Abstract
Excessive consumption of unhealthy foods is a major public health problem. While many people attempt to control their food intake through dieting, many relapse to unhealthy eating habits within a few months. We have begun to study this clinical condition in rats by adapting the reinstatement model, which has been used extensively to study relapse to drug seeking. In our adaptation of the relapse model, reinstatement of palatable food seeking by exposure to food-pellet priming, food-associated cues, or stress is assessed in food-restricted (to mimic dieting) rats after operant food-pellet self-administration training and subsequent extinction of the food-reinforced responding.
In this review, we first outline the clinical problem and discuss a recent study in which we assessed the predictive validity of the reinstatement model for studying relapse to food seeking during dieting by using the anorexigenic drug fenfluramine. Next, we summarize results from our initial studies on the role of several stress- and feeding-related peptides (corticotropin-releasing factor, hypocretin, melanin- concentrating hormone, peptide YY3-36) in reinstatement of palatable food seeking. We then present results from our studies on the role of dopamine and medial prefrontal cortex in stress-induced reinstatement of food seeking. We conclude by discussing potential clinical implications.
We offer two main conclusions: (1) the food reinstatement model is a simple, reliable, and valid model to study mechanisms of relapse to palatable food seeking during dieting, and to identify medications to prevent this relapse; (2) mechanisms of relapse to food seeking are often dissociable from mechanisms of ongoing food intake.
Keywords: food, relapse, stress, priming, cue, reinstatement, dieting, self-administration
Introduction
Excessive consumption of unhealthy foods is a major public health problem and weight gain after dieting is associated with increased preference and consumption of such foods (McGuire et al., 1999; Torres and Nowson, 2007). While many people attempt to control their food intake through dieting, most of them relapse to the unhealthy eating habits within a few months (Kramer et al., 1989; Peterson and Mitchell, 1999; Skender et al., 1996). This relapse to unhealthy eating habits often occurs after acute exposure to palatable foods, food-associated cues, or stress (Grilo et al., 1989; Kayman et al., 1990; McGuire et al., 1999; Polivy and Herman, 1999; Torres and Nowson, 2007). Recently, we have begun to study this clinical situation by using a rat reinstatement model (Ghitza et al., 2006), which is commonly used to study relapse to abused drugs (See, 2002; Self and Nestler, 1998; Shaham et al., 2003).
In this model (Fig. 1), rats are given limited access to home-cage food (Box 1) and are trained to lever-press or nose-poke for 45 mg palatable food (Box 2) pellets. After extinction of the food-reinforced responding, rats are tested for reinstatement of food seeking induced by exposure to pellet priming (non-contingent pre-session delivery of several food pellets), food-associated cues (tone-light discrete cues that were previously paired with pellet delivery during training), or the pharmacological stressor yohimbine (Nair et al., 2009a). Yohimbine is a prototypical alpha-2 adrenoceptor antagonist that induces stress- and anxiety-like states in both laboratory animals and humans (Bremner et al., 1996a, b); yohimbine also induces heroin and alcohol craving in drug addicts (Stine et al., 2002; Umhau et al., 2011). Interestingly, as discussed in Box 3 both noradrenergic and non-noradrenergic components appear to contribute to yohimbine-induced reinstatement of food and drug seeking.
Figure 1. The adaptation of the drug reinstatement model to study relapse to palatable food seeking during dieting.
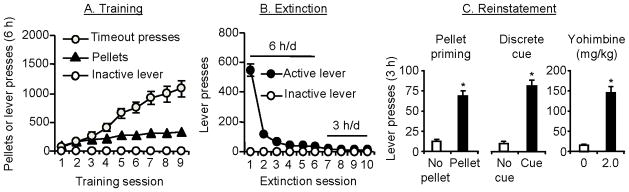
(A) Training phase. Rats are trained to lever press for palatable food pellets every other day under a fixed-ratio-1 (FR-1) 20 sec timeout reinforcement schedule for 9–10 sessions; pellet delivery is paired with the presentation of a discrete tone-light cues. Data are mean±sem number of pellets earned, timeout lever presses, and inactive lever presses during the training sessions. (B) Extinction phase. Lever presses are extinguished in the presence of tone–light cues (for pellet- and yohimbine-induced reinstatement) or without tone-light cues (for cue-induced reinstatement) until rats reach an extinction criterion. Data are mean±sem number of presses on the previously active lever or the inactive lever. (C) Reinstatement testing. Tests are conducted under extinction conditions. During tests for pellet-priming-induced reinstatement rats are given several (typically 4) non-contingent food pellets at the beginning of the test session. During tests for discrete cue-induced reinstatement, lever responding leads to contingent presentation of the tone-light cues. During tests for yohimbine-induced reinstatement, rats are injected with yohimbine (2 mg/kg, i.p.) 30–45 min prior to the start of the test sessions. Data are mean±sem number of presses on the previously active lever in the presence or absence of the reinstating stimuli. Data are based on results from Ghitza (2007). Note: the food reinstatement model has also been successfully used to study mechanisms of context-induced reinstatement of food seeking (Bossert et al., 2006; Hamlin et al., 2006).
Box 1. The food reinstatement model: some unresolved issues and future directions.
While the reinstatement model used to study relapse to food seeking is based on the reinstatement model of drug relapse, there are important differences to consider when relating the different versions of the model to their respective human conditions. The most critical difference relates to the nature of the rewards. Food is critical to sustain life, whereas drugs of abuse are not. Because of this simple fact, relapse is not identical in the two human conditions or in their respective model. Relapse to drug use has a clearly defined criterion, the resumption of drug use after a period of abstinence. In contrast, relapse to unhealthy food consumption after a period of dieting is less clearly defined, because dieting involves food restriction and not complete abstinence.
Dieting in the human condition has two main components, abstaining from consumption of unhealthy foods and consuming a limited amount of nutritionally balanced foods for sustenance. The relapse to palatable food seeking model attempts to capture these two aspects of dieting during the extinction and testing phases, which are described in detail below. But first we suggest that the model might account for the unhealthy eating phase that occurs before dieting.
Generally speaking, humans with unhealthy eating habits consume limited amounts of nutritionally balanced foods and greater amounts of calorically dense palatable foods. During the training phase of our model, rats are given limited access to nutritionally balanced (healthy) chow in the home-cage and intermittent access to palatable food pellets in the operant conditioning chambers. Notably, the rats consume similar amounts of food on training days (~16 g chow and 10–12 g palatable food pellets) as compared to their free-access daily intake (approximately 26–28 g/day) when only home-cage chow is available.
Subsequently, during the extinction and testing phases, the rats are food-restricted (receiving ~20 g of nutritionally balanced home-cage chow, which is ~75% of their normal daily intake) and no longer have access to the palatable pellets, mimicking the human dieting condition in which subjects not only restrict the amount of food they eat but also tend to consume nutritionally balanced foods. The level of restriction used in the model is on par with that used in human studies of caloric restriction (Stewart et al., 2013). As discussed in the review, there is some evidence across studies of a differential effect of pharmacological agents on reinstatement in food-restricted versus food-sated rats. In the future, a valuable approach would be to use a within-study design to examine whether stress-, cue-, or pellet-priming-induced reinstatement are mechanistically similar or different in food-restricted versus food-sated rats (Cason and Aston-Jones, 2013a, b). In our experience, such studies are less straightforward than one might assume, because the effect of both pellet priming and yohimbine on reinstatement is significantly weaker and more variable in food-sated versus food-restricted rats (unpublished observations).
Finally, the target population seeking treatment for relapse to unhealthy eating after dieting is primarily comprised of overweight and obese individuals. To date, however, the food reinstatement model has been primarily used to examine relapse to palatable food seeking in normal weight rats. Therefore, an important direction for future research would be to use the food reinstatement model in rats previously made overweight by exposure to a high-fat diet in the home-cage (Johnson and Kenny, 2010). Such studies would increase the clinical relevance of the model and would also allow investigators to study factors such as weight loss during dieting on relapse vulnerability.
Box 2. Palatable food in operant reinstatement studies.
A large body of literature, primarily derived from home-cage feeding studies, has demonstrated that rats prefer high-fat food over regular more nutritionally-balanced food (Dallman et al., 2003). Thus, in our initial studies we have used custom-made (BioServ) pellets with moderate to high percentage of fat (25–35%) (Ghitza et al., 2006; Ghitza et al., 2007; Nair et al., 2009b; Nair et al., 2008). Our assumption was that these pellets are the preferred food for the rats during the operant training phase. However, some rats in these studies did not show reliable pellet-priming-induced reinstatement. This observation prompted us to perform more formal preference tests, using different pellet types (obtained from TestDiet and Bioserv) with different compositions of fat (0 to 35%) and carbohydrate (45% to 91% [sugar pellets]) and different flavors (no flavor, banana, chocolate, grape) (Pickens et al., 2012). The ‘winner’ in these preference tests was 45-mg food pellets that contain 12.7% fat, 66.7% carbohydrate and 20.6% protein (Catalogue # 1811155, TestDiet). Therefore, in our recent published studies (Calu et al., 2013; Cifani et al., 2012; Pickens et al., 2012) and our ongoing studies we have been using this pellet type.
