Abstract
Discoidin Domain Receptor 1 (DDR1) is a widely expressed receptor tyrosine kinase (RTK) which regulates cell differentiation, proliferation and migration and remodeling of the extracellular matrix. Collagen(s) are the only known ligand for DDR1. We have previously reported that collagen stimulation leads to oligomerization of the full length receptor. In this study we investigated the effect of oligomerization of the DDR1 extracellular domain (ECD) pre and post ligand binding. Solid phase binding assays showed that oligomers of recombinant DDR1-Fc bound more strongly to collagen compared to dimeric DDR1-Fc alone. In addition, DDR1-Fc itself could oligomerize upon in-vitro binding to collagen when examined using atomic force microscopy. Inhibition of dynamin mediated receptor endocytosis could prevent ligand induced endocytosis of DDR1-YFP in live cells. However inhibition of receptor endocytosis did not affect DDR1 oligomerization. In summary our results demonstrate that DDR1 ECD plays a crucial role in receptor oligomerization which mediates high-affinity interactions with its ligand.
Keywords: Collagen, discoidin domain receptor, extracellular domain, oligomerization, atomic force microscopy
1. Introduction
Discoidin domain receptors (DDRs) are widely expressed receptor tyrosine kinases (RTKs) that bind to and get activated by collagen(s), the major component of the extracellular matrix (Vogel et al., 1997; Shrivastava et al., 1997). There are two known members of the DDR family; namely, DDR1 and DDR2, both distinguished from other RTKs by the presence of an extracellular discoidin (DS) domain and an unusually long juxtamembrane (JM) region. DDRs have an unusually slow activation process compared to other RTKs requiring longer stimulation to achieve full scale ligand-induced tyrosine phosphorylation, the reasons behind which are not completely understood (Vogel et al., 1997; Shrivastava et al., 1997).
In vitro work by us and others revealed that high affinity interaction with collagen requires dimerization and/or pre-oligomerization of DDR1 (Agarwal et al., 2007; Abdulhussein et al., 2008; Noordeen et al., 2006). It has also been reported that a significant percentage of the DDR1 population forms ligand independent dimers on the cell-surface (Abdulhussein et al., 2008; Noordeen et al., 2006; Mihai et al., 2009). Using fluorescence microscopy and live cell imaging we had also shown that ligand binding results in receptor oligomerization and endocytosis (Mihai et al., 2009). The specific sites in DDR1 responsible for receptor dimerization have been described to be the leucine zipper motif in the transmembrane domain (Noordeen et al., 2006) and the cysteine residues in the JM region of the DDR1 extracellular domain (ECD) (Abdulhussein et al., 2008). While it is speculated that regions in DDR1 ECD may contribute to receptor dimerization (Carafoli et al., 2012), the role of the ECD in receptor oligomerization is not completely understood.
In this study we investigated how oligomerization of DDR1 ECD pre- and post- binding to collagen impacts its ligand-binding ability. Solid phase binding assays were used to compare how pre-oligomerization of recombinant DDR1 ECD (dimeric DDR1-Fc) impacts its collagen binding ability. Atomic force microscopy (AFM) was used to ascertain oligomerization of DDR1-Fc post ligand binding. A YFP tagged full length DDR1 was used to examine receptor oligomerization on the cell surface. Our results generate new insights into how oligomerization of the DDR1 ECD is crucial for this ligand-receptor interaction.
2. Materials and Methods
2.1. Reagents
Fc-tagged ECDs of human DDR1 and TrkB were purchased as recombinant protein from R&D Biochemicals, MN and reconstituted in sterile phosphate buffered saline (PBS) at a stock concentration of 100 μg/ml. Bovine dermal collagen type I was obtained from Advanced BioMatrix. Mouse monoclonal anti-DDR1 (against ECD) was from R&D Biochemicals, MN. Anti-Fc antibody was from Jackson Immunoresearch, (West Grove, PA). Anti-mouse and anti-goat IgG horseradish-peroxidase-conjugated antibodies were obtained from Santa Cruz Biotech. The DDR1–YFP construct was generated using a plasmid containing the entire mouse DDR1b sequence (obtained from Regeneron Pharmaceuticals, Tarrytown, NY), as previously described (Mihai et al., 2009). Dynasore, an inhibitor for dynamin mediated endocytosis (Macia et al., 2006) was purchased from Sigma-Aldrich, St. Louis, MO. Glass-bottom culture dishes for live cell microscopy were obtained from MatTek Glassware (Ashland, MA).
