Abstract
Purpose
To demonstrate a novel swept source optical coherence tomography (SS-OCT) imaging device employing a vertical cavity surface-emitting laser (VCSEL) capable of imaging the full eye length and to introduce a method employing this device for non-contact optical ocular biometry. To compare the measurements of intraocular distances using this SS-OCT instrument with commercially available optical and ultrasound biometers. To evaluate the intersession reproducibility of measurements of intraocular distances using SS-OCT.
Design
Evaluation of technology
Participants
Twenty eyes of 10 healthy subjects imaged at the New England Eye Center at Tufts Medical Center and Massachusetts Institute of Technology between May and September 2012.
Methods
Averaged central depth profiles were extracted from volumetric SS-OCT datasets. The intraocular distances such as central corneal thickness (CCT), aqueous depth (AD), anterior chamber depth (ACD), crystalline lens thickness (LT), vitreous depth (VD), and axial eye length (AL) were measured and compared with a partial coherence interferometry (PCI) device (IOL Master; Carl Zeiss Meditec, Inc.), as well as an immersion ultrasound (IUS) A-scan biometer (Axis-II PR; Quantel Medical, Inc.).
Main Outcome Measures
Reproducibility of the measurements of intraocular distances, correlation coefficients, intraclass correlation coefficients
Results
The standard deviations of the repeated measurements of intraocular distances using SS-OCT were: 6 μm (CCT), 16 μm (ACD), 14 μm (AD), 13 μm (LT), 14 μm (VD) and 16 μm (AL). Strong correlations between all three biometric instruments were found for AL (r > 0.98). AL measurement using SS-OCT correlates better with IOL Master (r = 0.998) than with immersion ultrasound (r = 0.984). SS-OCT and IOL Master measured higher AL values than ultrasound (175 μm and 139 μm). No statistically significant difference of ACD between optical (SS-OCT or IOL Master) and ultrasound method was detected. High intersession reproducibility of SS-OCT measurements of all intraocular distances was observed with intraclass correlation coefficients >0.99.
Conclusions
SS-OCT using VCSEL technology enables full eye length imaging as well as high precision, non-contact ocular biometry. The measurements with the prototype SS-OCT instrument correlate well with commercial biometers. SS-OCT biometry has the potential to provide clinically useful comprehensive biometric parameters for pre- and post-operative eye evaluation.
Measurements of intraocular distances, known as ocular biometry, are essential for accurate outcomes in cataract and refractive surgeries. Precise measurements of axial intraocular distances and keratometric parameters are critical for proper intraocular lens (IOL) power calculation and post-operative patient refractive outcomes 1.
Shortly after the introduction of ultrasound in medical imaging in the 1950’s, ophthalmologists began to employ the technology for measuring intraocular distances and imaging the posterior segment of the eye 2. Ocular biometry with ultrasound rapidly became the gold standard. Ultrasonic devices can perform axial length measurements with the resolution of ~100 μm. However, ultrasound requires direct contact of the eye with a transducer or a coupling medium in order to transmit the sound waves into the eye and measure axial distance.
Early optical methods to measure intraocular distances were based on photography 3. In the 1980s, measurement utilizing femtosecond pulse light sources was developed 4. Eye length measurement using partial coherence interferometry and modified methods were demonstrated by Fercher et al. 5–7. The most important feature of optical biometry is that it is non-contact. Thus, it can enable assessment of surgical outcomes immediately after the operation. Moreover, optical methods offer higher resolution (10–20 um) compared with ultrasound techniques. Two commercial optical devices based on time-domain interferometry were introduced to the market: the IOL Master (Carl Zeiss Meditec Inc.) and Lenstar (Haag-Streit AG) 8–11. Both instruments use low coherence light sources, and the length of the reference arm of the interferometer is varied during the scanning procedure. Other modalities used clinically for post-operative axial eye length measurements include magnetic resonance imaging and x-ray tomography 12, 13.
Fourier-domain optical coherence tomography (OCT) or reflectometry both have performance advantages over time-domain techniques 14. Fourier-domain detection can be performed using a broadband light source with a spectrometer (spectral / Fourier domain OCT, SD-OCT) or a frequency swept light source with high-speed detector (swept source / Fourier domain OCT, SS-OCT). Clinical OCT instruments typically have an imaging range of 2–3 mm, and are designed to separately image the posterior segment (retina, choroid, and optic nerve) or parts of the anterior segment (cornea or anterior chamber angle). Visualization of the entire anterior segment of the eye (including the crystalline lens) can be done with OCT, but previous techniques required application of specific methods to extend the axial measurement range, and include full-range approaches 15 or using two or more reference paths 16.
It is even more challenging to image the eye from the cornea to the retina with a single OCT device. Full eye length imaging with OCT requires an axial measurement range (maximum optical path difference) of at least ~40 mm in air, accounting for refractive index of ocular components and patient eye length variations. Such a long imaging range typically cannot be achieved by SD-OCT instruments, since the range is limited by the spectrometer resolution. However, Fourier-domain reflectometry with two reference arms (well-defined offset) has been demonstrated for in vivo eye length measurement 17. Additionally, SS-OCT with extended coherence length has also been reported for full eye imaging in swine 18. Finally, spectral and swept source OCT systems for simultaneous anterior segment and retinal imaging have also been demonstrated 19, 20.
