Abstract
Animals tend to reject bitter foods. However, long-term exposure to some unpalatable tastants increases acceptance of the foods. Here, we showed that dietary exposure to the unappealing food but safe additive, camphor, caused the fruit fly, Drosophila melanogaster, to decrease camphor rejection. The TRPL cation channel was a direct target for camphor in gustatory receptor neurons (GRNs), and long-term feeding on a camphor diet led to reversible down-regulation of TRPL protein levels. The turnover of TRPL was controlled by an E3 ubiquitin ligase, Ube3a. The decline in TRPL levels and increased acceptance of camphor reversed after returning the flies long-term to a camphor-free diet. We propose that dynamic regulation of taste receptor levels by ubiquitin-mediated protein degradation comprises an important molecular mechanism that allows an animal to alter taste behavior in response to a changing food environment.
Introduction
The decision as to whether to eat a particular food is determined by an animal’s physiological status, genetic make-up and the features of the food, including nutrient value, palatability and toxicity1. Exposure to new types of foods can also alter taste preferences. This phenomenon of dietary experience impacting on food selection is documented in many animals, including, humans2, 3, mice4 and several insects such as moths5, 6.
The attraction and aversion to different foods promotes survival since it allows for discrimination between nutrient-rich sources, many of which are sweet, from toxic chemicals, which are typically perceived as bitter. However, many beneficial nutrients taste bitter to animals, and are avoided needlessly7. Aversion to less appealing but safe foods is not a problem if an animal has the option to consume alternatives. If this is not the case, dietary experience can cause animals to reduce their aversion to safe foods, which they formerly found unappealing. This decrease in gustatory avoidance can be reversed upon returning to a more varied diet with intrinsically more palatable options5, 7.
Changes in taste preference could occur through alterations either in the peripheral taste receptor cells, or in higher-order neural circuits controlling food intake in the brain. Brain regions such as the mammalian gustatory cortex8 and mushroom body in insects are implicated in some forms of taste associated learning and memory9, 10. However, there are limited molecular insights into whether plastic alterations in taste receptor cells influence diet-induced changes in taste aversion.
Here, we found that we could recapitulate dietary-dependent taste desensitization in fruit flies. The flies decreased their distaste to the unpalatable but nontoxic tastant camphor after feeding on camphor diet. This change depended on TRPL, a member of the TRPC family11, 12, which was activated directly by camphor in vitro. TRPL13 is related to the classical Drosophila TRP channel14, both of which function in phototransduction15, 16. Prolonged exposure to a camphor diet caused selective down-regulation of TRPL in the primary taste organ, the proboscis. The decrease in TRPL expression in GRNs was caused by Ube3a-mediated protein degradation. Persistent feeding on camphor also triggered a subsequent reduction in the number of synapses formed by TRPL-expressing GRNs. We propose that the increased behavioral acceptance of a formerly unpalatable tastant is mediated by Ube3a-mediated ubiquitination and degradation of TRPL.
Results
Dietary exposure to camphor attenuated gustatory aversion
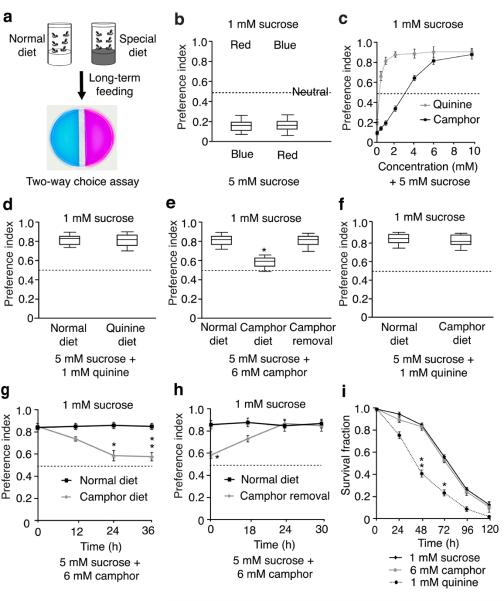
To determine whether diet could cause flies to selectively reduce their distaste for some unpalatable compounds, we continuously exposed the animals to diets containing aversive chemicals for 12 – 36 hours. To test their gustatory preferences, we used a simplified two-way choice assay (Fig. 1a). One tastant was mixed with either red or blue food coloring, and the other tastant was mixed with the alternative dye. We then scored the flies with red, blue or purple abdomens. Flies chose 5 mM over 1 mM sucrose, and the preference index (PI) was not affected by the food coloring (Fig. 1b). To test the validity of the assay, we performed spectrophotometry to quantitatively measure the color of dissected guts. The preference for 5 mM over 1 mM sucrose was similar to that obtained using the visual scoring system (Supplementary Fig. 1a). Upon mixing 5 mM sucrose with aversive chemicals such as quinine or camphor, the animals exhibited reduced preferences for the 5 mM sucrose (Fig. 1c). The flies exhibited similar distastes for 1 mM quinine and 6 mM camphor (Fig. 1c).
Figure 1. Behavioral and electrophysiological responses of wild-type flies after feeding on a normal diet or a camphor-containing diet.
(a) Behavioral assay for taste learning analysis. Flies were fed 1 mM sucrose only (normal diet) or 1 mM sucrose plus aversive tastants (special diet), and then subjected to two-way food choice assays. One side contained 1 mM sucrose and the other side had 5 mM sucrose/bitter tastant mixtures laced with either blue or red food coloring. (b) Testing the effects of red and blue food coloring on taste discrimination using the two-way choice test. The red and blue dyes were switched as indicated. ~70 flies per trial. n=10 trials. (c) Flies were given a choice between 1 mM and 5 mM sucrose mixed with the indicated concentrations of camphor or quinine. ~70 flies per trial, n=5 trials. (d–f) Two-way choice tests using flies raised on a normal (camphor- and quinine-free) diet or on a diet comprised of either 1 mM quinine or 6 mM camphor (plus 1 mM sucrose) for 24 hours. Shown in e are the responses after moving flies from a camphor diet to a normal diet for 24 hours (camphor removal). n=10 trials. *p=0.00012. (g) Relationship between the length of time on a 6 mM camphor diet, and increased acceptance of camphor. After maintaining the flies on a camphor diet for the indicated times, their preferences for 1 mM sucrose versus 5 mM sucrose plus 6 mM camphor were assessed. n=5 trials. *p=0.00093. **p=0.00011. (h) Flies were maintained on a normal or camphor diet for 24 hours and then switched to a camphor-free diet for the indicated times before conducting the two-way choice tests. n=5 trials. *p=0.00014. (i) Relative toxicities of 6 mM camphor and 1 mM quinine. The flies were fed 1 mM sucrose or 1 mM sucrose plus either 6 mM camphor or 1 mM quinine. The fraction of viable flies were assessed at the indicated times. n=3 trials. ~20 flies per trial. *p=0.00047. **p=0.00012. Error bars indicate SEMs. One-way ANOVA tests with Bonferroni post-hoc analysis.
To test whether fruit flies modify their taste bias due to prior experience, we feed the animals aversive chemicals, combined with 1 mM sucrose, for 24 hours. Continual exposure did not reduce their distaste for 1 mM quinine (Fig. 1d). Of significance here, pre-exposure to a 6 mM camphor diet for 24 hours significantly attenuated the flies’ camphor aversion (Fig. 1e). This desensitization was dependent on the length of time on the camphor diet (Fig. 1g) and was maximal once the camphor concentration reached 6 mM (Supplementary Fig. 2a). Camphor-exposed flies showed the same repulsion as naïve flies to quinine and other bitter tastants such as caffeine, strychnine and lobeline (Fig. 1f and Supplementary Fig. 2b), indicating that the camphor diet did not cause a global reduction in aversion to bitter chemicals.
To determine whether this experience-dependent change in taste preference was reversible, we transferred the camphor-exposed flies for 18—30 hours to normal, camphor-free food. The original bias against camphor was restored fully after 24 hours (Fig. 1h). Using the spectrophotometric assay, we obtained similar reversible changes to exposure to a camphor diet, but not to a quinine diet (Supplementary Fig. 1b – e).
