Abstract
Background
A large subset of patients who enter treatment for alcohol dependence report nonabstinent drinking goals (e.g., reduction in drinking) rather than abstinence, and this pretreatment goal choice may be associated with drinking outcomes and alcohol-related problems.
Methods
An analysis of the 16-week Combined Pharmacotherapies and Behavioral Interventions (COMBINE) study was conducted to determine the association between self-reported pretreatment drinking goal and drinking outcomes and alcohol-related problems. Participants who reported a nonabstinent drinking goal (n=340) were matched with participants who reported an abstinent drinking goal (n=340) on three variables believed to contribute to treatment outcomes: COMBINE experimental group, gender, and number of prebaseline heavy drinking days.
Results
Analyses revealed no interaction between the COMBINE experimental group and drinking goal on outcome measures, so results were collapsed and examined as a function of drinking goal group. Participants who chose an abstinent drinking goal had significantly more weeks with no drinking or no heavy drinking, reported fewer heavy drinking days, reported fewer days with >1 drink, and were more likely to have a ≥50% decrease in drinks per day between baseline and week 16 of the intervention. However both groups reported reductions over time in percent drinking days, mean drinks per day, number of heavy drinking days, and number of drinking days per week, and participants in both groups experienced significant reductions in alcohol-related problems and improvements in psychosocial functioning.
Conclusions
Results replicate and expand upon previous studies examining the association between drinking goal and treatment outcome. These data also provide support for the standard inclusion of drinking treatment goal as a stratification variable in study interventions, or as a covariate in outcome analyses, and highlight several areas that warrant additional research regarding patients who enter alcohol treatment with a nonabstinent drinking goal.
Keywords: COMBINE, alcohol, treatment goal, controlled drinking, abstinence, naltrexone, acamprosate
Introduction
Alcohol is the most widely abused substance in the United States and is responsible for more substance abuse-related treatment admissions than all other forms of drug use combined (Substance Abuse and Mental Health Services Administration, 2010). Alcohol dependence is associated with a number of adverse individual and societal consequences (Cargiulo, 2007; Gutjahr and Gmel, 2001) and high rates of morbidity and mortality (Rehm et al., 2003).
Historically, alcohol treatment studies have identified alcohol abstinence as the primary treatment endpoint. However, alcohol is unique from other drugs of abuse because it can be consumed legally, it bears large cultural significance, and it may, in small and controlled quantities, even yield health benefits (Collins et al., 2009; Nova et al., 2012). Thus, it is reasonable that some individuals seeking alcohol treatment may prefer to continue drinking alcohol in a controlled manner rather than abstain from alcohol completely. The recognition that reductions in drinking (as opposed to complete abstinence) can serve as a valuable treatment endpoint is supported by a recent National Institute on Alcohol Abuse and Alcoholism (NIAAA) recommendation to evaluate percent heavy drinking days, versus complete abstinence, as a clinically important drinking outcome measure in alcohol intervention studies (Allen, 2003; Falk et al., 2010; Gastfriend et al., 2007). However, there is little empirical data available regarding the prevalence of patients who enter treatment with the explicit intention of reducing their alcohol consumption but not abstaining from alcohol completely.
There is also a dearth of information regarding the contribution that drinking goal (abstinence vs. nonabstinence) may have on treatment outcomes. An analysis of the United Kingdom Alcohol Treatment Trial (UKATT) multi-site, randomized trial compared drinking goal with 3 and 12-month alcohol treatment outcomes (Adamson et al., 2010). Participants who defined complete abstinence as a treatment goal at baseline were significantly more likely to report abstinence at a 3 and 12-month follow-up and were more likely to have been classified as a successful treatment outcome, as compared to participants who indicated a preference for a nonabstinence goal. However, the nonabstinence goal group was significantly more likely to report non-problem drinking at the 3 and 12-month follow-ups, consistent with their initial drinking goal. Thus, although both patient populations may have achieved their predefined drinking goals, only the abstinence group would have been considered a treatment success based upon the traditional abstinence-only outcome measure. Although drinking goal may be dynamic (e.g., patients may not have a clear goal in mind upon entering treatment, or may change their goal after treatment exposure), these data suggest that studies that do not take individual drinking goal into account may be failing to identify successful treatment outcomes among patients who reduced drinking in a manner consistent with their individual treatment goal, and at a level that is socially acceptable and non-problematic.
The current study is an analysis from a multi-site controlled study of interventions for alcohol dependence that seeks to determine whether pretreatment drinking goal is predictive of several clinically-relevant drinking-related outcomes and alcohol-related problems. The Combined Pharmacotherapies and Behavioral Interventions (COMBINE) study was a multi-site, randomized, controlled trial that was conducted between January 2001-January 2004 to evaluate the individual and combined effects of acamprosate and naltrexone vs. placebo, with or without a concurrently-delivered behavioral intervention, on alcohol abstinence (Anton et al., 2006). For the current analysis, participants from the COMBINE study were dichotomized posthoc, based on the self-reported abstinence goal they specified at study intake, into abstinence and nonabstinence drinking goal groups to determine whether pretreatment drinking goal was associated with outcome measures. These data will provide important information regarding the association between pretreatment drinking goal and treatment outcomes. Ultimately, these data have potentially important implications for the design and analysis of clinical trials for the treatment of alcohol dependence.