Recently introduced frequency-swept light sources, known as vertical cavity surface-emitting lasers (VCSEL), offer superior performance for OCT imaging. One of the major advantages is the extremely long coherence length of VCSELs (CLEO: Science and Innovations 2011 paper: PDPB2; link http://www.opticsinfobase.org/abstract.cfm?URI=CLEO_SI-2011-PDPB2. Accessed March 25, 2013.) 21. In addition, since VCSEL technology enables adjustable sweep speed and tuning range, SS-OCT with VCSELs is a versatile modality that can generate cross-sectional images and volumetric scans with variable and imaging range resolution 22. This feature extends the versatility of OCT applications. Current signal detection and acquisition systems for SS-OCT can acquire signals at GHz sampling rates. These high bandwidths, in conjunction with long coherence length of VCSELs significantly improve performance of OCT instruments, enabling imaging far beyond standard depth ranges.
The purpose of this study was to demonstrate a novel full eye length SS-OCT imaging device and introduce a method for performing comprehensive, non-contact optical biometry. The measurements of intraocular distances using SS-OCT were compared with commercially available optical and ultrasound biometers. The reproducibility of ocular biometry was also evaluated.
Methods
The procedures were approved by the Institutional Review Boards at Massachusetts Institute of Technology and Tufts Medical Center. The research adhered to the tenets of the Declaration of Helsinki. Signed informed consent was obtained from subject before the study.
Twenty eyes of 10 healthy subjects with no ocular surface pathology, previous intraocular surgery or keratorefractive laser treatment (10 men, age 27.3±3.6 yrs, range 22–33 yrs) were investigated. The mean spherical equivalent refractive error was −3.9±3.1 diopters (D) (range −9.25–0 D). We performed ocular biometry with three instruments: the IOL Master (V5.5, Carl Zeiss Meditec Inc., Dublin, CA), an ultrasound A-scan biometer (Axis-II PR, Quantel Medical Inc., Cournon d’Auvergne Cedex, France) and a prototype SS-OCT instrument developed by our group.
The IOL Master is the current gold standard for biometry and its operation is based on partial coherence interferometry (PCI, equivalent to time-domain OCT) at a wavelength of 780 nm to obtain the depth profile of the eye and measure axial eye length (AL). The device can perform keratometric measurements using a camera image of corneal reflections of a hexagonal spot pattern illumination. Anterior chamber depth (ACD) is determined by a slit illumination technique and relies on previous keratometry measurements. The instrument calculates AL as an average of 5 measurements, and ACD is an average of 5 measurements. In this study, each eye was scanned three times by the IOL Master. The subject removed their head from the chinrest between each session.
Immersion ultrasound A-scan biometry (IUS) is a traditional biometric technique and requires contact of the eye with a saline solution that couples ultrasound waves into the eye. The echo time delay of ultrasound waves reflected from ocular surfaces is detected and intraocular distances (ACD, lens thickness LT, vitreous depth VD and AL) are calculated using the following sound velocities: aqueous – 1532 m/s, crystalline lens – 1641 m/s and vitreous – 1532 m/s. Topical anesthetic (Proparacaine) was used before the measurement and each eye was measured once using IUS biometry (Axis-II PR, Quantel Medical Inc.). We performed the IUS measurement last for each subject in order to avoid possible confounding effects of eye contact.
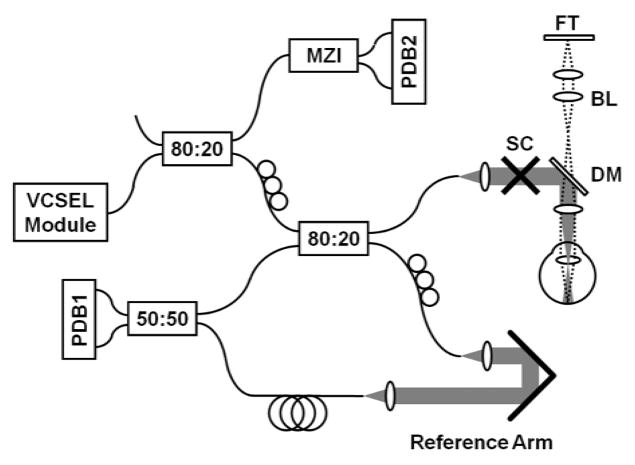
The prototype SS-OCT instrument with VCSEL swept light source is shown schematically in Fig 1. The incident power on the eye was 1.9 mW at 1065 nm center wavelength, which was consistent with the American National Standard Institute (ANSI Z136.1-2007) for safe exposure 22. The patient interface of the OCT instrument was configured for anterior segment imaging and performed a telecentric beam scan with a 73 μm spot size with a ~4 mm focal position behind the cornea. The VCSEL was swept at a 50 kHz sweep repetition rate. The wavelength sweep range was ~30 nm around a 1065 nm center wavelength, which provided an axial resolution of 19 μm and an imaging range of 60 mm in tissue. The measured sensitivity of the system was 105 dB. Precise depth calibration was performed, but since this method is highly technical it is described in detail elsewhere 23. During actual measurement, the subjects fixated on a back-illuminated target (Maltese cross) such that visual axis of the eye was aligned to the optical axis of the instrument (Fig 1). The OCT design of the interferometer configuration and dual-channel acquisition enabled precise depth calibration for each A-scan. We obtained five volumetric OCT data sets consisting of 300×300 axial scans and covering 8.5 mm × 8.5 mm area for each eye. The operator centered the pupil with respect to the OCT scanned field using a real-time preview. The measurement time to acquire a single volumetric data set was 1.9 seconds. The subject removed their head from the chinrest between each session. After processing the volumetric data, we selected 100 A-scans from the center of the pupil (area 280 μm × 280 μm) and averaged them to generate an axial profile of the eye.
