Abstract
AIM: To investigate whether electroacupuncture (EA) at Zusanli (ST36) prevents intestinal barrier and remote organ dysfunction following prolonged hemorrhagic shock through a vagus anti-inflammatory mechanism.
METHODS: Sprague-Dawley rats were subjected to about 45% of total blood volume loss followed by delayed fluid replacement (DFR) with Ringer lactate 3h after hemorrhage. In a first study, rats were randomly divided into six groups: (1) EAN: EA at non-channel acupoints followed by DFR; (2) EA: EA at ST36 after hemorrhage followed by DFR; (3) VGX/EA: vagotomy (VGX) before EA at ST36 and DFR; (4) VGX/EAN: VGX before EAN and DFR; (5) α-bungarotoxin (α-BGT)/EA: intraperitoneal injection of α-BGT before hemorrhage, followed by EA at ST36 and DFR; and (6) α-BGT/EAN group: α-BGT injection before hemorrhage followed by EAN and DFR. Survival and mean arterial pressure (MAP) were monitored over the next 12 h. In a second study, with the same grouping and treatment, cytokine levels in plasma and intestine, organ parameters, gut injury score, gut permeability to 4 kDa FITC-dextran, and expression and distribution of tight junction protein ZO-1 were evaluated.
RESULTS: MAP was significantly lowered after blood loss; EA at ST36 improved the blood pressure at corresponding time points 3 and 12 h after hemorrhage. EA at ST36 reduced tumor necrosis factor-α and interleukin (IL)-6 levels in both plasma and intestine homogenates after blood loss and DFR, while vagotomy or intraperitoneal injection of α-BGT before EA at ST36 reversed its anti-inflammatory effects, and EA at ST36 did not influence IL-10 levels in plasma and intestine. EA at ST36 alleviated the injury of intestinal villus, the gut injury score being significantly lower than that of EAN group (1.85 ± 0.33 vs 3.78 ± 0.59, P < 0.05). EA at ST36 decreased intestinal permeability to FITC-dextran compared with EAN group (856.95 ng/mL ± 90.65 ng/mL vs 2305.62 ng/mL ± 278.32 ng/mL, P < 0.05). EA at ST36 significantly preserved ZO-1 protein expression and localization at 12 h after hemorrhage. However, EA at non-channel acupoints had no such effect, and abdominal vagotomy and α-BGT treatment could weaken or eliminate the effects of EA at ST36. Besides, EA at ST36 decreased blood aminotransferase, MB isoenzyme of creatine kinase and creatinine vs EAN group at corresponding time points. At the end of 12-h experiment, the survival rate of the EA group was significantly higher than that of the other groups.
CONCLUSION: EA at ST36 attenuates the systemic inflammatory response, protects intestinal barrier integrity, improves organ function and survival rate after hemorrhagic shock via activating the cholinergic anti-inflammatory mechanism.
Keywords: Hemorrhagic shock, Zusanli, Electro-acupuncture, Intestinal permeability, Tight junction
Core tip: The most important novel findings from this study are that when delayed resuscitation is inevitable during emergency situations such as hemorrhagic shock occurring in war without sufficient fluids, electroacupuncture at ST36 can be performed and it can successfully attenuate systemic inflammation, decrease gut injury and permeability and improve blood pressure and outcomes, which is consistent with preserved intestinal barrier function after hemorrhage and delayed fluid resuscitation.