2.2. Solid Phase Binding Assays
Collagen was immobilized in 96-well micro-titer plates by incubating the wells with 25 μg/ml of collagen in phosphate buffer saline (PBS), overnight at 37ºC. Thereafter, the plates were washed three times with 200μl TBS (tris buffered saline) (Bio-Rad, Hercules, CA) containing 0.05% tween (GE Healthcare, Uppsala, Sweden), followed by blocking with 300μl of 1% bovine serum albumin (Santa Cruz Biotechnology, Santa Cruz, CA) with 0.05% tween overnight at 4 ºC. The wells were washed again three times with TBS-tween and incubated with 100 μl of recombinant DDR1 or TrkB ECDs at concentrations ranging from 0 to 10 μg/ml, overnight at 4ºC. To pre-oligomerize DDR1 ECD, the protein was incubated with an equal mass of anti-Fc antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) in PBS at 4ºC overnight as previously described (Agarwal et al., 2007). Collagen-coated wells were incubated with non- or pre-oligomerized proteins containing equal amounts of DDR1 ECD. To detect binding of recombinant proteins to collagen, the plates were washed and thereafter probed with anti-Fc or anti-DDR1 antibodies, followed by washing and incubating with HRP conjugated secondary antibodies. Bound protein was detected by adding 100μl of 3,3′,5,5′-tetramethylbenzidine, (TMB) to each well for 20 min at room temperature protected from light. The reaction was stopped using 1N HCl (Sigma Aldrich, St. Louis, MO) solution, and the absorbance of the plates was recorded at 450 nm using a spectrophotometer. All experiments were performed at least three times.
2.3. Atomic Force Microscopy
Collagen (1 μg/ml) was mixed with recombinant DDR1-Fc (0.2 μg/ml) in ice cold PBS and incubated at 4ºC. As a control DDR1-Fc and collagen alone samples were also incubated in PBS under similar conditions. At specific time points (0, 1, 2, 4 and 24 hrs), the samples were aliquoted onto chilled and freshly cleaved mica substrates, incubated for 5 minutes, washed and air dried and subjected to AFM imaging using the Multimode AFM (Digital Instruments, Santa Barbara, CA). AFM imaging was performed in tapping mode in ambient air using NSC15 cantilevers (Micromasch, Estonia) with a nominal spring constant of 40 Nm−1. Both height and amplitude images were recorded at 512 lines per scan direction. Topographic heights of DDR1-Fc in samples with or without collagen were measured from AFM images, by the section analysis feature of the Nanoscope software. At least n= 50 particles were analyzed per sample.
2.4. Fluorescence Microcopy
HEK 293 cells were seeded on glass bottom culture dishes and transiently transfected with DDR1-YFP construct using Fugene 6 transfection reagent (Roche, Boston, MA). After 24 hours of transfection the cells were incubated with 80 μM Dynasore for 30 min prior to stimulation with collagen type 1 (10 μg/ml) for 30 min at 37º C. At the end of the stimulation interval, the cells were membrane stained using CellMask Deep Red (Molecular Probes, Eugene, OR) at 37º C for 5 min. Following membrane staining the cells were fixed in 2% formalin buffered in PBS for 10 min and imaged in PBS. Similar samples were prepared using cells not treated with dynasore, and with cells not stimulated with collagen. Imaging was done using a Zeiss LSM 510 confocal microscope with a 63x water immersion objective. YFP was imaged using the 488 nm line of an Argon laser and the membrane stain was imaged using a 633 nm HeNe laser. For each channel a z stack of 1 μm optical slices was acquired in 1 μm steps, covering the entire z range of the cell monolayer.
3. Results
DDR1-Fc dimers and antibody induced DDR1-Fc oligomers have previously been characterized by us using atomic force microscopy (AFM) and SDS PAGE (Agarwal et al., 2007). DDR1-Fc appears as 91 and 197 kD in reducing and non-reducing conditions respectively on SDS PAGE. The anti-Fc mediated oligomers are approximately 600kD as ascertained using size exclusion chromatography (Mihai et al, 2006). AFM analysis on samples in a fluid environment revealed that DDR1-Fc dimers and antibody mediated oligomers had an average topographic height of 3.24(±0.7) and 4.02(±0.8) nm, respectively.