Materials and Methods
Participants
Participants met DSM-IV criteria for alcohol-dependence, and endorsed drinking (≥14 drinks/week for women and ≥21 drinks/week for men) for a 30-day consecutive period and ≥2 heavy drinking days (defined as ≥4 drinks/day for women and ≥5 drinks/day for men) in the 90 days preceding study enrollment, with a minimum of 4 (but no more than 21) days of abstinence prior to randomization and no significant signs of alcohol withdrawal. Eligible participants (n=1,383) were randomized into one of nine groups for the 16-week outpatient trial. Twenty-five percent (n=340) of participants selected a nonabstinent drinking goal at treatment intake. For the present analyses, the total participant sample was initially dichotomized into abstinent and nonabstinent drinking goal groups, however the large discrepancy in sample sizes between the groups resulted in an unequal distribution of power that prevented meaningful comparisons from being made. Therefore, for the purpose of this analysis, participants in the nonabstinent drinking goal group were individually matched with a participant from the abstinent drinking goal group, resulting in a total sample of 680 participants. Matching was conducted based on 3 a priori-defined variables believed to contribute to treatment success (ordered here in priority for matching): COMBINE experimental group, gender, and number of prebaseline heavy drinking days. There was 100% agreement between the groups regarding COMBINE experimental group and gender. The prebaseline number of heavy drinking days variable was matched to ±20% between the matching pairs. All but two of the participant pairs matched on that criterion; the two outliers matched within ±30% of each other. As seen in Table 1, there were no significant between-group differences on these variables.
Table 1.
Demographic, Pretreatment, and Treatment Characteristics
| Abstinent Drinking Goal (n=340) | Nonabstinent Drinking Goal (n=340) | p-value | |
|---|---|---|---|
| Demographic Characteristics | |||
| Caucasian (%) | 77 | 83 | 0.07 |
| Currently working (%) | 68 | 78 | <.01 |
| High School Diploma (%) | 93 | 97 | 0.08 |
| Male (%) | 61 | 61 | 0.99 |
| Pretreatment Characteristics | |||
| Nicotine Use (%) | 81 | 77 | 0.29 |
| Cocaine Use (%) | 10 | 7 | 0.99 |
| Any Drug Use (%) | 19 | 17 | 0.48 |
| Heavy drinking days (mean %) | 67 ± 24 | 68 ± 26 | 0.76 |
| Drinks per day (mean) | 8.3 ± 4.5 | 8.0 ± 5.5 | 0.37 |
| Treatment Characteristics | |||
| Acamprosate (%) | 45 | 45 | |
| Naltrexone (%) | 49 | 49 | |
| CBI Counseling (%) | 40 | 40 | |
| Naltrexone Dose (mean) | 89.5 ± 18.6 | 88.2 ± 21.0 | 0.24 |
| Acamprosate Dose (mean) | 2595.1 ± 584.3 | 2526.8 ± 664.3 | 0.06 |
| Adherence to medication (mean %) | 91.1 ± 14.8 | 87.5 ± 20.6 | <.01 |
| Withdrew from treatment (%) | 19 | 17 | 0.24 |
| Days to first drink | 99.9 ± 14.5 | 100.9 ± 14.6 | 0.29 |
| BAC first peak drinking day | 0.29 ± 0.15 | 0.26 ± 0.15 | <.01 |
Chi-square for categorical variables, t-tests for continuous variables
COMBINE Study Procedures
Participants were assigned to one of nine treatment groups. Medications were provided to treatment groups in a double-blind, double-dummy format, and consisted of placebo/placebo, acamprosate/placebo, placebo/naltrexone, and acamprosate/naltrexone. Eight treatment groups received platform medical management therapy to enhance medication compliance and alcohol abstinence. Four groups received an additional combined behavioral intervention (CBI) designed to promote greater alcohol abstinence. A ninth group received CBI but no medication, to evaluate the role of placebo effects in treatment success. The primary COMBINE study outcome measures were percent drinking days and time to first heavy drinking day. Results revealed no main effect of naltrexone, acamprosate, or CBI on the primary outcome measure of percent drinking days, though a significant main effect of naltrexone was observed on time to first heavy drinking day. Combined naltrexone and CBI treatment (in the presence of concurrent medical management) was significantly associated with beneficial effects for several treatment outcomes, including greater percent abstinent days, fewer drinks per days, and fewer heavy drinking days per month (Anton et al., 2006).
Measures
Only the measures most relevant to this analysis are described here; a complete list and schedule of COMBINE assessments can be found elsewhere (Anton et al., 2006).
Abstinence goals
As part of the study intake, participants were asked to endorse one of seven different treatment goals. Five of these goals were dichotomized into abstinent and nonabstinent drinking goals (Table 2). Participants who selected one of the two remaining goals (“I don’t really have a clear goal in mind” and “None of the above”) were excluded from this analysis.