Box 3. Do noradrenaline and alpha-2 adrenoceptors play a role in yohimbine-induced reinstatement?
Yohimbine has been used for many years in the stress/anxiety field as a pharmacological stressor whose presumed stress-related mechanism of action is blockade of presynaptic alpha-2 adrenoceptors in noradrenergic cell body regions (locus coeruleus, lateral tegmental nuclei), resulting in increased brain noradrenaline cell firing and release in terminal areas (Abercrombie et al., 1988; Aghajanian and VanderMaelen, 1982; Bremner et al., 1996a, b). Yohimbine, however, also binds at varying degrees of affinity to D2 dopamine receptors (Scatton et al., 1980), alpha-1 adrenoceptors (Doxey et al., 1984), benzodiazepine binding sites (Matsunaga et al., 2001), and 5-HT1A receptors (Winter and Rabin, 1992). Nevertheless, based on the stress/anxiety literature, and the findings that the prototypical alpha-2 adrenoceptor agonist clonidine and related agonists (lofexidine, guanbenz) potently inhibit intermittent-footshock-induced reinstatement of drug seeking (Erb et al., 2000; Le et al., 2005; Shaham et al., 2000b), our working hypothesis was that yohimbine-induced reinstatement of food or drug seeking is mediated by its action on alpha-2 adrenoceptors and noradrenergic transmission (Ghitza et al., 2006; Shepard et al., 2004). As discussed below, empirical evidence for this notion is very mixed.
We found little evidence for a role of alpha-2 adrenoceptors in yohimbine-induced reinstatement of food seeking (Fig. 3 in Nair et al. (2009a): yohimbine’s effect on reinstatement was neither decreased by clonidine nor mimicked by the selective alpha-2 adrenoceptor antagonist RS 79948 (Milligan et al., 1997). We also found that the alpha-2 adrenoceptor agonist guanfacine has no effect on yohimbine-induced reinstatement of food seeking (Le et al., 2011). On the other hand, a noradrenergic component of yohimbine’s effect on reinstatement of food seeking is suggested by our finding that the selective alpha-1 postsynaptic adrenoceptor antagonist prazosin blocks yohimbine-induced reinstatement at doses that have no effect on ongoing food self-administration (Le et al., 2011) (Fig. 3B). Prazosin also blocked both yohimbine- and footshock-induced reinstatement of alcohol seeking (Le et al., 2011).
The evidence for a role of alpha-2 adrenoceptors and noradrenaline transmission in yohimbine-induced reinstatement of drug seeking is also mixed. Clonidine decreases yohimbine-induced reinstatement of alcohol seeking in rats (Le et al., 2009) and cocaine seeking in monkeys (Lee et al., 2004). However, clonidine has no effect on yohimbine-induced reinstatement of cocaine seeking in rats (Brown et al., 2009) or yohimbine-induced reinstatement of cocaine CPP in mice (Mantsch et al., 2010). Additionally, 6-hydroxydopamine lesions of ventral or dorsal noradrenergic bundles have no effect on yohimbine-induced reinstatement of alcohol seeking in rats (Le et al., 2009). Furthermore, yohimbine’s effect on reinstatement of alcohol seeking in rats is not mimicked by RS79948 (Le et al., 2009). On the other hand, yohimbine’s effect on reinstatement in monkeys is mimicked by RS79948 (Lee et al., 2004); additionally, the selective alpha-2 adrenoceptor antagonist BRL44408 reinstates cocaine CPP in mice (Mantsch et al., 2010). Finally, the postsynaptic beta-adrenoceptor antagonist propranolol decreases yohimbine-induced reinstatement of cocaine CPP in mice (Mantsch et al., 2010).
Taken together, the observations that postynaptic alpha1- and beta-adrenoceptor antagonists block yohimbine-induced reinstatement of food and drug seeking (Le et al., 2011; Mantsch et al., 2010) suggest an important role of noradrenaline in this reinstatement. Surprisingly, however, it appears that alpha-2 adrenoceptors are not involved in yohimbine’s effect of reinstatement of food seeking, and evidence for a role of these receptors in yohimbine-induced reinstatment of drug seeking is mixed.
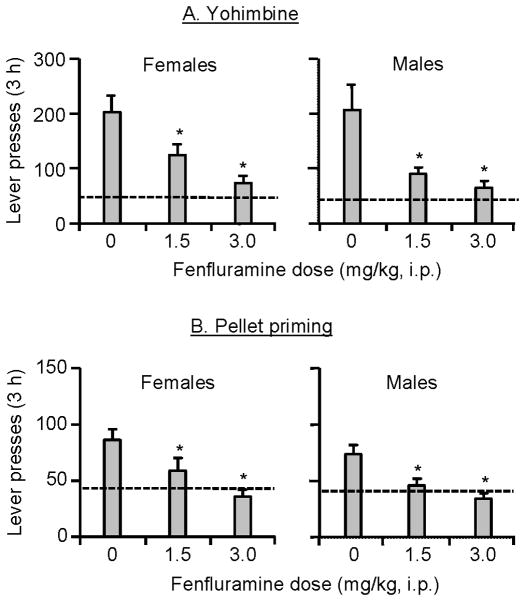
The use of the reinstatement procedure to model relapse to unhealthy eating habits during dieting has posed an important question: is this a valid model for the human situation? This question is not unique to food reinstatement studies; the validity of the reinstatement model to assess drug relapse has been a focus of intense debate in the addiction field (Epstein and Preston, 2003; Epstein et al., 2006b; Katz and Higgins, 2003). The demonstration of predictive validity, or the ability of an animal model to identify drugs with potential therapeutic value, is considered to be important criterion in determining the validity of an animal model (Markou et al., 1993; Willner, 1984). In this regard, we recently demonstrated that fenfluramine, a serotonin releaser known to have anorectic effects in both humans and laboratory animals (Rothman and Baumann, 2002; Rowland and Charlton, 1985), decreases both yohimbine- and pellet-priming-induced reinstatement of food seeking in male and female rats (Fig. 2) (Pickens et al., 2012). This finding supports the predictive validity (or more accurately the ‘postdictive validity’ as human results preceded the laboratory animal results) of the reinstatement model to study relapse to food seeking during dieting. In addition to the predictive validity of the reinstatement model, the model’s experimental procedures are simple, and more importantly, reinstatement is reliably induced by pellet priming, food-associated cues, or the pharmacological stressor yohimbine (Nair et al., 2009a). Thus, the reinstatement model can serve as a useful animal model to study mechanisms of relapse to palatable food seeking during dieting, and to identify novel medications for prevention of this relapse.
Figure 2. Demonstration of the predictive validity of the reinstatement model: effect of fenfluramine on yohimbine- and pellet-priming-induced reinstatement of food seeking in female and male rats.
Data are mean±sem number of active lever presses during the reinstatement tests after (A) yohimbine injections or (B) pellet priming; rats were pretreated with vehicle and fenfluramine before the reinstatement tests. Dashed arrows: represent baseline extinction responding in the fenfluramine vehicle condition in the absence of the reinstating stimuli. * Different from the fenfluramine vehicle condition, p<0.05. Data are based results from Pickens et al. (2012).
In the following sections, we first summarize results from our studies on the role of several stress- and feeding-related peptides, including corticotropin-releasing factor (CRF), hypocretin/orexin, melanin-concentrating hormone (MCH), and peptide YY3-36 (PYY3-36). We then present recent studies that implicate dopamine and dorsal medial prefrontal cortex (mPFC) in stress-induced reinstatement of food seeking. We conclude by discussing potential clinical implications of our results. Table 1 provides a summary of results from our neuropharmacological and optogenetic studies*. Unless otherwise noted, the rats in the studies reviewed are food restricted in their home-cage (see Box 1 for a discussion on the degree to which food restriction in the model mimics dieting in humans).
Table 1.
Effect of pharmacological agents or brain manipulations on ongoing food self-administration and reinstatement.
| Peptide receptor, agonist/antagonist or brain manipulation | Food self-administration | Reinstatement of food seeking | Reference | ||
|---|---|---|---|---|---|
| Pellet priming | Food cues | Yohimbine | |||
|
CRF1 receptor antagonist Antalarmin (20, 40 mg/kg) |
— | — | ↓ | (Ghitza et al., 2006) | |
|
Hypocretin 1 receptor antagonist SB 334867 (10, 20 mg/kg) |
↓ | — | — | — | (Nair et al., 2008) |
|
MCH 1 receptor antagonist SNAP 94847 (10–30 mg/kg) |
↓ | — | — | — | (Nair et al., 2009b) |
|
Y2 receptor agonist PYY3-36 (0.1, 0.2 mg/kg) |
— | ↓ | ↓ | — | (Ghitza et al., 2007) |
|
Alpha-1 adrenoceptor antagonist Prazosin (2 mg/kg) |
— | ↓ | (Le et al., 2011) | ||
|
5-HT releaser Fenfluramine (0.75–3.0 mg/kg) |
↓ | ↓ | ↓ | (Pickens et al., 2012) | |
|
D1 receptor antagonist SCH 23390 (5, 10 μg/kg, SC) |
— | ↓ | ↓ | (Nair et al., 2009a; Nair et al., 2011) | |
| SCH 23390 (0.5, 1 μg/side, dorsal mPFC) | — | ↓ | — | ↓ | |
| Optogenetic inhibition of dorsal mPFC | — | — | ↓ | (Calu et al., 2013) | |
Abbreviations/symbols: ↓, decrease; ↑, increase; -, no effect; blank space, not tested.