Because the flies reduced their aversion to camphor but not quinine, we tested the possibility that quinine, but not camphor, was toxic when combined with 1 mM sucrose. 1 mM sucrose is a minimal diet and resulted in decreased survival over time (Fig. 1i; LT50, 74.9 ±4.5 hours). Addition of 1 mM quinine to the sucrose resulted in more rapid lethality (Fig. 1i; LT50 , 41.6 ±2.3 hours). In contrast, 6 mM camphor had no adverse effect on viability (Fig. 1i; LT50 , 71.6 ±4.2 hours). To exclude that the increased lethality caused by 1 mM quinine resulted from starvation due to insufficient intake of the quinine/sucrose mixture, rather than toxicity of quinine, we injected 1 mM quinine and 6 mM camphor into the abdomens. Flies injected with 1 mM quinine died faster than the control animals injected with H2O alone, while injection of 6 mM camphor had no effect on survival (Supplementary Fig. 3a). Thus, decreased aversion occurred due to pre-exposure to a diet consisting of the innocuous tastant, camphor, but not after exposure to a diet containing the toxic chemical, quinine.
We tested whether long-term exposure to other bitter tastants would lead to increased gustatory acceptance. To conduct this analysis, we used concentrations of caffeine, strychnine, and lobeline that elicited similar avoidances in normally fed flies as 1 mM quinine and 6 mM camphor (Supplementary Fig. 3b). Exposure to caffeine for 24 hours caused a decrease in caffeine avoidance while prefeeding on strychnine or lobeline did not result in a decline in aversion to these tastants (Supplementary Fig. 3b). We tested whether toxicity was associated with these other bitter compounds, and found that 1 mM strychnine and 1 mM lobeline, which did not lead to desensitization, caused more severe lethality than 6 mM caffeine (Supplementary Fig. 3c).
The changes in taste preferences could reflect modifications in the peripheral GRNs or in post-synaptic neurons that comprise the taste circuit. To address whether the activity of the GRNs was altered, we monitored camphor-induced action potentials by performing tip recordings on taste sensilla. GRNs are housed in hair-like sensilla distributed on the main taste organ, the labellum, and several other body parts17, 18. Sensilla are distinguished by size (short, S; intermediate, I; long, L), and avoidance compounds are detected principally by S-type sensilla19.
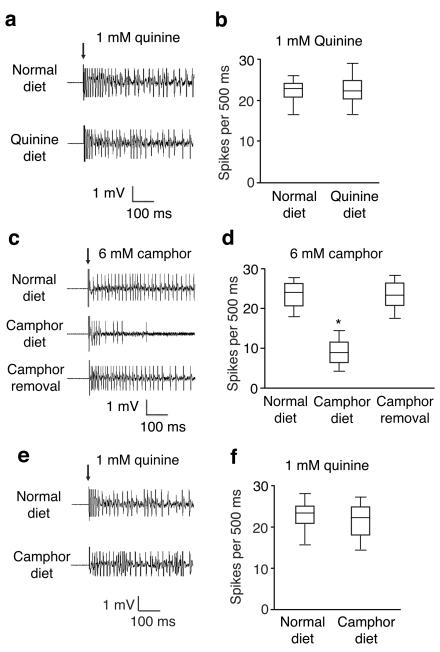
We focused on S6 sensilla and recorded quinine- and camphor-induced action potentials using naïve animals, and flies that have been pre-fed quinine or camphor diets. There were no significant differences in action potential frequencies between flies raised on normal food or maintained on a quinine diet (Fig. 2a,b). Camphor elicited a high frequency of action potentials in animals that had not tasted camphor previously (Fig. 2c,d; 25.2 ±1.9/500 ms). However, if the flies were pre-exposed to camphor for 24 hours, the frequency of camphor-induced action potentials declined (Fig. 2c,d; 9.8 ±1.1/500 ms). Decrements in firing frequencies occurred over a range of camphor concentrations (Supplementary Fig. 4a). These changes correlated with the reduced camphor avoidance over the same doses of camphor (Supplementary Fig. 4b), suggesting that the diminished camphor-induced action potentials contributed to the behavioral changes. After returning the flies to normal camphor-free food, the original higher frequency of camphor-induced action potentials was restored (Fig. 2c,d; 24.6 ±2.5/500 ms). Pre-exposure to a camphor diet had no effect on the frequencies of action potentials to other tastants, such as quinine (Fig. 2e,f; action potentials/500 ms: normal diet, 24.2 ±2.1; quinine diet, 24.8 ± 1.7).
Figure 2. Electrophysiological responses of wild-type flies after feeding on a normal diet or a camphor-containing diet.
(a and b) Action potentials in S6 sensilla in response to 1 mM quinine after feeding the flies a normal or quinine-containing diet. n= 5 animals. (c and d) Action potentials in S6 sensilla in response to 6 mM camphor. The flies were fed a normal diet, a diet containing 6 mM camphor for 24 hours, or the camphor diet for 24 hours and then a normal diet for 24 hours. n=15 animals. *p=0.00011. (e and f) Action potentials in S6 sensilla in response to 1 mM quinine after feeding the flies a normal or camphor-containing diet. n=10 animals. The arrows indicate the application of the recording electrode to the sensilla. Error bars indicate SEMs. One-way ANOVA tests with Bonferroni post-hoc analysis.
The findings that the experience-induced changes in camphor preferences were paralleled by alterations in action potentials support the model that the GRNs contribute to the desensitization. Moreover, the reversibility of the behavior and action potential frequency indicate that pre-exposure to a camphor diet did not result in death of the GRNs.
TRPL was required for camphor taste
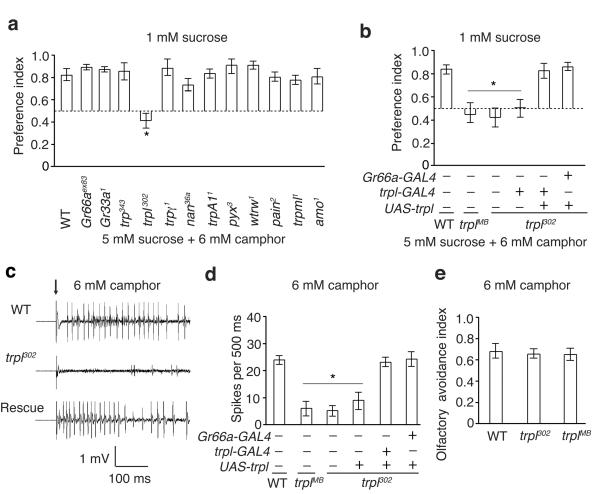
To characterize the mechanisms underlying experienced-induced taste modifications, we considered whether the concentration of a camphor receptor or a camphor-responsive ion channel might vary due to camphor exposure. Therefore, we tested the requirement for gustatory receptors (GRs) and TRP channels. Mutations affecting two GRs (Gr66aex83, Gr33a1), which are broadly required for avoiding an array of bitter tastants20, 21 had no impact on camphor aversion (Fig. 3a). We also tested mutations affecting TRP channels, since at least one member of this channel family, TRPA1, senses aversive tastants22, 23. Mutations affecting most TRP channels had no significant impact on camphor aversion (Fig. 3a). In contrast, both trpl alleles tested (trpl302 and trplMB03075) displayed a deficit in camphor avoidance (Fig. 3a,b and Supplementary Fig. 5a). The preference index elicited by trpl mutant flies was not as low as in wild-type flies that were allowed to choose between 1 mM and 5 mM sucrose alone (Fig. 1b), because bitter compounds suppress the activity of sugar-responsive GRNs24, 25. Loss of trpl greatly reduced camphor-induced action potentials in S6 sensilla (Fig. 3c,d), indicating that activation of GRNs by camphor was impaired. The trpl302 mutant showed normal avoidances to other aversive tastants (Supplementary Fig. 5b,c), and a wild-type ability to respond to sucrose (Supplementary Fig. 5d,e).
Figure 3. Requirement for trpl for responding to the taste of camphor.