Figure 1.
Experimental set-up for swept source optical coherence tomography full eye length imaging (VCSEL – vertical cavity surface-emitting laser, MZI – Mach-Zehnder interferometer, SC – galvanometer scanners, DM – dichroic mirror, BL – Badal lens, FT – fixation target, PDB – balanced photodetector).
The depth positions of ocular structures and measured ocular distances were identified. The physical (geometric) intraocular distances were obtained by dividing optical distances by the corresponding refractive index at 1065 nm wavelength of the structural component of the eye. Since most reports on optical properties of ocular structures include only wavelengths up to 900 nm, the Cauchy chromatic dispersion formula and parameters given by Atchison and Smith were used to estimate the indices at 1065 nm 24–26. The following group refractive indices at the central wavelength of 1065 nm were obtained: cornea – 1.3755, aqueous – 1.3356, crystalline lens – 1.4048, vitreous – 1.3354. The following intraocular distances were measured: central corneal thickness (CCT), aqueous depth (AD), anterior chamber depth (ACD), lens thickness (LT), vitreous depth (VT), and axial eye length (AL). The aqueous depth is the distance between posterior surface of the cornea and anterior surface of the crystalline lens. The anterior chamber depth is defined here as the distance from anterior surface of the cornea to the anterior surface of the crystalline lens, i.e. ACD = CCT + AD.
The data was entered into a Microsoft Excel 2010 spreadsheet (Microsoft Corp., Redmond, WA) and was analyzed by SAS statistical software (v. 9.3; SAS Institute, Inc., Cary, NC). To estimate inherent precision of the instrument, the standard deviation of repeated measurements of the same subject within one imaging session was calculated. The correlations between instruments were assessed using Pearson correlation coefficients. Statistical significance was taken to be a level of α = 0.05. Additionally, the agreement between the methods by performing Bland-Altman analysis was assessed. Both the bias and 95% confidence interval were calculated. We used a paired t-test to analyze statistical significance. Because the two eyes of a given subject are not independent, a randomly selected eye from each subject was employed for data analysis. Reproducibility was assessed using one-way analysis of variance (ANOVA) with a random-effects model 27. Variance analysis enabled computation of intraclass correlation coefficient for each measured parameter.
Results
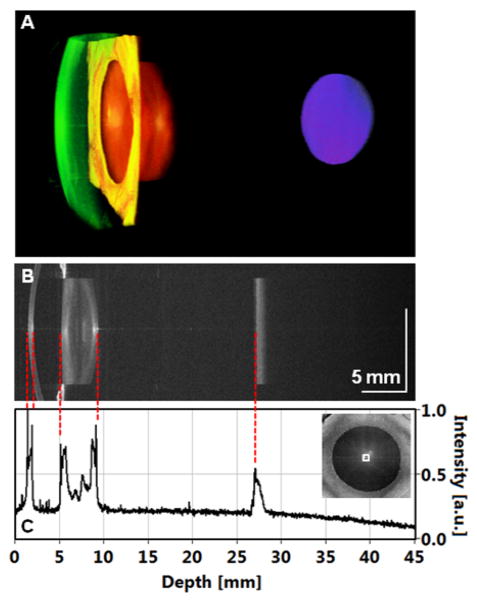
OCT raster scanning of the pupil area to acquire volumetric data sets spanning the entire eye length was performed. Since each volume consisted of 300×300 A-scans, and axial scan rate was 50 kHz, each data set could be acquires in <2 seconds. Fig 2A demonstrates an example rendering of a volumetric OCT data set. Fig 2B shows a central cross-sectional OCT image. The anterior chamber as well as the retina can be visualized. A central averaged depth profile (A-scan) is presented in Fig 2C. The averaged A-scan shows light intensity reflected from different structures of the eye. The first two peaks arise from the anterior and posterior surfaces of the cornea. Aqueous humor is not scattering. Deeper reflections are from the crystalline lens. Different optical properties of the lens nucleus and cortex can be distinguished. The vitreous humor is low scattering and behind the crystalline lens. The deepest signal is from retinal and choroidal structures.
Figure 2.
Full eye length imaging using swept source optical coherence tomography. A, 3-D rendering of the volumetric data set (cornea – green, iris – yellow, crystalline lens – orange, retina – blue). B, Central cross-section. C, Extracted profile enables identification of ocular surfaces allowing for measurements of intraocular distances.
