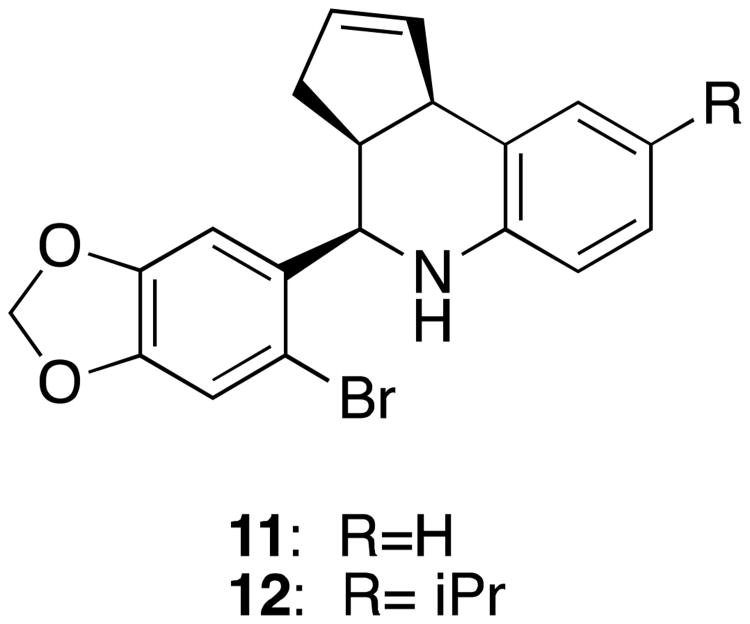
An effort to identify antagonists of GPR30 was initiated based on the structure of compound 7. Focused testing of a small set of analogs led to the identification of compound 11 (G15) (Figure 4). This close structural analog of 7 exhibits high affinity (20 nM) to GPR30, but little or no affinity for ER-α or ER-β at concentrations up to 10 μM. In contrast to compound 7, however, it produced no calcium mobilization response and blocked the agonists effects of 7 and classical estrogens. This antagonist tool compound has been evaluated in in vivo models to further understand the role of GPR30, distinguish it from the classical estrogen receptors, and to evaluate its potential as a drug target. Results using both the agonist and antagonist probe point to the potential of targeting this newly characterized GPCR to treat depression. In a tail suspension test that is used to characterize anti-depressant effects, compound 7 reduced immobility time in a dose-dependent manner, while 11 alone had no effect. However, pre-treatment with the antagonist (compound 11) attenuated the effects of 7 thereby confirming the involvement of GPR30.51 The GPR30 antagonist was used in a provocative study that suggested that the neuroprotective effects of benzothiophene selective estrogen receptor modulators (SERM's), such as raloxifene, work through GPR30 rather than through ER-dependent or anti-oxidant mechanisms.52 Second-generation antagonists have been developed such as compound 12 (G36) shown in Figure 4.53 The design of this molecule was based on docking studies of compounds 7 and 11 to ER-α that suggested the ethanone moiety in 7 made critical H-bonding contacts with the GP30 receptor that were responsible for receptor activation, and also sterically prevented binding to ER-α. This structural feature is lacking in compound 11, thereby removing an important selectivity determinant. To increase selectivity, the sterically demanding, but H-bonding incompetent, isopropyl substituent was incorporated to afford compound 12. This compound, as designed, maintains the antagonist activity towards GPR30, yet is highly selective over the classical estrogen receptors.
Figure 4.
Structure of GPR30 antagonists 11 and 12.
A number of factors contributed to the successful identification and characterization of GPR30 tools, as well as their widespread use by the research community. One major influence was the co-localization of key investigators with highly specialized expertise. The use of flow cytometry as a screening methodology, expertise in GPCR biology, as well as access to sophisticated computational chemistry and informatics tools allowed for focused and efficient screening, and the rapid characterization of compound 7.46 Furthermore, the academic setting allowed prompt publication of the finding, and gave external researchers rapid access to these tools.54 A search of Pubmed using the term “GPR30” lists 327 references, the first of which was published in 1997 and describes the identification of the orphan receptor GPR30.37 Between 1997 and 2006, when the report of the agonist appeared in the literature, only 23 papers were published on GPR30. Clearly the availability of tool compounds contributed to the rapid interest and work in this area. Finally, the high risk nature of this project – the identification of a probe molecule whose target, the first G-protein coupled estrogen receptor was poorly understood - was ideal to be pursued in an academic setting.
Probes of Molecular Chaperones: Chemical Modulators of Heat Shock Protein 70 (Hsp70)
Molecular chaperones are essential for protein maturation. They play critical roles in cellular processes, including protein folding, degradation, and transport, as well as the pathogenesis of many human diseases. Heat shock proteins (Hsp's) are induced upon cellular heat treatment and were also first discovered in this fashion. Since Hsp's prevent protein misfolding and undesirable protein aggregation, they form part of the cytoprotective response mechanism, counteracting apoptosis and autophagy. As a consequence of their antagonism of cell death, Hsp's are often overexpressed in malignancies,55 and their inhibition can increase the sensitivity of cancer cell lines to antitumor agents.56,57,58
Hsp's are generally subdivided by their molecular weight into several families, including Hsp110, Hsp90, Hsp70, Hsp60, Hsp40, and other smaller congeners. Often, they are recruited as multiprotein complexes with other cofactors and co-chaperones, and their expression levels are interdependent.59 In contrast to Hsp90, which has attracted a large amount of interest from cancer researchers in pharmaceutical companies and academia, Hsp70 has remained a secondary drug target for many years. Recently, however, attention has started to shift, in realization of the many potential benefits that the selective modulation of this chaperone might bring to human health.60
In addition to overexpression in various cancer types, Hsp70's are implicated in a variety of pathological conditions. In the stressed environment of cells infected by parasites, such as Trypanosoma or Plasmodium falciparum, or by viral strains, such as hepatitis B, papillomavirus, HSV, or polyoma virus, Hsp70's are required for cell viability, and any inhibition of their function impairs the spread of parasite or virus. Finally, Hsp70 function has also been linked to neurological disorders, such as cystic fibrosis, Parkinson's and Huntington's disease, Alzheimer's disease, and various ataxias.
Among the 13 known human Hsp70's, the constitutively expressed Hsc70, the endoplasmic reticulum-localized GRP70, and the mitochondrial mtHsp70 are not considered as druggable as the highly stress-inducible Hsp70, which is often not observed under normal conditions. Two structural subunits in Hsp70, a C-terminal peptide-binding domain (PBD) and an N-terminal ATPase domain, collaborate in substrate binding, refolding, and client release after ATP hydrolysis. The main co-chaperones that are critical to Hsp70 function are J-domain co-chaperones, such as Hsp40, that stimulate ATPase activity, nucleotide exchange factors that catalyze the release of ADP, such as Hsp110 or HspBP1, and co-chaperones that stabilize complexes with Hsp90 and Hsp90 client proteins, such as Hop and CHIP.
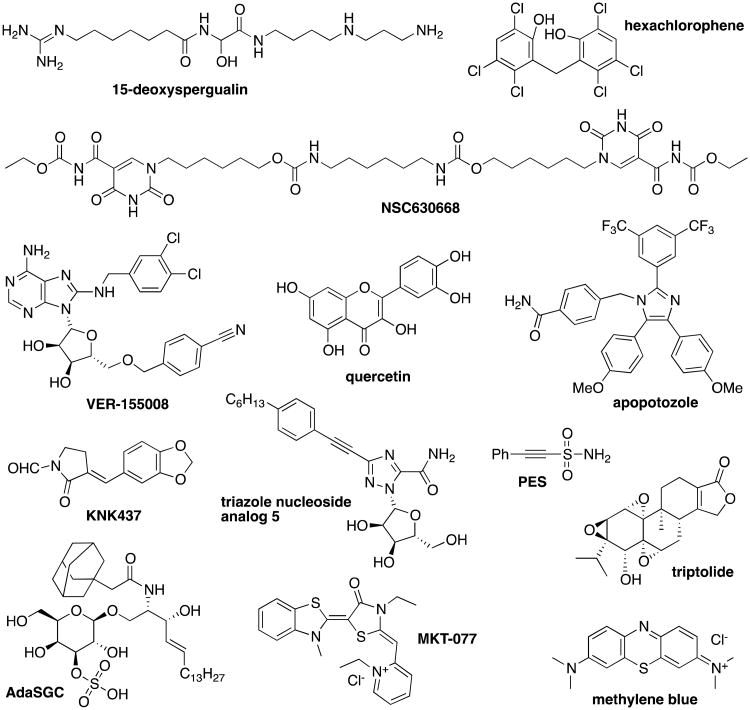
Due to the predominant therapeutic focus on Hsp90, the considerable complexity of multi-protein chaperone complexes, and the slowly emerging knowledge on the multiple roles of Hsp70, only a handful of Hsp70 agonists or antagonists have been developed to date. A majority of these are either nonspecific, chemically unstable, or not amenable to further improvements using medicinal chemistry strategies (Figure 5). Decoy targets of Hsp70, such as the peptide ADD-70,61 have also been used to neutralize its function.