(a) Two-way choice assays showing survey of potential requirements for GRs or for TRP channels for avoiding consumption of 6 mM camphor. w1118 was used as the “wild-type” control (WT). n=3 trials. *p=0.000089. (b) Rescue of behavioral deficits in response to 6 mM camphor using UAS-trpl and the indicated GAL4 line. The trplMB03075 allele is abbreviated as trplMB. n=10 trials. *p=0.0001. (c and d) Action potentials in S6 sensilla in response to 6 mM camphor. n=15 animals. *p=0.0001. The “rescue” in c indicates trpl302 flies expressing UAS-trpl expressed under the control of the Gr66a-GAL4. The arrow indicates the application of the recording electrodes to the sensilla in c. (e) DART assays26 using 6 mM camphor. n=5 trials. Error bars indicate SEMs. One-way ANOVA tests with Bonferroni post-hoc analysis.
We tested for rescue of the trpl302 mutant phenotype, by employing the GAL4/UAS system to express a wild-type trpl transgene (UAS-trpl) in Gr66a-positive GRNs (Gr66a-GAL4), which respond to aversive tastants. Expression of UAS-trpl under the control of either the Gr66a-GAL4 or a trpl-GAL4 restored normal camphor avoidance (Fig. 3b), and camphor-induced action potentials in GRNs (Fig. 3c,d).
Camphor not only has a bitter taste but also has an aversive aroma to flies. To test whether TRPL functioned in olfactory avoidance of camphor, we used the direct airborne repellent test (DART)26. Wild-type flies and the trpl mutants were both repelled similarly by camphor (Fig. 3e). Thus, trpl was required for avoiding the taste and not the smell of camphor.
Camphor directly activates TRPL
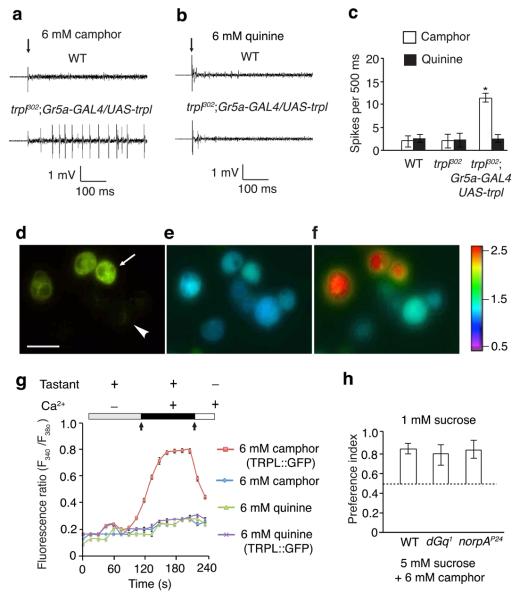
To address whether TRPL was sufficient to act as a taste sensor for camphor in vivo, we misexpressed trpl in sugar responsive GRNs of trpl302 flies, using the Gr5a-GAL4 and UAS-trpl transgenes. We performed tip recordings and found that misexpressing trpl in L-type (L2) sensilla conferred camphor responses (Fig. 4a,c). In contrast, misexpression of trpl in the L-type sensilla failed to generate physiological responses to other bitter tastants such as quinine and strychnine (Fig. 4b and Supplementary Fig. 6a,b). Misexpression of trpl in L-type sensilla had no effect on the sucrose response (Supplementary Fig. 6c,d). These data suggested that TRPL was activated by camphor. Consistent with these physiological results, misexpressing trpl in L-type sensilla increased the attractiveness of camphor relative to trpl302 mutants that did not misexpress trpl+ (Supplementary Fig. 6e). The attraction was relatively mild, consistent with the finding that ectopic expression of TRPL in Gr5a-expressing GRNs resulted in fewer camphor-induced action potentials than in bitter-responsive GRNs that express TRPL. The moderate camphor response by the transgenic flies might be the due to the absence of a protein in Gr5a GRNs, which in normal TRPL-expressing cells contributes to full TRPL expression levels or activity.
Figure 4. Camphor directly activated TRPL.
(a–c) Sample tip recordings and quantification showing that camphor, but not quinine, induced action potentials after misexpression of trpl in Gr5a GRNs (L2 sensilla). n=10 animals. *p=0.00063. (d–f) Ca2+ photometry showing increased Ca2+ influx in S2 cells transiently expressing a TRPL::GFP fusion protein. (d) GFP-positive cells (expressing TRPL::GFP) identified by fluorescent microscopy. The arrow and arrowhead indicate examples of GFP-positive and negative cells, respectively. The scale bar indicates 10 μm. (e) F340/F380 ratio prior to addition of 6 mM camphor, 6 mM quinine or Ca2+ to the bath solution (left arrow in g). (f) F340/F380 after adding 6 mM camphor and then 2 mM CaCl2 to the bath solution (right arrow in g). The color scale indicates the F340/F380 ratios. (g) Changes in F340/F380 after adding either 6 mM camphor or 6 mM quinine, and then 2 mM CaCl2 to the bath solution. n=3 trials (20 cells/trial). (h) Comparing camphor avoidance among wild type, dGq1 and norpAP24 animals using two-way choice tests. n=3 trials. Error bars indicate SEMs. One-way ANOVA tests with Bonferroni post-hoc analysis.
To investigate whether camphor directly activated TRPL, we expressed a TRPL::GFP fusion protein in Drosophila S2 cells and performed Ca2+ imaging. Application of camphor to TRPL::GFP-positive cells induced a robust increase in intracellular Ca2+ (Fig. 4d – g). This was due to Ca2+ influx rather than Ca2+ release from intracellular stores since it required the presence of Ca2+ in the bath solution. S2 cells that did not express TRPL::GFP were unresponsive to camphor (Fig. 4d – f). Application of quinine failed to produce a significant increase in intracellular Ca2+ in TRP::GFP positive cells (Fig. 4g). Consistent with these results, an independent analysis reported that camphor activated a TRPL::GFP fusion protein in S2 cells27. The combination of in vivo and in vitro misexpression data indicated that TRPL was sufficient to confer a response to camphor. In further support of this conclusion, loss of either the dGqα or the phospholipase C (NORPA) that function upstream of TRPL in photoreceptors cells had no impact on camphor avoidance (Fig. 4h).
TRPL was localized to the dendrites of GRNs
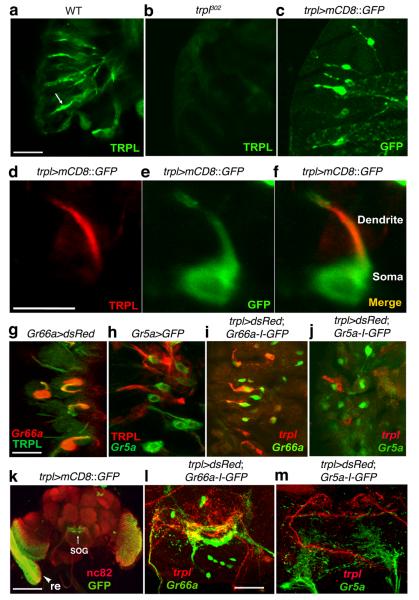
We examined the expression pattern of TRPL and found that TRPL was enriched in the dendrites of GRNs (Fig. 5a,d). The immunoreactivity was missing in trpl302 mutant animals (Fig. 5b). To obtain a more sensitive reagent to examine the cellular distribution of trpl, we generated a trpl-GAL4 line to drive expression of GFP (UAS-mCD8::GFP). Anti-GFP stained ~10 GRNs per labellum (Fig. 5c), and the signal labeled the same GRNs as anti-TRPL (Fig. 5d – f).
Figure 5. TRPL was localized to GRN dendrites.
