Abstract
Atopic dermatitis (AD) is characterized by epidermal barrier defects and recurrent microbial skin infections. AD patients with a history of eczema herpeticum (ADEH+) have more severe skin disease and more highly TH2 polarized immune responses as compared to uncomplicated AD (ADEH−). However, the mechanisms linking epidermal barrier defects and viral skin infection are not well understood. Recently, it has been reported that interleukin (IL)-25 may play a role in augmenting TH2 responses. We examined protein expression of IL-25 in the skin biopsies from normal subjects (n=10), ADEH− (n=18), ADEH+ (n=7) and psoriasis (n=9). IL-25 expression was increased in the skin from ADEH−, ADEH+ and psoriasis compared to normal skin, and was significantly greater in lesional ADEH+ skin than in lesional ADEH- skin. Importantly, we demonstrated that IL-25 enhances herpes simplex virus (HSV)-1 and vaccinia virus replication by inhibiting filaggrin expression, and IL-25 acts synergistically with IL-4 and IL-13 to enhance HSV-1 replication in vitro. In contrast, interferon-γ inhibited HSV-1 replication in vitro. Additionally, we demonstrate that filaggrin is a critical protein to inhibit HSV-1 replication because filaggrin small interfering RNA knockdown enhances HSV-1 replication in vitro. Filaggrin breakdown products, however, inhibited HSV-1 replication in vitro.
INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disease that affects up to 20% of children and 3% of adults (Bieber, 2010; Zheng et al., 2011). Epidermal barrier defects and recurrent microbial skin infections are characteristic findings in AD (Irvine et al., 2011; Kim and Leung, 2012). Previously our laboratory reported that TH2 cytokines are over-expressed in AD skin (Hamid et al., 1994) and down-regulate epidermal barrier proteins (Howell et al., 2011; Howell et al., 2009; Kim et al., 2008). Additionally, we have reported that TH2 cytokines promote disseminated viral skin infections such as eczema herpeticum (EH) by inhibiting the expression of antimicrobial peptides (AMPs) in AD skin (Howell et al., 2006a; Howell et al., 2006b; Howell et al., 2011). It is known that AD patients with a history of EH (ADEH+) have more TH2-polarized immune responses (Beck et al., 2009) and a higher prevalence of filaggrin (FLG) null mutations compared with patients with AD not complicated by EH, i.e. ADEH−, (Gao et al., 2009). FLG gene mutations only affect a minority of AD patients. Therefore, additional mechanisms linking epidermal barrier defects and susceptibility to viral skin infections remain to be elucidated.
Recently, it has been proposed that IL-25 might play an important role in augmenting TH2 responses in allergic diseases (Barlow et al., 2011; Hvid et al., 2011). IL-25 and its receptor IL-17Rh1 are expressed in AD skin (Hvid et al., 2011; Lee et al., 2001), and IL-25 down-regulates FLG mRNA (Hvid et al., 2011). There have been no previous studies, however, investigating whether IL-25 modulation of epidermal barrier proteins enhances viral replication. Moreover, it has not been investigated whether TH2 cytokines act synergistically with IL-25 to modulate epidermal barrier protein expression and to enhance viral replication.
In this study, we examined IL-25 expression in human skin and compared the relative effects of IL-25, TH2 cytokines and interferon (IFN)-γ on the expression of filaggrin. Additionally, we demonstrate that IL-25 functionally enhances herpes simplex virus (HSV)-1 and vaccinia virus (VV) replication by inhibiting filaggrin expression, and found that TH2 cytokines act synergistically with IL-25 to enhance HSV-1 replication via their inhibitory effects on filaggrin expression.