3.1. Pre-oligomerization of DDR1 ECD increases its collagen binding affinity
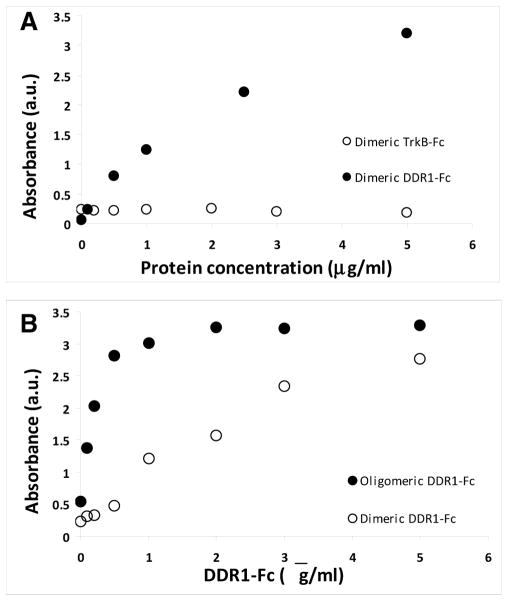
To confirm the ability of dimeric DDR1-Fc to bind to collagen, DDR1-Fc or TrkB-Fc were incubated over immobilized collagen in solid-phase binding assays and the bound protein detected using anti-Fc antibodies. As shown in Figure 1A, DDR1-Fc specifically bound to collagen while the control protein TrkB-Fc showed no significant binding. To examine if pre-oligomerization of DDR1 ECD impacts its ability to bind to collagen, similar assays were performed by incubating DDR1-Fc before and after pre-oligomerization to collagen coated wells and the bound protein detected using anti-DDR1 antibodies. The DDR1-Fc dimers and antibody-induced oligomers of DDR1-Fc used in our samples had identical concentrations of DDR1 ECD. Our solid-phase binding assays show a rapid saturation of the binding signal for oligomerized DDR1-Fc as compared to the dimeric samples (Figure 1B). The IC50 for binding of dimeric and oligomeric DDR1-Fc to immobilized collagen type 1 was determined to be 553 ± 179 ng/ml (7.85 ± 2.54 nM) and 176 ± 44.9 ng/ml (2.5 ± 0.64 nM) respectively using the approach of Orosz and Ovádi (2002).
Figure 1.
Binding of DDR1-Fc to immobilized collagen type 1. (A) DDR1-Fc dimers bind to collagen but the control protein TrkB-Fc does not. The bound proteins were detected using anti-Fc antibodies. (B) Antibody (anti-Fc) induced oligomers of DDR1-Fc bound to collagen with a higher affinity than the dimers alone. The amount of DDR1-Fc was identical in dimeric and oligomeric samples; bound proteins were detected using monoclonal anti-DDR1 antibodies.