Role of stress- and feeding-related peptides
In four pharmacological studies we assessed the role of CRF, PYY3-36, hypocretin, and MCH in reinstatement of food seeking. We sought to examine these peptides based on their involvement in stress- and feeding-related behaviors. For each of these peptides, we first provide background information and then describe our findings related to the role of each peptide in reinstatement of palatable food seeking.
Study 1: CRF (Ghitza et al., 2006)
CRF is a 41 amino-acid peptide that binds to CRF1 and CRF2 receptors (Chang et al., 1993; Lovenberg et al., 1995). The peptide CRF, which was first isolated from the hypothalamus (Vale et al., 1981), plays a critical role in the regulation of endocrine and behavioral responses to stress (Bale and Vale, 2004).
In an initial study we determined the effect of the selective CRF1 receptor antagonist antalarmin (Bornstein et al., 1998) on yohimbine-induced reinstatement of food seeking (Ghitza et al., 2006). This study followed previous findings on the effect of antalarmin on intermittent footshock-induced reinstatement of heroin, cocaine, and alcohol seeking (Le et al., 2000; Shaham et al., 1998). We have used yohimbine instead of intermittent footshock, the more commonly used stressor in drug reinstatement studies (Lu et al., 2003; Shaham et al., 2000a), because previous studies demonstrate that intermittent footshock is not an effective stimulus for reinstatement of food (regular food or sucrose) seeking (Ahmed and Koob, 1997; Buczek et al., 1999). Additionally, in a direct comparison between intermittent footshock versus yohimbine in rats with a history of methamphetamine or alcohol self-administration, yohimbine induced more reliable and stronger effect on reinstatement of drug seeking than footshock (Le et al., 2005; Shepard et al., 2004).
We found that systemic injections of antalarmin decrease yohimbine-induced reinstatement of palatable food seeking (Ghitza et al., 2006) (Fig. 3a). This effect was not due to motor deficits, because antalarmin had no effect on pellet-priming-induced reinstatement or ongoing food-reinforced lever pressing (Table 1). It is likely that antalarmin’s effect on yohimbine-induced reinstatement of food seeking is mediated by CRF1 receptors located at extrahypothalamic brain sites (Van Pett et al., 2000). In a subsequent study with alcohol-trained rats, antalarmin decreased yohimbine-induced reinstatement but had no effect on yohimbine-induced elevation of plasma corticosterone, an index of hypothalamic-pituitary adrenal (HPA) axis activation (Dallman et al., 1995; Selye, 1976). This conclusion is in agreement with results from many studies indicating that intermittent footshock-induced reinstatement of drug seeking is mediated by extrahypothalamic brain sites (Shalev et al., 2010). The brain sites involved in the inhibitory effect of antalarmin on yohimbine-induced reinstatement of food seeking are unknown.
Figure 3. Effect of a CRF1 receptor antagonist and an alpha-1 adrenoceptor antagonist on yohimbine-induced reinstatement of food seeking.

Data are mean±sem number of active lever presses during the tests for vehicle- or yohimbine-induced reinstatement tests after pretreatment with (A) antalarmin and (B) prazosin. * Different from the vehicle condition, p <0.05. Data are based results from Ghitza et al. (2006) and Le et al. (2011).
Finally, an important observation in our study was that yohimbine (at a dose that robustly reinstates food seeking; 2 mg/kg, see figures) induces anxiety/stress-like responses in the social interaction test (File and Seth, 2003), an effect that was reversed by antalarmin pretreatment (Ghitza et al., 2006). These findings suggest that, under our experimental conditions, yohimbine induces a CRF-dependent stress state.
Study 2: PYY3-36 (Ghitza et al., 2007)
PYY3-36, the predominant circulatory derivative of Peptide YY, is a gastrointestinal hormone released from intestinal L-cells in response to food intake (Adrian et al., 1985; Eberlein et al., 1989). PYY3-36 binds to receptors of neuropeptide Y, an orexigenic peptide expressed in the hypothalamus (Leibowitz, 1995), with high affinity for presynaptic inhibitory Y2 receptors and low affinity for postsynaptic excitatory Y1 and Y5 receptors (Ballantyne, 2006; Grandt et al., 1992).
Our study was inspired by several papers on the effect of PYY3-36 on food intake in laboratory animals and humans (Batterham et al., 2003; Batterham et al., 2002; Batterham et al., 2007). We found that systemic injections of PYY3-36 decrease pellet-priming- and cue-induced reinstatement of food seeking but not yohimbine-induced reinstatement. Contrary to our expectation (based on the studies of Batterham and colleagues), PYY3-36 injections had no effect on ongoing food self-administration. The inhibitory effect of PYY3-36 on pellet-priming-induced reinstatement was blocked by systemic injections of the Y2 receptor antagonist BIIE0246, suggesting that Y2 receptors mediate this effect. The brain sites underlying the Y2-receptor mediated effect of PYY3-36 remain unclear, because while PYY3-36 injections into the arcuate nucleus of the hypothalamus decreased pellet-priming-induced reinstatement, these injections had no effect on cue-induced reinstatement. Additionally, arcuate nucleus BIIE0246 injections did not reverse the inhibitory effect of systemic PYY3-36 on pellet-priming-induced reinstatement (Ghitza et al., 2006). Finally, systemic PYY3-36 injections had no effect on drug-priming- or cue-induced reinstatement of heroin seeking, suggesting a selective effect of PYY3-36 on reinstatement of food seeking.
Study 3: Hypocretin (Nair et al., 2008)
Hypocretin 1 and 2 are neuropeptides synthesized by neurons of the lateral hypothalamus and perifornical area (de Lecea et al., 1998; Sakurai et al., 1998) that project to many brain areas (Peyron et al., 1998). Hypocretin 1 has similar affinities for hypocretin 1 and 2 receptors while hypocretin 2 has greater affinity for the hypocretin 2 receptor (Smart et al., 2000; Smart et al., 1999). Hypocretins are well known for their role in arousal and feeding (de Lecea et al., 1998; Sakurai et al., 1998).
The inspiration for our study came from two influential studies in the addiction field on the role of hypocretins in morphine reward and reinstatement of morphine conditioned place preference (CPP) (Harris et al., 2005), and intermittent-footshock-induced reinstatement of operant cocaine seeking (Boutrel et al., 2005). However, in our studies we have found little evidence for a role of hypocretins in reinstatement of food seeking during dieting (Nair et al., 2008). While ventricular injections of hypocretin 1 reinstated food seeking after extinction (extending Boutrel et al. (2005) results with cocaine), systemic injections of the hypocretin 1 receptor antagonist SB 334867 had no effect on pellet-priming, cue-, and yohimbine-induced reinstatement. In contrast, SB 334867 injections decreased ongoing food self-administration.
However, results from two other studies suggest a role for hypocretin 1 receptors in reinstatement of food seeking. Richards et al. (2008) reported that in food-sated rats trained to self-administer 5% sucrose solution, systemic injections of SB 334867 decrease yohimbine-induced reinstatement of sucrose seeking. Cason and Aston-Jones (Cason and Aston-Jones, 2013b) reported that in food-restricted rats, and to a lesser degree in food-sated rats, systemic SB 334867 injections decrease cue-induced reinstatement of sucrose pellet seeking.
The discrepancy between these studies may be attributable to several methodological differences between the experimental procedures used. One possibility is the difference in non-operant (home-cage) feeding conditions. For example, in the Richards et al. (2008) study, rats had free access to regular chow, while rats in our study (Nair et al., 2008) were restricted to ~65% of their normal daily home-cage regular chow intake. However, this methodological difference cannot account for the finding of Cason and Aston-Jones (2013b) that SB 334867 injections decrease cue-induced reinstatement of sucrose seeking in food-restricted but not food-sated rats. Another potential reason for the discrepant findings is the maximal dose of SB 334867 used in the different studies. The positive results from Cason and Aston-Jones (2013b) are primarily based on the finding with a dose of 30 mg/kg, while in our study the maximal dose was 20 mg/kg (Nair et al., 2008). However, the maximal SB 334867 dose possibility cannot explain Richards et al. (2008) results where yohimbine-induced reinstatement was blocked by lower SB 334867 doses (5 or 10 mg/kg). Finally, a potential reason for the conflicting findings is that the hypocretin system may be sensitive to the nutritive content of the food rewards used in the different studies: Richards et al. (2008) and Cason and Aston-Jones (2013b) used sucrose (solution or pellets), while we used 35% high-fat pellets (Nair et al., 2008).