RESULTS
IL-25 expression is increased in skin with AD and psoriasis
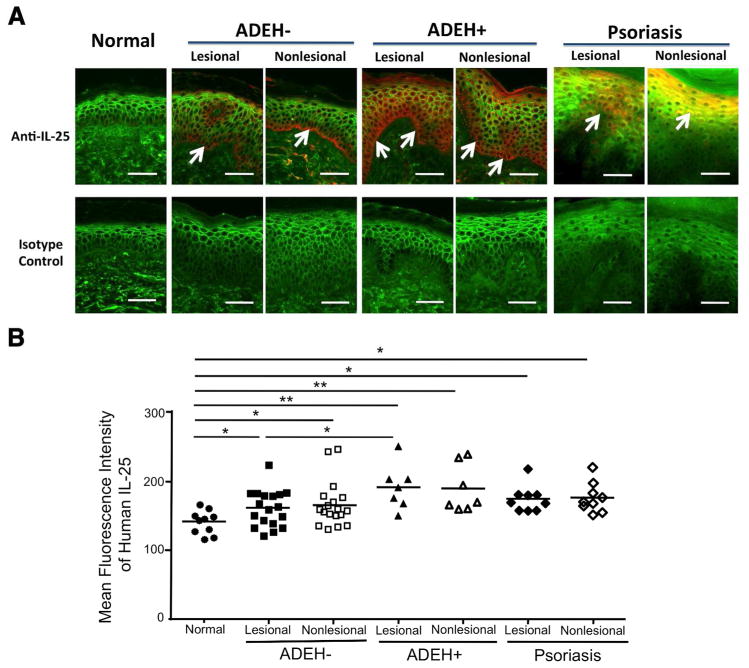
A recent study showed that IL-25 protein is expressed in AD skin (Hvid et al., 2011). However, there have been no previous studies demonstrating protein expression of IL-25 in normal subjects versus patients with ADEH− and ADEH+. In this study, we examined the protein expression of IL-25 in skin biopsies from 10 normal subjects, 18 ADEH− patients and 7 ADEH+ patients. Additionally, we examined the expression of IL-25 in the skin from 9 psoriasis patients as a disease control. As shown in Figure 1a, IL-25 protein expression was increased in the skin of patients with ADEH−, ADEH+ and psoriasis compared with skin from normal subjects. The composite data for IL-25 immunostaining in all samples are shown in Figure 1b. The staining intensity of IL-25 was significantly increased in lesional and non-lesional skin from ADEH− (P < 0.05, P < 0.05), ADEH+ (P < 0.01, P < 0.01) and psoriasis (P < 0.05, P < 0.05, respectively) patients compared with skin from normal subjects. However, it is important to note that the staining intensity of IL-25 in lesional ADEH+ skin was significantly increased (P < 0.05) compared with lesional ADEH- skin. Furthermore, we performed genotypic analysis for common filaggrin mutations including R501X, 2282del4, R2447X, S3247X, and 3702delG in all samples. 1 of 10 normal subjects (10%), 6 of 18 ADEH− (33.3%) and 1 of 7 ADEH+ (14.3%) showed heterozygotic mutations, and no homozygotic mutations were reported.
Figure 1. The expression of IL-25 in human skin.
(a) Representative paraffin embedded skin biopsies from normal subjects (n=10) and patients with ADEH− (n=18), ADEH+ (n=7) and psoriasis (n=9) stained for IL-25 (red) are shown. Wheat germ agglutinin-conjugated fluorescein isothiocyanate (green) stained the cytoskeleton. Images were collected at x 400 magnification. Arrows point to IL-25 expression. Bar=50 μm. (b) The mean fluorescent intensity of IL-25 is shown in the epidermis of each biopsy. *P < 0.05, **P < 0.01.
IL-25 inhibits the expression of filaggrin and acts synergistically with TH2 cytokines to inhibit filaggrin expression
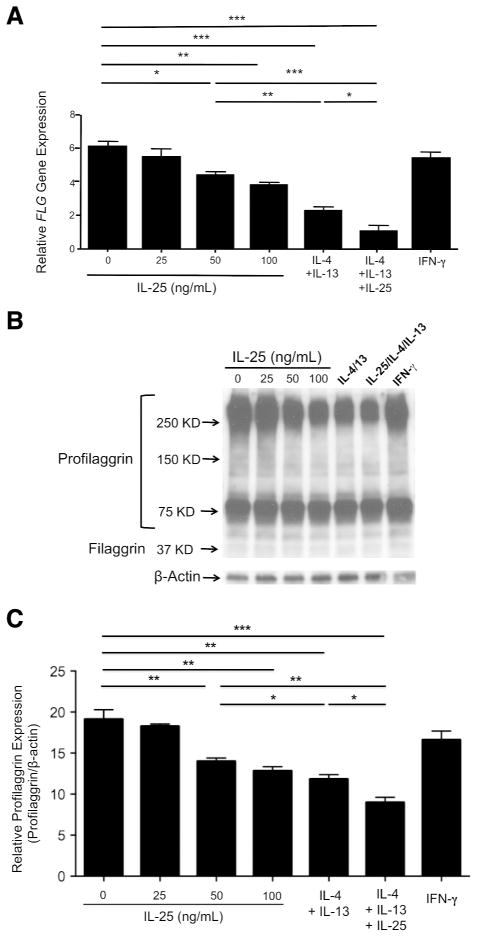
A recent study found that IL-25 inhibits mRNA expression of FLG (Hvid et al., 2011), but these investigators did not study protein expression of filaggrin. Therefore, we examined whether IL-25 modulates both mRNA and protein expression of filaggrin. In addition, we compared the effects of IL-25 with those of TH2 cytokines (IL-4 and IL-13) on filaggrin expression. We differentiated normal human keratinocytes (KCs) with 1.3 mmol/L CaCl2 in various concentrations of IL-25, TH2 cytokines, IFN-γ or a combination of IL-25 and TH2 cytokines for 5 days. Gene expression of FLG was significantly inhibited (P < 0.05) by 50 ng/mL of IL-25 (4.35 ± 0.18 ng of FLG/ng of 18s) compared with media alone (6.02 ± 0.33 ng of FLG/ng of 18s) (Figure 2a). In addition, the gene expression of FLG was significantly decreased in KCs treated with a combination of IL-25 and TH2 cytokines (1.27 ± 0.22 ng) as compared to KCs treated with IL-25 (P < 0.001, 4.35 ± 0.18 ng) or TH2 cytokines (P < 0.05, 2.25 ±0.20 ng) alone. Moreover, this was confirmed at the protein level using western blot analysis (Figure 2b and 2c). We also performed FLG genotypic analysis for the KCs we used. None of the KCs genotyped had the mutations of R501X, 2282del4, R2447X, S3247X and R3702delG.