Table 2.
Definition of Pretreatment Goals
| Abstinent Drinking Goal |
|
| Nonabstinent Drinking Goal |
|
Self-report of drinking
Drinking was assessed via a timeline follow-back self-report interview at study weeks 8 and 16 (Sobell and Sobell, 1992). Drinks were converted into standard drink units (SDUs), defined as 0.5 ounces of pure alcohol, for data analysis.
Drinker inventory of consequences (DrInC, Miller et al., 1995)
The DrInC questionnaire is a 50-item self-report measure of alcohol-related problems. Answers are rated on a 4-point likert scale (never, once or a few times, once or twice a week, daily or almost daily). The DrInC yields 5 subscales (physical, relationship, intrapersonal, impulsive action, and social responsibility), with higher values indicating more problem areas, and provides a sensitive assessment of different domains of potential alcohol-related problems. The DrInC was administered at intake, mid, and end-of-treatment to evaluate drinking-related consequences; only results from intake and end-of-treatment administrations were evaluated here.
Brief Symptom Inventory (BSI, Derogatis, 1993)
The BSI is a 53 item self-report of psychosocial functioning. Answers are rated on a 5-point likert scale (not at all, a little bit, moderately, quite a bit, extremely). The BSI yields 9 symptom subscales and 3 global scales, with higher values representing more severe problems. The BSI was administered at intake, mid, and end-of-treatment to evaluate psychosocial functioning; only results from intake and end-of-treatment administrations were evaluated here.
Self-reported treatment ratings
COMBINE participants completed several questions regarding their satisfaction with treatment. The following questions were evaluated in this analysis: “How would you rate the quality of your treatment”, “Did you get the kind of treatment you wanted”, “Has treatment helped you to deal more effectively with your drinking problem”, “How satisfied are you with the treatment you received”, “How have you changed your drinking behavior since you began treatment”, “How helpful was having a say in what happened in my own treatment”, and “How helpful was having a therapist who emphasized stopping drinking altogether (abstinence)”. Each question was rated on 5-point Likert scales (anchored by excellent/very satisfied/yes definitely and poor/very dissatisfied/no definitely) and was completed at the end-of-treatment visit (week 16).
Data Analysis
Participant characteristics at intake were analyzed using Fisher’s Exact tests for dichotomous variables and independent t-tests for continuous variables. Main effects and interactions of COMBINE experimental group and drinking goal on percent drinking days were evaluated using a 5-way (COMBINE experimental conditions: acamprosate, naltrexone, CBI x drinking goals: nonabstinent, abstinent) Analysis of Covariance (ANCOVA). The primary study analyses reported a significant effect of treatment site; therefore, treatment site was included in the analyses of main COMBINE experimental group effects as a covariate (Anton et al., 2006). Time to first heavy drinking day was evaluated using a Kaplan-Meier model for continuous variables. No interactions between experimental group and drinking goal were observed, so participants were collapsed across experimental groups to isolate the main effects of drinking goal on treatment outcomes.
Several clinically-relevant drinking related outcomes were evaluated as a function of drinking goal group. A dichotomous variable representing heavy drinking (defined as ≥5 drinks/day for men and ge;4 drinks/day for women) was provided within the COMBINE dataset for each drinking day. Independent t-tests were used to compare between-group differences on total percent drinking days, total number of heavy drinking days, number of study weeks with no drinking, number of study weeks with no heavy drinking, breath alcohol concentration (BAC) on peak drinking days, and number of days with ge;1 drink. Fisher’s exact tests were used to compare between-group differences on the percent of participants with any heavy drinking during treatment, the percent of participants with any heavy drinking during the final week of treatment, and the percent of participants who reported a ≥50% reduction in drinking from baseline to week 16. Repeated measures regression analyses were used to examine longitudinal effects of abstinence group, time, and group x time interactions on four clinically relevant outcome measures, including percent drinking days, number of drinks per day, number of heavy drinking days, and number drinking days per week over the 16-week intervention. Retention was provided as a dichotomous variable within the COMBINE dataset and was defined as having withdrawn from all active medications or treatments. The dichotomous nature of the retention data precluded a Kaplan-Meier survival analysis of retention data; therefore, percent participants retained in treatment was compared between groups using a Fisher’s exact test. Finally, Kaplan-Meier survival models were used to examine between-group differences in time to first heavy drinking day and time to peak drinking day.
DrInC and BSI derived subscale scores were provided within the COMBINE dataset and were evaluated using repeated measures regression analyses to examine longitudinal effects of drinking group, time, and group x time interactions, to yield a measure of alcohol-related problems. Treatment satisfaction ratings were dichotomized (excellent or good vs. neutral, bad, or very bad) and compared between groups using a Fisher’s exact test.
Site was not included as a covariate in any of these analyses because the site that drove the effects in the primary analysis (site 2) was equally distributed between the abstinent and nonabstinent drinking goal groups (n=29 vs. n=26 from site 2, respectively). Significant between-group demographic differences were not controlled for in these analyses because there was no a priori reason to believe the specific differences that emerged would contribute to treatment outcomes. Two-tailed tests were used and all results were considered statistically significant if P≤.05. Statistical analyses were conducted using SPSS software 19.0 and SAS software version 9.2.