Figure 5.
Structures of inhibitors of Hsp70 and related heat shock pathway modulators.
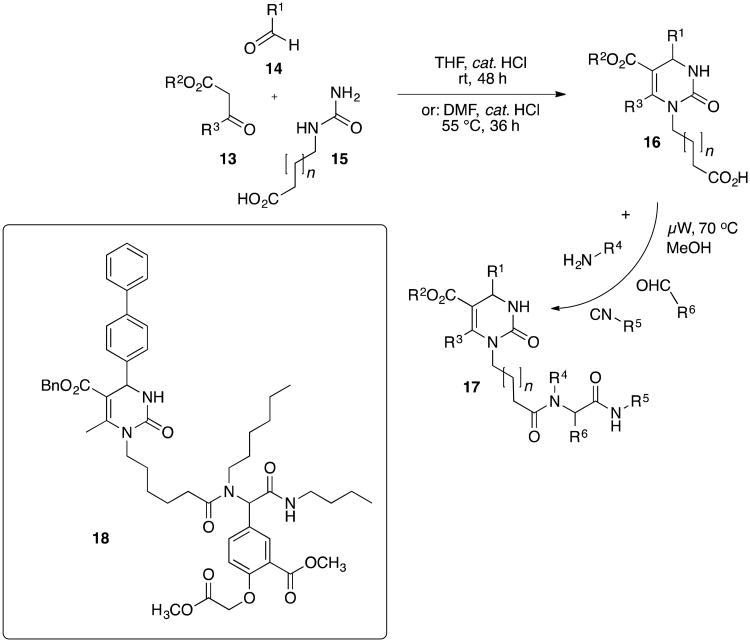
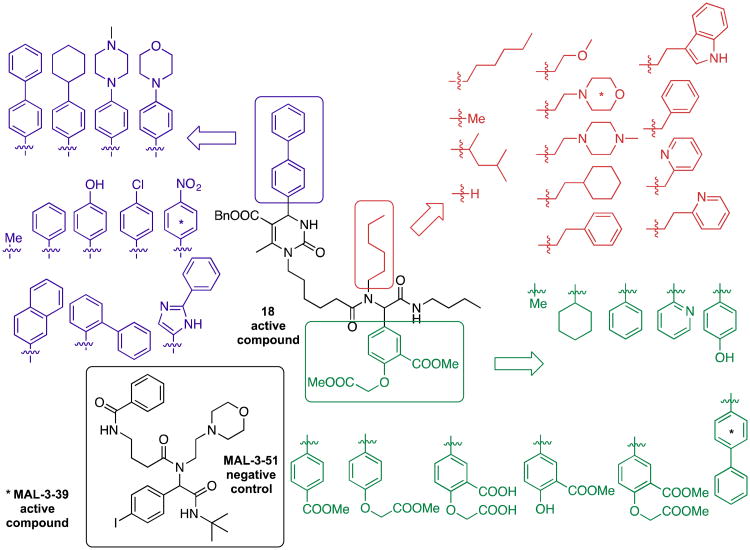
In a search for small molecule structural mimics of 15-deoxyspergualin and, especially, NSC 630668, two known, albeit non-specific and not very potent Hsp70 inhibitors, a group at the University of Pittsburgh identified a new class of Hsp70 modulators in 2004.56 These synthetic compounds contain a pyrimidinone core, which makes them amenable for diversification by multicomponent and cascade reactions. As a result, small molecule libraries were readily obtained for screening purposes (Schemes 4 and 5). The Biginelli dihydropyrimidine synthesis with β-ketoesters 13, aldehydes 14, and ureas 15 generated heterocycles 16, which could further be subjected to an Ugi four-component condensation to attach the peptoid side chains in Biginelli-Ugi hybrids such as 17.62 The novel Hsp-70 modulator 18 (MAL3-101) was one of the first compounds prepared in this ca. 360-membered library. As of December 2012, 800 diverse bioassays on 158 of these dihydropyrimidinones have been reported in PubChem,63 and 105 compounds met active hit criteria. Both active hits and inactive control reagents were thus available and allowed the tailoring of the SAR in a diverse range of biological systems with potential involvement of Hsp70. Mechanism of action studies established that lead structure 18 had no effect on the KCAT for ATP hydrolysis but blocked the ability of Hsp70 co-chaperones to enhance Hsp70 ATPase activity. Compound 18 also compromised the transport of a nascent polypeptide into the yeast endoplasmic reticulum (ER), a cellular process that requires the interaction between Hsp70 and a co-chaperone.
Scheme 4.
Preparation of Hsp70 modulators by consecutive multicomponent reactions: Tandem Biginelli-Ugi process and structure of lead compound 18.
Scheme 5.
Dihydropyrimidinone library synthesis.
Select agents from these libraries exhibited notable activities, including: [1] inhibition of breast cancer cell proliferation with no effect on normal cells,64 [2] synergistic effects on multiple myeloma cell growth when combined with Hsp90 or proteasome inhibitors,50 and [3] interference with polyomavirus replication,65 which requires the recruitment of Hsp70 to a viral-encoded oncoprotein. Compound 18 and its congeners have therefore proven valuable as in vitro probes for Hsp70 function under conditions in which knock-down strategies are unavailable or are not readily transferable. For example, unique pyrimidinones inhibited the insertion of tail-anchor proteins into biological membranes and thwarted protein translocation and parasite replication in a Trypanosoma model.66 Other members of this chemical family prevented the growth of P. falciparum in infected red blood cells, an event that requires Hsp70 and co-chaperone function, thus further confirming the role of Hsp70 in parasite replication.67 Finally, the specificity of one inhibitor class was established by NMR and by multiplex expression profiling studies, which measure distinct cellular responses to small molecule modulators. It was found that the pyrimidinone interacted with the Hsp40 binding domain of the Hsp70 ATPase.68
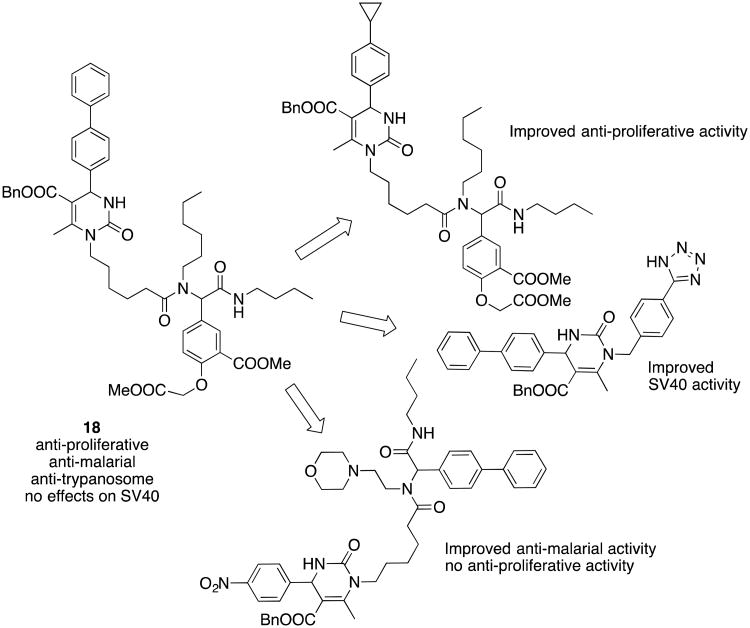
The probe character of compound 18 is illustrated by activities in >50 different international research laboratories which have been using it as a selective Hsp70 modulator and by >30 research papers that apply it as a tool compound. These studies have further advanced the understanding of the role of Hsp70 in cancer cell, viral and parasite replication. Furthermore, as shown in Scheme 6, members of this chemical library can be used as a platform to develop more selective lead structures against Hsp70-induced pathologies.69 The lower molecular weight analogs of 18 have improved aqueous solubility properties and lower lipophilicity, potentially moving this scaffold from the realm of tool compounds closer to drug-like properties. Not surprisingly, preliminary PK studies have demonstrated rapid metabolism in rodents, and work continues to increase potency and decrease the metabolic liabilities in this series.