Figure 2. IL-25 downregulates filaggrin expression, and acts synergistically with TH2 cytokines to inhibit filaggrin expression.
KCs were stimulated in the various concentrations of IL-25, TH2 cytokines (50 ng/mL of IL-4 and 50 ng/mL of IL-13), a combination of IL-25 (50 ng/mL) and TH2 cytokines or IFN-γ (20 ng/mL) for 5 days. (a) The gene expression of FLG was examined using real-time RT-PCR. (b) The protein expression of filaggrin was evaluated by western blot analysis. (c) Relative expression of profilaggrin in 250 KD and bigger profilaggrin bands. Data from 1 representative experiment of 3 independent experiments performed are shown. *P < 0.05, **P < 0.01, ***P < 0.001.
IL-25 enhances HSV-1 replication and acts synergistically with TH2 cytokines to enhance HSV-1 replication
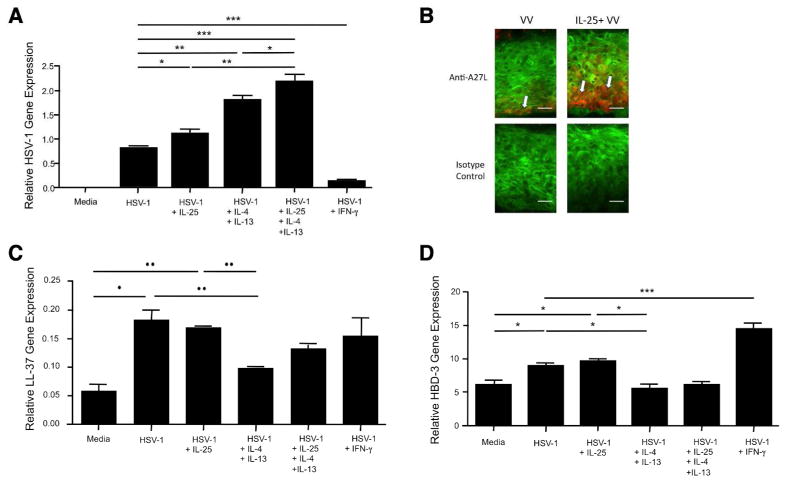
Both IL-25 and TH2 cytokines are over-expressed in the skin of patients with ADEH− and ADEH+, and it is known that TH2 cytokines enhance viral replication (Howell et al., 2006b; Howell et al., 2007). Since IL-25 is known to enhance TH2 responses, we investigated whether IL-25 also enhances viral replication. We differentiated KCs with 1.3 mmol/L CaCl2 in the presence or absence of IL-25, TH2 cytokines, IFN-γ or a combination of IL-25 and TH2 cytokines for 5 days. Then, the cells were incubated with HSV-1 or VV for an additional 24 hours. Indeed, we found that the gene expression of HSV-1 was significantly increased (P < 0.05) in KCs pretreated with IL-25 (1.12 ± 0.06 ng) as compared to KCs without IL-25 pretreatment (0.83 ± 0.01 ng) (Figure 3a). Similarly, VV gene expression was significantly increased in KCs pretreated with IL-25 as compared to KCs without IL- 25 pretreatment (data not shown). In addition, HSV-1 expression was further increased in KCs pretreated with a combination of IL-25 and TH2 cytokines (2.18 ± 0.13 ng) as compared to cells pretreated with IL-25 (1.12 ± 0.06 ng, P < 0.01) or TH2 cytokines (1.80 ± 0.07 ng, P < 0.05) alone. In contrast, HSV-1 expression was significantly decreased (P < 0.001) in KCs pretreated with IFN-γ (0.12 ± 0.02 ng) compared with cells without IFN-γ pretreatment (0.83 ± 0.01 ng). We also found that VV staining intensity was increased in organotypic skin pretreated with IL-25 compared with organotypic skin without IL-25 pretreatment (Figure 3b).
Figure 3. IL-25 enhances viral replications, and acts synergistically with TH2 cytokines to enhance viral replication.
KCs were differentiated in the absence or presence of IL-25 (50 ng/mL), TH2 cytokines (50 ng/mL of IL-4 and 50 ng/mL of IL-13), a combination of IL-25 and TH2 cytokines or IFN-γ (20 ng/mL) for 5 days. Then, the cells were incubated with HSV-1 (MOI, 0.1) for an additional 24 hours. The gene expression of HSV-1 (a), LL-37 (c), and HBD-3 (d) was examined using real-time RT-PCR. (b) Organotypic skin sections were stained for vaccinia virus (red) and the cytoskeleton (green). Arrows point to vaccinia virus. Bar=50 μm. Data from 1 representative experiment of 3 independent experiments performed are shown. *P < 0.05, **P < 0.01, ***P < 0.001.