(a and b) Anti-TRPL stained dendrites from wild-type but not trpl302 labella. The images shown are stacks of confocal sections. The arrow in a indicates a GRN dendrite. (c) Expression of UAS-mCD8::GFP in GRNs in the labella, under control of the trpl-GAL4 (trpl>mCD8::GFP). (d–f) Co-localization of endogenous TRPL with the trpl>mCD8::GFP reporter. TRPL was enriched in dendrites of the GRN. Shown is a single confocal section. (g) Labella expressing UAS-dsRed under the control of the Gr66a-GAL4 (Gr66a>dsRed) with anti-TRPL (green) and anti-dsRed (red). (h) Labella expressing Gr5a>GFP with anti-TRPL (red) and anti-GFP (green). (i) Labella expressing the trpl-GAL4 and UAS-dsRed (trpl>dsRed) and Gr66a-I-GFP with dsRed (red) and anti-GFP (green). (j) Labella expressing trpl>mCD8::dsRed and Gr5a-I-GFP with anti-mCD8 (red) and anti-GFP (green). (k) A wild-type fly head (expressing trpl>mCD8::GFP) stained with anti-GFP and anti-nc82, which labeled the whole brain. The arrow and arrowhead indicate the SOG and the retina, respectively. (l) SOG region from a fly expressing trpl>mCD8::dsRed and Gr66a-I-GFP stained with anti-mCD8 (red) and anti-GFP (green). (m) SOG region from a fly expressing trpl>mCD8::dsRed and Gr5a-I-GFP stained with anti-mCD8 (red) and anti-GFP (green). The scale bars in a, d and g (10 μm) apply to the entire rows. The scale bar in k indicates 50 μm, and the scale bar in l (10 μm) applies to l and m.
To determine the spatial distribution of trpl+ GRNs relative to bitter and sugar GRNs, we compared the pattern of the trpl reporter with either the Gr5a-I-GFP or Gr66a-I-GFP transgenes, respectively, which expressed GFP under the direct control of either the Gr5a or Gr66a promoter28. An average of four GRNs that stained with either anti-TRPL (Fig. 5g) or the trpl reporter (trpl-GAL4 and UAS-dsRed) overlapped with the Gr66a-positive cells (Fig. 5i). None of the trpl-expressing GRNs overlapped with theGr5a reporter (Fig. 5g—j).
We also examined the central brain expression pattern of trpl. Consistent with its established role in phototransduction, trpl was enriched in the retina and in the lamina (Fig. 5k), the latter of which was innervated by photoreceptor cell axons. The reporter also stained the subesophageal ganglion (SOG; Fig. 5k), an area of the brain that received projections from the GRNs. The SOG region targeted by trpl GRNs partially overlapped with Gr66a GRNs (Fig. 5l), and there was no colocalization with the Gr5a GRNs (Fig. 5m). Thus, the innervation patterns in the SOG mirrored the relative spatial distribution of trpl, Gr66a and Gr5a in the GRNs.
TRPL was selectively down-regulated by a camphor diet
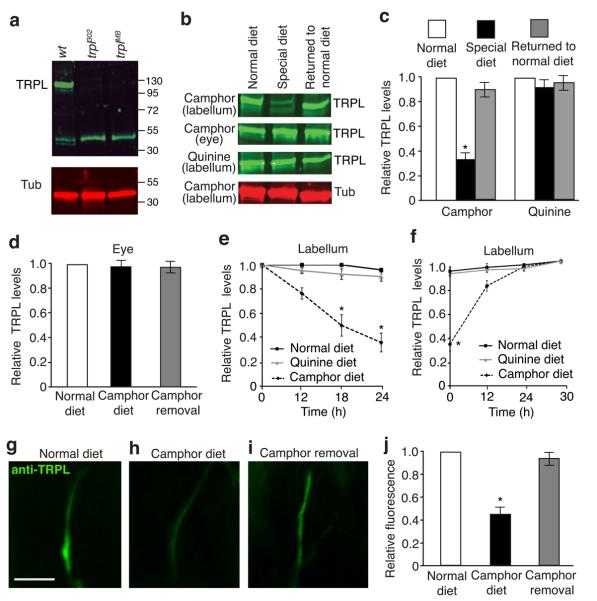
We tested whether changes in TRPL protein levels could provide a mechanism through which taste desensitization occurs in response to persistent exposure to a tastant. We fed wild-type flies camphor for 12—24 hours, and compared the level of TRPL to that in flies maintained on a normal diet. We found that the concentration of TRPL in the labellum declined in flies continuously exposed to camphor, and was reduced to 36.3 ± 3.1% the original level after 24 hours (Fig. 6a—c,e). Heterozygous trpl302/+ flies, which displayed normal behavioral and physiological responses to camphor (Supplementary Fig. 7a – c), expressed 78.4% ± 3.3% of the wild-type TRPL levels (Supplementary Fig. 7d, e). TRPL in the eyes was unaffected by the camphor diet (Fig. 6b,c; Supplementary Fig. 8d). 24 hours after camphor withdrawal, the amount of TRPL in the labellum returned to the original levels (Fig. 6b,c,f; Supplementary Fig. 8d). Consistent with the Western blot data, anti-TRPL staining in the GRN dendrites was reduced when the animals were maintained on a camphor diet. The expression level of TRPL in the GRNs was restored to normal 24 hours after camphor removal (Fig. 6g—j).
Figure 6. Effects of a camphor or normal diet on TRPL protein expression in GRNs.
(a) Western blot containing adult head extracts from wild-type and trpl mutant flies probed with anti-TRPL and anti-Tubulin. TRPL is ~123 kD. The ~55 kD band was detected non-specifically and therefore provided an additional loading control. (b) Western blots probed with anti-TRPL or anti-Tubulin. The flies were maintained on: 1) a normal diet, 2) a camphor or a quinine diet for 24 hours (special diet, indicated to the left), or 3) a special diet and then a normal diet for 24 hours (returned to normal diet). The labella and eye extracts were prepared from the same flies. The full-length blots are shown in Supplementary Fig 8d. (c) Quantification of TRPL levels in the labella after the flies were exposed for 24 hours to a 6 mM camphor diet or a 1 mM quinine diet. n=3 trials. *p=0.00012. (d) Quantification of TRPL levels in the eyes after the flies were exposed for 24 hours to a 6 mM camphor diet. (e) TRPL levels in the labella of flies maintained for 12—24 hours on a normal diet, a camphor diet, or a quinine diet. n=3 trials. *p=0.002 and 0.001 (18 and 24 h, respectively), (f) TRPL levels in flies kept on a camphor diet or a quinine diet for 24 hours followed by a normal diet for the indicated times. n=3 trials. *p=0.00011. (g–i) Immunocytochemical analysis of TRPL levels in the dendrites of GRNs after the flies were exposed to the following diets: (g) normal diet for 24 hours, (h) a camphor diet for 24 hours, (i) a camphor diet for 24 hours followed by a normal diet for 24 hours. The scale bar in f indicated 10 μm. (j) Quantification of the data in g-i. n=10 animals. *p=0.00076. The error bars indicate SEMs. One-way ANOVA tests with Bonferroni post-hoc analysis.
E3 ubiquitin ligase required for taste desensitization
To test whether TRPL down-regulation occurred via transcriptional repression, we examined the effect of a camphor diet on GFP levels (UAS-GFP) that were expressed under control of the trpl promoter (trpl-GAL4). The trpl-Gal4 appeared to reflect endogenous trpl expression as the TRPL protein was eliminated in the labellum, after expressing a cell death gene (UAS-hid) under the control of the trpl-Gal4 (Supplementary Fig. 8a). Of significance here, the concentration of GPF was indistinguishable in trpl-GAL4/UAS-GFP labella prepared from flies maintained on normal or camphor diets (Supplementary Fig. 8b,c). This suggested that there was no significant reduction in trpl transcriptional activity. Thus, we considered a post-transcriptional mechanism.
Ubiquitination is a widely used mechanism to control protein turnover and contributes to neuronal plasticity29. To address whether the reduction in TRPL occurred through ubiquitin-mediated protein degradation, we tested whether we could block taste adaptation and down-regulation of TRPL by misexpressing a yeast ubiquitin-specific protease, UBP230 in trpl GRNs. Remarkably, misexpressing UAS-UBP2 using the trpl-Gal4 abolished the reduction in TRPL protein and prevented taste adaptation in response to a camphor diet (Supplementary Fig. 9a,b,e). Expression of UBP2 also prevented the diet-induced decline in camphor-induced action potentials (Supplementary Fig. 9c,d). These data indicated that ubiquitin modification drove TRPL degradation.