In order to assess instrument precision, one eye of a subject was measured 17 times without removing the head from the headrest. Intraocular distances were determined for each measurement. Mean values and standard deviations of intraocular distances are given in Table 1. The inherent precision of the instrument was estimated by the standard deviation of intraocular distances. Standard deviation of AL measurement is 16 um. The highest coefficient of variation (CV) is observed in CCT, ACD and LT measurement.
Table 1.
Precision of intraocular distances with swept source optical coherence tomography
| Parameter | Mean [mm] | Standard Deviation [mm] | Coefficient of Variation |
|---|---|---|---|
| Central corneal thickness | 0.519 | 0.006 | 1.08% |
| Anterior chamber depth | 3.743 | 0.016 | 0.43% |
| Aqueous depth | 3.225 | 0.014 | 0.42% |
| Lens thickness | 3.810 | 0.013 | 0.34% |
| Vitreous depth | 18.304 | 0.014 | 0.08% |
| Axial length | 25.857 | 0.016 | 0.06% |
In different sessions, we measured 20 eyes of 10 subjects using SS-OCT, PCI and IUS. Correlations of measured biometric parameters between all three instruments are given in Table 2. For each eye, the mean values of intraocular distances from 3 measurements with PCI (IOL Master), 3 randomly chosen measurements with SS-OCT and a measurement with IUS were used. Additionally, in each case correlation was assessed separately for right and left eyes. For example, correlation plots for AL are presented in Fig 3. Generally, SS-OCT showed stronger correlation with PCI than with IUS, although IUS is also strongly correlated with PCI. Lower correlation coefficient was observed for ACD and LT measurements by optical and ultrasound methods. However, strong correlation of VD between SS-OCT and IUS was obtained. All correlation coefficients are statistically significant (p < 0.05).
Table 2.
Pearson correlation coefficients of the intraocular distances measured by swept source optical coherence tomography, partial coherence interferometry and immersion ultrasound A-scan biometry.
| Parameter | SS-OCT vs. PCI | SS-OCT vs. IUS | PCI vs. IUS |
|---|---|---|---|
| Axial length | OU – 0.9972 (OD – 0.9969 OS – 0.9975) | OU – 0.9846 (OD – 0.9928 OS – 0.9742) | OU – 0.9911 (OD – 0.9947 OS – 0.9865) |
|
| |||
| Anterior chamber depth | OU – 0.8997 (OD – 0.8717 OS – 0.9235) | OU – 0.7686 (OD – 0.7918 OS – 0.7465) | OU – 0.6991 (OD – 0.7521 OS – 0.6537) |
|
| |||
| Lens thickness | - | OU – 0.7141 (OD – 0.7270 OS – 0.7046) | - |
|
| |||
| Vitreous depth | - | OU – 0.9779 (OD – 0.9897 OS – 0.9704) | - |
SS-OCT = swept source optical coherence tomography; PCI = partial coherence interferometry (IOL Master); IUS = immersion ultrasound A-scan biometry; OD = oculus dexter (right eye); OS = oculus sinister (left eye); OU = oculus uterque (both eyes).
All cases are statistically significant with p < 0.05.
Figure 3.
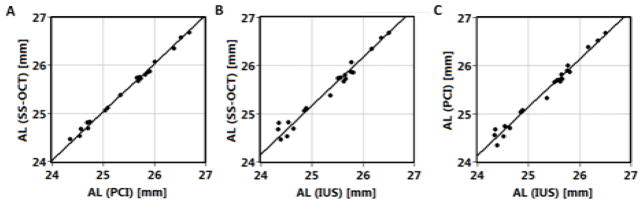
Correlation of axial length measured with swept source optical coherence tomography (SS-OCT), partial coherence interferometry (PCI; IOL Master) and immersion ultrasound A-scan biometry (IUS).
In the following analysis, we used a randomly selected eye from each subject to avoid bias due to left and right eye correlations. An example of Bland-Altman analysis is presented in Fig 4 for axial eye length comparison. The means and standard deviations (SD) of differences as well as 95% confidence intervals (CI) were calculated and are shown in Table 3. AL measured with SS-OCT is 35 μm longer than with IOL Master. Higher values are also obtained for ACD measurements. Both optical biometric modalities (SS-OCT and PCI) measure higher values of AL and ACD than ultrasound (IUS). The paired t-test for means revealed significant differences in biometric parameters, except for ACD measurements performed by any optical method (PCI or SS-OCT) and IUS.
Figure 4.
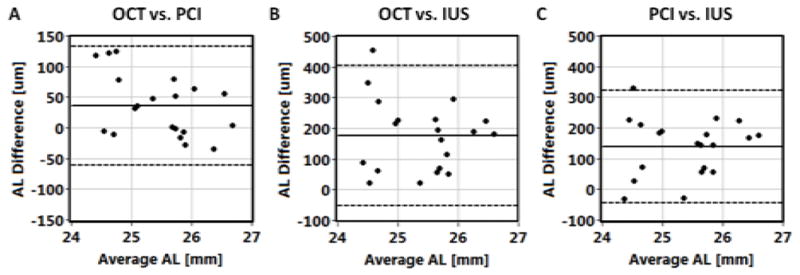
Agreement of axial eye length (AL) measurements between swept source optical coherence tomography (OCT), partial coherence interferometry (PCI; IOL Master) and immersion ultrasound A-scan biometry (IUS). Solid horizontal line indicates bias, and dashed lines show 95% confidence interval.