In summary, it appears that the role of hypocretin 1 receptors in reinstatement of food seeking is dependent on the type of food used during the training phase. Based on the fact that hypocretin 1, which reliably reinstates both food and drug seeking (Boutrel et al., 2005; Nair et al., 2008), has similar affinity to hypocretin 1 and 2 receptors, a question for future research is the role of hypocretin 2 receptors in reinstatement of food seeking. This is an interesting question in light of evidence for a role of this receptor in food and drug reward (Funato et al., 2009; Malherbe et al., 2009; Tabaeizadeh et al., 2013; Zhang et al., 2007), and drug-priming-induced reinstatement of alcohol CPP in mice (Shoblock et al., 2011).
Study 4: MCH (Nair et al., 2009a)
MCH is a 19 amino-acid cyclic peptide produced by neurons in the lateral hypothalamus, perifornical region, and nearby zona incerta (Bittencourt et al., 1992; Nahon et al., 1989). This peptide binds to MCH1 (the only functional receptor in rodents) and MCH2 receptors (Chambers et al., 1999; Saito et al., 2001), and is an important regulator of feeding and energy balance (Qu et al., 1996; Shimada et al., 1998).
Our study on the role of the MCH system in reinstatement of food seeking was inspired by the finding that nucleus accumbens MCH1 receptors play an important role in food-taking behavior (Georgescu et al., 2005). This finding is potentially relevant to reinstatement of food seeking, because the nucleus accumbens plays a critical role in reinstatement of drug seeking induced by drug priming, drug cues, and stress (Crombag et al., 2008; Kalivas and McFarland, 2003; See, 2005; Shaham et al., 2003), as well as cue-induced food seeking (Floresco et al., 2008). We were also interested in the particular role of MCH1 receptors in yohimbine-induced reinstatement, because systemic injections of the MCH1 receptor antagonist SNAP 94847 (the drug used in our study) decrease behavioral and physiological stress responses (David et al., 2007; Smith et al., 2009).
The pattern of results we have obtained with MCH and the MCH1 receptor antagonist SNAP 94847 was very similar to our results with hypocretin 1 and the selective hypocretin 1 receptor antagonist SB 334867. Specifically, while ventricular injections of MCH reinstated food seeking and systemic injections of SNAP 94847 decreased food self-administration, the MCH1 receptor antagonist had no effect on pellet-priming-, cue-, and stress-induced reinstatement of food seeking (Nair et al., 2009b). In agreement with these findings, Mul et al. (2011) reported that knockdown of the rat MCH-precursor Pmch (resulting in a MCH-deficient rat) decreases high-fat pellet (45% fat) self-administration on both fixed-ratio and progressive ratio reinforcement schedules, but has no effect on pellet-priming, cue-, or yohimbine-induced reinstatement of food seeking in food-restricted rats. However, contrary to our findings and the findings of Mul et al. (2011), Karlsson et al. (2012) recently found that systemic injections of a different MCH1 receptor antagonist, GW803430, decrease cue-induced reinstatement of sucrose seeking in rats given free access to food in their home-cage. Methodological differences related to the use of different MCH1 receptor antagonists (SNAP 94847 versus GW803430), different types of food reward (high-fat pellet versus sucrose), or the feeding conditions (food-restricted versus food-sated) may account for these different results.
Conclusions
The results from the studies described above have advanced our understanding of mechanisms of reinstatement of food seeking. But perhaps more importantly, these results, and those described in the section below, lead to two tentative general conclusions: (1) the mechanisms of pellet-priming- and cue-induced reinstatement are largely dissociable from those of stress-induced reinstatement, and (2) the mechanisms of reinstatement of food seeking are largely dissociable from those underlying ongoing food self-administration (Table 1).
The first conclusion is supported by the finding that the CRF1 receptor antagonist antalarmin decreases yohimbine-induced reinstatement but not pellet-priming-induced reinstatement, while PYY3-36 attenuates food-priming- and cue-induced reinstatement, but not yohimbine-induced reinstatement (Ghitza et al., 2006; Ghitza et al., 2007). The second conclusion is supported by the finding that the hypocretin 1 receptor antagonist SB 334867 and the MCH1 receptor antagonist SNAP 94847 decrease food self-administration but have no effect on food-priming- or cue-induced reinstatement. Additionally, PYY3-36 attenuates food-priming- and cue-induced reinstatement, but has no effect on food self-administration (Ghitza et al., 2007; Nair et al., 2009b; Nair et al., 2008).
Finally, an unexpected conclusion based on our studies, and other studies reviewed above, is that the mechanism of a given reinstating stimulus (e.g., food cues, yohimbine) might differ depending on the food type used and/or the non-operant basal feeding conditions (restricted feeding versus freely available food). Thus, the hypocretin 1 receptor antagonist SB 334867 decreases yohimbine-induced reinstatement in food-sated rats trained to self-administer sucrose, but not in food-restricted rats trained to self-administer high-fat food pellets (Nair et al., 2008; Richards et al., 2008). Additionally, pharmacological antagonism of MCH1 receptors decreases cue-induced reinstatement in food-sated rats trained to self-administer sucrose, but not in food-restricted rats trained to self-administer high-fat food pellets (Karlsson et al., 2012; Nair et al., 2009b). Finally, another example in the literature is that the cannabinoid CB1 receptor antagonist rimonabant decreases cue-induced reinstatement after a history of sucrose or Ensure, but not corn-oil, self-administration (De Vries et al., 2005; Ward et al., 2007).
Role of dopamine and medial prefrontal cortex (mPFC)
The mPFC and dopamine transmission in this brain area play a critical role in reinstatement of drug seeking induced by drug priming, different types of cues (discrete, discriminative, contextual), and stress (Bossert et al., 2013; Kalivas and McFarland, 2003; See, 2005). This literature, along with a preliminary study in which we found that low doses of the D1-family receptor antagonist SCH 23390 (0.005 and 0.01 mg/kg), which have no effect on food self-administration, decrease yohimbine- and pellet-priming-induced reinstatement [see Fig. 2 in (Nair et al., 2009a)], have inspired three recent studies in which we studied the role of mPFC and local dopamine transmission in reinstatement of food seeking. Our emphasis was on yohimbine-induced reinstatement in these three studies, because dorsal mPFC injections of SCH 23390 or the mixed D1/D2 receptor antagonist fluphenazine decrease footshock-stress-induced reinstatement of cocaine seeking (Capriles et al., 2003; McFarland et al., 2004) and immobilization stress-induced reinstatement of cocaine CPP (Sanchez et al., 2003). Additionally, stressors preferentially activate the mesocortical dopaminergic projection from ventral tegmental area (VTA) to mPFC (Deutch and Roth, 1990; Thierry et al., 1976). Finally, yohimbine increases mPFC extracellular dopamine levels (Tanda et al., 1996) and expression of the immediate early gene c-fos [a neuronal activity marker, (Morgan and Curran, 1991)] (Bing et al., 1992; Funk et al., 2006; Singewald et al., 2003).
We have used male rats in the first study (Nair et al., 2011) and female rats in the two subsequent studies (Calu et al., 2013; Cifani et al., 2012). We have expanded our studies to female rats, as well as to the role of ovarian hormones in reinstatement of food seeking, because the proportion of women who use dietary supplements and seek dietary treatment is more than twice that of men (Davy et al., 2006; Pillitteri et al., 2008). Additionally, there is evidence for both sex differences and for a role of ovarian hormones in food intake (Asarian and Geary, 2006; Nance, 1983) and reinstatement of drug seeking (Becker and Hu, 2008; Carroll et al., 2004).
Study 1: Role of mPFC dopamine in stress-induced reinstatement (Nair et al., 2011)
In this study, we first replicated our previous preliminary observation that systemic injections of low doses of SCH 23390 block yohimbine-induced reinstatement of food seeking (Fig. 4A). We then assessed Fos immunoreactivity in the dorsal and ventral mPFC of rats injected with vehicle or SCH 23390 prior to yohimibine-induced reinstatment. We found that yohimbine causes a strong increase in Fos expression in dorsal mPFC (Fig. 4B) and a relatively modest increase in ventral mPFC (Nair et al., 2011). We followed up on these findings by demonstrating that SCH 23390 injections into dorsal (Fig. 4C) but not ventral mPFC decreases yohimbine-induced reinstatement. In subsequent experiments, we found that dorsal mPFC SCH 23390 injections modestly decrease pellet-priming-induced reinstatement but znot cue-induced reinstatement or ongoing food self-administration; the modest effect of SCH 23390 on pellet-priming-induced reinstatement is in agreement with results from a previous study (Sun and Rebec, 2005).
Figure 4. Dorsal mPFC dopamine D1-family receptors mediate yohimbine-induced reinstatement of food seeking.
(A) Systemic SCH23390 (0.01 mg/kg) injections block yohimbine-induced reinstatement; data are mean±sem number of active lever presses during the reinstatement tests. (B) Systemic SCH23390 (0.01 mg/kg) injections block yohimbine-induced Fos expression in dorsal mPFC; data are mean±sem Fos immunoreactive neurons per mm2. (C) Dorsal mPFC SCH23390 injections (0.5 or 1.0 μg/side) decrease yohimbine-induced reinstatement; data are mean±sem number of active lever presses during the reinstatement tests. * Different from the SCH 23390 vehicle condition, p <0.05. Data are based on results from Nair et al. (2011).