Scheme 6.
Structural modifications of the Hsp70 probe molecule 18 lead to changes in the biological profile.
The development of compound 18 and its analogs to probe Hsp70 function took advantage of a unique combination in chemistry and biology expertise that was available at the University of Pittsburgh. At the time of the initial work, little was known about the role of Hsp70 compared to Hsp90, and therefore research projects associated with Hsp70 would have been considered high risk and purely academic. Nonetheless, early efforts to obtain a tool compound to probe for biological pathways influenced by Hsp70 and to chemically modulate the cellular activity of this chaperone have provided a chemical platform to explore Hsp70 functions in cancer and other Hsp70-linked diseases, and inspired new starting points for drug discovery efforts in the cellular stress pathway.
Deciphering Cannabinoid Receptor Biology: A Selective CB2 Agonist
Cannabinoid receptor (CB) agonists have been used as therapeutics throughout time in a number of cultures. CB receptor agonists include Δ-9-tetrahydrocannabinol (Δ9-THC), the active component of marijuana, and anandamide, an endogenous CB receptor agonist. The therapeutic indications of cannabinoids include pain relief, appetite stimulation and suppression of nausea. However, their psychoactive component, which includes euphoria, cognitive dysfunction, drowsiness, and motor incoordination, limit their legal and practical use as a therapeutic. To date, two cannabinoid receptors have been identified, CB1, which is found primarily in the central nervous system, and CB2, which is mainly expressed on immune cells in the periphery.70 The distribution pattern of CB2 led to the hypothesis that a therapeutic that targets CB2 selectively may exhibit desirable therapeutic effects, including pain relief, without the psychoactive side effects.71
In order to test the theory of the differentiated therapeutic effects of activation of CB1 versus CB2, agents that selectively activate CB2 were sought. One such agent, compound AM1241, was developed at the University of Connecticut.72 AM1241 was derived from the known non-selective indole class of cannabinoid receptor agonists represented by WIN55212-2 and JWH-015 (Figure 6). Compared to earlier agonists that exhibited modest selectivities, AM1241 exhibited 82-fold selectivity for CB2 over CB1 based on binding affinity assays (Table 1).73 AM1241 bears significant structural similarity to the earlier, less selective agonists, but is differentiated by the indole nitrogen substituent and the specific benzoyl aromatic moiety.
Figure 6.
Structures of CB2 Agonists.
Table 1. Binding Affinities of CB2 Agonists.
| Compound | CB2 Ki (nM) | CB1 Ki (nM) | Fold-Selectivity |
|---|---|---|---|
| AM1241 | 3.4 | 280 | 82 |
| WIN 55212-2 | 0.3 | 1.9 | 6 |
| JWH-015 | 14 | 383 | 27 |
Significantly, using AM1241, it was demonstrated that a selective CB2 receptor agonist provides relief of pain without the psychotropic effects produced by a pan-cannabinoid receptor agonist.72 After injection of AM1241 into the hindpaw of a mouse, analgesic activity toward a thermal stimulus applied to the same paw was observed. Co-administration of a CB2 receptor antagonist blocked the effect, while a CB1 antagonist did not. The results of this initial study led researchers to investigate the use of a CB2 receptor selective agonist for the treatment of pain as well as other disease states that involve the CB2 receptor. To provide evidence that the anti-nociceptive activity of AM1241 is caused by direct association with CB2 in vivo, the effects of AM1241 in several pain models comparing wild type (CB2+/+) mice and genetically modified CB2+/+ mice were studied. It was found that AM1241 inhibited nociception in CB+/+ mice, but not CB2+/+ littermates, providing strong evidence of its direct activation of CB2.74
Since its initial disclosure, AM1241 has been used by many groups to evaluate the role of cannabinoid receptors in various models of pain, including in animal models of inflammatory pain,75,76 neuropathic pain,77 capsaicin-induced pain,78 postoperative pain,79 and pain caused by cancer.80 Amongst these studies, there were no reports of psychotropic side effects, further confirming the differentiation between the CB1 and CB2 receptors.
Although initial studies suggested that CB2 is only expressed in the periphery, AM1241 was used as a probe to complement the findings that CB2 receptor gene expression is up-regulated in both the spinal cord and dorsal root ganglia (DRG) tissues in rats under inflammatory and neuropathic pain models.81 In a study conducted by Beltramo et al., AM1241 was shown to dose-dependently inhibit capsaicin-induced release of the pain biomarker calcitonin gene-related peptide (CGRP) in rat spinal cord slices, a result that also suggests the presence of CB2 in neurons of the spinal cord.82
The availability of AM1241 enabled experiments that elucidated the mechanism of action of CB2 in the periphery, outside the CNS. One research group demonstrated that the antinociceptive response of AM1241 is caused by released endogenous opioid peptides, specifically β-endorphin, acting on neurons.83 Interestingly, other CB2 agonists including GW405833, exhibited antinociception without the release of endogenous opioids.84 In another study, it was shown that the nociceptive behavior of cannabinoids, WIN 55212-2 and AM1241, is mediated in the periphery through activation of TRPA1, a cation channel that is known to play an important role in pain modulation.85
The ready availability of AM1241 has allowed its pharmacology to be studied thoroughly, and this work has led to the conclusion that its effects are complex. Studies by Yao et al.86 and Bingham76 revealed that AM1241 has varied pharmacological effects, depending on the assay conditions. Yao et al. concluded that AM1241 is a protean agonist, meaning that the ligand can act as an agonist, antagonist or inverse agonist at the same receptor and its functional activity is dependent upon the relative level of constitutive activity of the system. Confounding the complexity of its pharmacology is that AM1241 is a racemate. Researchers at Wyeth separated the enantiomers and compared activities of (R,S)-AM1241, (R)-AM1241, and (S)-AM1241 in both affinity assays and efficacy (cAMP) assays. Interestingly, the R-enantiomer exhibits a 44-fold higher affinity to the human CB2 (hCB2) receptor than the S-enantiomer, with similar results for the rat and mouse CB2 receptors (rCB2 and mCB2). In the cAMP assay, agonist activity is observed for (R,S)-, (R)- and (S)-AM1241 against hCB2, whereas with rCB2 and mCB2, racemic and (R)-AM1241 are reverse agonists and (S)-AM1241 is an agonist. These experiments demonstrate the differential effects of the enantiomers of AM1241 as well as their difference in activity in rodent versus human CB2.
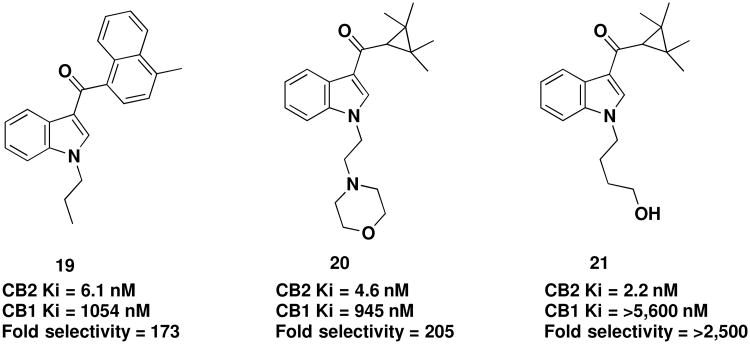
Since the discovery of AM1241, the development of selective cannabinoid agonists and antagonists has been a very active area of research, with additional CB2 selective agonists such as 19 (JWH-120) and a series from Abbott Laboratories (Figure 7, compounds 20 & 21) described.73,87 As evident by these examples, continued pursuance of this series of compounds over the years has led to further significant improvements in selectivity.
Figure 7.
Recent examples of selective CB2 agonists
Non-indole CB2 agonists have since been identified. Notably, pyrimidine 22 (Figure 8), developed by GSK, was studied in a phase 2 clinical trial to evaluate its analgesic efficacy in patients following third molar tooth extraction. Unfortunately, the compound was not effective in this trial.88,89 Compound 23 (Cannabinor), another selective CB2 agonist, was tested in a phase 2a trial for the treatment of capsaicin-induced pain and also found to be ineffective. The lack of efficacy despite promising in vivo activity is not well understood.
Figure 8.