It is known that TH2 cytokines enhance viral replication by inhibiting expression of AMPs such as cathelicidin (LL-37) and human beta defensin (HBD)-3 (Howell et al., 2006b; Howell et al., 2007). Therefore, we further investigated whether IL-25 modulates the expression of LL-37 and HBD-3 using the same samples. As shown in Figure 3c and 3d, the gene expression of LL-37 and HBD-3 was significantly up-regulated in KCs stimulated with HSV-1 (LL-37: 0.18 ±0.02 ng, P < 0.05; HBD-3: 8.98 ±0.43 ng, P < 0.05) compared with cells treated with media alone (LL-37: 0.06 ±0.01 ng; HBD-3: 6.11 ±0.84 ng). HSV-1 induced gene expression of LL-37 and HBD-3 was significantly inhibited in cells treated with TH2 cytokines (LL- 37: 0.10 ±0.01 ng, ±< 0.05; HBD-3: 5.64 ±0.62 ng, P < 0.05) compared with cells treated with IL-25 (LL-37: 0.17 ±0.01 ng; HBD-3: 9.64 ±0.38 ng) or HSV-1 alone (LL-37: 0.18 ±0.02 ng, HBD-3: 8.98 ±0.43 ng). However, IL-25 did not inhibit HSV-1 induced gene expression of LL-37 and HBD-3, suggesting that IL-25 does not enhance HSV-1 replication by inhibiting AMPs.
FLG silencing enhances HSV-1 replication
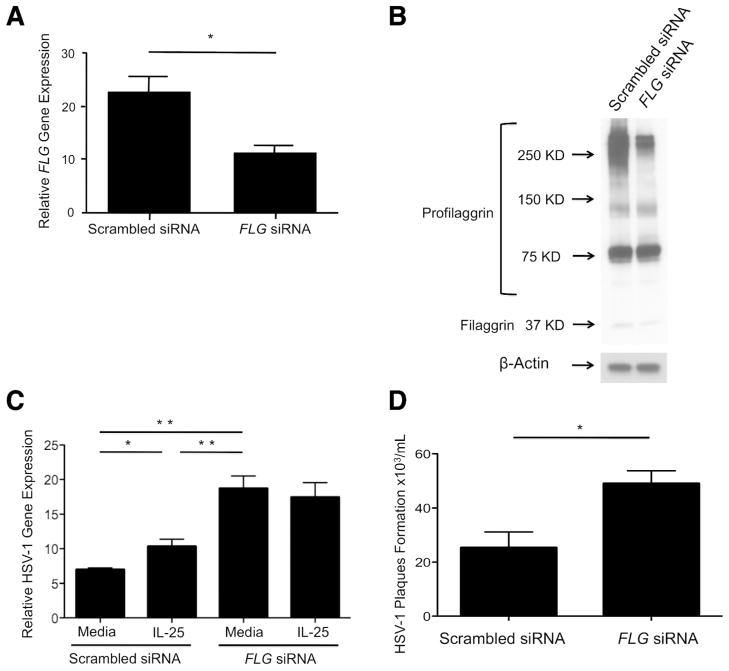
It has been suggested that filaggrin is a critical protein to maintain epidermal integrity and plays an important role in preventing Staphylococcus aureus infection (Miajlovic et al., 2010; O’Regan et al., 2008). Therefore, we further investigated the importance of filaggrin against HSV-1 replication using a small interfering RNA (siRNA) technique. We transfected KCs with scrambled siRNA or FLG siRNA, and the KCs were differentiated in the presence or absence of IL-25 for 5 days. The cells were then stimulated with HSV-1 for an additional 24 hours. As shown in Figure 4a, FLG gene expression was significantly inhibited (P < 0.05) in KCs transfected with FLG siRNA (11.30 ± 1.28 ng) compared with cells transfected with scrambled siRNA (22.52 ± 3.09 ng). This was confirmed at the protein level using western blot technique (Figure 4b). Importantly, HSV-1 gene expression was significantly enhanced (P < 0.01) in KCs transfected with FLG siRNA (18.72 ± 1.79 ng) compared with cells transfected with scrambled siRNA (6.99 ± 0.24 ng) (Figure 4c). We further investigated using HSV-1 viral plaque-forming assay. As shown in Figure 4d, HSV-1 plaques were significantly (P < 0.05) increased in KCs transfected with FLG siRNA (49.00 ± 4.73 × 103 plaques/mL) compared with cells transfected with scrambled siRNA (25.33 ± 5.81 × 103 plaques/mL). However, HSV-1 gene expression was not affected by IL-25 in KCs transfected with FLG siRNA (Figure 4c).
Figure 4. FLG silencing enhances HSV-1 replication.
KCs were transfected with scrambled siRNA or FLG siRNA, and the cells were differentiated for 5 days. Then, the cells were incubated with HSV-1 (MOI, 0.1) for an additional 24 hours. (a) The gene expression of FLG in the KCs was examined using real-time RT-PCR. (b) The protein expression of filaggrin and profilaggrin was evaluated by western blot technique. (c) The gene expression of HSV-1 was examined using real-time RT-PCR. (d) Viral plaque formation was determined by using the viral plaque assay. Data from 1 representative experiment of 3 independent experiments performed are shown. *P < 0.05, **P < 0.01.