SCH 23390 also binds to 5-HT2C (formerly 5-HT1C) and 5-HT2A receptors where it acts as an agonist (Briggs et al., 1991; Millan et al., 2001) and antagonist (Monti et al., 1990; Neumeyer et al., 2003; Porter et al., 2000; Schreiber et al., 1995), respectively. Therefore, we assessed the effect of dorsal mPFC injections of the 5-HT2C receptor agonist MK212 (Ramos et al., 2005) and the 5-HT2A receptor antagonist M100907 (McMahon et al., 2001) on yohimbine-induced reinstatement of food seeking. These 5-HT ligands had no effect on this reinstatement, indicating that the effect of dorsal mPFC SCH 23390 injections on yohimbine-induced reinstatement are mediated by local D1-family receptors.
Taken together, our study established a critical role of dorsal but not ventral mPFC dopamine in yohimbine-induced reinstatement of food seeking, extending previous results on the critical role of this brain area in stress-induced reinstatement of drug seeking (Kalivas and McFarland, 2003; Shaham et al., 2003). The pattern of effects of dorsal mPFC SCH 23390 injections, reliable inhibition of yohimbine-induced reinstatement, modest effect on pellet-priming-induced reinstatement, and no effect on cue-induced reinstatement or ongoing food self-administration (Table 1), support our conclusions of dissociable or partially dissociable mechanisms of reinstatement induced by stress versus pellet priming and cues, and reinstatement of food seeking versus ongoing food self-administration.
Study 2: mPFC activation and synaptic alterations after stress-induced reinstatement: a study using c-fos-GFP transgenic female rats (Cifani et al., 2012)
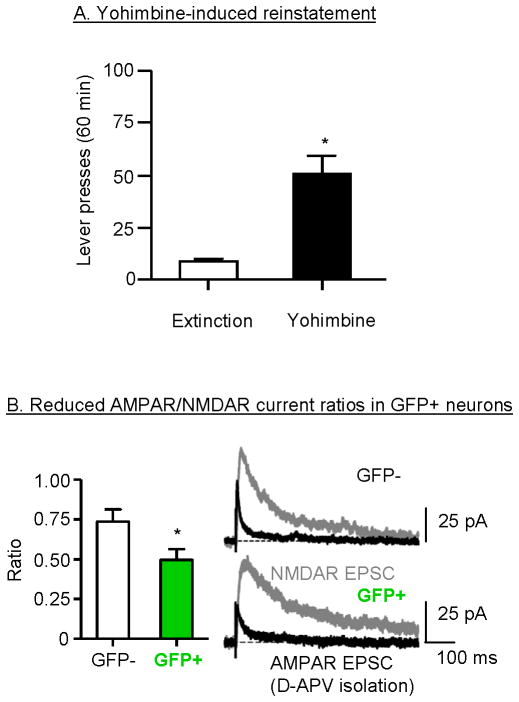
In this study we addressed three questions. The first was whether yohimbine-induced reinstatement of food seeking is associated with mPFC activation (as assessed by Fos expression) in female rats. The second was whether this reinstatement, as well as pellet-priming-induced reinstatement, is influenced by ovarian hormone fluctuations. The third was whether yohimbine-induced reinstatement is associated unique synaptic plasticity changes in mPFC activated (Fos-positive) versus non-activated (Fos-negative) neurons. For this purpose, we used a novel c-fos-GFP transgenic rat developed by Drs. Bruce Hope, Brandon Harvey (NIDA), and James Pickel (NIMH) (Cifani et al., 2012). The c-fos-GFP rat contains a transgene with the c-fos promoter (Morgan and Curran, 1991) that induces rapid transcription of the gfp (green fluorescent protein) coding sequence in response to strong neuronal activation (Barth et al., 2004). Strongly activated Fos neurons in these rats can be identified by their GFP expression in electrophysiological slice preparations to compare synaptic properties of activated (GFP-positive) neurons and nearby non-activated (GFP-negative) neurons.
The results of our study demonstrate that yohimbine- and pellet-priming-induced reinstatement in female rats is associated with increased Fos (and GFP) expression in both ventral and dorsal mPFC, confirming our findings with male rats (Nair et al., 2011). Of note, the effect of yohimbine on mPFC activation was substantially stronger than that of pellet priming. Additionally, yohimbine-induced reinstatement (Fig. 5A) was associated with reduced AMPA receptor/NMDA receptor current ratios (Fig 5B) and increased paired-pulse facilitation in activated GFP-positive but not in GFP-negative neurons (Cifani et al., 2012). These findings indicate reduced synaptic glutamate transmission in the activated neurons, possibly reflecting a delayed compensatory response to strong initial neuronal activation during the reinstatement tests, which occurs several hours after testing (the time of electrophysiological assessment).
Figure 5. Yohimbine-induced reinstatement is associated with glutamatergic plasticity in activated (GFP positive) neurons in dorsal mPFC.
(A) Yohimbine (2 mg/kg)-induced reinstatement of food seeking; data are mean±sem number of active lever presses during the reinstatement tests. * Different from baseline extinction condition, p<0.05. (B) Reduced AMPA/NMDA current ratios in GFP-positive but not GFP-negative neurons after yohimbine-induced reinstatement. Mean±sem of AMPAR/NMDAR current ratios were determined using an NMDA receptor antagonist (D-APV) application procedure. Example traces show AMPAR (black) and NMDAR-mediated EPSCs (gray) in GFP+ and GFP- dorsal mPFC neurons. * Different from the GFP-negative condition, p<0.05. Data are based on results from Cifani et al. (2012).
We also found little evidence for a role of ovarian hormones in yohimbine- or pellet-priming-induced reinstatement and mPFC activation. Ovariectomy (to eliminate circulating estrogen and progesterone) had no effect on yohimbine-induced reinstatement and mPFC Fos expression; yohimbine-induced reinstatement was also not affected by the estrous cycle phase. For pellet-priming-induced reinstatement, ovariectomy modestly decreased this reinstatement but in a follow-up experiment, pellet-priming-induced reinstatement was not affected by the estrous cycle phase. These largely negative findings are consistent with the finding from our fenfluramine study (Pickens et al., 2012), in which we did not observe sex differences in the magnitude of yohimbine- and pellet-priming-induced reinstatement of food seeking.
Taken together, the results of our study indicate that ovarian hormones do not appear to play a role in stress-induced reinstatement of food seeking and that this reinstatement is associated with unique glutamatergic synaptic alterations in strongly activated mPFC neurons. The functional significance of reduced glutamatergic plasticity in activated neurons after stress-induced reinstatement of food seeking requires further investigation using tools (that are yet to be developed) to selectively manipulate synaptic plasticity events in GFP-positive (activated) neurons but not in GFP-negative (non-activated) neurons.
Finally, based on data regarding sex differences and the role of ovarian hormones in reinstatement of drug seeking (Carroll et al., 2004), including yohimbine-induced reinstatement of cocaine seeking (Anker and Carroll, 2010; Feltenstein et al., 2011), it is somewhat surprising that neither sex differences (Pickens et al., 2012) nor a significant role of ovarian hormones (Cifani et al., 2012) in reinstatement of food seeking have been demonstrated in our studies. In reconciling our data with the drug reinstatement results, it should be noted that conclusions regarding sex differences and the role of ovarian hormones in reinstatement of drug seeking are primarily based on studies using cocaine. There are differences between the mechanisms of drug reward and relapse/reinstatement across drug classes (Badiani et al., 2011; Bossert et al., 2013). Relevant to our negative findings are data demonstrating that manipulations of ovarian hormones have no effect on heroin self-admistration (Stewart et al., 1996) or footshock-stress-induced reinstatement of heroin seeking (Shaham and Stewart, 1995). Additionally, Feltenstein et al. (2011) recently demonstrated that the estrous cycle phase has no effect on yohimbine-induced reinstatement of nicotine seeking. A tentative conclusion from the above studies is that while ovarian hormones play an important role in reinstatement of cocaine seeking, they likely play a minimal role in reinstatement of reward seeking for other drugs and palatable food.