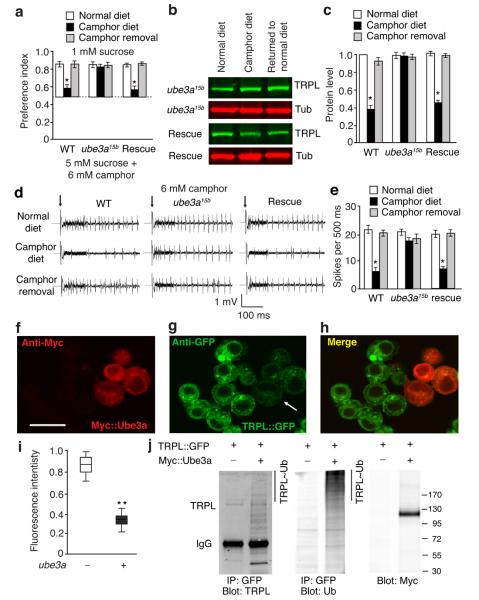
To identify which E3 ubiquitin ligase was required for TRPL turnover, we used RNAi knock-down to survey eight known E3 ubiquitin ligases31, 32. We found that selective RNAi knock-down of ube3a in trpl GRNs disrupted taste adaptation in response to a camphor diet (Supplementary Fig. 9f). A mutation disrupting Drosophila ube3a, affects dendritic remodeling of periphery sensory neurons, such as multi-dendritic neurons33, 34, suggesting a role for Ube3a in sensory systems. Indeed, mutation of ube3a (ube3a15b) rendered the animals unable to undergo taste adaptation, after being maintained on a camphor diet (Fig. 7a). Furthermore, pre-feeding a camphor diet to ube3a15a mutants did not reduce camphor-induced action potentials (Fig. 7d,e). Consistent with these findings, TRPL levels were unchanged in the proboscis of ube3a15a flies fed a camphor diet (Fig. 7b,c; Supplementary Fig 10d). We rescued the behavioral, physiological and TRPL turnover abnormalities in ube3a15a mutants with a genomic ube3a+ transgene. In support of the finding that Ube3a functions in camphor-induced down-regulation of TRPL, the ube3a reporter was co-expressed with trpl in GRNs (Supplementary Fig. 10a—c).
Figure 7. ube3a was required to form camphor-induced taste desensitization.
(a) Behavioral responses to 1 mM sucrose versus 5 mM sucrose plus 6 mM camphor. The tests were performed using wild-type flies, ube3a15b, or the mutant animals harboring a genomic ube3a+ rescue transgene. The animals were fed a normal diet, a camphor diet for 24 hours, or a camphor diet followed by returning to a normal diet for 24 hours. n=5 trials. *p=0.00082 (WT). (b and c) TRPL protein levels in the proboscis of wild-type, ube3a15b and rescued flies after feeding a normal or camphor diet. The full-length blots are shown in Supplementary Fig 10d. n=3 trials. *p=0.00078 (WT). (d and e) Tip recordings showing responses of S6 sensilla to 6 mM camphor. The recordings were performed using wild type, ube3a15b and ube3a15b flies containing the genomic ube3a+ rescue transgene (rescue). The animals were maintained on a normal diet, a camphor diet for 24 hours, or a camphor diet for 24 hours and then a camphor-free diet for 24 hours. n=8 animals. *p=0.0022 (WT). (f-h) Immunocytochemical analysis of TRPL expression in a TRPL::GFP S2 cell line transfected with a vector encoding Ube3a with an N-terminal Myc tag (Myc::Ube3a). Myc::Ube3a and TRPL::GFP expression was revealed by anti-Myc and anti-GFP staining, respectively. The scale bar indicates 10 μm. (i) Quantification of the fluorescence intensity of TRPL::GFP in the TRPL::GFP S2 cell line transiently-expressing Myc::Ube3a. n=3 trials with ~10 cells for each trial. **p=0.00013. (j) Cell lysates of the TRPL::GFP S2 cell line with or without Ube3a::Myc. TRPL::GPF was immunoprecipitated with anti-TRPL and the blot was probed with anti-Ubiquitin. The loading controls were blotted with anti-Myc and anti-TRPL. Error bars indicate SEMs. One-way ANOVA tests with Bonferroni post-hoc analysis.
To test if Ube3a directly regulated TRPL stability through ubiquitination, we transiently expressed Myc-tagged Ube3a in S2 cells that stably expressed TRPL::GFP, since the TRPL levels in the labellum were too low to perform biochemical analyses. As revealed by immunocytochemistry, there was a reduction in TRPL::GFP in S2 cells co-expressing Ube3a, as compared to S2 cells that did not express Ube3a (Fig. 7f—i). This suggested that Ube3a targeted TRPL::GFP for degradation in vitro. To test whether TRPL::GFP was ubiquitinated by Ube3a in S2 cells, we co-immunoprecipitated TRPL::GFP with GFP antibodies and probed the Western blot with ubiquitin antibodies. After expressing Ube3a, ubiquitination of TRPL::GFP was elevated (Fig. 7j). The ladder of bands was due to differential ubiquitination (Fig. 7j).
Synaptic connections reduced by long-term camphor feeding
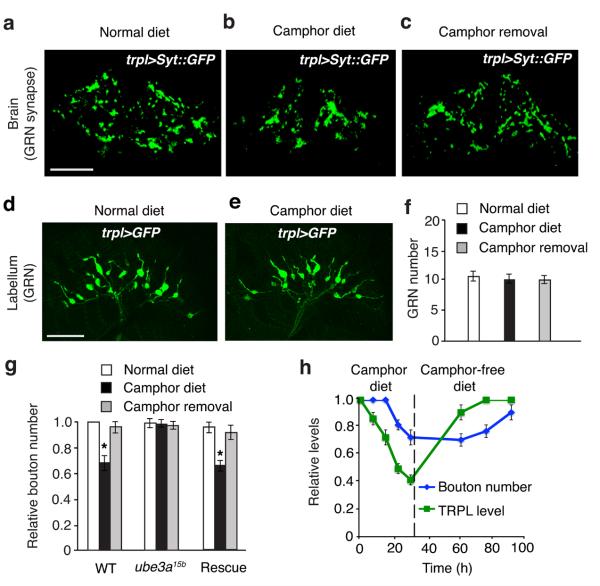
As synapses in adult animals including Drosophila can be shaped by neural activity and experience35-37, we asked if there were changes in the synaptic connections formed by trpl GRNs in the SOG. To observe synaptic boutons, we expressed a GFP-tagged synaptic vesicle protein, synaptotagamin (Syt::GFP)38, in trpl GRNs using the trpl-Gal4 and UAS-syt::GFP. Syt::GFP was localized selectively to the synaptic connection sites of the axonal terminals of TRPL GRNs (Fig. 8a). This synaptic reporter highlighted the overall morphology of individual boutons, which appeared as discrete punctate structures. As trpl was expressed in only a small subset of GRNs, the boutons formed by trpl GRNs in the SOG were structurally well defined and spaced, thereby facilitating quantification of bouton numbers.
Figure 8. Effects of camphor exposure on the number of synaptic boutons formed by trpl-expressing GRNs in the SOG.
(a–c) Confocal fluorescent microscopy showing boutons (stained with GFP) in trpl+ animals maintained on a: (a) normal diet, (b) camphor diet for 24 hours, and (c) camphor-free diet after pre-camphor exposure for 24 hours. The boutons were labeled by expressing UAS-syt::GFP under the control of the trpl-GAL4. (d—f ) GRN morphology in the labella of wild-type animals maintained for 24 hours on a normal or camphor diet, respectively. The GRNs were labeled by expressing UAS-mCD8::GFP under the control of the trpl-GAL4. (g) Quantification of bouton numbers formed by trpl GRNs in wild type and ube3a15b animals. n=7 trials. *p=0.0018 (WT). One-way ANOVA tests with Bonferroni post-hoc analysis. (h) The relative time course of TRPL turnover and synapse remodeling. Error bars indicate SEMs. The scale bars in a and d indicate 10 μm.