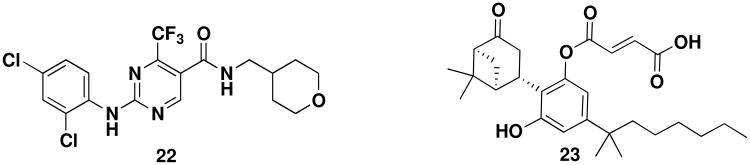
Non-indole CB2 agonists.
AM1241 has been used as a probe in more than 50 peer-reviewed publications, by academic and pharmaceutical laboratories. The discovery and availability of this compound opened new avenues to study the effects of selective CB2 agonism, especially in relation to pain relief. In particular, it allowed rapid developments in the understanding of CB receptors and provided proof of concept that the known beneficial and problematic effects of THC can be separated. Its discovery in the academic setting was advantageous to cannabinoid and therapeutic research areas in that its rapid disclosure in the literature followed by its commercial availability from at least 10 vendors allowed for its wide use as a tool compound.
Probes of β-Amyloid: Pittsburgh Compound B (PiB)
Alzheimer's disease (AD), the most prevalent form of dementia, affects over 18 million people worldwide. It and other dementias are characterized by progressive memory loss and cognitive decline. The distinguishing feature of AD, compared to other causes of dementia, is the presence of neuronal plaques that consist of β-amyloid peptide (Aβ). This characteristic, evident only on autopsy, provides the definitive diagnosis of the disease.90 Due to a lack of useful biomarkers, diagnosis of AD in patients relies on a battery of mental status exams, medical history, physical and neurological exams and laboratory tests to rule out other causes of dementia. A non-invasive detection method for the presence of Aβ would aid in the diagnosis of the disease at an early stage, before extensive neuronal damage occurs, and allow researchers to study the progression of the disease as it relates to production of Aβ.
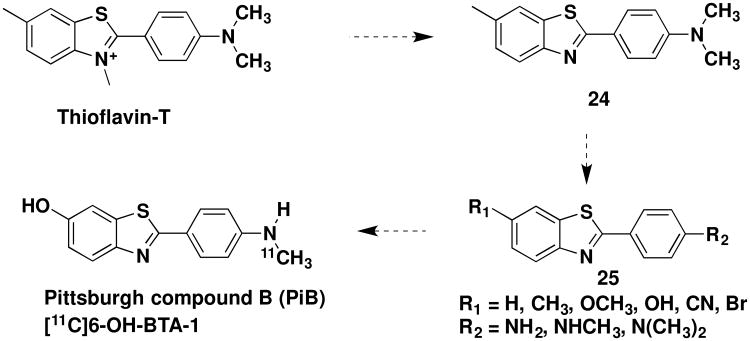
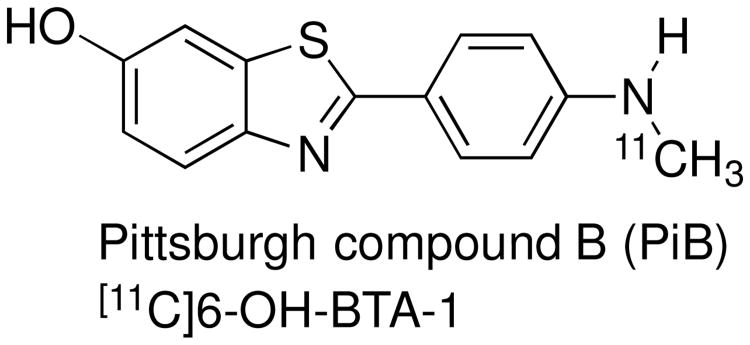
One method to detect the presence of Aβ in live patients is positron emission tomography (PET), whereby a radiolabeled ligand that binds selectively to Aβ acts as an imaging agent.91 One of the first such β-amyloid (Aβ) imaging agents, Pittsburgh Compound B (PiB), was developed at the University of Pittsburgh Medical Center. It is a carbon-11-radiolabeled analog of thioflavin-T.92 After its report in 2003, more than one hundred studies in which this agent was used were published.93
From a medicinal chemistry stand point, the inspiration for the discovery of PiB arose from thioflavin T (Scheme 7), a dye that binds to amyloid fibrils. The use of such a compound in vivo, however, is thwarted by its lack of brain penetration, presumably due to the charged nitrogen atom. Thus, researchers from the University of Pittsburgh Medical School prepared the neutral demethylated analog of thioflavin T, 24 (6-Me-BTA-2)92,94 and found that this compound had both improved binding affinity to Aβ fibrils and improved brain penetration compared to thioflavin-T. SAR studies, through preparation of numerous analogs including those with varied R1 and R2 substitutions (Structure 25, Scheme 7), provided compounds with further improved binding affinity and brain permeability. Amongst these, PiB (also referred to as N-methyl-[11C]6-OH-BTA-1), was identified as an analog that exhibited optimal properties as an imaging agent, including potent and specific binding, high brain penetration and rapid brain clearance rate.
Scheme 7.
Evolution of PiB from Thioflavin T.
PiB binds to aggregated Aβ(1-40) fibrils with a binding affinity (Ki) value of 4.3 nM as measured by its ability to displace [N-methyl-3H]BTA-1. As predicted by its logPC18 (1.2) and logPC8 values (1.3), at two minutes after injection, PiB exhibits adequate brain penetration in mouse and baboon (0.21% and 0.27% of injected dose per gram of brain normalized to body weight, respectively) for use as a radiotracer. Furthermore, as compared to the other analogs tested, PiB had the fastest clearance rate in both mouse and baboon with a t1/2 from baboon brain of 13 min, a value that is similar to known PET radioligands, and an important safety characteristic.
Additional in vitro characterization of PiB was performed to confirm its utility. Binding studies with a tritiated analog of PiB indicated that it binds to homogenates of postmortem AD frontal cortex with a Kd value of 1.4 nM and does so with 1:1 stoichiometry to Aβ under saturating conditions. Alternatively, there was no appreciable binding to homogenates of postmortem frontal cortex from a non-diseased control brain. Utilizing non-radiolabled PiB, it was found that amyloid plaques and cerebrovascular amyloid were stained in a similar manner to that of an Aβ antibody. These data confirm the binding affinity and specificity of PiB for Aβ deposits. The cumulative in vitro and in vivo data collected for PiB led to the selection of this compound for evaluation in humans.
The first human study using PiB was reported in 2004, less than two years after the initial publication.95 In this proof-of-concept study, involving 16 patients with a diagnosis of mild AD and 9 healthy controls, increased retention of PiB was observed in the frontal cortex, a brain region where Aβ accumulates, in AD patients compared to most healthy controls. By the year 2008, four years after the first reported study of PiB in humans, the compound had been used in approximately 3,000 people in over 40 medical centers centers, and was considered the “gold standard” as an amyloid PET tracer.91
Assuming a disease modifying therapy for AD is imminent, and that the presence of Aβ is predictive of the onset of the disease, an Aβ imaging agent would be a valuable diagnostic tool for predicting the onset of AD before symptoms of memory loss and dementia occur. As such, studies with PiB have involved healthy elderly individuals and patients diagnosed with mild cognitive impairment (MCI), as well as AD. In one study, PiB uptake in non-demented elderly subjects was associated with decreased episodic memory performance, a characteristic that may be suggestive of incipient AD. In a study that involved 206 subjects over 3 years, MCI subjects and healthy individuals who had high PiB retention were more likely to progress to AD than those with low PiB retention.96 The same study revealed that amyloid burden increases gradually as patients progress from cognitive normality to late stage AD, and that extensive Aβ deposition precedes cognitive impairment. The findings from this study and others indicate that there may be a window of opportunity to reverse the course of Alzheimer's disease following a finding of PiB retention and before the onset of symptoms, and further underlines the importance of this molecule, and others like it.