Study 3: Effect of optogenetic inhibition of mPFC on stress-induced reinstatement (Calu et al., 2013)
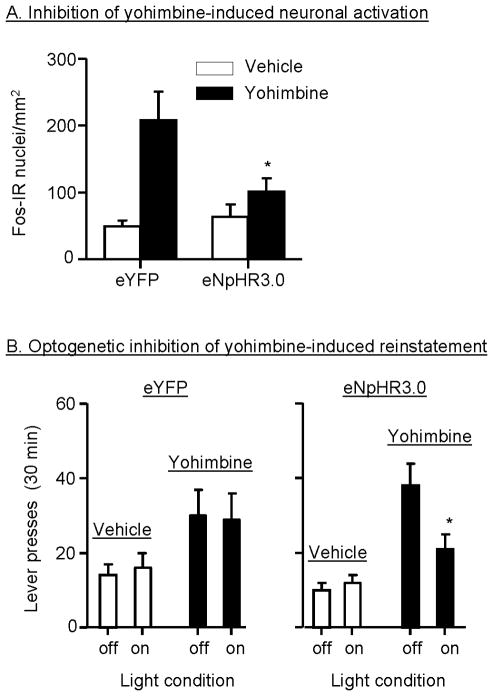
In our most recent study, we have used an optogenetic approach to demonstrate that neuronal activity in the dorsal mPFC mediates yohimbine-induced reinstatement of food seeking in female rats (Calu et al., 2013). In this study, we employed a neuronal silencing strategy in which we used adeno-associated virus (AAV)-mediated gene transfer to transfect dorsal mPFC neurons with microbial light-sensitive protein, enhanced Natronomonas pharaonis halorhodopsin (eNpHR), which is a light-sensitive electrogenic Cl- pump that hyperpolarizes transfected neurons when activated by ~590 nm yellow light (Han and Boyden, 2007; Yizhar et al., 2011). We found that optical stimulation of dorsal mPFC expressing eNpHR3.0 reduces endogenous and stress-induced neuronal activity, as assessed by slice electrophysiology, in vivo electrophysiology in awake rats, and Fos immunohistochemistry. When yellow light was delivered bilaterally, there was a significant reduction in yohimbine-induced neuronal activation in the hemisphere containing the halorhodopsin construct as compared to the hemisphere expressing the control construct (Fig. 6A). This finding confirms the ability of the optogenetic silencing method to reduce yohimbine-induced neuronal activity, and thus we proceeded to test the causal role of dorsal mPFC in yohimbine-induced reinstatement. We found that optogenetic inhibition of this region decreases yohimbine-induced reinstatement of food seeking (Fig 6B), but not ongoing food self-administration or pellet-priming-induced reinstatement. These data provide additional evidence for the notion that mechanisms underlying reinstatement by the different reinstating stimuli are often dissociable, and for dissociation between mechanisms of reinstatement of food seeking versus ongoing food self-administration (Table 1).
Figure 6. Optogenetic inhibition of dorsal mPFC decreases yohimbine-induced neuronal activation and reinstatement of food seeking.
(A) Yellow light stimulation inhibits yohimbine (2 mg/kg)-induced Fos activation in the dorsal mPFC hemisphere injected with the halorhodopsin construct (eNpHR3.0). The light stimulation had no effect on yohimbine-induced Fos activation in the other hemisphere injected with the control viral construct (eYFP); data are mean±sem Fos immunoreactive neurons per mm2. (B) Yellow light stimulation inhibits yohimbine-induced reinstatement, but not pellet-priming-induced reinstatement; data are mean±sem number of active lever presses during the reinstatement tests. * Different from the eYFP condition, p<0.05. Data are based on results from Calu et al. (2013).
Finally, our finding that optogenetic inhibition of dorsal mPFC has no effect on pellet-priming-induced reinstatement is consistent with results from an earlier study of McFarland and Kalivas (2001) who reported that muscimol+baclofen (GABAergic agonists) inactivation of the dorsal mPFC has no effect on this reinstatement. However, our negative data are not consistent with our previous results (Nair et al., 2011) and with those of Sun and Rebec (2005) that blockade of D1-like receptors in dorsal mPFC modestly decreased pellet-priming-induced reinstatement. In considering the different effects of muscimol+baclofen reversible inactivation by either GABAergic agonists or optogenetic inhibition versus dopamine receptor blockade on pellet-priming-induced reinstatement, it should be noted that others have also obtained different results in reinstatement studies depending on whether reversible inactivation or dopamine receptor antagonists were used. Floresco et al. (2008) reported that muscimol+baclofen reversible inactivation of accumbens shell potentiates cue-induced food seeking. In contrast, Guy et al. (2011) reported that SCH 23390 injections (primarily in shell and the border between core and shell) inhibit this reinstatement. Another example is that McFarland and Kalivas (2001) reported that cocaine-priming-induced reinstatement is decreased by muscimol+baclofen injections in accumbens core but not shell. In contrast, Anderson et al. (2003) reported that this reinstatement is decreased by SCH 23390 injections into accumbens shell but not core.
In conclusion, our optogenetic study has identified a role of dorsal mPFC in stress-induced reinstatement of food seeking in female rats. This study, together with recent studies examining mPFC role in reinstatement of cocaine seeking (Stefanik et al., 2013) and VTA neurons in reinstatement of food seeking (Adamantidis et al., 2011), demonstrate the utility of combining the traditional reinstatement model (Epstein et al., 2006a) with optogenetic approaches to identify brain circuits of relapse to drug and food seeking.
Conclusions
The results from the three studies described above indicate a critical role of dorsal but not ventral mPFC and dopamine transmission in this brain area in yohimbine-induced reinstatement of food seeking. It also appears that dorsal mPFC dopamine plays some role in pellet-priming-induced reinstatement but not cue-induced reinstatement. However, the negative results for cue-induced reinstatement in our study (Nair et al., 2011) should be interpreted with caution, because of the relatively weak reinstatement response to the cues (compared with yohimbine and pellet priming). The results from our two most recent studies further demonstrate the feasibility of using optogenetics to study mechanisms of reinstatement of food seeking (Calu et al., 2013) and the new c-fos-GFP transgenic rats to identify unique synaptic plasticity changes in a small population of neurons that are activated during the reinstatement tests and thus potentially mediate pellet-priming-, cue-, and stress-induced reinstatement (Cifani et al., 2012).
Conclusions and implications for medication development
We have reviewed results from our studies and related studies on mechanisms of reinstatement of palatable food seeking during dieting. As mentioned above, we have offered two main conclusions. The first is that the food reinstatement model is a simple, reliable, and valid model for studying mechanisms of relapse to palatable food seeking during dieting, and for identifying medications to prevent this relapse. The second is that mechanisms of relapse to food seeking are often dissociable from mechanisms of ongoing food intake.
The second conclusion may have important implications for translational research aimed at identifying novel medications for prevention of relapse to unhealthy eating habits during dieting. In particular, medications for dietary treatment have been primarily developed based on their effects on physiological mechanisms that regulate ongoing food intake or food metabolism (Adan, 2013; Bray and Greenway, 2007). Yet it has been known for many years that physiological states of hunger and satiety are often dissociable from food seeking and consumption, which are to a significant extent under the control of external stimuli such as food-associated cues and stressors (Carels et al., 2001; Schachter, 1968). Therefore, the use of the reinstatement model allows for the identification of pharmacological agents that prevent relapse to palatable food seeking and consumption induced by these stimuli during dieting. However, to the degree that the food reinstatement model mimics relapse to unhealthy food-taking behavior in humans, these potential medications are unlikely to reach clinical development if the targeted outcome in animal models is reduction of ongoing food intake (Table 1). Additionally, the available results suggest that medications effective against food-priming-induced or cue-induced reinstatement may not be effective against stress-induced reinstatement (Table 1). Thus, results from the food reinstatement model suggest that clinical pharmacological regimens intended to prevent dietary lapses will likely require combination drug therapy.
Many dieters relapse to unhealthy eating habits during dieting.
We have begun to study this human condition in rats by using a reinstatement model
We present results on the role of several peptides in reinstatement of food seeking
We also present results on the role of mPFC in reinstatement of food seeking
We discuss potential clinical implications.
Acknowledgments
Preparation of this review was supported by the National Institute on Drug Abuse, Intramural Research Program.
Footnotes
Due to space limitations of the special issue, in the current review we primarily describe data from our own studies, as well as selected food reinstatement studies that are directly relevant to our findings. We refer readers to our comprehensive earlier review (Nair et al. Prog Neurobiol 2009) in which we provided a literature review of mechanisms of reinstatement of food seeking and also compared mechanisms of reinstatement of food seeking to those of reinstatement of drug seeking.