Table 3.
Bland-Altman analysis of the biometric measurements using swept source optical coherence tomography, partial coherence interferometry and immersion ultrasound A-scan biometry.
| Parameter | SS-OCT vs. PCI | SS-OCT vs. IUS | PCI vs. IUS | |||
|---|---|---|---|---|---|---|
| Mean±SD of Differences [μm] | 95% Confidence Interval [μm] | Mean±SD of Differences [μm] | 95% Confidence Interval [μm] | Mean±SD of Differences [μm] | 95% Confidence Interval [μm] | |
| AL | 35±50 (p=0.005) | −61 – 132 | 175±117 (p<0.001) | −54 – 403 | 139±94 (p<0.001) | −44 – 322 |
| ACD | 85±75 (p<0.001) | −61 – 232 | 37±164 (p=0.325) | −284 – 358 | 48±181 (p=0.248) | −402 – 306 |
| LT | - | - | −268±236 (p<0.001) | −731 – 195 | - | - |
| VD | - | - | 415±155 (p<0.001) | 111 – 719 | - | - |
SS-OCT = swept source optical coherence tomography; PCI = partial coherence interferometry (IOL Master); IUS – immersion ultrasound A-scan biometry; AL = axial eye length; ACD = anterior chamber depth; LT = lens thickness; VD = vitreous depth; SD = standard deviation.
Finally, the reproducibility of SS-OCT was evaluated using one-way ANOVA with a random-effects model. As shown in Table 4, all parameters show very good intersession reproducibility with statistical significance (p < 0.001). The highest ICC is obtained for AL.
Table 4.
Intersession reproducibility of biometric measurements using swept source optical coherence tomography.
| Parameter | Intraclass Correlation Coefficient | 95% Confidence Interval for ICC | Intersession Standard Deviation [μm] |
|---|---|---|---|
| Central corneal thickness | 0.9913 | 0.9638 – 0.9965 | 5 |
| Anterior chamber depth | 0.9980 | 0.9916 – 0.9992 | 13 |
| Aqueous depth | 0.9981 | 0.9920 – 0.9992 | 14 |
| Lens thickness | 0.9946 | 0.9868 – 0.9987 | 25 |
| Vitreous depth | 0.9996 | 0.9982 – 0.9998 | 26 |
| Axial length | 0.9998 | 0.9991 – 0.9999 | 16 |
ICC = intraclass correlation coefficient.
All cases are statistically significant with p < 0.001.
Discussion
Ocular biometry with the SS-OCT system showed comparable precision to the current “gold standard” clinical systems. PCI laboratory systems and commercial optical biometers are reported to have repeatability of intraocular distances measurements ranging from 0.3–30 μm 6, 8, 28–31. In this study, the largest coefficient of variation is in CCT measurements, due to the finite digital resolution of axial measurements, although precision is comparable to other studies. Higher CVs for ACD and LT than for AL are probably attributed to microfluctuations in eye accommodation 32. This phenomenon occurs even when a stationary fixation target is used. Very precise AL measurements are possible with the SS-OCT instrument.
The measurements performed in this study demonstrated excellent correlation of AL between all instruments, in agreement with earlier reports 7, 10, 33. Generally, optical biometers (IOL Master and SS-OCT) show better correlation among themselves, since they are both optical measurements, than between their ultrasound counterpart. Additionally, the immersion ultrasound used in this study can be uncomfortable and affects the subject’s ability to control fixation. Among all of the factors, inaccurate AL measurements seem to have the biggest role in refractive errors after cataract surgery. Lower values of correlation coefficients for ACD coefficients were also obtained in other studies 30, 34, 35.
The Bland-Altman method enabled assessing agreement between the methods. Results indicate that PCI (IOL Master) and SS-OCT measure longer AL than IUS. This result was already observed and the statistical difference can be explained by the measurement technique 7, 8, 29. The IOL Master measures the AL from the cornea to retinal pigment epithelium. The AL measurement using SS-OCT was performed in a similar way to maintain consistency. On the other hand, ultrasound measurements define AL as distance between the cornea and internal limiting membrane of the retina. Both optical devices measure similar AL values in this study. The small differences between SS-OCT and IOL Master may be caused by uncertainties in the estimated group refractive indices of ocular components. In addition, the IOL Master can measure light reflection from both apexes of the eye (anterior corneal surface and the retina) and therefore treats the entire eye as homogeneous structure with an effective refractive index. Correction factor was also applied in the IOL Master algorithm to match optical and high-precision ultrasonic measurements of AL 36. The prototype SS-OCT device used in this study determines all intraocular distances separately, and AL is the sum of all ACD, LT and VD. The precision of SS-OCT device depth calibration might be another factor contributing to the observed difference in AL between IOL Master and SS-OCT. The difference in ACD measurements between optical biometers probably arises from the fact the IOL Master uses an optical section image of the eye taken with slit-lamp illumination at the angle of 38° with res pect to visual axis to determine ACD 35, 37.