A notable use of PiB was in a phase 2 clinical trial of the immunotherapeutic bapineuzumab, an antibody of Aβ.97 PiB PET was used to measure reductions of cortical Aβ fibrils in patients with mild to moderate AD. The outcome of the trial indicated that bapineuzumab treatment over a 78-week period reduced cortical fibrillar Aβ by approximately 25% compared to placebo. It was noted that while bapineuzumab binds to Aβ oligomers as well as diffuse and complex plaques, PiB binds only to fibrillar Aβ. Therefore the effect of the drug using this measurement might be underestimated. Nevertheless, this trial represents the use of PiB to monitor Aβ reductions as a result of an anti-Aβ drug in a clinical trial. In addition to its purposes for studying amyloid production in relation to AD, PiB, also has been studied in individuals with other diseases or disorders with amyloid pathology including fronto-temporal lobar dementia, Parkinson's disease,98 dementia with Lewy bodies,99 cerebral amyloid angiopathy, and prion disease.93
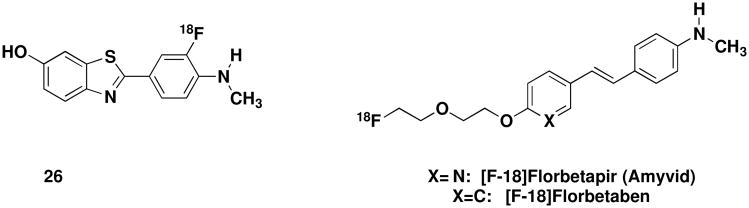
Carbon-11's half-life of 20 minutes poses both advantages and disadvantages when it comes to the practical use of PiB as a PET tracer. The short half-life allows for sequential imaging studies in the same participant to be performed on the same day. The major disadvantage, however, is that very few sites worldwide have a cyclotron, the instrument required for the on-site production of short-lived C-11 radiolabeled PET tracers. Fluorine-18 (F-18) labeled tracers, with a significantly longer half-life addresses this shortcoming. To make this probe more widely accessible, severalF-18 labeled tracers that target Aβ have been developed including compound 26 (also referred to as [F-18]3'F-PiB and F-18-Flutemetamol, Figure 10), also developed at the University of Pittsburgh Medical Center.100 This compound successfully completed phase 1 and 2 clinical trials101,102 and is currently being evaluated in phase 3 trials, and has comparable patterns of binding in in vivo PET retention studies and should have much wider accessibility compared to [C-11]PiB.
Figure 10.
Structures of compound 26, [F-18]Floretapir and [F-18]Florbetaben
The discovery of PiB has influenced the design of other amyloid tracers including [F-18]-florbetapir (Amyvid)103 and [F18]-florbetaben (Figure 10).104 Florbetapir F-18 was discovered in the lab of Hank F. Kung at the University of Pennsylvania, and licensed for development by Avid Radiopharmaceuticals Inc., now a subsidiary of Eli Lilly. Recently, the FDA approved Amyvid as the first approved PET imaging agent to estimate Aβ plaques in patients with cognitive impairment. Differing by only one atom, [F18]-florbetaben is being developed by Bayer Schering Pharma AG and is currently in Phase 3 clinical trials.
The academic research environment provided an ideal setting for the discovery of PiB for a variety of reasons. First, the researchers had access to highly specialized facilities, resources and expertise – including access to a cyclotron at the University of Pittsburgh Medical Center, which is available at relatively few institutions, as well as a access to tissue and brain samples that made the development of PiB possible. Secondly, at the time these efforts started, the value and use of a β-amyloid imaging agent was questionable because the amyloid hypothesis was not well accepted and research for Aβ lowering therapies was in an early stage of discovery. The development of other imaging agents targeting β-amyloid attests to the value of this work. The large number of Aβ targeting therapeutics in clinical trials makes the need for these diagnostics urgent.
Conclusion
The examples in this perspective highlight five diverse probe and tool compounds that emanated from academic laboratories. ROS sensors probe a biological response that has helped to define the timing and elucidate the pathways of stress responses and cellular degeneration. Their development in an academic setting was fostered by a focus on identifying tools to understand basic biology and cellular metabolism, rather than launching a commercial therapeutic. Compound 7 (G-1) and subsequent analogs were crucial in de-orphaning a new GPCR and understanding its role in estrogen-mediated pharmacology, and are representative of high-risk projects that are well suited for an academic environment. Their discovery relied on specific expertise in computational chemistry, GPCR biology and flow cytometry that was co-localized. Similarly compound 18 (MAL3-101) and its analogs were essential to interrogate the role of Hsp70 in a range of biological systems as well as identify chaperone involvement in normal and abnormal cellular stress response. Their development relied on a unique combination of expertise in synthetic organic chemistry and fundamental Hsp biology at Pittsburgh, and a willingness to pursue high-risk exploratory projects. The academic setting allowed ready access for many research groups to AM1241, which in turn contributed to the thorough understanding of cannabinoid receptor biology and served as a starting point for drug discovery efforts. PiB is an atypical tool compound in that it detects a specific structure and is useful as a diagnostic agent, rather than a therapeutic. As such, it has provided important understanding about the time course of AD pathology, potential risk factors, and the effectiveness of certain therapies. The unique access to cyclotron technology and Alzheimer's patients, as well as AD tissue and brain samples was crucial to the development of PiB. By continuing to take advantage of the special expertise resident in university settings, and the ability to pursue novel projects that may have limited commercial value, probes from academic researchers can continue to provide valuable tools for biomedical researchers. Furthermore, the current environment in the commercial drug discovery arena may lead to even greater reliance on academia for identifying suitable probe and lead structures, and other tools to interrogate biological phenomena. We believe that the collaboration of chemists who apply sound chemical concepts and innovative structural design, biologists who are fully committed to mechanism of action studies, institutions that understand portfolio building and risk sharing in IP licensing, and funding mechanisms dedicated to provide resources leading to the launch of Phase I studies will provide many future successful case studies toward novel therapeutic breakthroughs.
Figure 2.
Structure of 17-β-estradiol and 7.
Figure 9.
Structure of Pittsburgh Compound B (PiB).
Acknowledgments
The authors are grateful to the National Institutes of Health, in the Institute of General Medical Sciences (NIGMS) Chemical Methodologies and Library Development Center Program (GM067082 to P.W.) for support of their work.
Funding Sources: GM067082
Abbreviations
- Aβ
beta-amyloid peptide AD, Alzheimer's disease
- BODIPY
boron-dipyrromethene
- DOTA
2,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
- ER
endoplasmic reticulum
- ER-α
estrogen receptor alpha
- ER-β
estrogen receptor beta
- GPER1
G-protein coupled estrogen receptor 1
- MCI
mild cognitive impairment
- OxPhos
oxidative phosphorylation
- PiB
Pittsburgh Compound B
- PBD
peptide binding domain
- RNS
reactive nitrogen species
- Δ9-THC
Δ-9-tetrahydrocannabinol
- TPP
triphenylphosphonium
Biographies
Donna Huryn is currently Research Professor in the Department of Pharmaceutical Sciences at the University of Pittsburgh and Adjunct Professor Chemistry at the University of Pennsylvania. She is a graduate of Cornell University, and received a Ph.D. in Chemistry from the University of Pennsylvania. She spent 18 years as a medical chemist in the pharmaceutical industry working in CNS, cancer, anti-viral and inflammation therapeutic areas. Her current research interests include the development of probes and therapeutics for cancer, neurodegenerative disorders and infectious disease.
Lynn Resnick is currently a medicinal chemist at Knopp Biosciences in Pittsburgh, PA. Before joining Knopp she was a research assistant professor at the University of Pittsburgh, where she acted as the scientific coordinator for the University of Pittsburgh Chemical Diversity Center. Prior to that she was a Principle Research Scientist at Wyeth Research for 10 years, where she worked as a medicinal chemist with a focus on diseases and disorders of the central nervous system. She obtained a Ph.D. from Brown University and a B.A. from Rutgers University.
Peter Wipf is currently Distinguished University Professor of Chemistry and Professor of Pharmaceutical Sciences at the University of Pittsburgh, where he spent the last 23 years working on the total synthesis of natural products, organometallic and heterocyclic methodology, medicinal chemistry, and computational analysis. He spent 2 years as a Swiss National Science Foundation postdoctoral fellow at the University of Virginia, and he obtained his Ph.D. and his Diploma in Chemistry from the University of Zürich in 1987 and 1984, respectively.
Footnotes
Author Contributions: The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript
References
- 1.Stevens AJ, Jensen JJ, Wylier K, Kilgore PC, Chatterjee S, Rohrbaugh ML. The role of pubic-sector research in the discovery of drugs and vaccines. N Engl J Med. 2011;364:535–541. doi: 10.1056/NEJMsa1008268. [DOI] [PubMed] [Google Scholar]
- 2.Frye S, Crosby M, Edwards T, Juliano R. U.S. academic drug discovery. Nature Rev Drug Disc. 2011;10:409–410. doi: 10.1038/nrd3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zerhouni E. The NIH Roadmap. Science. 2003;302:63–64. doi: 10.1126/science.1091867. [DOI] [PubMed] [Google Scholar]
- 4.Austin CP, Brady LS, Insel TR, Collins FS. NIH Molecular Libraries Initiative. Science. 2004;306:1138–1139. doi: 10.1126/science.1105511. [DOI] [PubMed] [Google Scholar]
- 5.Collins FS. Reengineering translational science: the time is right. Sci Transl Med. 2011;3:90cm17. doi: 10.1126/scitranslmed.3002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfizer. [accessed January 20, 2013];Centers for Therapeutic Innovation. http://www.pfizer.com/research/rd_works/centers_for_therapeutic_innovation.jsp.