The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the data presented in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Keller RW, Jr, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, Tourino C, Bonci A, Deisseroth K, de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adan RA. Mechanisms underlying current and future anti-obesity drugs. Trends Neurosci. 2013 doi: 10.1016/j.tins.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, VanderMaelen CP. alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology. 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology. 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010;107:264–267. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Ballantyne GH. Peptide YY(1-36) and peptide YY(3-36): Part I. Distribution, release and actions. Obes Surg. 2006;16:651–658. doi: 10.1381/096089206776944959. [DOI] [PubMed] [Google Scholar]
- Barth AL, Gerkin RC, Dean KL. Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse. J Neurosci. 2004;24:6466–6475. doi: 10.1523/JNEUROSCI.4737-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Frontiers Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing G, Chen S, Zhang Y, Hillman D, Stone EA. Noradrenergic-induced expression of c-fos in rat cortex: neuronal localization. Neurosci Lett. 1992;140:260–264. doi: 10.1016/0304-3940(92)90116-o. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Webster EL, Torpy DJ, Richman SJ, Mitsiades N, Igel M, Lewis DB, Rice KC, Joost HG, Tsokos M, Chrousos GP. Chronic effects of a nonpeptide corticotropin-releasing hormone type I receptor antagonist on pituitary-adrenal function, body weight, and metabolic regulation. Endocrinology. 1998;139:1546–1555. doi: 10.1210/endo.139.4.5938. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (accepted pending minor revisions) 2013 doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Sheffler-Collins SI, Ghitza UE. The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav Brain Res. 2006;173:148–152. doi: 10.1016/j.bbr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA, Greenway FL. Pharmacological treatment of the overweight patient. Pharmacol Rev. 2007;59:151–184. doi: 10.1124/pr.59.2.2. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996a;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies Synapse. 1996b;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Briggs CA, Pollock NJ, Frail DE, Paxson CL, Rakowski RF, Kang CH, Kebabian JW. Activation of the 5-HT1C receptor expressed in Xenopus oocytes by the benzazepines SCH 23390 and SKF 38393. Br J Pharmacol. 1991;104:1038–1044. doi: 10.1111/j.1476-5381.1991.tb12546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D’Souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology. 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Buczek Y, Le AD, Wang A, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology. 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Calu DJ, Kawa AB, Marchant NJ, Navarre BM, Henderson MJ, Chen B, Yau HJ, Bossert JM, Schoenbaum G, Deisseroth K, Harvey BK, Hope BT, Shaham Y. Optogenetic inhibition of dorsal medial prefrontal cortex attenuates stress-induced reinstatement of palatable food seeking in female rats. J Neurosci. 2013;33:214–226. doi: 10.1523/JNEUROSCI.2016-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Carels RA, Hoffman J, Collins A, Raber AC, Cacciapaglia H, O’Brien WH. Ecological momentary assessment of temptation and lapse in dieting. Eat Behav. 2001;2:307–321. doi: 10.1016/s1471-0153(01)00037-x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G. Attenuation of saccharin-seeking in rats by orexin/hypocretin receptor 1 antagonist. Psychopharmacology (Berl) 2013a doi: 10.1007/s00213-013-3051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology (Berl) 2013b;226:155–165. doi: 10.1007/s00213-012-2902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J, Ames RS, Bergsma D, Muir A, Fitzgerald LR, Hervieu G, Dytko GM, Foley JJ, Martin J, Liu WS, Park J, Ellis C, Ganguly S, Konchar S, Cluderay J, Leslie R, Wilson S, Sarau HM. Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature. 1999;400:261–265. doi: 10.1038/22313. [DOI] [PubMed] [Google Scholar]
- Chang CP, Pearse RV, 2nd, O’Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- Cifani C, Koya E, Navarre BM, Calu DJ, Baumann MH, Marchant NJ, Liu QR, Khuc T, Pickel J, Lupica CR, Shaham Y, Hope BT. Medial prefrontal cortex neuronal activation and synaptic alterations after stress-induced reinstatement of palatable food seeking: a study using c-fos-GFP transgenic female rats. J Neurosci. 2012;32:8480–8490. doi: 10.1523/JNEUROSCI.5895-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag H, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Trans R Soc Lond B: Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Strack AM, Hanson ES, Sebastian RJ. The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Ann N Y Acad Sci. 1995;771:730–742. doi: 10.1111/j.1749-6632.1995.tb44724.x. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci (U S A) 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Klemenhagen KC, Holick KA, Saxe MD, Mendez I, Santarelli L, Craig DA, Zhong H, Swanson CJ, Hegde LG, Ping XI, Dong D, Marzabadi MR, Gerald CP, Hen R. Efficacy of the MCHR1 antagonist N-[3-(1-{[4-(3,4-difluorophenoxy)phenyl]methyl}(4-piperidyl))-4-methylphen yl]-2-methylpropanamide (SNAP 94847) in mouse models of anxiety and depression following acute and chronic administration is independent of hippocampal neurogenesis. J Pharmacol Exp Ther. 2007;321:237–248. doi: 10.1124/jpet.106.109678. [DOI] [PubMed] [Google Scholar]
- Davy SR, Benes BA, Driskell JA. Sex differences in dieting trends, eating habits, and nutrition beliefs of a group of midwestern college students. J Am Diet Assoc. 2006;106:1673–1677. doi: 10.1016/j.jada.2006.07.017. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, de Vries W, Janssen MC, Schoffelmeer AN. Suppression of conditioned nicotine and sucrose seeking by the cannabinoid-1 receptor antagonist SR141716A. Behav Brain Res. 2005;161:164–168. doi: 10.1016/j.bbr.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990;85:367–403. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- Doxey JC, Lane AC, Roach AG, Virdee NK. Comparison of the alpha-adrenoceptor antagonist profiles of idazoxan (RX 781094), yohimbine, rauwolscine and corynanthine. Naunyn Schmiedebergs Arch Pharmacol. 1984;325:136–144. doi: 10.1007/BF00506193. [DOI] [PubMed] [Google Scholar]
- Eberlein GA, Eysselein VE, Schaeffer M, Layer P, Grandt D, Goebell H, Niebel W, Davis M, Lee TD, Shively JE, et al. A new molecular form of PYY: structural characterization of human PYY(3-36) and PYY(1-36) Peptides. 1989;10:797–803. doi: 10.1016/0196-9781(89)90116-2. [DOI] [PubMed] [Google Scholar]
- Epstein D, Preston K, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006a;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology. 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006b;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology. 2011;216:53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience. 2008;154:877–884. doi: 10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, Yanagisawa M. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009;9:64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF(1) receptors. Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Nair SG, Golden SA, Gray SM, Uejima JL, Bossert JM, Shaham Y. Peptide YY3-36 decreases reinstatement of high-fat food seeking during dieting in a rat relapse model. J Neurosci. 2007;27:11522–11532. doi: 10.1523/JNEUROSCI.5405-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandt D, Teyssen S, Schimiczek M, Reeve JR, Jr, Feth F, Rascher W, Hirche H, Singer MV, Layer P, Goebell H, et al. Novel generation of hormone receptor specificity by amino terminal processing of peptide YY. Biochem Biophys Res Commun. 1992;186:1299–1306. doi: 10.1016/s0006-291x(05)81547-5. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Shiffman S, Wing RR. Relapse crises and coping among dieters. J Consult Clin Psychol. 1989;57:488–495. doi: 10.1037//0022-006x.57.4.488. [DOI] [PubMed] [Google Scholar]
- Guy EG, Choi E, Pratt WE. Nucleus accumbens dopamine and mu-opioid receptors modulate the reinstatement of food-seeking behavior by food-associated cues. Behav Brain Res. 2011;219:265–272. doi: 10.1016/j.bbr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Blatchford KE, McNally GP. Renewal of an extinguished instrumental response: neural correlates and the role of D1 dopamine receptors. Neuroscience. 2006;143:25–38. doi: 10.1016/j.neuroscience.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS One. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Karlsson C, Zook M, Ciccocioppo R, Gehlert DR, Thorsell A, Heilig M, Cippitelli A. Melanin-concentrating hormone receptor 1 (MCH1-R) antagonism: reduced appetite for calories and suppression of addictive-like behaviors. Pharmacol Biochem Behav. 2012;102:400–406. doi: 10.1016/j.pbb.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kayman S, Bruvold W, Stern JS. Maintenance and relapse after weight loss in women: behavioral aspects. Am J Clin Nutr. 1990;52:800–807. doi: 10.1093/ajcn/52.5.800. [DOI] [PubMed] [Google Scholar]
- Kramer FM, Jeffery RW, Forster JL, Snell MK. Long-term follow-up of behavioral treatment for obesity: patterns of weight regain among men and women. Int J Obes. 1989;13:123–136. [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Fletcher PJ. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology. 2009;204:477–488. doi: 10.1007/s00213-009-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology. 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotropin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF. Brain peptides and obesity: pharmacologic treatment. Obes Res. 1995;3(Suppl 4):573S–589S. doi: 10.1002/j.1550-8528.1995.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Scott Hall F, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Malherbe P, Borroni E, Pinard E, Wettstein JG, Knoflach F. Biochemical and electrophysiological characterization of almorexant, a dual orexin 1 receptor (OX1)/orexin 2 receptor (OX2) antagonist: comparison with selective OX1 and OX2 antagonists. Mol Pharmacol. 2009;76:618–631. doi: 10.1124/mol.109.055152. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–2178. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Tsukada H, Nishiyama S, Sekine Y, Kakiuchi T, Iyo M, Mori N. Yohimbine increases the binding potential for [11C]flumazenil in the monkey brain. J Neural Transm. 2001;108:1375–1382. doi: 10.1007/s007020100014. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire MT, Wing RR, Klem ML, Lang W, Hill JO. What predicts weight regain in a group of successful weight losers? J Consult Clin Psychol. 1999;67:177–185. doi: 10.1037//0022-006x.67.2.177. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Filip M, Cunningham KA. Differential regulation of the mesoaccumbens circuit by serotonin 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Neurosci. 2001;21:7781–7787. doi: 10.1523/JNEUROSCI.21-19-07781.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Quentric Y, Cussac D. The “selective” dopamine D1 receptor antagonist, SCH23390, is a potent and high efficacy agonist at cloned human serotonin2C receptors. Psychopharmacology. 2001;156:58–62. doi: 10.1007/s002130100742. [DOI] [PubMed] [Google Scholar]