INTRODUCTION
The current treatment for hypovolemic shock focuses on maintaining sufficient tissue perfusion and vital organ function with early and adequate fluid replenishment. Delayed fluid resuscitation for hemorrhagic shock usually occurs when mass casualties happen in austere environments such as battlefield, earthquake, or accidents, where intravenous fluid resuscitation is often difficult or even impossible. Subsequent to delayed resuscitation of hypovolemic shock, a high mortality and an increase in incidence of serious complications such as systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) may thus befall to these victims[1]. Although delayed resuscitation have been demonstrated to result in a more profound shock insult than early resuscitation[2,3], its pathological mechanisms remain poorly understood. One potential pathogenic mechanism appears to be associated with proinflammatory cytokine response[4,5] and, gut plays a key role in the development of intestinal and systemic inflammatory response following hemorrhagic shock and severe burn[6]. Gut becomes a source of proinflammatory mediators resulting from impairment of intestinal mucosal barrier that may amplify SIRS, produce a systemic response state and distant organ failure, and lead to MODS or even death[7-9]. Thus, interventions to prevent intestinal barrier breakdown, excessive inflammation and subsequent organ dysfunction when hemorrhagic shock prolonged due to delayed resuscitation are crucial in controlling hemorrhagic shock without sufficient fluid infusion, especially during evacuation and transportation.
The cholinergic anti-inflammatory pathway is a neural mechanism that inhibits the expression of pro-inflammatory cytokines through the interaction of the principle vagus nerve neurotransmitter, acetylcholine, and the cholinergic α7 nicotinic acetylcholine receptor (α7nAChR) subunit located on cytokine-expressing cells by stimulating the vagus nerve by either electrical or pharmacological methods[10,11]. Activation of the cholinergic anti-inflammatory pathway by vagus nerve stimulation can prevent cytokine release and tissue injury[12], prolong survival and protect against the development of hypotension in rats during lethal hemorrhagic shock[13]. Recently, researchers have demonstrated an expanded role for vagus nerve stimulation and the cholinergic anti-inflammatory mechanism that provides a protective effect on the gut against epithelial barrier dysfunction and alleviates inflammatory injury in intestine and remote organs[14-16]. However, due to complicated manipulation and untoward side effects, including serious tissue injury, it is still difficult to apply electrical stimulation to the vagus nerve in clinical practice. Therefore, a more clinically desirable alternative therapy needs to be established during the resuscitative phase of trauma care.
Acupuncture as one of the therapeutic maneuvers in traditional Chinese medicine (TCM) has been applied in clinics for thousands of years, and it has been found to have a bidirectional neuron-endocrine-immune system regulating effect, and antagonize systemic inflammatory response without side effects. We have demonstrated that electroacupuncture (EA) at ST36 had a significantly positive effect on hemorrhagic shock[2] in rats with delayed fluid resuscitation, however, its mechanism remains unknown. We have recently furthermore proved that EA alleviated intestinal barrier insult and system inflammation in a rat ischemia model through activating the cholinergic anti-inflammatory pathway[17]. Therefore, we investigated whether EA at ST36 protected intestinal barrier function, thus preventing remote organ injury after prolonged hemorrhagic shock in rats with delayed fluid replacement (DFR) through activating the cholinergic anti-inflammatory-dependent mechanism.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (8-10 wk, 240-260 g) were purchased from Experimental Animal Center of Military Medical Sciences of the Chinese PLA. Rats were acclimatized for a while in mesh cages in a temperature-controlled room with a 12-h light-dark cycle in the animal quarter of our laboratory and fasted overnight, but allowed free access to water until 4 h before surgery. The research protocols were approved by the Committee of Scientific Research of the First Hospital Affiliated to General Hospital of PLA, China. The experiment was conducted in compliance with the Guide for Care and Use of Laboratory Animals of National Research Council, China.
Surgical procedures
Rats were anesthetized and instrumented with 3% isoflurane inhalation (Yeeran Technology Limited, Beijing, China). Ketamine 10 mg/kg was hypodermically injected for local anesthesia. Isoflurane (0.7%) was used to maintain anesthesia during the experiments. Animals were allowed to breath spontaneously under a nose cone scavenging system using a veterinary anesthesia delivery system (Kent Scientific TOPO, Torrington, CT, United States). With aseptic technique, poly-ethylene (PE50) catheters were placed in the right carotid artery for continuous artery blood pressure monitoring, in the left femoral artery for blood withdrawal, and in the right femoral vein for fluid infusion. A 2-cm upper-midline laparotomy incision was made to identify gastroesophageal junction and expose the dorsal and ventral vagus nerve on the distal esophagus with a Phenix XLT165-LB stereomicroscope (Phenix Optical Instrument Group Company, Jiangxi Province, China). Rectal temperature was maintained at 37 °C with a heating pad and a heating lamp.