Results
Participants
Table 1 presents the characteristics of study participants at treatment intake. Compared to the abstinent drinking group participants, participants with a nonabstinent drinking goal were more likely to be employed (68% vs. 78%, respectively; p<.01) and to have a high school education (92% vs. 96%, respectively; p<.05), however were less likely than abstinent drinking goal participants to adhere to their study medications (91% vs. 88%, respectively; p<.01). The two groups did not differ on any of the prebaseline drinking variables that were examined (Table 1). There were no differences in percent of participants retained in treatment (p=.24).
Effects of drinking goal and COMBINE experimental group on COMBINE primary drinking outcomes
A 5-way ANCOVA revealed between-group main effects of treatment site (F(10,27)=2.84, p<.01), drinking goal (F(1,27)=37.41, p<.001), acamprosate (F(1,27)=3.88, p<.05), and naltrexone (F(1,27)=4.35, p<.05) on percent drinking days; however, no significant interactions between site, drinking goal, and experimental groups were observed. A Cox Regression revealed a main effect of site (p=.001) that was driven by one site (p=.01) on time to first heavy drinking day. No site x drinking group or drinking group x experimental condition interactions were observed.
Effects of drinking goal group on drinking-related outcomes
When collapsed across treatment groups, several significant associations between drinking goal group and drinking-related outcomes were observed. Abstinent drinking goal group participants were significantly different from nonabstinent drinking goal group participants on several domains that suggested better treatment outcome, such as number of weeks with no drinking (8.5 vs. 4.9, respectively; p<.001); number of weeks with no heavy drinking (10.6 vs. 9.0, respectively; p<.001), percent participants that reported any heavy drinking day during the study (66% vs. 78%, respectively; p<.001), mean percent of days with ≥1 drink (22% vs. 38%, respectively; p<.001), and percent participants reporting greater than 50% decrease in drinks per day between baseline and week 16 (61% vs. 43%, respectively; p<.001). However, abstinent drinking goal group participants did provide higher BAC readings than nonabstinent goal group participants on their peak drinking day (.29 vs. .25, respectively; p<.01). No group differences were observed on the percent of participants reporting a greater than 50% decrease in heavy drinking days between baseline and week 16.
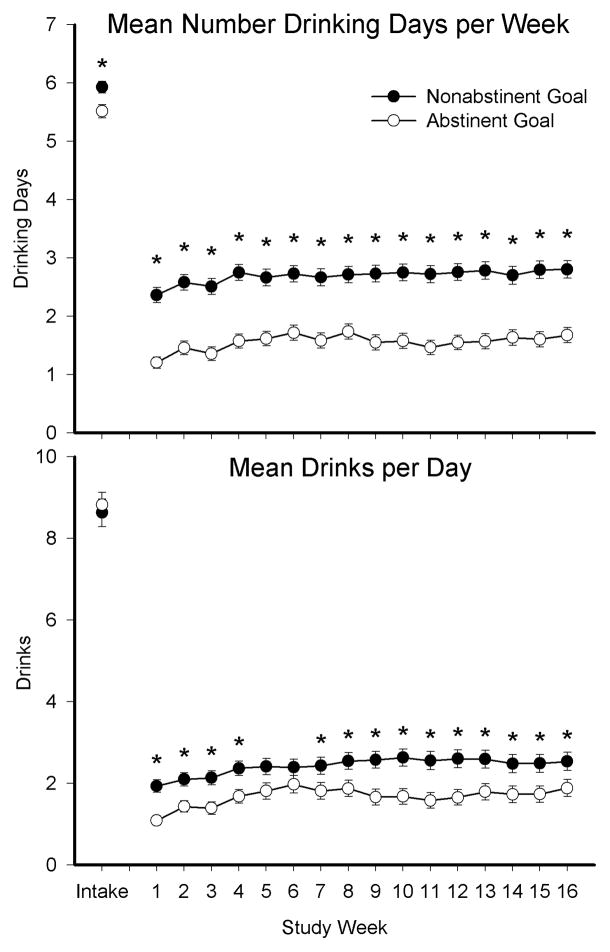
A significant main effect of abstinent drinking goal group was observed on all four repeated measure variables examined. Specifically, there was a significant main effect on percent drinking days (F(1,678)=8.38, p<.01), mean drinks per day (F(1,678)=11.69, p<.01), number of heavy drinking days (F(1,678)=8.35, p<.01), and number drinking days per week (F(1,678)=84.14, p<.0001). Significant effects of time were also observed on each measure, however a drinking goal x time interaction was only observed on mean drinks per day (Figure 1, top panel; F(16,11000)=2.51, p<.001) and number drinking days per week (Figure 1, bottom panel; F(16,11000)=3.43, p<.001). Finally, Kaplan-Meier survival analyses revealed no significant main effect of drinking goal group on days to first heavy drinking day (p=.17) or time to peak drinking day (p=.90)
Figure 1.