- Milligan CM, Linton CJ, Patmore L, Gillard N, Ellis GJ, Towers P. [3H]-RS-79948-197, a high affinity radioligand selective for alpha 2-adrenoceptor subtypes. Ann N Y Acad Sci. 1997;812:176–177. doi: 10.1111/j.1749-6632.1997.tb48164.x. [DOI] [PubMed] [Google Scholar]
- Monti JM, Fernandez M, Jantos H. Sleep during acute dopamine D1 agonist SKF 38393 or D1 antagonist SCH 23390 administration in rats. Neuropsychopharmacology. 1990;3:153–162. [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Mul JD, la Fleur SE, Toonen PW, Afrasiab-Middelman A, Binnekade R, Schetters D, Verheij MM, Sears RM, Homberg JR, Schoffelmeer AN, Adan RA, DiLeone RJ, De Vries TJ, Cuppen E. Chronic loss of melanin-concentrating hormone affects motivational aspects of feeding in the rat. PLoS One. 2011;6:e19600. doi: 10.1371/journal.pone.0019600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahon JL, Presse F, Bittencourt JC, Sawchenko PE, Vale W. The rat melanin-concentrating hormone messenger ribonucleic acid encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinology. 1989;125:2056–2065. doi: 10.1210/endo-125-4-2056. [DOI] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009a;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Pickens CL, Smith DG, Shaham Y. Effects of the MCH1 receptor antagonist SNAP 94847 on high-fat food-reinforced operant responding and reinstatement of food seeking in rats. Psychopharmacology (Berl) 2009b;205:129–140. doi: 10.1007/s00213-009-1523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008 doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Navarre BM, Cifani C, Pickens CL, Bossert JM, Shaham Y. Role of dorsal medial prefrontal cortex dopamine D1-family receptors in relapse to high-fat food seeking induced by the anxiogenic drug yohimbine. Neuropsychopharmacology. 2011;36:497–510. doi: 10.1038/npp.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance DM. The developmental and neural determinants of the effects of estrogen on feeding behavior in the rat: a theoretical perspective. Neurosci Biobehav Rev. 1983;7:189–211. doi: 10.1016/0149-7634(83)90015-5. [DOI] [PubMed] [Google Scholar]
- Neumeyer JL, Kula NS, Bergman J, Baldessarini RJ. Receptor affinities of dopamine D1 receptor-selective novel phenylbenzazepines. Eur J Pharmacol. 2003;474:137–140. doi: 10.1016/s0014-2999(03)02008-9. [DOI] [PubMed] [Google Scholar]
- Peterson CB, Mitchell JE. Psychosocial and pharmacological treatment of eating disorders: a review of research findings. J Clin Psychol. 1999;55:685–697. doi: 10.1002/(sici)1097-4679(199906)55:6<685::aid-jclp3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Cifani C, Navarre BM, Eichenbaum H, Theberge FR, Baumann MH, Calu DJ, Shaham Y. Effect of fenfluramine on reinstatement of food seeking in female and male rats: implications for the predictive validity of the reinstatement model. Psychopharmacology (Berl) 2012;221:341–353. doi: 10.1007/s00213-011-2585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri JL, Shiffman S, Rohay JM, Harkins AM, Burton SL, Wadden TA. Use of dietary supplements for weight loss in the United States: results of a national survey. Obesity (Silver Spring) 2008;16:790–796. doi: 10.1038/oby.2007.136. [DOI] [PubMed] [Google Scholar]
- Polivy J, Herman CP. Distress and eating: why do dieters overeat? Int J Eat Disord. 1999;26:153–164. doi: 10.1002/(sici)1098-108x(199909)26:2<153::aid-eat4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Porter JH, McCallum SE, Varvel SA, Vann RE. The discriminative stimulus properties of the atypical antipsychotic olanzapine in rats. Psychopharmacology. 2000;148:224–233. doi: 10.1007/s002130050046. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Ramos M, Goni-Allo B, Aguirre N. Administration of SCH 23390 into the medial prefrontal cortex blocks the expression of MDMA-induced behavioral sensitization in rats: an effect mediated by 5-HT2C receptor stimulation and not by D1 receptor blockade. Neuropsychopharmacology. 2005;30:2180–2191. doi: 10.1038/sj.npp.1300735. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology. 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Therapeutic and adverse actions of serotonin transporter substrates. Pharmacol Ther. 2002;95:73–88. doi: 10.1016/s0163-7258(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Charlton J. Neurobiology of an anorectic drug: fenflurmaine. Prog Neurobiol. 1985;27:13–62. doi: 10.1016/0301-0082(86)90011-0. [DOI] [PubMed] [Google Scholar]
- Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435:26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sanchez CJ, Bailie TM, Wu WR, Li N, Sorg BA. Manipulation of dopamine d1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience. 2003;119:497–505. doi: 10.1016/s0306-4522(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Scatton B, Zivkovic B, Dedek J. Antidopaminergic properties of yohimbine. J Pharmacol Exp Ther. 1980;215:494–499. [PubMed] [Google Scholar]
- Schachter S. Obesity and eating. Internal and external cues differentially affect the eating behavior of obese and normal subjects. Science. 1968;161:751–756. doi: 10.1126/science.161.3843.751. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Selye H. Forty years of stress research: principal remaining problems and misconceptions. Can Med Assoc J. 1976;115:53–56. [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology. 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000a;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs JM, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of the locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000b;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, Morton K, Palmer J, Bonaventure P, Carruthers NI, Lovenberg TW, Boggs J, Galici R. Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology. 2011;215:191–203. doi: 10.1007/s00213-010-2127-x. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Skender ML, Goodrick GK, Del Junco DJ, Reeves RS, Darnell L, Gotto AM, Foreyt JP. Comparison of 2-year weight loss trends in behavioral treatments of obesity: diet, exercise, and combination interventions. J Am Diet Assoc. 1996;96:342–346. doi: 10.1016/S0002-8223(96)00096-X. [DOI] [PubMed] [Google Scholar]
- Smart D, Jerman JC, Brough SJ, Neville WA, Jewitt F, Porter RA. The hypocretins are weak agonists at recombinant human orexin-1 and orexin-2 receptors. Br J Pharmacol. 2000;129:1289–1291. doi: 10.1038/sj.bjp.0703257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Jerman JC, Brough SJ, Rushton SL, Murdock PR, Jewitt F, Elshourbagy NA, Ellis CE, Middlemiss DN, Brown F. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br J Pharmacol. 1999;128:1–3. doi: 10.1038/sj.bjp.0702780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DG, Hegde LG, Wolinsky TD, Miller S, Papp M, Ping X, Edwards T, Gerald CP, Craig DA. The effects of stressful stimuli and hypothalamic-pituitary-adrenal axis activation are reversed by the melanin-concentrating hormone 1 receptor antagonist SNAP 94847 in rodents. Behav Brain Res. 2009;197:284–291. doi: 10.1016/j.bbr.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, Lalumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, Woodside BC, Shaham Y. Changes in ovarian hormones do not affect the initiation of intravenous self-administration of heroin in the female rat. Psychobiology. 1996;24:154–159. [Google Scholar]
- Stewart TM, Bhapkar M, Das S, Galan K, Martin CK, McAdams L, Pieper C, Redman L, Roberts S, Stein RI, Rochon J, Williamson DA, Group CS. Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy Phase 2 (CALERIE Phase 2) screening and recruitment: methods and results. Contemp Clin Trials. 2013;34:10–20. doi: 10.1016/j.cct.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol Psychiatry. 2002;51:642–651. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;177:315–323. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Tabaeizadeh M, Motiei-Langroudi R, Mirbaha H, Esmaeili B, Tahsili-Fahadan P, Javadi-Paydar M, Ghaffarpour M, Dehpour AR. The differential effects of OX1R and OX2R selective antagonists on morphine conditioned place preference in naive versus morphine-dependent mice. Behav Brain Res. 2013;237:41–48. doi: 10.1016/j.bbr.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Tanda G, Bassareo V, Di Chiara G. Mianserin markedly and selectively increases extracellular dopamine in the prefrontal cortex as compared to the nucleus accumbens of the rat. Psychopharmacology. 1996;123:127–130. doi: 10.1007/BF02246169. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of mesocortical DA system by stress. Nature. 1976;263:242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, Heilig M. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology. 2011;36:1178–1886. doi: 10.1038/npp.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Walker EA, Dykstra LA. Effect of cannabinoid CB1 receptor antagonist SR141716A and CB1 receptor knockout on cue-induced reinstatement of Ensure and corn-oil seeking in mice. Neuropsychopharmacology. 2007;32:2592–2600. doi: 10.1038/sj.npp.1301384. [DOI] [PubMed] [Google Scholar]
- Willner P. The validity of animal models of depression. Psychopharmacology. 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rabin RA. Yohimbine as a serotonergic agent: evidence from receptor binding and drug discrimination. J Pharmacol Exp Ther. 1992;263:682–689. [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang GC, Mao LM, Liu XY, Wang JQ. Long-lasting up-regulation of orexin receptor type 2 protein levels in the rat nucleus accumbens after chronic cocaine administration. J Neurochem. 2007;103:400–407. doi: 10.1111/j.1471-4159.2007.04748.x. [DOI] [PubMed] [Google Scholar]