Intersession reproducibility studies use multiple measurements of the subjects. The statistical analysis revealed very high ICCs for all intraocular distances. Similar reproducibility results were observed by Vogel et al. for IOL Master as well as by Buckhurst et al., for the Lenstar (Haag-Streit) 11, 30. The reproducibility studies reported here show that SS-OCT can provide reliable biometry measurements.
The main limitation of the present study is the relatively low sample size. Additionally, all subjects were men, no hyperopic eyes were measured and no subjects had cataracts or other ocular pathologies. Although we obtained promising results, they should be interpreted carefully until studies with larger sample sizes and broader subject demographics are performed.
The system operates at the central wavelength of 1065 nm which has reduced scattering and attenuation from ocular opacities compared with shorter wavelengths (~800 nm) used in commercial instruments 38. Moreover, the deeper light penetration along with high SS-OCT system sensitivity may improve imaging performance in patients with severe cataracts, which is a challenge for the IOL Master. As a consequence, determination of biometric parameters in cataract patients may be easier and more accurate with this technology. Recent studies show that up to 17% of cataract patients cannot be evaluated by available commercial optical biometers due to lens density and/or fixation ability 7, 39–42. Future studies will investigate cataract patients with different grades of cataract density in order to determine the performance and success rate of SS-OCT biometry 38.
The potential advantages of volumetric SS-OCT are significant. Apart from obtaining the depth profile of the eye for ocular biometry, the system also enables long range OCT imaging of posterior segment ocular structures with a single instrument, which can be used for non-invasive post-operative determination of surgical outcome. Another advantage is that, in principle, all of the parameters necessary for modern IOL power formulas (i.e. AL, ACD, CCT, K1, K2, white-to-white distance) can be obtained from a single data set using a single instrument. The patient can be scanned by a single instrument saving time and money. Consequently, volumetric OCT biometry ultimately promises to improve IOL power calculation and the refractive outcomes of cataract surgery. Finally, OCT biometry is non-contact so topical anesthesia or pupil dilation is not required, improving patient comfort.
Volumetric full eye length OCT imaging provides not only information on intraocular distances, but also enables the measurement of off-axis biometric parameters that are used in modern IOL formulas. Whereas white-to-white distance can be determined from en-face images when larger area is scanned, reliable keratometry is challenging and requires motion-free volumetric data sets or different scanning protocols. Therefore, volumetric full eye length OCT imaging has the potential to provide comprehensive biometric information in pre- and post-operative assessment of the eye in a single, non-contact scanning procedure.
Acknowledgments
Financial Support: Supported by grants from the National Institutes of Health (R01-EY011289-27, R01-EY013178-12, R01-EY013516-09, R01-EY019029-04, R44EY022864-01, R01-CA075289-16, R01-NS057476-05, R44CA101067-05), Air Force Office for Scientific Research (FA9550-10-1-0551 and FA9550-10-1-0063), and Thorlabs matching funds to Praevium Research Inc.. Additional support from an unrestricted Research to Prevent Blindness grant and the Massachusetts Lions Clubs. The funding organizations had no role in the design or conduct of this research.
Footnotes
Conflict of Interest: James G. Fujimoto – Royalities – from intellectual property owned by M.I.T and licensed to Carl Zeiss Meditec Inc and Optovue Inc.; Stock Options – Optovue Inc.
Jay S. Duker – Research Support – Carl Zeiss Meditec Inc., Optovue Inc., and Topcon Medical Systems Inc.; Stock – Hemera Biosciences Inc., EyeNetra, and Ophthotech Corp.
Benjamin Potsaid – Royalities – from intellectual property owned by M.I.T and licensed to Optovue Inc.; Employment – Thorlabs Inc.
Vijaysekhar Jayaraman – Stock and Employment – Praevium Inc.; Royalities – Thorlabs Inc.