Hemorrhagic shock protocol
Each animal’s estimated blood volume was calculated using the formula[18]: Total blood volume (TBV) (mL) = body weight (g) × 0.06 (mL/g) × 0.77. Hemorrhagic shock was induced by withdrawing 45% of the calculated TBV within 20 min (30% was withdrawn over the first 3 min and suspended for 7 min, and the rest 15% was withdrawn over another 10 min), using an infusion or a withdrawal pump (Kelifeng Apparatus, Beijing, China). The completion of the hemorrhagic shock model concluded the preparation phase, and the time was metered 0. Mean arterial pressure (MAP) was monitored using a PICCO-PLUS cardio-pulmonary volume monitor (PULSION, Feldkirchen, Germany) after exsanguinations were initiated, and recorded 0.5 h before bleeding, immediately upon completion of the hemorrhagic shock (0), 3 h and 12 h after blood loss. The early survival rate was recorded at 12 h after bleeding. Fluid resuscitation was initiated 3 h after exsanguinations.
Animal grouping and treatment
All the animals underwent the same surgical procedure and hemorrhagic shock protocol, and the experimental rats were randomly assigned to six groups: (1) EAN group: Rats underwent EA at non-channel acupoints located 0.5 cm lateral and distal from ST36 points[19], followed by DFR similar to EA group; EA parameters and delayed rehydration were the same as the EA group; (2) EA group: Animals underwent EA at ST36 points, which were located at posterior and lateral side of the knee joint, 5 mm below capitulum fibulae[19], immediately after the blood loss followed by DFR 3 h after hemorrhage. EA at ST36 with an electro-acupuncture apparatus (HANS, LH202H) was performed as described before[17]. Briefly, both hind limbs were shaved and the skin was disinfected. ST36 acupuncture point was punctured with a depth of 7 mm, and then the needle was connected with an electro-acupuncture apparatus. The electric current with the intensity of 2 mA and 2-100Hz was continued for 1.5 h immediately after hemorrhage. Three hours after blood loss, rats were given a femoral vein infusion with Ringer lactate (2 times the amount of blood loss); (3) VGX/EA group: Animals underwent vagotomy of the dorsal and ventral vagus nerve on the distal esophagus prior to EA at ST36 immediately after blood loss followed by DFR; EA parameters and delayed rehydration were the same as the EA group; (4) VGX/EAN group: Animals underwent vagotomy similar to VGX/EA group before EA at non-channel acupoints similar to EAN group immediately after blood loss followed by DFR. EA parameters and delayed rehydration were the same as the EA group; (5) α-BGT/EA group: α-bungarotoxin (α-BGT 1 μg/kg, an antagonist of α7 subunit of cholinergic nicotinic receptor, which inhibits the α7 subunit of acetylcholine receptors by blocking a pivotal communication pathway between the efferent vagus and intestinal immune cells[20,21]) was injected intraperitoneally prior to hemorrhage and followed by EA at ST36 and DFR similar to EA group. EA parameters and delayed rehydration were the same as the EA group; and (6) α-BGT/EAN group: α-BGT was injected intraperitoneally prior to hemorrhage and followed by EA at non-channel acupoints and DFR similar to EAN group.
Each group was then randomly divided into two subgroups: one subgroup (n = 12) for investigation of survival rate and MAP; the other one (n = 18) for cytokine levels, organ parameters, gut injury score, ZO1 detection and intestinal permeability to FITC-dextran test; blood and intestine for cytokine levels were harvested at 0, 3 and 12 h after blood loss (3-5 animals per group); blood for organ parameters test, and intestine for gut injury score, ZO1 detection and intestinal permeability to FITC-dextran test were harvested at 12 h after blood loss (3-5 animals per group).