3.2. Collagen induces oligomerization of DDR1-ECD post ligand binding
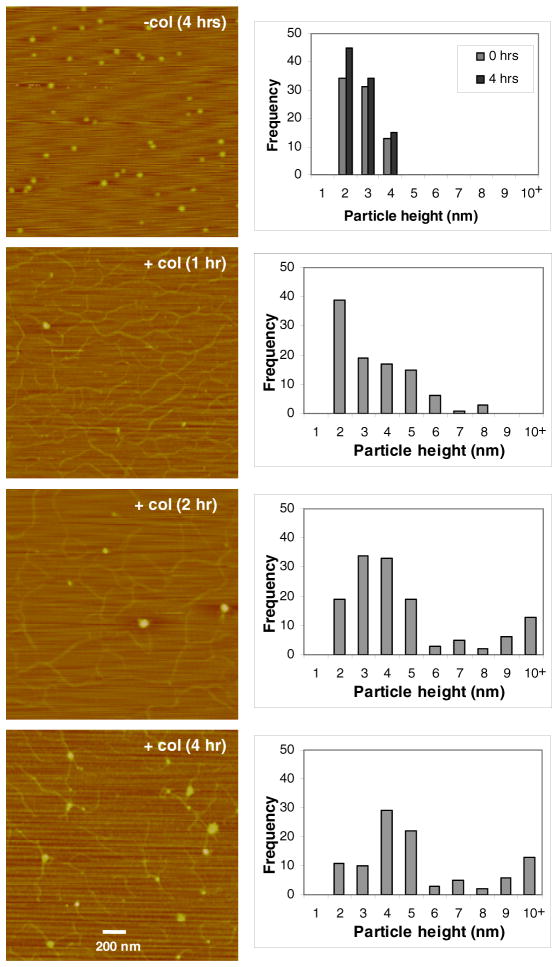
To examine the oligomeric state of DDR1 ECD upon collagen binding, we performed single-molecule studies using AFM. DDR1-Fc dimers were incubated with collagen in solution and thereafter immobilized on a mica surface for AFM studies. For comparison, DDR1-Fc and collagen alone samples were also imaged using AFM. Dimeric DDR1-Fc imaged as a globular protein with topographic height ranging from 1 to 4 nm, with an average height of 2.23 ± 0.6 nm. Incubation of DDR1-Fc alone for upto 4 hrs in solution did not change the size distribution or average height (2.14±0.72) of particles (Figure 2). Upon collagen binding a significant alteration in the morphology of DDR1-Fc was observed in a time-dependent manner. Very few binding events were observed in AFM images when DDR1-Fc and collagen samples were incubated for less than 1 hr (data not shown). The number of binding events increased with incubation times > 1 hr. Particle size analysis from AFM images showed that the DDR1-Fc particles bound to collagen were not uniform in size and exhibited heterogeneity in both topographical height as well as lateral width. The topographic height of globular structures bound to collagen was analyzed for various time points as shown in Figure 2. Our AFM analysis confirmed that dimeric DDR1-Fc binds to collagen as the majority of particles at the 1 hr time point exhibited a topographic height corresponding to that of DDR1-Fc alone. However, a small percentage of particles exhibited a particle size > 4 nm, suggesting the formation of DDR1 oligomers. At longer incubation times (>2hrs) the particle size distribution shifted towards larger sizes and particles 10–15 nm in height were also observed binding to collagen. The particle size distribution reached a saturation after 4 hrs of incubation with no significant differences observed between 4 hrs and 24 hrs (data not shown) of incubation. Table I summarizes how the average height of DDR1-Fc increased upon collagen binding. Our results thus show that collagen binding led to oligomerization of the dimeric DDR1-Fc post ligand-binding.
Figure 2.
DDR1-Fc oligomerizes upon collagen binding. AFM height images and topographic height measurements of pure DDR1-ECD (DDR1-Fc) (0.2 μg/ml) without and with incubation with collagen type 1 for times indicated. DDR1-Fc images as a globular protein 2–4 nm in size. Collagen binding resulted in oligomerization of DDR1-Fc (which can be seen as white globular structures binding to filaments of collagen). Quantitative analysis of particle height of DDR1-Fc bound to collagen is presented at various time points. Collagen-bound DDR1-Fc exhibited a significant increase in particle height as compared to DDR1-Fc alone.
Table 1.
Topographic height of DDR1-Fc from AFM images
| Collagen | Time (hrs) | Average height (nm) |
|---|---|---|
|
| ||
| − | 0 | 2.23±0.60 |
| − | 4 | 2.14±0.72 |
| − | 24 | 2.36±0.82 |
|
| ||
| + | 1 | 2.87±1.62 |
| + | 2 | 4.19±2.66 |
| + | 4 | 4.88±2.76 |
| + | 24 | 4.78±2.55 |
3.3. DDR1 oligomerization occurs on the cell-surface
Based on our observations that DDR1 ECD preserves the capacity to oligomerize upon ligand binding, we further examined if receptor oligomerization occurred on the cell surface. Cells transfected with YFP labeled full-length DDR1 enabled us to follow receptor trafficking in intact cells using confocal fluorescence microscopy. Treatment with dynasore, an inhibitor of dynamin, was used to block dynamin mediated endocytosis. Dynamin is one of the main proteins catalyzing the scission of the endocytic vesicles from the plasma membrane, and it was shown to be involved in both clathrin and caveolae-mediated endocytosis (McClure and Robinson, 1996). As shown in Figure 3A, prior to collagen stimulation DDR1-YFP was found to be uniformly distributed along the plasma membrane in HEK 293 cells. Collagen stimulation resulted in endocytosis of DDR1-YFP, which localized as aggregates (oligomers) present in the cytoplasmic region (Figure 3B). In another set of samples, dynasore treatment was used to block dynamin mediated receptor endocytosis. Dynasore treatment was found to inhibit collagen induced endocytosis of DDR1-YFP, with little YFP signal recorded in the cytoplasmic region (Figure 3C). However, in dynasore treated samples, DDR1-YFP displayed a punctuate distribution as oligomers along the cell membrane upon collagen stimulation, in contrast with the uniform distribution recorded in the non stimulated samples. Thus, our results demonstrate that oligomerization of DDR1 occurs on the cell surface upon collagen stimulation consistent with the plausible role of DDR1 ECD in mediating receptor oligomerization post ligand binding.