The camphor-adapted animals showed a mild decrease in bouton numbers in the SOGs, relative to flies maintained on a normal diet (Fig. 8b,c,f). In contrast, exposure of ube3a15b animals to a camphor diet did not cause a reduction in the number of boutons (Fig. 8f). We compared the number of GRNs between naïve and camphor fed animals and found that there was no significant difference in total GRNs in the proboscis (Fig. 8d—f). Bouton elimination occurred later than TRPL down-regulation (Fig. 8g, Supplementary Fig 10e). After camphor removal, there was prominent bouton regrowth, which took place following the recovery of TRPL expression (Fig. 8g, Supplementary Fig 10e).
Discussion
Modification of taste preference by food experience
Depending on food properties, an animal decides to accept or reject the food. In most terrestrial animals, sweet substances are assumed to provide nutrients, whereas many bitter compounds are correlated with poisons. However, this latter assumption is flawed, as many bitter foods are safe and nutritious7. Consequently, many animals learn to accept formerly unpalatable, bitter tasting foods, but only if they are safe, and if more appealing options are unavailable. While changes in the mammalian gustatory cortex are associated with diet-induced changes in taste preference1, prior to the current work, it was unclear the nature of the molecular modifications in taste receptor cells that contribute to environmentally-induced modifications of food selection.
We found that fruit flies, like many other animals, including insects such as locusts, and moths5, decrease food avoidance to certain bitter foods after prolonged exposure. The flies decreased their aversion to the unappealing tastant, camphor, in response to dietary experience. However, the flies did not form adaptation to all bitter tastants, including quinine, strychnine or lobeline.
Taste preference modified by TRPL down-regulation in GRNs
The decline in rejection of camphor was controlled in peripheral sensory neurons through a reversible decline in the concentration of the camphor-activated TRPL channel in dendrites. Because TRPL was activated by camphor but not other unpalatable tastants such as quinine, down-regulation of this channel selectively affected the aversion to camphor.
Two observations support the conclusion that the down-regulation of TRPL contributes to taste adaptation. First, removal of camphor from the diet resulted in a return to the original TRPL levels, and a restoration of the former aversion to camphor. Second, during camphor exposure, an E3 ubiquitin ligase, Ube3a, targeted TRPL for degradation, thereby decreasing TRPL expression levels in the GRNs. Loss of Ube3a eliminated the camphor-diet induced down-regulation of TRPL, and prevented taste desensitization. This finding also highlighted that the diet-induced reduction in TRPL was mediated by protein turnover. Consistent with this mechanism underlying the decline in TRPL levels, rather than a reduction in trpl transcription, the activity of the trpl reporter was indistinguishable in flies maintained on normal or camphor diets. Thus, this work identifies a molecular mechanism in peripheral sensory neurons that underlies plastic, diet-induced alterations in food preference. An open question concerns the link between TRPL activation and down-regulation of the channel. Given that TRPL is a Ca2+ permeable channel39-41, Ube3a activity might be directly or indirectly activated by a rise in Ca2+.
Taste preference modification and synapse remodeling
During the formation of taste adaptation at the behavioral level, there was a second change that occurred. Following the down-regulation of TRPL, the number of boutons at the GRN axonal terminals in the SOG declined. Thus, the synapse loss appeared to be a secondary consequence of the decline in TRPL. In further support of this conclusion, the number of synapses was unchanged in the ube3a mutant, which did not show a reduction in TRPL protein. Since the morphological change was reversible upon withdrawal of a sustained camphor diet, GRNs in adult Drosophila undergo cellular modification. An important future question concerns the identity of the molecular pathway bridging the long-distance communication between the decline of TRPL in the dendrites and bouton pruning in the axons.
We suggest that the synapse elimination may be insufficient to cause the camphor desensitization, but might synergize with TRPL down-regulation to decrease the distaste for camphor. In summary, our work revealed that food experience can modify behavior by altering signaling at both the dendrites and axons of the GRNs.
Mechanisms similar to those described here may represent an evolutionarily conserved strategy that contributes to chemosensory desensitization. It is noteworthy that a worm TRPV channel, OSM-9, functions in both olfactory adaptation42 and in adaptation to NaCl43. Thus, a similar mechanism of ubiquitination-mediated down-regulation of OSM-9 might contribute to chemosensory desensitization in C. elegans. Finally, diet induced morphological changes in mammalian taste buds have been reported44. The current work raises the possibility that changes in taste preference in other animals including vertebrates may be mediated by alterations in the concentration of receptors and channels in taste receptor cells and subsequent modifications in synaptic connections.
Methods
Drosophila stocks
We raised all flies at 22—25°C on standard cornmeal/molasses/yeast media unless specified otherwise. The fly stocks used were: w1118, Gr66a-Gal445, Gr5a-Gal445, Gr66aex8320, Gr33a121, Gr66a-I-GFP28, Gr5a-I-GFP28, UAS-mCD8::GFP46, ube3a15b (Wu et al. 2008), g[6myc::ube3a+] (Wu et al., 2008), trpl30216, trplMB03075 (Bloomington Stock Center), trp34347, trpγ1 (Q. Ren, Y. Lee, M. Fowler and C. Montell unpublished), nan36a48, trpA1149, pyx250, wtrw149, pain251, trpml152, amo153, norpAP24 (also known as norpA36)54and dGqα155. We backcrossed the trpl302 stock and all the other mutants subjected to behavioral tests for five generations into a w1118 background. We used w1118 as the control flies. For brevity, the wild-type (wt) designation refers to w1118. UAS-UBP2 30 and the RNAi lines used to knock down expression of eight ubiquitin ligases [neurolized, su(dx), ari-1, ari-2, highwire, slmb, ube3a and parkin] were obtained from the Bloomington Stock Center and the VDRC, respectively.
Generation of transgenic flies
We generated the UAS-trpl transgene by subcloning the trpl cDNA into the pUAST vector. To generate the trpl-Gal4 construct, we subcloned a 4327 base pair region flanking the 5’ end of the trpl gene (2R: 5636895—5641221, http://flybase.org/cgi-bin/gbrowse/dmel/?ref=2R;start=5638509;stop=5654330;nav4=1;plugin=) into the pCasPeR vector. The transgenic flies were generated by P-element-mediated germ-line transformation (BestGene Inc.).
Immunocytochemistry
To perform the immunocytochemistry, we dissected fly tissues were in 0.1 M phosphate buffer (pH 7.2) and fixed in 4% paraformaldehyde at room temperature for ~30 minutes. The primary antibodies used were: rabbit anti-TRPL (1:200), rabbit anti-GFP (1:500, Invitrogen, A-11122), mouse anti-GFP (1:500, Invitrogen, A-11121) and mouse anti-nc82 (1:50, Developmental Studies Hybridoma Bank). The secondary antibodies used were: donkey anti-rabbit 488 (1:200, Jackson ImmunoResearch, 711-545-152) and goat anti-mouse 594 (1:200, Jackson ImmunoResearch, 115-585-003).
To perform the immunocytochemistry using S2 cells, we transfected the cells, which were grown on coverslips in Petri dishes. 24 hours later, we fixed the cells with 4% paraformaldehyde for 30 min at room temperature. After washing with 0.2% Triton-X100 in 1x phosphate buffer, we double-stained the cells with mouse anti-Myc (1:10, Invitrogen, R950-25) and rabbit anti-GFP (1:400, Invitrogen, A-11122) at 4°C overnight. The secondary antibodies were donkey anti-mouse 594 (1:200, Jackson ImmunoResearch, 715-585-150) and goat anti-rabbit 488 (1:200, Jackson ImmunoResearch, 111-545-003). We repeated all immunostaining experiments at three times.
We acquired the images using a Zeiss LSM510-Meta confocal microscopy (Zeiss Axiovert 200 with 510-Meta confocal module, JHU SOM Microscopy Facility).