Association between pretreatment drinking goals and treatment outcomes at intake and throughout the 16-week intervention. Significant between-group differences on mean number drinking days per week (top panel) and mean drinks per day (bottom panel) between participants who self-reported an abstinent (open circles) or nonabstinent (closed circles) drinking treatment goal during the intake visit. Asterisks represent significant between-group differences (p≤.05).
Effects of drinking goal group on alcohol-related problems
As seen in Table 3, no significant differences on several different ratings of treatment satisfaction were observed between the abstinent and nonabstinent drinking goal groups. There was a significant between-subject effect of drinking goal group (F(1,555)=58.3, p<.001), within-subject effects of time (F(1)=688.9, p<.001), and drinking goal group x time interaction (F(1.2)=27.9, p<.001) on DrInC subscale scores. There was a significant between-subject effect of drinking goal group (F(1,488)=8.83, p=.003), and a significant within-subject effect of time (F(1)=41.62, p<.001) on BSI subscale scores. No significant drinking goal group x time interaction (p=.51) on BSI subscales was observed. The significant effects of drinking goal group were driven largely by the abstinent goal group (Table 3), which entered treatment with higher ratings on the DrInC and BSI subscale scores as compared to the nonabstinent goal group. Both groups reported reductions over time in alcohol related problems and reported comparable levels of alcohol-related problems and psychosocial functioning, as assessed via the DrInC and BSI, at the end-of-treatment visit.
Table 3.
Assessment of treatment satisfaction, alcohol-related problems, and psychosocial functioning.
| Abstinent Drinking Goal
|
Nonabstinent Drinking Goal
|
|||
|---|---|---|---|---|
| Baseline | End-of-Treatment | Baseline | End-of-Treatment | |
| Treatment Satisfaction (% rating excellent or good) | ||||
| Quality of treatment | 77 | 81 | ||
| Received kind of treatment wanted | 70 | 70 | ||
| Satisfied with treatment received | 76 | 79 | ||
| How helpful was having say in what happened in treatment | 64 | 66 | ||
| How helpful was having a therapist emphasize abstinence | 51 | 48 | ||
| Treatment Satisfaction (% agreeing) | ||||
| Treatment helped to deal more effectively with drinking | 73 | 74 | ||
| Drinking behavior better since beginning treatment | 78 | 82 | ||
| Drinker Inventory of Consequences Scales | ||||
| Physical (0–22) | 9.6 ± 0.23 | 2.9 ± 0.24 | 6.9 ± 0.23 | 2.4 ± 0.23 |
| Relationship (0–30) | 10.1 ± 0.32 | 3.1 ± 0.29 | 6.8 ± 0.31 | 2.3 ± 0.28 |
| Intrapersonal (0–24) | 15.3 ± 0.31 | 5.0 ± 0.37 | 11.1 ± 0.30 | 4.2 ± 0.35 |
| Impulsive Action (0–31) | 4.6 ± 0.24 | 2.5 ± 0.21 | 5.8 ± 0.24 | 2.0 ± 0.21 |
| Social Responsibility (0–21) | 6.4 ± 0.22 | 2.0 ± 0.19 | 3.9 ± 0.21 | 1.4 ± 0.19 |
| Total Drinking Consequences (0–124) | 49.4 ± 1.10 | 15.4 ± 1.20 | 34.5 ± 1.04 | 12.3 ± 1.20 |
| Control Scale (0–3) | 1.6 ± 0.03 | 0.7 ± 0.04 | 1.8 ± 0.03 | 0.7 ± 0.04 |
| Brief Symptom Inventorya | ||||
| Somatization | 53.8 ± 0.63 | 53.3 ± 0.60 | 51.6 ± 0.32 | 51.1 ± 0.59 |
| Obsessive Compulsive | 59.2 ± 0.66 | 57.8 ± 0.66 | 55.1 ± 0.65 | 54.1 ± 0.65 |
| Intrapersonal | 57.5 ± 0.68 | 55.1 ± 0.67 | 54.1 ± 0.67 | 52.7 ± 0.66 |
| Depression | 61.7 ± 0.66 | 59.7 ± 0.68 | 57.7 ± 0.65 | 56.3 ± 0.67 |
| Anxiety | 58.6 ± 0.69 | 56.0 ± 0.72 | 54.0 ± 0.68 | 51.9 ± 0.71 |
| Hostility | 55.3 ± 0.62 | 54.7 ± 0.63 | 51.8 ± 0.61 | 50.8 ± 0.62 |
| Phobic Anxiety | 54.1 ± 0.57 | 50.7 ± 0.52 | 52.4 ± 0.56 | 50.4 ± 0.52 |
| Paranoid | 54.4 ± 0.64 | 53.0 ± 0.60 | 53.1 ± 0.63 | 52.0 ± 0.59 |
| Psychoticism | 63.9 ± 0.64 | 61.2 ± 0.68 | 58.6 ± 0.63 | 57.8 ± 0.67 |
| Positive Symptoms Total | 60.1 ± 0.64 | 58.7 ± 0.73 | 55.8 ± 0.63 | 54.4 ± 0.72 |
| Positive Symptoms Distress Index | 55.4 ± 0.50 | 53.1 ± 0.50 | 51.7 ± 0.49 | 51.3 ± 0.50 |
| Global Severity Index | 60.4 ± 0.66 | 58.3 ± 0.72 | 55.5 ± 0.65 | 54.1 ± 0.71 |
Values represent mean ± SEM unless otherwise specified
Values are t-scores
Discussion
This analysis provides evidence that individual alcohol drinking goal is associated with drinking-related outcome measures following exposure to a treatment intervention. Participants in this study who intended to achieve abstinence in treatment demonstrated significantly greater reductions in drinking throughout the intervention when compared to participants who entered treatment with the intention of reducing but not abstaining from drinking, independent of the experimental condition to which they had been assigned. These results are not explained by differential rates of retention and are consistent with a growing number of studies that have reported associations between baseline treatment drinking goals and alcohol intervention outcomes (Adamson et al., 2010; At-Otaiba et al., 2008; Duckert, 1993).