Figure 3.
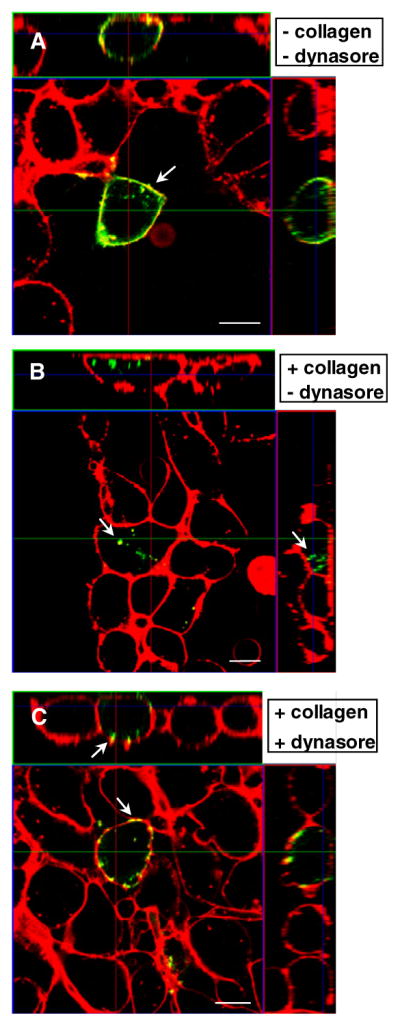
DDR1 oligomerization occurs on cell surface. HEK 293 cells transiently transfected with DDR1-YFP were stimulated with collagen type 1 (as indicated), and imaged using confocal microscopy after membrane staining and fixation. Each panel depicts the projection of a z-stack on the xy (central panel), xz (left panel) and yz planes (upper panel). A) Nonstimulated samples show uniform DDR1-YFP distribution (green) localized in the cell membrane (red). B) Collagen stimulation induces aggregation of DDR1-YFP and translocation to the cytoplasmic region. C) Treatment with dynasore prior to collagen stimulation prevents DDR1-YFP endocytosis; at the same time DDR1-YFP distribution changes from a uniform to a discrete, aggregated one. Scale bars are 10 μm.
4. Discussion
Our results demonstrate that oligomerization of DDR1 ECD is crucial for high-affinity receptor-ligand binding. Previous studies by us (Agarwal et al., 2007) and others (Noordeen et al., 2006) have shown conflicting results where DDR1-Fc dimers failed to bind to collagen in one study and did bind in another. These discrepancies were likely due to differences in the techniques used (surface plasmon resonance (SPR) vs. solid-phase binding assays) for analyzing receptor binding. In this study, by utilizing solid-phase binding assays we confirm that dimeric DDR1-Fc does indeed bind to collagen. The IC50 determined for binding of DDR1-Fc dimers (~ 7.5 nM) to bovine dermal collagen type 1 in this study was comparable to that for rat tail collagen type 1 (~ 10 nM) as reported earlier (Noordeen et al., 2006). We also demonstrate that pre-oligomerization of DDR1-Fc enhances its binding to collagen (IC50 for oligomers was three times lower than that for DDR1 dimers) consistent with our earlier SPR results (Agarwal et al., 2007).