- 7.Lessl M, Bryans JS, Richards D, Asadullah K. Crowd sourcing in drug discovery. Nature Rev Drug Disc. 2011;10:241–242. doi: 10.1038/nrd3412. [DOI] [PubMed] [Google Scholar]
- 8.For several recent examples see: Bayer HealthCare Grants4Targets. [accessed January 20, 2013]; www.grants4targets.com/scripts/pages/en/index.php.Lessl M, Schoepe S, Sommer A, Schneider M, Asadullah K. Grants4Targets- An Innovative Approach to Translate Ideas from Basic Research to Novel Drugs. Drug Disc Today. 2011;16:288–292. doi: 10.1016/j.drudis.2010.11.013.GlaxoSmithKline Partnerships. [accessed January 20, 2013];Academic Collaborations. http://www.gsk.com/partnerships/academic-collaborations.html.Eli Lilly. Open Innovation Drug Discovery. [accessed January 20, 2013]; https://openinnovation.lilly.com/dd/
- 9.Borman S. Drugs from academia. Chem Eng News. 2007;85(16):42–47. [Google Scholar]
- 10.Huryn DM. Drug discovery in an academic setting: Playing to the strengths. Med Chem Lett. 2013;4:313–315. doi: 10.1021/ml400012g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meggers E. Exploring biologically relevant chemical space with metal complexes. Curr Opin Chem Biol. 2007;11:287–292. doi: 10.1016/j.cbpa.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber SL. Organic synthesis toward small-molecule probes and drugs. Proc Natl Acad Sci USA. 2011;108:6699–6702. doi: 10.1073/pnas.1103205108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer RA, Wurst JM, Tan DS. Expanding the range of ‘druggable’ targets with natural product-base libraries: an academic perspective. Curr Opin Chem Biol. 2010;14:308–314. doi: 10.1016/j.cbpa.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitty A. Growing PAINS in academic drug discovery. Future Med Chem. 2011;3:797–801. doi: 10.4155/fmc.11.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conn PJ, Roth BL. Opportunities and challenges of psychiatric drug discovery: roles for scientists in academic, industry, and government settings. Neuropsychopharm. 2008;33:2048–2060. doi: 10.1038/sj.npp.1301638. [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen WL. Challenges for academic drug discovery. Angew Chem Int Ed. 2012;51:11680–11684. doi: 10.1002/anie.201204625. [DOI] [PubMed] [Google Scholar]
- 17.FitzGerald GA. Can Intellectual property save drug development? Science. 2012;338:483–484. doi: 10.1126/science.1231170. [DOI] [PubMed] [Google Scholar]
- 18.Oberlies NH, Kroll DJ. Camptothecin and Taxol: Historic achievements in natural products research. J Nat Prod. 2004;67:129–135. doi: 10.1021/np030498t. [DOI] [PubMed] [Google Scholar]
- 19.Kingston DGI. Taxol, a molecule for all seasons. Chem Comm. 2001:867–880. [Google Scholar]
- 20.Horwitz SB. Personal recollections on the early development of taxol. J Nat Prod. 2004;67:136–138. doi: 10.1021/np0304464. [DOI] [PubMed] [Google Scholar]
- 21.Frye SV. The art of the chemical probe. Nature Chem Biol. 2010;6:159–161. doi: 10.1038/nchembio.296. [DOI] [PubMed] [Google Scholar]
- 22.Workman P, Collins I. Probing the probes: Fitness factors for small molecule tools. Chem & Biol. 2010;17:561–577. doi: 10.1016/j.chembiol.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pak YK, Jeong JH. Mitochondria: the secret chamber of therapeutic targets for age-associated degenerative diseases. Biomol Ther. 2010;18:235–245. [Google Scholar]
- 24.Silverman RB. Design of selective neuronal nitric oxide synthase inhibitors for the prevention and treatment of neurodegenerative diseases. Acc Chem Res. 2009;42:439–451. doi: 10.1021/ar800201v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kagan VE, Bayir HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang J, Stoyanovsky DA, Wipf P, Kochanek PM, Greenberger JS, Pitt B, Shvedova AA, Borisenko G. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radical Biol Med. 2009;46:1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji J, Kline AE, Amoscato A, Samham-Arias AK, Sparvero LJ, Tyurin VA, Tyurina YY, Fink B, Manole MD, Puccio AM, Okonkwo DO, Cheng JP, Alexander H, Clark RSB, Kochanek PM, Wipf P, Kagan VE, Bayir H. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat Neurosci. 2012;15:1407–1413. doi: 10.1038/nn.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z, Celotto AM, Romero G, Wipf P, Palladino MJ. Genetically encoded redox sensor identifies the role of ROS in degenerative and mitochondrial disease pathogenesis. Neurobiol Dis. 2012;45:362–368. doi: 10.1016/j.nbd.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuznetsov AV, Kehrer I, Kozlov AV, Haller M, Redl H, Hermann M, Grimm M, Troppmair J. Mitochondrial ROS production under cellular stress: comparison of different detection methods. Anal Bioanal Chem. 2011;400:2383–2390. doi: 10.1007/s00216-011-4764-2. [DOI] [PubMed] [Google Scholar]
- 29.Soh N. Recent advances in fluorescent probes for the detection of reactive oxygen species. Anal Bioanal Chem. 2006;386:532–543. doi: 10.1007/s00216-006-0366-9. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S, Wu T, Fan J, Li Z, Jiang N, Wang J, Dou B, Sun S, Song F, Peng XA. BODIPY-based fluorescent dye for mitochondria in living cells, with low cytotoxicity and high photostability. Org Biomol Chem. 2013;11:555–558. doi: 10.1039/c2ob26911b. [DOI] [PubMed] [Google Scholar]
- 31.Dickinson BC, Srikun D, Chang CJ. Mitochondrial-targeted fluorescent probes for reactive oxygen species. Curr Opin Chem Biol. 2010;14:50–56. doi: 10.1016/j.cbpa.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci USA. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickinson BC, Chang CJ. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J Am Chem Soc. 2008;130:11561–11561. doi: 10.1021/ja802355u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao W. Lighting up H2O2: the molecule that is a “necessary evil” in the cell. Angew Chem Int Ed Engl. 2009;48:3022–3024. doi: 10.1002/anie.200805651. [DOI] [PubMed] [Google Scholar]
- 35.Yuan L, Lin W, Xie Y, Chen B, Zhu S. Single fluorescent probe responds to H2O2, NO, and H2O2/NO with three different sets of fluorescence signals. J Am Chem Soc. 2012;134:1305–1315. doi: 10.1021/ja2100577. [DOI] [PubMed] [Google Scholar]
- 36.Caldorera-Moore ME, Liechty WB, Peppas NA. Responsive theranostic systems: integration of diagnostic imaging agents and responsive controlled release drug delivery carriers. Acc Chem Res. 2011;44:1061–1070. doi: 10.1021/ar2001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–617. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- 38.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nature Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 39.Prossnitz ER, Sklar LA, Oprea TI, Arterburn JB. GPR30: a novel therapeutic target in estrogen-related disease. Trends Pharmacol Sci. 2008;29:116–123. doi: 10.1016/j.tips.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 41.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nature Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]; Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid BiochemMol Biol. 2008;109:350–353. doi: 10.1016/j.jsbmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. Chronic treatment with the G protein–coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology. 2009;150:3753–3758. doi: 10.1210/en.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayashida K, Shoji I, Deng L, Jiang DP, Ide YH, Hotta H. 17β-estradiol inhibits the production of infectious particles of hepatitis C virus. Microbiol Immunol. 2010;54:684–690. doi: 10.1111/j.1348-0421.2010.00268.x. [DOI] [PubMed] [Google Scholar]