Filaggrin breakdown products induce acidic environment and inhibit HSV-1 replication
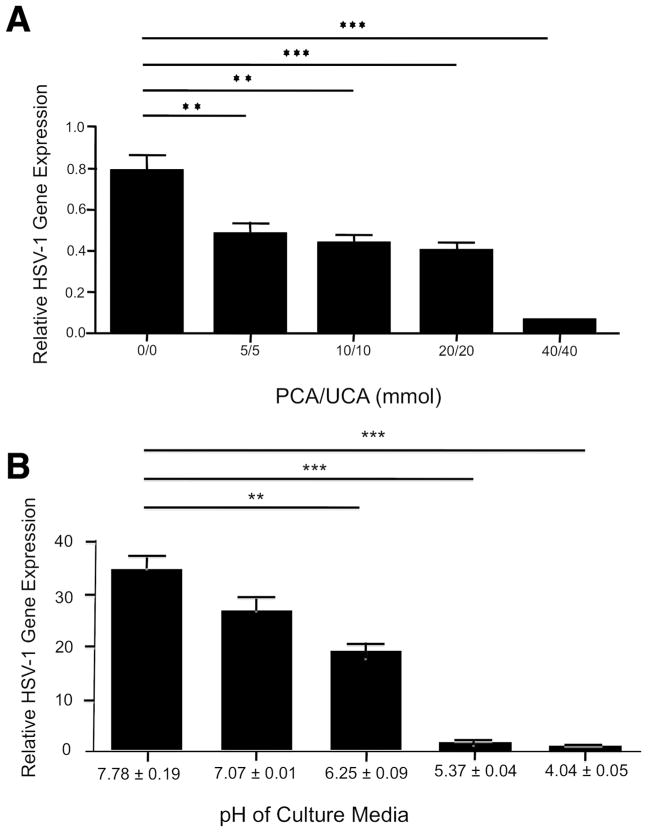
Filaggrin breakdown products such as urocanic acid (UCA) and pyrrolidone carboxylic acid (PCA) are important in maintaining an acidic environment in the skin (O’Regan et al., 2008; Kezic et al., 2012), and an acidic pH inhibits the entry of HSV-1 into cells (Rosenthal et al., 1989). In addition, it has been shown that UCA and PCA inhibit growth of Staphylococcus aureus (Miajlovic et al., 2010). Therefore, we further investigated whether UCA and PCA induce acidic pH conditions and inhibit HSV-1 replication. We incubated KCs with HSV-1 in the presence of various concentrations of UCA and PCA for 24 hours. The pH of KCs culture media was decreased in a dose dependent manner (data not shown). In addition, as shown in Figure 5a, HSV-1 gene expression was significantly inhibited (P < 0.01) by UCA and PCA with a concentration as low as 5 mmol/L (0.48 ± 0.04 ng) compared with media alone (0.78 ± 0.09 ng). We further investigated whether an acidic environment inhibit HSV-1 replication. We incubated KCs with HSV-1 in the presence of various concentrations of hydrochloric acid for 24 hours. Importantly, HSV-1 gene expression was significantly (P < 0.01) inhibited by medium with pH 6.25 ± 0.09 (16.14 ±2.26 ng) compared with medium with pH 7.78 ± 0.19 (35.14 ±3.46 ng) (Figure 5b).
Figure 5. Filaggrin breakdown products and an acidic environment inhibit HSV-1 replication.
KCs were incubated with HSV-1 (MOI, 0.1) in the presence of various concentrations of UCA and PCA (a) or hydrochloric acid (b) for 24 hours. The gene expression of HSV-1 was examined using real-time RT-PCR. Data from 1 representative experiment of 3 independent experiments performed are shown. **P < 0.01, ***P < 0.001.
DISCUSSION
In our current study, we found that IL-25 is increased in lesional and non-lesional skin of patients with ADEH−, ADEH+ and psoriasis as compared to normal skin. This finding confirms a previous study that IL-25 is expressed in the skin from normal subjects, and patients with AD and psoriasis (Hvid et al., 2011). Importantly, we also demonstrated that the level of IL-25 protein expression is higher in lesional ADEH+ skin than it is in lesional ADEH- skin, suggesting IL-25 may play a role in the enhancement of HSV-1 replication in ADEH+ skin. On the other hand, viral infection is not common in psoriasis skin (Christophers et al., 1987) even though IL-25 expression is increased in psoriasis skin compared to normal subject. This could be explained that psoriasis skin does not have increased TH2 cytokines, which inhibit expression of AMPs and epidermal barrier proteins, and shows increased TH1 cytokines known to inhibit viral replication (Leung et al., 2011).