Two-way choice feeding assay
To perform the two-way choice assays, we developed a modified assay that resulted in improved reproducibility. The assay employed a 10 × 35 mm Petri dishes, which were divided into two equal zones by a 3 × 3 × 35 mm (height × width × length) plexiglass strip melted along the bottom of the dish. We mixed the bitter or sweet tastants with 0.4% agar as well as red dye (sulforhodamine B, Sigma, S9012; 0.02% final concentration) or blue dye (brilliant blue FCF, Wako chemical, 027-18142; 0.01% final concentration). 1 ml food mixture was added to each side of the dish. Once the food solidified, we gently transferred two-day old flies that had been pre-starved for 24 hours into each dish. We used ~70 flies per dish since larger populations of animals caused anomalous effects due to overcrowding in the Petri dishes. The two-way food choice assays were conducted in the dark for 90 minutes. If the animals were fed a special diet consisting of 1 mM sucrose and a bitter compound (see below), the animals were not pre-starved before performing the assays since the flies were still in a state of hunger. We scored flies with blue (NB), red (NR) or purple (NP) abdomens using light microscopy in a blind fashion. The preference indexes (PI) were calculated using the formula as follows: PI= (NB + ½ NP)/(NB + NR + NP) or (NR + ½ NP)/(NB + NR + NP).
To verify the reliability of the visually based scoring of abdominal colors, we performed spectrophotometric analysis. Specifically, immediately after conducting the two-way choice assays, the midguts were dissected. We used 20 animals per assay, since this provided an adequate concentration of dye for quantification. We homogenized the midguts in 50 μl 50% ethanol, removed the debris by high-speed centrifugation, and used the supernatants were used for the colormetric assays. The absorbances of blue and red were measured using a NanoDrop spectrophotometer at 630 nm and 565 nm, respectively.
Effect of diet on taste aversion
To test for diet-induced changes in taste aversion, we added 2 ml of each tastant solution to a folded piece of Kimwipe paper (10 × 10 cm, KIMTECH), which was placed in a vial (outer diameter × height, 28.5 × 95 mm, VWR). The special diets consisted of 1 mM sucrose combined with one of the following bitter substances: 6 mM camphor, 1 mM quinine, 6 mM caffeine, 1 mM strychnine and 1 mM lobeline. ~70 two-day old flies were added to the vials and allowed to feed for 24 hours unless indicated otherwise. Since the 1 mM sucrose/bitter diets provided poor sources of nutrients, the flies were not satiated at the conclusion of the 24 hour feeding period. Therefore, they were immediately subjected to the two-way choice feeding assay as described above, without the inclusion of a starvation period.
Direct airborne repellent test (DART) assays
The set up for performing the DART assays was as described previously26. Briefly, we dissolved 6 mM camphor in DMSO, which we placed at the end of one 15 ml Falcon tube (tube A), and the DMSO control at the end of the other tube (Tube B). We then inserted screens near the ends of the tubes to prevent contact between the chemicals and the flies. We transferred 50 – 70 two-day old flies to the tubes, and connected the two tubes with tape. The flies were then incubated for 30 minutes, photographed, and the numbers of flies near the ends of the tubes (between the bottoms and the 5 ml marks) were tabulated. The avoidance indexes (A.I.s) were calculated according to the following formula: A.I = N B – NA /NB + NA.
Toxicity assays
We used two assays to determine the toxicities of unpalatable tastants. Both tests used the following final concentrations, which elicited similar avoidance responses in naïve animals: 6 mM camphor, 1 mM quinine, 6 mM caffeine, 1 mM strychnine and 1 mM lobeline. In one assay, each of these tastants was diluted in 1 mM sucrose. We soaked a paper ball folded from a piece of Kimwipe paper (length × width, 10 × 10 cm, KIMTECH) with 3 ml of each of the tastant solutions. We placed the wet paper ball snuggly at the bottom of the vials (outer diameter × height, 28.5 × 95 mm, VWR). We used 20 two-day old flies per assay, which provided sufficient numbers for quantification over the five day assay. Every 24 hours we scored the number of dead flies, which we assessed as the flies that remained immobile after shaking the vials. As a control, flies were fed 1 mM sucrose only. In a second assay, we injected 5 μl of one of the chemicals directly into the abdomen of an adult fly (15 animals/chemical). 5 μl of water was injected as a negative control. Following the injections, the animals were maintained on 1 mM sucrose food. The viability of the animals was monitored every 24 hours.
Tip recordings
We dissolved the bitter tastants in 1 mM KCl electrolyte, which we then added to the recording pipets. The tip recordings were performed as described20. We used S6 sensilla to perform the recordings with bitter tastants. After ectopic expression of trpl in sugar GRNs, we recorded the responses to 6 mM camphor in L2 sensilla. For the responses to 50 mM sucrose, we performed tip recordings in L4 or L2 sensilla with 30 mM tricholine citrate as the electrolyte.
Ca2+ imaging
We expressed TRPL in Drosophila S2 cells by transfecting the cells with a vector (pMT-EGFP-trpl) encoding a TRPL::eGFP fusion protein. Copper sulfate was added to the media for 24 hours to a final concentration of 300 μM to induce TRPL::GFP expression. The transfected cells were plated on a glass bottom Petri dish (10 × 35 mm, MatTek) and incubated for 24 hours. After washing with Ca2+ imaging buffer (140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM D-glucose and 10 mM HEPES, pH 7.4), the cells were incubated with 2 μM Fura-2AM (Invitrogen) in Ca2+ imaging buffer for 20 minutes. After washing with the imaging buffer without CaCl2, we mounted the glass bottom dishes on the stage of a Nikon Eclipse TE 300 inverted microscope. We applied either 6 mM camphor or 6 mM quinine to the Ca2+ imaging buffer, and subsequently added CaCl2 to the bath solution to a final concentration of 2 mM. An increase in free intracellular Ca2+ was monitoring by assaying changes in the A340/A380 absorption ratio (F340/F380) in conjunction with Nikon NIS-element imaging software. S2 cells expressing TRPL::eGFP were identified by on the basis of positive GFP signals, which were detected using a GFP filter installed in the microscope. The data were acquired using a CCD camera (Roper Scientific) attached to the microscope.
Ubiquitination of TRPL in S2 cells
The S2 cell line stably expressing TRPL::GFP was cultured using Schneider’s medium containing 300 μg/ml hygromycin B (Roche). After growing to a confluency of ~70%, the cells were transiently co-transfected with constructs encoding UAS-Ube3a::Myc and Act-Gal4 DNA (1:1 molar ratio) using Effectene transfection reagent (Qiagen).
To perform co-IP experiments, 24 post-transfection, we harvested the cells, which we then quickly lysed with 1x RIPA buffer [50 mM Tris-HCl; 150 mM NaCl; 1.0% Igepal CA-630 (NP-40), 0.5% sodium deoxycholate and 0.1% sodium dodecyl sulfate. pH 8.0] containing protease inhibitor (Roche). Normally, 100 μl RIPA buffer was used to lyse 1×106 cells. After spinning down the cells, we incubated the supernatant with protein A agarose beads for 1 hour at 4°C (Santa Cruz Biotechnology, sc-2001) to reduce non-specific binding. Then the pre-cleaned cell lysate was incubated with primary antibodies (0.5 μg antibodies/100μl lysate) and protein A agarose beads (10 μl beads/100 μl lysate) for 24 hours at 4°C. After washing with 1X RIPA buffer, the proteins were fractionated by SDS-PAGE and blotted with anti-ubiquitin (Santa Cruz Biotechnology, sc-8017).
Labella and eyes were dissected from the same flies. Extracts from either 20 labella or 2 compound eyes were fractioned per lane. To perform the Western blots, we used the following antibodies: 1) primary antibodies: rabbit anti-TRPL (1:1000) and mouse anti-tubulin (1:4000, DSHB), and 2) secondary antibodies (infrared fluorescent probes conjugated secondary antibodies; LI-COR Bioscience): IRDye 800CW goat anti-Mouse (1:2000, LI-Core, 926-32213) and IRDye 680 goat anti-rabbit (1:2000, LI-Core, 926-68071). To detect and analyze the fluorescent signals we used The Odyssey® Infrared Imaging System (LI-COR Bioscience). We performed the Western blots at least three times.
Statistical analyses
We used two-way unpaired Student’s t-tests and one-way or two-way ANOVA with Bonferroni post-hoc analysis to determine the statistical significances of pair wise and multiple comparisons, respectively. The data were collected in a random manner and no data were excluded. Data distribution was assumed to be normal but this was not formally tested. No statistical methods were used to predetermine sample sizes but our sample sizes are similar to those reported in previous publications20, 24, 26, 56.