Participants in both groups also reported significant reductions in alcohol-related problems and improvements in psychosocial functioning. As seen in Table 3, the significant between-group effects were driven largely by the abstinent drinking goal group, which entered treatment with significantly more alcohol-related problems. Despite this difference, participants in both groups reported comparable levels of problems upon ending treatment. Treatment satisfaction ratings also did not differ between the groups, suggesting the effects of drinking goal group on abstinence and alcohol-related problems were not associated with perception of treatment quality. These data suggest that patients who enter treatment with a nonabstinent drinking goal may be able to successfully maintain low levels of drinking without experiencing a high rate of associated alcohol-related problems.
Together with these other reports, the present data provide strong support for the standard inclusion of drinking treatment goal as a stratification variable in study interventions, or as a covariate in outcome analyses. The present analyses revealed that a large subset of people entering treatment for alcohol dependence do not intend to seek abstinence and that pretreatment drinking goal was associated with several clinically meaningful drinking outcomes. Therefore, failure to measure and account for treatment goal may be obscuring outcome analyses in clinical evaluations of alcohol treatment, and may limit the generalizability of study findings if drinking goal is not suitably considered in an alcohol-related clinical trial. Relatedly, it may be more clinically relevant to assess treatment outcome in the context of individual treatment goals, rather than applying a uniform definition of success across all participants.
Including drinking goal in clinical trials appears to be an important factor to consider in study design, but there is currently no standardized method for assessing drinking goals. Previous studies have rated goals through a semi-structured interview, a brief questionnaire, or by asking participants to identify weekly drinking goals and assessing whether those goals were met (Adamson et al., 2010; At-Otaiba et al., 2008; Duckert, 1993). Standardizing this process would enable researchers to better generalize results across participant samples and isolate the effects of medications and behavioral interventions within abstinent drinking goal and nonabstinent drinking goal subpopulations. Table 2 shows the questions presented during the COMBINE study that were used to categorize participants into abstinent and nonabstinent drinking goal groups. Additional goals (“I don’t really have a clear goal in mind” and “None of the above”) were also presented but excluded from these analyses. Together these options represent a diverse range of treatment goals and, in absence of a standardized measure, may serve as a quick and easily administered measure for future studies seeking to categorize drinking goals.
It is interesting to note that the effects of pretreatment drinking goal were most prominent in analyses that evaluated drinking days, and were less likely to be detected in analyses of heavy drinking days. For example, significant abstinence goal x time interactions were observed for mean drinks per day and number of drinking days per week, but not number of heavy drinking days. Thus, it is possible that the heavy drinking days outcome measure may be less sensitive to the effect of drinking goal than drinking days. Therefore, studies that do not measure drinking goal at treatment intake may benefit from using heavy drinking days as an outcome measure, a strategy that is consistent with several recent recommendations (Allen, 2003; Falk et al., 2010; Gastfriend et al., 2007).
Approximately 25% of the total COMBINE study sample endorsed a nonabstinent drinking goal at study intake, which is consistent with other studies that have reported 16–46% of participants entering treatment for alcohol use disorders identify a nonabstinent drinking goal (At-Otaiba et al., 2008; Booth et al., 1984; Heather et al., 2010; Hodgens et al., 1997; Ojehagen and Berglund, 1989). Participants in the present study who reported a nonabstinent drinking goal were more likely to be employed, had higher educational levels, and reported lower rates of drinking-related problems and psychosocial issues than participants who entered treatment seeking abstinence, suggesting that abstinent drinking goal participants entered treatment with more problems. This finding has also been reported in previous studies (Heather et al., 2010; Pachman et al., 1978), and is consistent with data that suggests experiencing a greater prevalence of alcohol-related problems can motivate patients to seek abstinence from alcohol (Tucker, 1995). It would be important to understand what motivated patients with nonabstinent drinking goals to seek treatment before they acquired more severe alcohol-related problems. Characterizing reasons why these participants enter treatment would aid in the development of strategies to recruit patients into treatment prior to the development of severe alcohol-related problems.