Using AFM, we show that the recombinant DDR1 ECD (which lacks the transmembrane and intracellular domains) undergoes oligomerization upon binding to collagen in-vitro. Although we could not determine the stoichiometry of DDR1 oligomers formed, our AFM results show that the mode value for DDR1-Fc size distribution before and after collagen binding was 2–3 and 4–5 nm respectively. Based on the previously determined topographic heights of antibody-induced oligomers of DDR1 (Agarwal et al., 2007), we estimate that ligand binding results in formation of tetramers, hexamers or octamers consisting of two to four DDR1-Fc dimers. However, besides tetramers-octamers, higher order oligomers of DDR1-Fc (10–15 nm in height) are also formed upon collagen binding. Our cell based studies confirmed that DDR1 oligomerization occurs at the cell surface upon ligand binding and the oligomers thus formed were heterogeneous in size.
Several putative sites in the discoidin (DS) domain, DS-like domain and the JM region of DDR1 ECD may mediate oligomerization of DDR1-Fc dimers. In a recent study, Carafoli et al. (2012) have shown that monoclonal antibodies (mAbs) that bind to the DS-like domain of DDR1, inhibit collagen-induced receptor activation. They propose that mAbs prevent the proximity of the two DS-like domains and the JM regions in the collagen-bound, signaling, state of the DDR1 dimer. A conserved patch between the DS and DS-like domain is understood to mediate protomer contacts in the signaling DDR1 dimer, either by forming a direct DS-DS interface or by providing a secondary collagen-binding site. In addition, Arg32 and Leu152 in the DS domain were also shown to mediate dimer formation in the crystal state and were required for DDR1 signaling, even though they are not part of the primary collagen-binding site. Thus far, soluble versions of monomeric DDR1 ECD have shown little (Noordeen et al., 2006) or reduced (Abdulhussein et al., 2004) binding to collagen in solid-phase binding assays. It remains to be investigated if monomeric DDR1 ECD can undergo ligand-induced oligomerization as elucidated for DDR1-Fc dimers in this study.
It is interesting to note that oligomerization of recombinant DDR1 ECD only occurred when the protein was incubated with collagen in solution and not when collagen was pre-immobilized on a surface as reported in our earlier studies (Agarwal et al., 2007). We believe that pre-immobilization of collagen in these earlier studies prevented accessibility of binding sites along all the faces of the collagen triple helix, and thus restricted receptor oligomerization. The use of neutral pH (conducive to this receptor-ligand interaction) did not enable strong attachment of collagen to mica required for fluid imaging. Therefore we had to employ AFM imaging of dried samples. Dry AFM imaging has previously been used to study binding of SPARC (Wang et al., 2005) and vWF (Novak et al., 2002) to collagen type I and of laminin to collagen type IV (Chen and Hansma, 2000). The basic morphology of DDR1-Fc dimers and collagen was found to be very similar in fluid vs. dry state based on our previous studies and our current results.
Oligomerization of membrane receptors plays an important role in the receptor function and existing literature points toward specific downstream signaling that is unique to multivalent ligands (Cochran et al., 2000; Kiessling et al., 2006). Numerous receptors, including EGFR (Stabley et al., 2013), integrins (Boettiger, 2012) toll-like receptor (Triantafilou et al., 2006), ErbB family (Yarden and Sliwkowski 2001) and Ephrin (Salaita et al., 2010) have been found to assemble into higher-order structures where downstream signaling events are often correlated to cluster formation. Consistent with our observations, a large heterogeneity in oligomer size has been reported for other RTKs, like the epidermal growth factor receptor (EGFR) (Abulrob et al., 2010). A recent study using a nano-patterned supported lipid bilayer technique to control EGFR clustering levels in living cells found that large-scale clustering of EGFR dampens its phosphorylation and that the cell endocytosis machinery contributes to this clustering behavior (Stabley et al., 2013).