Supplementary Material
Acknowledgments
We thank Janice Fischer, Kristin Scott and the Bloomington Stock Center for providing fly stocks, and Baruch Minke for the pMT-trpl-eGFP construct. This work was supported by grants to C.M. from the NIDCD (DC007864) and the NEI (EY10852).
This project was supported by a grant to C.M. from the NIDCD (DC007864).
References
- 1.Galindo MM, Schneider NY, Stahler F, Tole J, Meyerhof W. Taste preferences. Prog. Mol. Biol. Transl. Sci. 2012;108:383–426. doi: 10.1016/B978-0-12-398397-8.00015-0. [DOI] [PubMed] [Google Scholar]
- 2.Mennella JA, Trabulsi JC. Complementary foods and flavor experiences: setting the foundation. Ann. Nutr. Metab. 2012;60(Suppl 2):40–50. doi: 10.1159/000335337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke L. The importance of exposure for healthy eating in childhood: a review. J. Hum. Nutr. Diet. 2007;20:294–301. doi: 10.1111/j.1365-277X.2007.00804.x. [DOI] [PubMed] [Google Scholar]
- 4.Ackroff K, Weintraub R, Sclafani A. MSG intake and preference in mice are influenced by prior testing experience. Physiol. Behav. 2012;107:207–217. doi: 10.1016/j.physbeh.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Glendinning JI, Domdom S, Long E. Selective adaptation to noxious foods by a herbivorous insect. J. Exp. Biol. 2001;204:3355–3567. doi: 10.1242/jeb.204.19.3355. [DOI] [PubMed] [Google Scholar]
- 6.del Campo ML, Miles CI, Caillaud MC. Effects of experience on the physiology of taste discrimination in insects. In: Newland PL, Cobb M, Mario-Poll F, editors. Insect Taste. Taylor & Francis Group; New York: 2009. pp. 205–242. [PubMed] [Google Scholar]
- 7.Glendinning JI. Is the bitter rejection response always adaptive? Physiol. Behav. 1994;56:1217–1227. doi: 10.1016/0031-9384(94)90369-7. [DOI] [PubMed] [Google Scholar]
- 8.Elkobi A, Ehrlich I, Belelovsky K, Barki-Harrington L, Rosenblum K. ERK-dependent PSD-95 induction in the gustatory cortex is necessary for taste learning, but not retrieval. Nat Neurosci. 2008;11:1149–1151. doi: 10.1038/nn.2190. [DOI] [PubMed] [Google Scholar]
- 9.Masek P, Scott K. Limited taste discrimination in Drosophila. Proc. Natl. Acad. Sci. USA. 2010;107:14833–14838. doi: 10.1073/pnas.1009318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber B, Stocker RF, Tanimura T, Thum AS. Smelling, tasting, learning: Drosophila as a study case. Results Probl. Cell Differ. 2009;47:1–47. doi: 10.1007/400_2008_9. [DOI] [PubMed] [Google Scholar]
- 11.Venkatachalam K, Montell C. TRP channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu. Rev. Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 13.Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- 14.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 15.Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- 16.Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- 17.Montell C. A taste of the Drosophila gustatory receptors. Curr. Opin. Neurobiol. 2009;19:345–353. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 19.Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon SJ, Köttgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr. Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr. Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SH, et al. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc. Natl. Acad. Sci. USA. 2010;107:8440–8445. doi: 10.1073/pnas.1001425107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang K, et al. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meunier N, Marion-Poll F, Rospars JP, Tanimura T. Peripheral coding of bitter taste in Drosophila. J. Neurobiol. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- 25.Jeong YT, et al. An Odorant Binding Protein required for suppression of sweet taste by bitter chemicals. Neuron. 2013 doi: 10.1016/j.neuron.2013.06.025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon Y, et al. Drosophila TRPA1 channel Is required to avoid the naturally occurring insect repellent citronellal. Curr. Biol. 2010;20:1672–1678. doi: 10.1016/j.cub.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parnas M, et al. Carvacrol is a novel inhibitor of Drosophila TRPL and mammalian TRPM7 channels. Cell Calcium. 2009;45:300–309. doi: 10.1016/j.ceca.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Hegde AN. The ubiquitin-proteasome pathway and synaptic plasticity. Learning & memory. 2010;17:314–327. doi: 10.1101/lm.1504010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiAntonio A, et al. Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature. 2001;412:449–452. doi: 10.1038/35086595. [DOI] [PubMed] [Google Scholar]
- 31.Watts RJ, Hoopfer ED, Luo L. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 2003;38:871–885. doi: 10.1016/s0896-6273(03)00295-2. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Nishimura I, Imai Y, Takahashi R, Lu B. Parkin suppresses dopaminergic neuron-selective neurotoxicity induced by Pael-R in Drosophila. Neuron. 2003;37:911–924. doi: 10.1016/s0896-6273(03)00143-0. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, et al. A Drosophila model for Angelman syndrome. Proc. Natl. Acad. Sci. USA. 2008;105:12399–12404. doi: 10.1073/pnas.0805291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Y, et al. The Drosophila homologue of the Angelman syndrome ubiquitin ligase regulates the formation of terminal dendritic branches. Hum. Mol. Genet. 2009;18:454–462. doi: 10.1093/hmg/ddn373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eddison M, et al. arouser reveals a role for synapse number in the regulation of ethanol sensitivity. Neuron. 2011;70:979–990. doi: 10.1016/j.neuron.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 37.Foeller E, Feldman DE. Synaptic basis for developmental plasticity in somatosensory cortex. Curr. Opin. Neurobiol. 2004;14:89–95. doi: 10.1016/j.conb.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Zhang YQ, Rodesch CK, Broadie K. Living synaptic vesicle marker: synaptotagmin-GFP. Genesis. 2002;34:142–145. doi: 10.1002/gene.10144. [DOI] [PubMed] [Google Scholar]
- 39.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 40.Xu XZ, Li HS, Guggino WB, Montell C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–1164. doi: 10.1016/s0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]
- 41.Hu Y, et al. Appearance of a novel Ca2+ influx pathway in Sf9 insect cells following expression of the transient potential-like (trpl) protein of Drosophila. Biochem. Biophys. Res. Commun. 1994;201:1050–1056. doi: 10.1006/bbrc.1994.1808. [DOI] [PubMed] [Google Scholar]
- 42.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J. Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jansen G, Weinkove D, Plasterk RH. The G-protein γ subunit gpc-1 of the nematode C.elegans is involved in taste adaptation. EMBO J. 2002;21:986–994. doi: 10.1093/emboj/21.5.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shuler MG, Krimm RF, Hill DL. Neuron/target plasticity in the peripheral gustatory system. J. Comp. Neurol. 2004;472:183–192. doi: 10.1002/cne.11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr. Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 46.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 47.Wang T, Jiao Y, Montell C. Dissecting independent channel and scaffolding roles of the Drosophila transient receptor potential channel. J. Cell Biol. 2005;171:685–694. doi: 10.1083/jcb.200508030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J, et al. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- 49.Kwon Y, Shim HS, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat. Neurosci. 2008;11:871–873. doi: 10.1038/nn.2170. [DOI] [PubMed] [Google Scholar]
- 50.Lee Y, et al. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat. Genet. 2005;37:305–310. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- 51.Tracey WD, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 52.Venkatachalam K, et al. Motor deficit in a Drosophila model of mucolipidosis type IV due to defective clearance of apoptotic cells. Cell. 2008;135:838–851. doi: 10.1016/j.cell.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watnick TJ, Jin Y, Matunis E, Kernan MJ, Montell C. A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr. Biol. 2003;13:2179–2184. doi: 10.1016/j.cub.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Bloomquist BT, et al. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 55.Scott K, Becker A, Sun Y, Hardy R, Zuker C. Gqα protein function in vivo: genetic dissection of its role in photoreceptor cell physiology. Neuron. 1995;15:919–927. doi: 10.1016/0896-6273(95)90182-5. [DOI] [PubMed] [Google Scholar]
- 56.Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat. Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.