It is important to note that, despite not seeking abstinence, participants in the nonabstinent drinking goal group did achieve significant decreases in alcohol consumption over time. This finding is consistent with previous studies that examined controlled drinking interventions (Miller, 1978; Sanchez-Craig, 1980; 1984) and further supports this population’s interest in receiving treatment for their alcohol use (Adamson et al., 2010; At-Otaiba et al., 2008; Duckert, 1993; Sobell et al., 1995). Yet, many alcohol dependent patients report unwillingness to enter treatment programs because the program may mandate abstinence, or because the patient is unsure whether the treatment will mandate abstinence (Saunders et al., 2006). There is little known regarding the long-term outcomes of patients with nonabstinent treatment goals, so it remains possible that these reductions in drinking are clinically meaningful and may be sufficient for a subpopulation of alcohol users. Tailoring treatments to patients who do not yet want to seek abstinence may also motivate those individuals to seek treatment before developing serious alcohol-related problems. Studies have also shown that a large number of patients who enter treatment initially reporting a nonabstinent drinking goal convert to an abstinent drinking goal after they begin treatment (Hodgins et al., 1997; Ojehagen and Berglund, 1989).
In the current analysis, only approximately 50% of participants in both groups were satisfied with the therapist emphasizing abstinence, which was the lowest of the satisfaction ratings we evaluated and may reflect the dynamic nature of treatment goals. A drinking intervention that targeted reductions in drinking but not necessarily abstinence may also promote early entry into treatment for patients who might otherwise be reluctant to seek assistance in controlling their drinking. This idea is not new; controlled drinking interventions have been conducted since the 1970s, though modern medicine has placed greater emphasis on abstinence-only approaches (Sobell and Sobell, 2011). Although the goal of the present analysis was to evaluate whether individual drinking goals may confound drinking outcome measures, these data may lend support to the controlled drinking perspective.
This analysis had several limitations. First, this was not a prospective evaluation of the effect of treatment goal on outcome. Participant groups for this analysis were matched posthoc on prebaseline drinking variables to circumvent issues with comparing discrepant sample sizes. Matching was also conducted by one individual rather than two independent observers. It remains possible that the study results are a consequence of the manner in which participants were matched, although this outcome is unlikely given the lack of significant differences between the two groups on any prebaseline drinking characteristics (Table 1), as well as the consistency between these results and prior studies (e.g., UKATT). Second, participants in this sample were all seeking and willing to participate in an intensive alcohol treatment intervention, therefore it is unknown how these results will generalize to participants who may want to reduce drinking but are not yet seeking treatment. Third, drinking outcome measures were collected via self-report and were not objectively verified.
Despite these limitations, these data indicate that pretreatment drinking goal is significantly associated with several clinically meaningful drinking-related outcomes. These data support the standard measurement and inclusion of pretreatment drinking goal in the development and analysis of clinical trials of alcohol treatment, to mitigate potential confounding effects of pretreatment goal on study outcomes. These data also highlight several areas that warrant additional research attention, such as the characteristics of patients who are entering treatment with nonabstinent drinking goals, including their motives for seeking treatment before the development of more severe problems, and the long-term outcomes of the patients who successfully achieve reductions in drinking, to determine whether reductions in drinking may be sufficient in a subpopulation of alcohol users. Ultimately, these data indicate that additional, prospective evaluations of the association between drinking goal and outcome are warranted.
Acknowledgments
We would like to thank Linda Felch for her statistical guidance and analyses. The authors also acknowledge that the reported results are, in whole or in part, based on analyses of the COMBINE data set. These data were collected as part of a multisite clinical trial of alcoholism treatments supported by a series of grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute of Health, and DHSS. This publication has not been reviewed or endorsed by NIAAA or the COMBINE research group, and does not necessarily reflect the opinions of its members or NIAAA, who are not responsible for its contents. This study was supported in part by NIDA T32 DA007209 (Bigelow) and NIDA K24 DA023186 (Strain).