Much less is known about the mechanism of DDR clustering and its role in the receptor function. It is understood that DDR1 exists as an inactive dimer on the cell surface and undergoes further oligomerization upon ligand binding ((Abdulhussein et al., 2008; Noordeen et al., 2006; Mihai et al., 2009). Thus oligomerization seems to be a prerequisite for receptor activation. At present we are unable to define the reasons behind the heterogeneity observed in DDR1 oligomer sizes. We speculate that like the EGFR receptor, the large-scale (>10 nm) clusters of DDR1 may dampen receptor activation and only the tetramer-octamer population (4–5 nm in height) may be responsible for signaling. It is interesting to note that the time taken (4 hrs) for formation of maximum number of DDR1 ECD tetramer-octamer in-vitro, coincides with that for maximal receptor phosphorylation (Vogel et al., 1997). An increase in oligomer size with collagen stimulation time was also observed for full length DDR1b-YFP in our earlier studies (Mihai et al., 2009). Further studies using antibodies against phophorylated DDR1 may help elucidate how oligomer size correlates with receptor phosphorylation.
Endocytosis of the oligomerized receptor may also contribute to receptor function. In our previous study (Mihai et al., 2009), DDR1b oligomers formed upon ligand binding underwent rapid internalization and incorporation into the early endosomal compartments, an important site of receptor-initiated signal transduction (von Zastrow and Sorkin 2007). In addition to their role as active signaling kinases it has been suggested (Fu et al., 2013) that DDRs, may act as molecular scaffolds similar to other kinases such as integrin-linked kinase (ILK) and kinase suppressor of ras1 (KSR1) (Hannigan et al., 2011; Zhang et al., 2012). Integrin clustering was shown to modulate downstream signaling at focal adhesion sites where these molecular scaffolds develop and it is possible that DDR clustering could play a similar role (Shi and Boettiger 2003; Ye et al., 2010). Further studies would be needed to fully characterize the role played by DDR oligomerization in receptor function and signaling.
In conclusion, DDR1 ECD was shown to be sufficient for collagen mediated DDR1 oligomerization, and the oligomerized form binds to collagen with increased affinity. In full length receptors expressed on live cells, DDR1 oligomerization occurred on the cell surface in agreement with the role of DDR1 ECD in mediating oligomer formation. Together with our previous observations using cells expressing DDR1-YFP (Mihai et al., 2009), that the kinetics of DDR1 oligomerization is very fast (minutes), we propose that the receptor oligomerization precedes its phosphorylation upon collagen stimulation. These insights into receptor oligomerization may help design strategies to modulate DDR1-collagen interaction, receptor function, and downstream signaling.
Acknowledgments
This work was supported in part by K25 HL81442 and NSF CMMI 1201111 award to GA
Abbreviations
- DS
Discoidin
- DDR1
Discoidin Domain Receptor 1
- ECD
Extra Cellular Domain
- RTK
Receptor Tyrosine Kinase
- AFM
Atomic Force Microscopy
- FRET
Förster Resonance Energy Transfer
- YFP
Yellow Fluorescent Protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdulhussein R, McFadden C, Fuentes-Prior P, Vogel WF. Exploring the collagen-binding site of the DDR1 tyrosine kinase receptor. J Biol Chem. 2004;279:31462–31470. doi: 10.1074/jbc.M400651200. [DOI] [PubMed] [Google Scholar]
- Abdulhussein R, Koo DH, Vogel WF. Identification of disulfide-linked dimers of the receptor tyrosine kinase DDR1. J Biol Chem. 2008;283:12026–33. doi: 10.1074/jbc.M704592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulrob A, Lu Z, Baumann E, Vobornik D, Taylor R, et al. Nanoscale imaging of epidermal growth factor receptor clustering: effects of inhibitors. J Biol Chem. 2010;285:3145–56. doi: 10.1074/jbc.M109.073338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal G, Mihai C, Iscru DF. Interaction of discoidin domain receptor 1 with collagen type 1. J Mol Biol. 2007;367:443–55. doi: 10.1016/j.jmb.2006.12.073. [DOI] [PubMed] [Google Scholar]