Alex Cable – Stock and Employment – Thorlabs Inc.; Stock – Praevium Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olsen T. Calculation of intraocular lens power: a review. Acta Ophthalmol Scand. 2007;85:472–85. doi: 10.1111/j.1600-0420.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 2.Jansson F. Measurements of intraocular distances by ultrasound. Acta Ophthalmol Suppl. 1963;(suppl 74):1–51. [PubMed] [Google Scholar]
- 3.Perkins ES, Hammond B, Milliken AB. Simple method of determining axial length of eye. Br J Ophthalmol. 1976;60:266–70. doi: 10.1136/bjo.60.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujimoto JG, Desilvestri S, Ippen EP, et al. Femtosecond optical ranging in biological systems. Opt Lett. 1986;11:150–2. doi: 10.1364/ol.11.000150. [DOI] [PubMed] [Google Scholar]
- 5.Fercher AF, Mengedoht K, Werner W. Eye-length measurement by interferometry with partially coherent light. Opt Lett. 1988;13:186–8. doi: 10.1364/ol.13.000186. [DOI] [PubMed] [Google Scholar]
- 6.Hitzenberger CK. Optical measurement of the axial eye length by laser Doppler interferometry. Invest Ophthalmol Vis Sci. 1991;32:616–24. [PubMed] [Google Scholar]
- 7.Hitzenberger CK, Drexler W, Dolezal C, et al. Measurement of the axial length of cataract eyes by laser Doppler interferometry. Invest Ophthalmol Vis Sci. 1993;34:1886–93. [PubMed] [Google Scholar]
- 8.Kiss B, Findl O, Menapace R, et al. Biometry of cataractous eyes using partial coherence interferometry: clinical feasibility study of a commercial prototype I. J Cataract Refract Surg. 2002;28:224–9. doi: 10.1016/s0886-3350(01)01272-x. [DOI] [PubMed] [Google Scholar]
- 9.Santodomingo-Rubido J, Mallen EA, Gilmartin B, Wolffsohn JS. A new non-contact optical device for ocular biometry. Br J Ophthalmol. 2002;86:458–62. doi: 10.1136/bjo.86.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eleftheriadis H. IOLMaster biometry: refractive results of 100 consecutive cases. Br J Ophthalmol. 2003;87:960–3. doi: 10.1136/bjo.87.8.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckhurst PJ, Wolffsohn JS, Shah S, et al. A new optical low coherence reflectometry device for ocular biometry in cataract patients. Br J Ophthalmol. 2009;93:949–53. doi: 10.1136/bjo.2008.156554. [DOI] [PubMed] [Google Scholar]
- 12.Takei K, Sekine Y, Okamoto F, Hommura S. Measurement of axial length of eyes with incomplete filling of silicone oil in the vitreous cavity using X ray computed tomography. Br J Ophthalmol. 2002;86:47–50. doi: 10.1136/bjo.86.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bencic G, Vatavuk Z, Marotti M, et al. Comparison of A-scan and MRI for the measurement of axial length in silicone oil-filled eyes. Br J Ophthalmol. 2009;93:502–5. doi: 10.1136/bjo.2008.147868. [DOI] [PubMed] [Google Scholar]
- 14.Choma M, Sarunic M, Yang C, Izatt J. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. [Accessed March 25, 2013];Opt Express [serial online] 2003 11:2183–9. doi: 10.1364/oe.11.002183. Available at: http://www.opticsinfobase.org/oe/abstract.cfm?uri=oe-11-18-2183. [DOI] [PubMed] [Google Scholar]
- 15.Baumann B, Pircher M, Gotzinger E, Hitzenberger CK. Full range complex spectral domain optical coherence tomography without additional phase shifters. [Accessed March 25, 2013];Opt Express [serial online] 2007 15:13375–87. doi: 10.1364/oe.15.013375. Available at: http://www.opticsinfobase.org/oe/abstract.cfm?uri=oe-15-20-13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonathan E. Dual reference arm low-coherence interferometer-based reflectometer for optical coherence tomography (OCT) application. Opt Commun. 2005;252:202–11. [Google Scholar]
- 17.Grajciar B, Pircher M, Hitzenberger CK, et al. High sensitive measurement of the human axial eye length in vivo with Fourier domain low coherence interferometry. [Accessed March 25, 2013];Opt Express [serial online] 2008 16:2405–14. doi: 10.1364/oe.16.002405. Available at: http://www.opticsinfobase.org/oe/abstract.cfm?uri=oe-16-4-2405. [DOI] [PubMed] [Google Scholar]
- 18.Chong CH, Suzuki T, Totsuka K, et al. Large coherence length swept source for axial length measurement of the eye. Appl Opt. 2009;48:D144–50. doi: 10.1364/ao.48.00d144. [DOI] [PubMed] [Google Scholar]
- 19.Dhalla AH, Nankivil D, Bustamante T, et al. Simultaneous swept source optical coherence tomography of the anterior segment and retina using coherence revival. Opt Lett. 2012;37:1883–5. doi: 10.1364/OL.37.001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruggeri M, Uhlhorn SR, De Freitas C, et al. Imaging and full-length biometry of the eye during accommodation using spectral domain OCT with an optical switch. [Accessed March 25, 2013];Biomed Opt Express [serial online] 2012 3:1506–20. doi: 10.1364/BOE.3.001506. Available at: http://www.opticsinfobase.org/boe/abstract.cfm?uri=boe-3-7-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potsaid B, Jayaraman V, Fujimoto JG, et al. MEMS tunable VCSEL light source for ultrahigh speed 60kHz-1MHz axial scan rate and long range centimeter class OCT imaging. In: Izatt JA, Fujimoto JG, Tuchin VV, editors. Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine XVI; Proceedings of SPIE; Bellingham, WA: SPIE; 2012. p. 82130M. [Google Scholar]