Bibliography
- Adamson SJ, Heather N, Morton V, Raistrick D. Initial preference for drinking goal in the treatment of alcohol problems: II. Treatment Outcomes. Alcohol Alcohol. 2010;45:36–142. doi: 10.1093/alcalc/agq005. [DOI] [PubMed] [Google Scholar]
- Allen JP. Measuring outcome in interventions for alcohol dependence and problem drinking: executive summary of a conference sponsored by the National Institute on Alcohol Abuse and Alcoholism. Alcohol Clin Exp Res. 2003;27:1657–1660. doi: 10.1097/01.ALC.0000091223.72517.13. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- At-Otaiba Z, Worden BL, McCrady BS, Epstein EE. Accounting for self-selected drinking goals in the assessment of treatment outcome. Psychol Addict Behav. 2008;22:439–443. doi: 10.1037/0893-164X.22.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth P, Dale B, Ansari J. Problem drinkers’ goal preference and treatment outcome: a preliminary study. Addict Behav. 1984;9:357–364. doi: 10.1016/0306-4603(84)90035-2. [DOI] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ, Mukamal KJ, Gray PO, Parks DA, Das DK, Korthuis RJ. Alcohol in moderation, cardioprotection, and neuroprotection: epidemiological considerations and mechanistic studies. Alcohol Clin Exp Res. 2009;33:206–219. doi: 10.1111/j.1530-0277.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckert F. Predictive factors for outcome of treatment for alcohol problems. J Subst Abuse. 1993;5:31–44. doi: 10.1016/0899-3289(93)90121-q. [DOI] [PubMed] [Google Scholar]
- Falk D, Wang XQ, Liu L, Fertig J, Mattson M, Ryan M, Johnson B, Stout R, Litten RZ. Percentage of subjects with no heavy drinking days: Evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol Clin Exp Res. 2010;34:2022–2034. doi: 10.1111/j.1530-0277.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- Gastfriend DR, Garbutt JC, Pettinati HM, Forman RF. Reduction in heavy drinking as a treatment outcome in alcohol dependence. J Subst Abuse Treat. 2007;33:71–80. doi: 10.1016/j.jsat.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Gutjahr E, Gmel G. Defining alcohol-related fatal medical conditions for social-cost studies in western societies: An update of the epidemiological evidence. J Subst Abuse. 2001;13:239–264. doi: 10.1016/s0899-3289(01)00086-4. [DOI] [PubMed] [Google Scholar]
- Heather N, Adamson S, Raistrick D, Slegg GP UKATT Research Team . Initial preference for drinking goal in the treatment of alcohol problems: I. Baseline difference between abstinence and non-abstinence groups. Alcohol Alcohol. 2010;45:128–135. doi: 10.1093/alcalc/agp096. [DOI] [PubMed] [Google Scholar]
- Hodgins DC, Leigh G, Milne R, Gerrish R. Drinking goal selection in behavioral self- management treatment of chronic alcoholics. Addict Behav. 1997;22:247–255. doi: 10.1016/s0306-4603(96)00013-5. [DOI] [PubMed] [Google Scholar]
- Miller WR. Behavioral treatment of problem drinkers: A comparative outcome study of three controlled drinking therapies. J Consult Clin Psychol. 1978;46:74–86. doi: 10.1037//0022-006x.46.1.74. [DOI] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS, Longabaugh R. DHHS Publication No 95-3911. Vol. 4. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1995. The Drinker Inventory of Consequences (DrInC): An Instrument for Assessing Adverse Consequences of Alcohol Abuse. Project MATCH Monograph Series. [Google Scholar]
- Nova E, Baccan GC, Zapatera B, Marcos A. Potential health benefits of moderate alcohol consumption: current perspectives in research. Proc Nutr Soc. 2012;71:307–315. doi: 10.1017/S0029665112000171. [DOI] [PubMed] [Google Scholar]
- Ojehagen A, Berglund M. Change of drinking goals in a two-year out-patient alcoholic treatment program. Addict Behav. 1989;14:1–9. doi: 10.1016/0306-4603(89)90011-7. [DOI] [PubMed] [Google Scholar]
- Pachman JS, Foy DW, Van Erd M. Goal choice of alcoholics: A comparison of those who chose total abstinence vs. those who choose responsible, controlled drinking. J Clin Psychol. 1978;34:781–783. doi: 10.1002/1097-4679(197807)34:3<781::aid-jclp2270340343>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Rehm J, Gmel G, Sempos CT, Trevisan M. Alcohol-related morbidity and mortality. Alcohol Res Health. 2003;27:39–51. [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Craig M. Random assignment to abstinence or controlled drinking in a cognitive-behavioral program: Short-term effects on drinking behavior. Addict Behav. 1980;5:35–39. doi: 10.1016/0306-4603(80)90019-2. [DOI] [PubMed] [Google Scholar]
- Sanchez-Craig M, Annis HM, Bornet AR, MacDonald KR. Random assignment to abstinence and controlled drinking: Evaluation of cognitive-behavioral program for problem drinkers. J Consult Clin Psychol. 1984;52:390–403. doi: 10.1037//0022-006x.52.3.390. [DOI] [PubMed] [Google Scholar]
- Saunders SM, Zygowicz KM, D’Angelo BR. Person-related and treatment-related barriers to alcohol treatment. J Subst Abuse Treat. 2006;30:261–270. doi: 10.1016/j.jsat.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Sobell MD, Sobell LC. Is it time for low-risk drinking goals to come out of the closet. Addiction. 2011;106:1715–1717. doi: 10.1111/j.1360-0443.2011.03509.x. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Gavin DR. Portraying alcohol treatment outcomes: different yardsticks of success. Behav Ther. 1995;26:643–669. [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. J Stud Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-41, HHS Publication No (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- Tucker JA. Predictors of help-seeking and the temporal relationship of help to recovery among treated and untreated recovered problem drinkers. Addiction. 1995;90:805–809. doi: 10.1046/j.1360-0443.1995.9068057.x. [DOI] [PubMed] [Google Scholar]