- Boettiger D. Mechanical control of integrin-mediated adhesion and signaling. Curr Opin Cell Biol. 2012;24:592–9. doi: 10.1016/j.ceb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Carafoli F, Mayer MC, Shiraishi K, Pecheva MA, Chan LY, et al. Structure of the discoidin domain receptor 1 extracellular region bound to an inhibitory Fab fragment reveals features important for signaling. Structure. 2012;20:688–97. doi: 10.1016/j.str.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Hansma HG. Basement membrane macromolecules: insights from atomic force microscopy. J Struct Biol 2000. 2000;131:44–55. doi: 10.1006/jsbi.2000.4252. [DOI] [PubMed] [Google Scholar]
- Cochran JR, Cameron TO, Stern LJ. The relationship of MHC-peptide binding and T cell activation probed using chemically defined MHC class II oligomers. Immunity. 2000;12:241–250. doi: 10.1016/s1074-7613(00)80177-6. [DOI] [PubMed] [Google Scholar]
- Fu HL, Valiathan RR, Arkwright R, Sohail A, Mihai C, Kumarasiri M, et al. Discoidin domain receptors: unique receptor tyrosine kinases in collagen-mediated signaling. J Biol Chem. 2013;288:7430–7437. doi: 10.1074/jbc.R112.444158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan GE, McDonald PC, Walsh MP, Dedhar S. Integrin-linked kinase: not so ‘pseudo’ after all. Oncogene. 2011;30:4375–4385. doi: 10.1038/onc.2011.177. [DOI] [PubMed] [Google Scholar]
- Kiessling LL, Gestwicki JE, Strong LE. Synthetic multivalent ligands as probes of signal transduction. Angew Chem Int Ed Engl. 2006;45:2348–2368. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–50. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- McClure SJ, Robinson PJ. Dynamin, endocytosis and intracellular signalling (review) Mol Membr Biol. 1996;13:189–215. doi: 10.3109/09687689609160598. [DOI] [PubMed] [Google Scholar]
- Mihai C, Iscru DF, Druhan LJ, Elton TS, Agarwal G. Discoidin domain receptor 2 inhibits fibrillogenesis of collagen type 1. J Mol Biol. 2006;361:864–76. doi: 10.1016/j.jmb.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Mihai C, Chotani M, Elton TS, Agarwal G. Mapping of DDR1 distribution and oligomerization on the cell surface by FRET microscopy. J Mol Biol. 2009;385:432–4. doi: 10.1016/j.jmb.2008.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordeen NA, Carafoli F, Hohenester E, Horton MA, Leitinger B. A transmembrane leucine zipper is required for activation of the dimeric receptor tyrosine kinase DDR1. J Biol Chem. 2006;281:22744–51. doi: 10.1074/jbc.M603233200. [DOI] [PubMed] [Google Scholar]
- Novák L, Deckmyn H, Damjanovich S, Hársfalvi J. Shear-dependent morphology of von Willebrand factor bound to immobilized collagen. Blood. 2002;99:2070–2076. doi: 10.1182/blood.v99.6.2070. [DOI] [PubMed] [Google Scholar]
- Orosz F, Ovádi J. A simple method for the determination of dissociation constants by displacement ELISA. J Immunol Methods. 2002;270:155–62. doi: 10.1016/s0022-1759(02)00295-8. [DOI] [PubMed] [Google Scholar]
- Salaita K, Nair PM, Petit RS, Neve RM, Das D, et al. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science. 2010;327:1380–1385. doi: 10.1126/science.1181729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Boettiger D. A novel mode for integrin-mediated signaling: tethering is required for phosphorylation of FAK Y397. Mol Biol Cell. 2003;14:4306–4315. doi: 10.1091/mbc.E03-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn, et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- Stabley D, Retterer S, Marshall S, Salaita K. Manipulating the lateral diffusion of surface-anchored EGF demonstrates that receptor clustering modulates phosphorylation levels. Integr Biol (Camb) 2013;5:659–668. doi: 10.1039/c3ib20239a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, et al. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem. 2006;281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Fertala A, Ratner BD, Sage EH, Jiang S. Identifying the SPARC binding sites on collagen I and procollagen I by atomic force microscopy. Anal Chem. 2005;77:6765–6771. doi: 10.1021/ac051349d. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Ye F, Hu G, Taylor D, Ratnikov B, Bobkov AA, et al. Recreation of the terminal events in physiological integrin activation. J Cell Biol. 2010;188:157–173. doi: 10.1083/jcb.200908045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Photiou A, Grothey A, Stebbing J, Giamas G. The role of pseudokinases in cancer. Cell Signal. 2012;24:1173–1184. doi: 10.1016/j.cellsig.2012.01.017. [DOI] [PubMed] [Google Scholar]