- 22.Grulkowski I, Liu JJ, Potsaid B, et al. Retinal, anterior segment and full eye imaging using ultrahigh speed swept source OCTwith vertical-cavity surface emitting lasers. [Accessed March 25, 2013];Biomed Opt Express [serial online] 2012 3:2733–51. doi: 10.1364/BOE.3.002733. Available at: http://www.opticsinfobase.org/boe/abstract.cfm?uri=boe-3-11-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grulkowski I, Liu JJ, Potsaid B, et al. High-precision, high-accuracy ultralong-range swept-source optical coherence tomography using vertical cavity surface emitting laser light source. Opt Lett. 2013;38:673–5. doi: 10.1364/OL.38.000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drexler W, Hitzenberger CK, Baumgartner A, et al. Investigation of dispersion effects in ocular media by multiple wavelength partial coherence interferometry. Exp Eye Res. 1998;66:25–33. doi: 10.1006/exer.1997.0401. [DOI] [PubMed] [Google Scholar]
- 25.Atchison DA, Smith G. Chromatic dispersions of the ocular media of human eyes. J Opt Soc Am A Opt Image Sci Vis. 2005;22:29–37. doi: 10.1364/josaa.22.000029. [DOI] [PubMed] [Google Scholar]
- 26.Sardar DK, Swanland GY, Yow RM, et al. Optical properties of ocular tissues in the near infrared region. Lasers Med Sci. 2007;22:46–52. doi: 10.1007/s10103-006-0421-y. [DOI] [PubMed] [Google Scholar]
- 27.Schuman JS, Hee MR, Puliafito CA, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography: a pilot study. Arch Ophthalmol. 1995;113:586–96. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- 28.Fercher AF, Hitzenberger C, Juchem M. Measurement of intraocular optical distances using partially coherent laser light. J Mod Opt. 1991;38:1327–33. [Google Scholar]
- 29.Drexler W, Findl O, Menapace R, et al. Partial coherence interferometry: a novel approach to biometry in cataract surgery. Am J Ophthalmol. 1998;126:524–34. doi: 10.1016/s0002-9394(98)00113-5. [DOI] [PubMed] [Google Scholar]
- 30.Vogel A, Dick HB, Krummenauer F. Reproducibility of optical biometry using partial coherence interferometry: intraobserver and interobserver reliability. J Cataract Refract Surg. 2001;27:1961–8. doi: 10.1016/s0886-3350(01)01214-7. [DOI] [PubMed] [Google Scholar]
- 31.Shammas HJ, Hoffer KJ. Repeatability and reproducibility of biometry and keratometry measurements using a noncontact optical low-coherence reflectometer and keratometer. Am J Ophthalmol. 2012;153:55–61. doi: 10.1016/j.ajo.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Monticone PP, Menozzi M. A review on methods used to record and analyze microfluctuations of the accommodation in the human eye. [Accessed March 25, 2013];J Eur Opt Soc Rapid Commun. 2011 6:11003. Available at: https://www.jeos.org/index.php/jeos_rp/article/view/11003/697. [Google Scholar]
- 33.Packer M, Fine IH, Hoffman RS, et al. Immersion A-scan compared with partial coherence interferometry: outcomes analysis. J Cataract Refract Surg. 2002;28:239–42. doi: 10.1016/s0886-3350(01)01259-7. [DOI] [PubMed] [Google Scholar]
- 34.Elbaz U, Barkana Y, Gerber Y, et al. Comparison of different techniques of anterior chamber depth and keratometric measurements. Am J Ophthalmol. 2007;143:48–53. doi: 10.1016/j.ajo.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 35.Rohrer K, Frueh BE, Walti R, et al. Comparison and evaluation of ocular biometry using a new noncontact optical low-coherence reflectometer. Ophthalmology. 2009;116:2087–92. doi: 10.1016/j.ophtha.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 36.Haigis W, Lege B, Miller N, Schneider B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol. 2000;238:765–73. doi: 10.1007/s004170000188. [DOI] [PubMed] [Google Scholar]
- 37.Buehl W, Stojanac D, Sacu S, et al. Comparison of three methods of measuring corneal thickness and anterior chamber depth. Am J Ophthalmol. 2006;141:7–12. doi: 10.1016/j.ajo.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 38.Povazay B, Hermann B, Unterhuber A, et al. Three-dimensional optical coherence tomography at 1050 nm versus 800 nm in retinal pathologies: enhanced performance and choroidal penetration in cataract patients. J Biomed Opt. 2007;12:041211. doi: 10.1117/1.2773728. [DOI] [PubMed] [Google Scholar]
- 39.Rajan MS, Keilhorn I, Bell JA. Partial coherence laser interferometry vs conventional ultrasound biometry in intraocular lens power calculations. Eye (Lond) 2002;16:552–6. doi: 10.1038/sj.eye.6700157. [DOI] [PubMed] [Google Scholar]
- 40.Hill W, Angeles R, Otani T. Evaluation of a new IOLMaster algorithm to measure axial length. J Cataract Refract Surg. 2008;34:920–4. doi: 10.1016/j.jcrs.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 41.Salouti R, Nowroozzadeh MH, Zamani M, et al. Comparison of the ultrasonographic method with 2 partial coherence interferometry methods for intraocular lens power calculation. Optometry. 2011;82:140–7. doi: 10.1016/j.optm.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 42.Mylonas G, Sacu S, Buehl W, et al. Performance of three biometry devices in patients with different grades of age-related cataract [report online] Acta Ophthalmol. 2011;89:e237–41. doi: 10.1111/j.1755-3768.2010.02042.x. [DOI] [PubMed] [Google Scholar]