On the basis of our current data and previous data (Hamid et al., 1994), both IL-25 and TH2 cytokines such as IL-4 and IL-13, are over-expressed in AD skin. However, ADEH+ patients are most TH2 polarized. Since TH2 cytokines reduce the expression of epidermal barrier proteins (Howell et al., 2009; Kim et al., 2008), we examined whether IL-25 modulates expression of filaggrin. We confirmed and extended the previous study showing that IL-25 inhibits mRNA expression of FLG (Hvid et al., 2011) by using both quantitative RT-PCR and western blot analysis. Filaggrin is produced as profilaggrin then breaks down into filaggrin monomers. Therefore, profilaggrin will be decreased first by IL-25 as we showed in current data. However, filaggrin monomer was not inhibited by IL-25 in our data. It is possible that prolonged incubation of KCs with IL-25 for greater than 5 days is needed before filaggrin monomer would also be reduced. In addition, there might be posttranslational events of filaggrin, which are hard to demonstrate in in vitro experiments. Additionally, we compared the inhibitory effects of IL-25 on filaggrin expression with those of TH2 cytokines. We showed that IL-25 is less potent than TH2 cytokines. However, IL-25 could potentially play an important role in inhibiting filaggrin expression in vivo because IL-25 promotes production of TH2 cytokines in vivo (Fort et al., 2001).
The current study studied whether IL-25 enhances both HSV-1 and VV replication. Importantly, IL-25 enhanced both HSV-1 and VV replication, but did not modulate the expression of AMPs, suggesting IL-25 does not enhance HSV-1 and VV replication by inhibiting the expression of AMPs. Therefore, we further investigated how IL-25 enhances viral replication using a siRNA technique. Of note, we found that HSV-1 replication was significantly enhanced in KCs transfected with FLG siRNA. This data strongly supports that filaggrin is a critical epidermal protein involved in the inhibition of HSV-1 replication. Moreover, this data is consistent with previous data that FLG KO mice are susceptible to VV infection (Geha and Oyoshi, 2012) and data that null mutations in the FLG gene are highly associated with ADEH+ (Gao et al., 2009). Additionally, we showed that IL-25 did not affect the HSV- 1 replication in the KCs transfected with FLG siRNA, suggesting that IL-25 enhances HSV-1 replication by inhibiting filaggrin expression, not by others.
We found that IL-25 acts synergistically with TH2 cytokines to inhibit filaggrin expression and enhance HSV-1 replication. This synergism is explained by the different mechanisms in which IL-25 and TH2 cytokines act. IL-25 inhibits filaggrin by activating nuclear factor kappa B (Lee et al., 2001). In contrast, TH2 cytokines inhibit expression of AMPs (Albanesi et al., 2007; Howell et al., 2006b; Howell et al., 2011) and epidermal barrier proteins (Kim et al., 2008; Sehra et al., 2010) through signal transducer and activator of transcription 6 activation. Therefore, IL-25 modulates filaggrin in a manner different than TH2 cytokines and acts synergistically with TH2 cytokines to inhibit filaggrin expression and enhance HSV-1 replication in vitro. In addition, as TH2 cytokines inhibit both filaggrin and AMPs, TH2 cytokines are more potent than IL-25 in vitro to enhance HSV-1 replication. Our data also showed that IFN-γ inhibits HSV-1 replication. This finding is consistent with previous data that IFN-γ induces AMPs (Albanesi et al., 2007; Nomura et al., 2003).
To understand how filaggrin deficiency enhances HSV-1 replication, we incubated KCs with HSV-1 in the presence of the filaggrin breakdown products. We found that UCA and PCA induce an acidic environment, and an acidic environment inhibits HSV-1 replication. These findings show consistency with previous data that filaggrin end products play an important role to maintain acidic environment in the skin (Kezic et al., 2012; O’Regan et al., 2008) and inhibit Staphylococcus aureus infection (Miajlovic et al., 2010; Rosenthal et al., 1989). Rosenthal et al reported that mild acidic environment inhibit viral entry into cells by blocking fusion of the viral envelope with a cellular membrane (Rosenthal et al., 1989). We do not have direct evidence that filaggrin itself inhibits HSV-1 replication. However, our data suggest that filaggrin deficiency modifies local pH and enhances viral entry into keratinocytes. Therefore, our data strongly suggest that filaggrin is a key epidermal barrier protein to prevent HSV-1 infection in skin modulated by IL-25, as well as IL- 4 and IL-13.
In summary, the current study demonstrates that IL-25 enhances viral replication by inducing filaggrin deficiency. Furthermore, PCA and UCA inhibit viral replication. Therefore, maintaining normal filaggrin expression may be important for control of viral skin infections, and topical application of PCA and UCA could be a useful strategy for prevention of viral infection in AD skin.
MATERIALS AND METHODS
Subjects
Subjects included ten healthy persons with no history of skin diseases, eighteen patients with ADEH−, seven patients with ADEH+ and nine patients with psoriasis. None of the patients had previously received systemic corticosteroids or cyclosporine, and none had received topical corticosteroid or calcineurin inhibitors for at least one week before enrollment. These studies were conducted according to the Declaration of the Helsinki Guidelines and were approved by the Institutional Review Board at National Jewish Health in Denver. All subjects gave written informed consent prior to participation in these studies. 2 mm punch skin biopsies were obtained from the upper arm or antecubital area. The skin biopsies were submerged in 1 mL of 10% buffered formalin for immunohistochemistry.