- 44.Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blasko E, Haskell CA, Leung S, Gualtieri G, Halks-Miller M, Mahmoudi M, Dennis MK, Prossnitz ER, Karpus WJ, Horuk R. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J Neuroimmunol. 2009;214:67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arterburn JB, Oprea TI, Prossnitz ER, Edwards BS, Sklar LA. Discovery of selective probes and antagonists for G protein-coupled receptors for FPR/FPRL1 and GPR30. Curr Topics Med Chem. 2009;9:1227–1236. doi: 10.2174/156802609789753608. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burai R, Ramesh C, Shorty M, Curpan R, Bologa C, Sklar LA, Oprea T, Prossnitz ER, Arterburn JB. Highly efficient synthesis and characterization of the GPR30-selective agonist G-1 and related tetrahydroquinoline analogs. Org Biomol Chem. 2012;8:2252–2259. doi: 10.1039/c001307b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramesh C, Nayak TK, Burai R, Dennis MK, Hathaway HJ, Sklar LA, Prossnitz ER, Arterburn JB. Synthesis and characterization of iodinated tetrahydroquinolines targeting the G protein-coupled estrogen receptor GPR30. J Med Chem. 2010;53:1004–1014. doi: 10.1021/jm9011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nayak TK, Dennis MK, Ramesh C, Burai R, Atcher RW, Sklar LA, Norenberg JP, Hathaway HJ, Arterburn JB, Prossnitz ER. Influence of charge on cell permeability and tumor imaging of GPR30-targetted 111In-labeled nonsteroidal imaging agents. ACS Chem Biol. 2010;5:681–690. doi: 10.1021/cb1000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burai R, Ramesh C, Nayak TK, Dennis MK, Bryant BK, Prossnitz ER, Arterburn JB. Synthesis and characterization of tricarbonyl-Re/Tc(I) chelate probes targeting the G protein-coupled estrogen receptor GPER/GPR30. PLOS One. 2012;7:e46861. doi: 10.1371/journal.pone.0046861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdelhamid R, Luo J, Vande Vrede L, Kundu I, Michalsen B, Litosh VA, Schiefer IT, Gherezghiher T, Yao P, Qin Z, Thatcher GRJ. Benzothiophene selective estrogen receptor modulators provide neuroprotection by a novel GPR30-dependent mechanism. ACS Chem Neurosci. 2011;2:256–268. doi: 10.1021/cn100106a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, Bologa CG, Oprea TI, Yamaguchi Y, Hayashi SI, Sklar LA, Hathaway H, Arterburn JB, Prossnitz ER. Identification of GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J Steroid Biochem Mol Biol. 2011;127:358–366. doi: 10.1016/j.jsbmb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edwards AM, Isserlin R, Baer GD, Frye SV, Willson TM, Yu FH. Too many roads not taken. Nature. 2011;470:163–165. doi: 10.1038/470163a. [DOI] [PubMed] [Google Scholar]
- 55.Braunstein MJ, Scott SS, Scott CM, Behrman S, Walter P, Wipf P, Coplan JD, Chrico W, Joseph D, Brodsky JL, Batuman O. Antimyeloma effects of the heat shock protein 70 molecular chaperone inhibitor MAL3-101. J Oncol. 2011:232037. doi: 10.1155/2011/232037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fewell SW, Smith CM, Lyon MA, Dumitrescu TP, Wipf P, Day BW, Brodsky JL. Small molecule modulators of endogenous and co-chaperone-stimulated Hsp70 ATPase activity. J Biol Chem. 2004;279:51131–51140. doi: 10.1074/jbc.M404857200. [DOI] [PubMed] [Google Scholar]
- 57.Rodina A, Vilenchik M, Moulick K, Aguirre J, Kim J, Chiang A, Litz J, Clement CC, Kang Y, She Y, Wu N, Felts S, Wipf P, Massague J, Jiang X, Brodsky JL, Krystal GW, Chiosis G. Selective compounds define Hsp90 as a major inhibitor of apoptosis in small-cell lung cancer. Nature Chem Biol. 2007;3:498–507. doi: 10.1038/nchembio.2007.10. [DOI] [PubMed] [Google Scholar]
- 58.Goloudina AR, Demidov ON, Garrido C. Inhibition of Hsp70: A challenging anticancer strategy. Cancer Lett. 2012;325:117–124. doi: 10.1016/j.canlet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Massey AJ. ATPases as drug targets: Insights from heat shock proteins 70 and 90. J Med Chem. 2010;53:7280–7286. doi: 10.1021/jm100342z. [DOI] [PubMed] [Google Scholar]
- 60.Powers MV, Jones K, Barillari C, Westwood I, van Montfort RLM, Workman P. Targeting Hsp70: The second potentially druggable heat shock protein and molecular chaperone? Cell Cycle. 2010;9:1542–1550. doi: 10.4161/cc.9.8.11204. [DOI] [PubMed] [Google Scholar]
- 61.Rerole AL, Gobbo J, De Thonel A, Schmitt E, Pais De Barros JP, Hammann A, Lanneau D, Fourmaux E, Deminov O, Micheau O, Lagrost L, Colas P, Kroemer G, Garrido C. Peptides and aptamers targeting Hsp70: A novel approach for anticancer chemotherapy. Cancer Research. 2011;71:484–495. doi: 10.1158/0008-5472.CAN-10-1443. [DOI] [PubMed] [Google Scholar]
- 62.Werner S, Turner DM, Lyon MA, Huryn DM, Wipf P. A focused library of tetrahydropyrimidinone amides via a tandem Biginelli-Ugi multi-component process. Synlett. 2006:2334–2338. [Google Scholar]
- 63.Wang Y, Xiao J, Suzek TO, Zhang J, Wang J, Zhou Z, Han L, Karapetyan K, Dracheva S, Shoemaker BA, Bolton E, Gindulyte A, Bryant SH. PubChem's bioassay database. Nucleic Acids Res. 2012;40:D400–D412. doi: 10.1093/nar/gkr1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright CM, Chovatiya RJ, Jameson NE, Turner DM, Zhu G, Werner S, Huryn DM, Pipas JM, Day BW, Wipf P, Brodsky JL. Pyrimidinone-peptoid hybrid molecules with distinct effects on molecular chaperone function and cell proliferation. Bioorg Med Chem. 2008;16:3291–3301. doi: 10.1016/j.bmc.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wright CM, Seguin SP, Fewell SW, Zhang H, Ishwad C, Vats A, Lingwood CA, Wipf P, Fanning E, Pipas JM, Brodsky JL. Inhibition of Simian Virus 40 replication by targeting the molecular chaperone function and ATPase activity of T antigen. Virus Res. 2009;141:71–80. doi: 10.1016/j.virusres.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rabu C, Wipf P, Brodsky JL, High SA. precursor-specific role for Hsp40/Hsc70 during tail-anchored protein integration at the endoplasmic reticulum. J Biol Chem. 2008;283:27504–27513. doi: 10.1074/jbc.M804591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.a) Botha M, Chiang AN, Needham PG, Stephens LL, Hoppe HC, Külzer S, Przyborski JM, Lingelbach K, Wipf P, Brodsky JL, Shonhai A, Blatch GL. Plasmodium falciparum encodes a single cytosolic type I Hsp40 that functionally interacts with Hsp70 and is upregulated by heat shock. Cell Stress Chap. 2011;16:389–401. doi: 10.1007/s12192-010-0250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chiang AN, Valderramos JC, Balachandran R, Chovatiya RJ, Mead BP, Schneider C, Bell SL, Klein MG, Huryn DM, Chen XS, Day BW, Fidock DA, Wipf P, Brodsky JL. Select pyrimidinones inhibit the propagation of the malarial parasite, Plasmodium falciparum. Bioorg Med Chem. 2009;17:1527–1533. doi: 10.1016/j.bmc.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wisen S, Bertelsen EB, Thompson AD, Patury S, Ung P, Chang L, Evans CG, Walter GM, Wipf P, Carlson HA, Brodsky JL, Zuiderweg ERP, Gestwicki JE. Binding of a small molecule at a protein-protein interface regulates the chaperone activity of hsp70-hsp40. ACS Chem Biol. 2010;5:611–622. doi: 10.1021/cb1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huryn DM, Brodsky JL, Brummond KM, Chambers PG, Eyer B, Ireland AW, Kawasumi M, LaPorte MG, Lloyd K, Manteau B, Nghiem P, Quade B, Seguin SP, Wipf P. Chemical methodology as a source of small-molecule checkpoint inhibitors and heat shock protein 70 (Hsp70) modulators. Proc Natl Acad Sci USA. 2011;108:6757–6762. doi: 10.1073/pnas.1015251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 71.Whiteside GT, Lee GP, Valenzano KJ. The role of the cannabinoid CB2 receptor in pain transmission and therapeutic potential of small molecule CB2 receptor agonists. Curr Med Chem. 2007;14:917–936. doi: 10.2174/092986707780363023. [DOI] [PubMed] [Google Scholar]
- 72.Malan TP, Jr, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, Porreca F, Makriyannis A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- 73.Marriott KSC, Huffman JW. Recent advances in the development of selective ligands for the cannabinoid CB2 receptor. Curr Topics Med Chem. 2008;8:187–204. doi: 10.2174/156802608783498014. [DOI] [PubMed] [Google Scholar]
- 74.Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, Porreca F, Buckley NE, Makriyannis A, Malan TP., Jr CB2 cannabinoid receptor mediation of antinociception. Pain. 2006;122:36–42. doi: 10.1016/j.pain.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 75.Nackley AG, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neurosci. 2003;119:747–757. doi: 10.1016/s0306-4522(03)00126-x. [DOI] [PubMed] [Google Scholar]
- 76.Bingham B, Jones PG, Uveges AJ, Kotnis S, Lu P, Smith VA, Sun SC, Resnick L, Chlenov M, He Y, Strassle BW, Cummons TA, Piesla MJ, Harrison JE, Whiteside GT, Kennedy JD. Species-specific in vitro pharmacological effects of the cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers. Br J Pharmacol. 2007;151:1061–1070. doi: 10.1038/sj.bjp.0707303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rahn EJ, Zvonok AM, Thakur GA, Khanolkar AD, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agent paclitaxel in rats. J Pharmacol Exp Ther. 2008;327:584–591. doi: 10.1124/jpet.108.141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hohmann AG, Farthing JN, Zvonok AM, Makriyannis A. Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin. J Pharmacol Exp Ther. 2004;308:446–453. doi: 10.1124/jpet.103.060079. [DOI] [PubMed] [Google Scholar]
- 79.LaBuda CJ, Koblish M, Little PJ. Cannabinoid CB2 receptor agonist activity in the hindpaw incision. Eur J Pharmacol. 2005;527:172–174. doi: 10.1016/j.ejphar.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 80.Curto-Reyes V, Llames S, Hidalgo A, Menendez L, Baamonde A. Spinal and peripheral analgesic effects of the CB2 cannabinoid receptor agonist AM1241 in two models of bone cancer-induced pain. Br J Pharmacol. 2010;160:561–573. doi: 10.1111/j.1476-5381.2009.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hsieh GC, Pai M, Chandran P, Hooker BA, Zhu CZ, Salyers AK, Wensink EJ, Zhan C, Carroll WA, Dart MJ, Yao BB, Honore P, Meyer MD. Central and peripheral sites of action for CB2 receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats. Br J Pharmacol. 2011;162:428–440. doi: 10.1111/j.1476-5381.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, Reggiani A. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 83.Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP., Jr CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whiteside GT, Gottshall SL, Boulet JM, Chaffer SM, Harrison JE, Pearson MS, Turchin PI, Mark L, Garrison AE, Valenzano KJ. A role for cannabinoid receptor, but not endogenous opioids, in the antinociceptive activity of the CB2-selective agonist, GW405833. Eur J Pharmacol. 2005;528:65–72. doi: 10.1016/j.ejphar.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 85.Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM. Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J Neurosci. 2008;28:1064–1075. doi: 10.1523/JNEUROSCI.1565-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yao BB, Mukherjee S, Fan Y, Garrison TR, Daza AV, Grayson GK, Hooker BA, Dart MJ, Sullivan JP, Meyer MD. In vitro pharmacological characterization of AM1241: a protean agonist at the cannabinoid CB2 receptor? Br J Pharmacol. 2006;149:145–154. doi: 10.1038/sj.bjp.0706838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El-Kouhen OF, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD. Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB2 cannabinoid receptor activity. J Med Chem. 2010;53:295–315. doi: 10.1021/jm901214q. [DOI] [PubMed] [Google Scholar]
- 88.Ostenfeld T, Price J, Albanese M, Bullman J, Guillard F, Meyer I, Leeson R, Costantin C, Ziviani L, Nocini PF, Milleri S. A randomized, controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist GW842166, ibuprofen or placebo in patients with acute pain following third molar tooth extraction. Clin J Pain. 2011;27:668–676. doi: 10.1097/AJP.0b013e318219799a. [DOI] [PubMed] [Google Scholar]
- 89.Giblin GMP, O'Shaughnessy CT, Naylor A, Mitchell WL, Eatherton AJ, Slingsby BP, Rawlings DA, Paul Goldsmith P, Brown AJ, Haslam CP, Clayton NM, Wilson AW, Chessell IP, Wittington AR, Green R. Discovery of 2-[(2,4-dichlorophenyl)amino]-N-[(tetrahydro-2H-pyran-4-yl)methyl]-4-(trifluoromethyl)-5-pyrimidinecarboxamide, a selective CB2 receptor agonist for the treatment of inflammatory pain. J Med Chem. 2007;50:2597–2600. doi: 10.1021/jm061195+. [DOI] [PubMed] [Google Scholar]
- 90.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 91.Rabinovici GD, Jagust WJ. Amyloid imaging in aging and dementia: testing the amyloid hypothesis in vivo. Behav Neurol. 2009;21:117–128. doi: 10.3233/BEN-2009-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 93.Cohen AD, Rabinovici GD, Mathis CA, Jagust WJ, Klunk WE, Ikonomovic MD. Using Pittsburgh compound B for in vivo PET imaging of fibrillar amyloid-beta. Adv Pharmacol. 2012;64:27–81. doi: 10.1016/B978-0-12-394816-8.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mathis CA, Bacskai BJ, Kajdasz ST, McLellan ME, Frosch MP, Hyman BT, Holt DP, Wang Y, Huang GF, Debnath ML, Klunk WE. A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain. Bioorg Med Chem Lett. 2002;12:295–298. doi: 10.1016/s0960-894x(01)00734-x. [DOI] [PubMed] [Google Scholar]
- 95.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 96.Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, Ackermann U, Jones G, Szoeke C, Salvado O, Martins R, O'Keefe G, Mathis CA, Klunk WE, Ames D, Masters CL, Rowe CC. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, Prossnitz ER, Rodriguez Martinez de Llano S, Liu E, Koller M, Gregg KM, Schenk D, Black R, Grundman M. 11C-PiB PET assessment of change in fibrillar amyloid-β load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 98.Silbert LC, Kaye J. Neuroimaging and cognition in Parkinson's disease dementia. Brain Pathol. 2010;20:646–653. doi: 10.1111/j.1750-3639.2009.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Claassen DO, Lowe VJ, Peller PJ, Petersen RC, Josephs KA. Amyloid and glucose imaging in dementia with Lewy bodies and multiple systems atrophy. Parkinsonism Rel Disord. 2011;17:160–165. doi: 10.1016/j.parkreldis.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 100.Klunk WE, Mathis CA, Jr, Wang Y. WO2004083195 A1. Benzothiazole derivative compounds, compositions and uses. :134.
- 101.Nelissen N, van Laere K, Thurfjell L, Owenius R, Vandenbulcke M, Koole M, Bormans G, Brooks DJ, Vandenberghe R. Phase 1 study of the Pittsburgh compound B derivative 18F-flutemetamol in healthy volunteers and patients with probable Alzheimer disease. J Nucl Med. 2009;50:1251–1259. doi: 10.2967/jnumed.109.063305. [DOI] [PubMed] [Google Scholar]
- 102.Vandenberghe R, van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, Hasselbalch S, Law I, Andersen A, Korner A, Minthon L, Garraux G, Nelissen N, Bormans G, Buckley C, Owenius R, Thurfjell L, Farrar G, Brooks DJ. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol. 2010;68:319–329. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- 103.Wong DF, Rosenberg PB, Zhou Y, Kumar A, Vanessa Raymont V, Ravert HT, Dannals RF, Nandi A, Brašić JR, Ye W, Hilton J, Lyketsos C, Kung HF, Joshi AD, Skovronsky DM, Pontecorvo MJ. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (flobetapir F 18) J Nucl Med. 2010;51:913–920. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barthel H, Sabri O. Florbetaben to trance amyloid-β in the Alzheimer brain by means of PET. J Alzheimers Dis. 2011;26:117–121. doi: 10.3233/JAD-2011-0068. [DOI] [PubMed] [Google Scholar]