Viruses
The Wyeth/ACAM2000 strain of vaccinia virus was obtained from the Centers for Disease Control and Prevention (Atlanta, GA). HSV-1 (VR-733) was purchased from American Type Culture Collection (Manassas, VA).
KC culture
KCs were grown in serum-free EpiLife cell culture medium (Cascade Biologics, Portland, OR) as described previously (Kim et al., 2011). KCs were seeded in 24-well plate overnight and then treated with various cytokines, PCA and UCA or hydrochloric acid for 24 hours or 5 days, followed by incubated with HSV-1 (MOI, 0.1) or VV (MOI, 0.1) for 24 hours.
Organotypic skin culture
To produce organotypic skin culture using KCs, human dermal fibroblast (Invitrogen, Carlsbad, CA) and culture insert (BD Bioscience, Bedford, MA) were used. Human dermal fibroblasts were cultured in Medium 106 (Invitrogen). A mixture of fibroblasts and dermal collagen type I (Advanced BioMatrix, San Diego, CA) were used as a dermal equivalent. KCs were plated on the dermal equivalent and cultured for 5 days in the presence or absence of IL-25 for 5 days. Then the cells were incubated with VV (MOI, 0.1) for an additional 24 hours.
Genotypic analysis of filaggrin
DNAs were isolated from human skin biopsies and KCs using a Roche MagNA Pure Compact with Nucleic Acid Isolation Kit I (Roche, Indianapolis, IN). The DNAs were subjected to genotyping using Taqman MGB probes (Roche) on a Roche Light Cycler 480 instrument. The fluorescent signals are collected in both VIC and FAM emission wavelengths by the LightCycler 480 real-time PCR machine (Roche) when the TaqMan MGB probes hybridized to the complementary sequence are cleaved by Fast Start Taq DNA polymerase during extension.
RNA preparation and real time RT-PCR
RNeasy Mini Kits (Qiagen, Valencia, CA) were used according to the manufacturer’s protocol to isolate RNA from cell cultures. RNA was reverse transcribed into cDNA and analyzed by RT-PCR by using an ABI Prism 7000 sequence detector (Applied Biosystems, Foster City, CA), as described earlier (Nomura et al., 2003). Primers and probes for human 18S, FLG, HSV-1, VV, HBD-3 and LL-37 were purchased from Applied Biosystems.
Immunofluorescent staining
Paraffin-embedded tissues from human skins or organotypic skin sections were stained as described earlier (Kim et al., 2011). Slides were stained with a polyclonal rabbit anti-human IL-25 antibody (1:500 dilution, EMD Millipore, Billerica, MA) or a monoclonal mouse anti-A27L antibody direct against VV (1:500, Abcam, Cambridge, MA).
Western blot analysis
Proteins were separated on an SDS-polyacrylamide gel, 4–20% (Bio-Rad, Hercules, CA) and blotted. The blots were blocked and incubated with primary antibodies. Monoclonal antibodies against filaggrin (1:200 dilution, Vector Laboratories Inc.) and β-actin (1: 1,500 dilution, Sigma Chemical, St Louis, MO) were used as primary antibodies. The densities of profilaggrin and β-actin were measured using ImageJ, version 1.43 (National Institution of Health).
Small interfering RNA silencing experiment
FLG and the control siRNA duplexes were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The KCs were plated in 24 well plates the day before transfection. Five pmol of siRNA duplexes was transfected into each well of cells using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). The following day, the cells were switched to serum free EpiLife Medium containing 1.3 mM CaCl2 to differentiate. After differentiating for 5 days, the cells were then incubated with HSV-1 (MOI: 0.1) for 24 hours.
Viral plaque assays
Cells and culture supernatants were harvested together after 24 hours of viral incubation to assess the production of infectious viral particles. Cells were disrupted by 3 freeze-thaw cycles. Viral yields were determined by using a titration plaque assay, as previously described (Howell et al., 2006c).
Statistical analysis
Statistical analysis was conducted using Graph Pad Prism, version 4.03 (San Diego, CA). Statistical differences in gene expression or protein expression between multiple groups was determined by using one-way analysis of variance (ANOVA), and significant differences were determined by a Tukey-Kramer test. In cases where two groups were compared, data were analyzed using an unpaired T test.
Acknowledgments
The authors wish to thank Shih-Yun Lyman for her assistance in the preparation of this manuscript and also wish to acknowledge The Edelstein Family Foundation for their generous support of this work. This work was in part supported by Atopic Dermatitis Research Network contract HHSN272201000020C and R01 AR41256. This research was also supported by Colorado Clinical and Translational Sciences Institute (CCTSI), CCTSI is supported in part by Colorado CTSA Grant UL1RR025780 from NCRR/NIH.
Abbreviations
- AD
atopic dermatitis
- ADEH−
AD without a history of eczema herpeticum
- ADEH+
AD associated with a history of eczema herpeticum
- AMP
antimicrobial peptide
- FLG
filaggrin
- HBD
human beta defensin
- HSV
herpes simplex virus
- IFN
interferon
- IL
interleukin
- KC
keratinocyte
- LL-37
cathelicidin
- PCA
pyrrolidone carboxylic acid siRNA, Small interfering RNA
- TH2
T Helper type 2
- UCA
urocanic acid
- VV
vaccinia virus
Footnotes
CONFLICT OF INTERESTS
The authors state no conflict of interest
References
- Albanesi C, Fairchild HR, Madonna S, et al. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol. 2007;179:984–992. doi: 10.4049/jimmunol.179.2.984. [DOI] [PubMed] [Google Scholar]
- Barlow JL, Flynn RJ, Ballantyne SJ, et al. Reciprocal expression of IL-25 and IL-17A is important for allergic airways hyperreactivity. Clin Exp Allergy. 2011;41:1447– 1455. doi: 10.1111/j.1365-2222.2011.03806.x. [DOI] [PubMed] [Google Scholar]
- Beck LA, Boguniewicz M, Hata T, et al. Phenotype of atopic dermatitis subjects with a history of eczema herpeticum. J Allergy Clin Immunol. 2009;124:260–269. 269 e261–267. doi: 10.1016/j.jaci.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophers E, Henseler T. Contrasting disease patterns in psoriasis and atopic dermatitis. Arch Dermatol Res. 1987;279(Suppl):S48–51. doi: 10.1007/BF00585919. [DOI] [PubMed] [Google Scholar]
- Fort MM, Cheung J, Yen D, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2- associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- Gao PS, Rafaels NM, Hand T, et al. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J Allergy Clin Immunol. 2009;124:507–513. 513 e501–507. doi: 10.1016/j.jaci.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha RS, Oyoshi MK. Filaggrin Deficiency Impairs Viral Containment in Mice Cutaneously Inoculated with Vaccinia Virus (VV) J Allergy Clin Immunol. 2012;129:AB37. doi: 10.1016/j.jaci.2014.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–876. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell MD, Boguniewicz M, Pastore S, et al. Mechanism of HBD-3 deficiency in atopic dermatitis. Clin Immunol. 2006a;121:332–338. doi: 10.1016/j.clim.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Howell MD, Gallo RL, Boguniewicz M, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006b;24:341–348. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Howell MD, Wollenberg A, Gallo RL, et al. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol. 2006c;117:836–841. doi: 10.1016/j.jaci.2005.12.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell MD, Gao P, Kim BE, et al. The signal transducer and activator of transcription 6 gene (STAT6) increases the propensity of patients with atopic dermatitis toward disseminated viral skin infections. J Allergy Clin Immunol. 2011;128:1006–1014. doi: 10.1016/j.jaci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009;124:R7–R12. doi: 10.1016/j.jaci.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Howell MD, Streib JE, Leung DY. Antiviral activity of human beta-defensin 3 against vaccinia virus. J Allergy Clin Immunol. 2007;119:1022–1025. doi: 10.1016/j.jaci.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvid M, Vestergaard C, Kemp K, et al. IL-25 in atopic dermatitis: a possible link between inflammation and skin barrier dysfunction? J Invest Dermatol. 2011;131:150– 157. doi: 10.1038/jid.2010.277. [DOI] [PubMed] [Google Scholar]
- Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- Kezic S, O’Regan GM, Lutter R, et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J Allergy Clin Immunol. 2012;129:1031–1039. e1031. doi: 10.1016/j.jaci.2011.12.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BE, Howell MD, Guttman-Yassky E, et al. TNF-alpha downregulates filaggrin and loricrin through c-Jun N-terminal kinase: role for TNF-alpha antagonists to improve skin barrier. J Invest Dermatol. 2011;131:1272–1279. doi: 10.1038/jid.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BE, Leung DY. Epidermal barrier in atopic dermatitis. Allergy Asthma Immunol Res. 2012;4:12–16. doi: 10.4168/aair.2012.4.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BE, Leung DY, Boguniewicz M, et al. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126:332–337. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Ho WH, Maruoka M, et al. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem. 2001;276:1660–1664. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- Leung DY, Gao PS, Grigoryev DN, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-gamma response. J Allergy Clin Immunol. 2011;127:965–73. doi: 10.1016/j.jaci.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miajlovic H, Fallon PG, Irvine AD, et al. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J Allergy Clin Immunol. 2010;126:1184–1190. e1183. doi: 10.1016/j.jaci.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura I, Goleva E, Howell MD, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- O’Regan GM, Sandilands A, McLean WH, et al. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008;122:689–693. doi: 10.1016/j.jaci.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Rosenthal KS, Killius J, Hodnichak CM, et al. Mild acidic pH inhibition of the major pathway of herpes simplex virus entry into HEp-2 cells. J Gen Virol. 1989;70 (Pt 4):857–867. doi: 10.1099/0022-1317-70-4-857. [DOI] [PubMed] [Google Scholar]
- Sehra S, Yao Y, Howell MD, et al. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184:3186–3190. doi: 10.4049/jimmunol.0901860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T, Yu J, Oh MH, et al. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011;3:67–73. doi: 10.4168/aair.2011.3.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]