The scheme for whole experiment is as follows in Figure 1.
Figure 1.
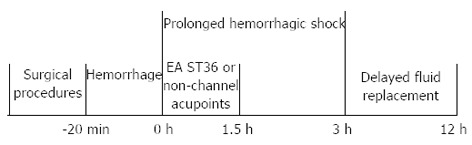
The scheme for whole experiment. EA: Electroacupuncture; ST36: Zusanli.
Samples of blood and intestine
Rats were anesthetized with 3% isoflurane inhalation. Blood was drawn through left femoral artery at 0, 3 and 12 h after blood loss, and then the animals were sacrificed for distal small intestine harvest. Plasma was obtained by centrifuging the blood at 10000 × g for 10 min at 4 °C. Organ functions were assessed by measuring blood aminotransferase (ALT), MB isoenzyme of creatine kinase (CK-MB) and creatinine (Cr) using a Cobas 6000 automatic biochemical analyzer (Roche Diagnostics, Basel, Switzerland). Segments of distal small intestine were harvested and immediately homogenized on ice with 1 mL denaturing lysis buffer or nondenaturing lysis buffer for Western blotting or ELISA. The homogenate was then centrifuged at 10000 × g for 10 min at 4 °C. Aliquots of the supernatants of plasma and tissue were stored at -80 °C until use. Segments of intestine were also harvested and fixed in 4% paraformaldehyde for histologic evaluation and immunofluorescence.
Detection of TNF-α, IL-6 and IL-10 levels in plasma and intestine
TNF-α, IL-6 and IL-10 levels in the plasma and intestine were assessed using commercially available ELISA kit according to the protocol provided by the manufacturer (Nanjing Jiancheng Corp., Nanjing, China). Supernatants were transferred into fresh tubes for the evaluation. Briefly, after adding 50 μL assay buffer, 50 μL samples or standard concentration for TNF-α, IL-6 or IL-10 were incubated with 50 μL diluted Biotin-Conjugate for 2 h at room temperature. After 3 washes, the plates were incubated with Streptavidin-HRP for 1 h at room temperature. After 3 washes, 3,3’,5,5’-Tetramethylbenzidine (TMB) substrate solution was added to the plates for 15 min and the reaction was stopped with stop solution. The absorbance rate was read at 450 nm. The concentrations of the samples were calculated according to the standard curve. The plasma TNF-α, IL-6 and IL-10 levels were expressed as pg/mL. Intestine TNF-α, IL-6 and IL-10 levels were expressed as picograms per milligram of protein.
Histopathologic score
The paraformaldehyde-fixed intestine was embedded in paraffin, and cut into 2-μm sections. Hematoxylin and eosin staining of the intestine was performed by the Pathology Department of the First Hospital Affiliated to the People’s Liberation Army General Hospital. Then the sections were viewed under a light microscope and evaluated by a pathologist blinded to the experimental groups. The injury to the intestinal mucosa was scored using the grading system as previously described[22].
Intestinal epithelial permeability
An in vivo intestinal permeability assay was performed to assess gut barrier function as described by Kao et al[23]. Briefly, 30 min before sacrifice, animals were anesthetized with inhaled isoflurane. A midline laparotomy incision was made and a 10-cm segment of distal ileum was isolated between silk ties. A solution of 1.0 mL phosphate-buffered saline (PBS, pH 7.2) containing 25 mg 4-kDa fluorescein isothiocyanate (FITC)-dextran (Sigma-Aldrich, St. Louis, MO, United States) was injected into the lumen of the isolated segment of intestine. The bowel was returned to the abdominal cavity and the abdomen was closed. Animals were maintained lightly under general anesthesia for 30 min, and systemic blood was drawn by left femoral artery puncture and placed in heparinized Eppendorf tubes on ice. Plasma was obtained by centrifuging the blood at 10000 × g for 10 min at -4 °C. Plasma fluorescence was measured by a fluorescence spectrophotometer (Synergy2; BioTek Multi-Detection Microplate reader, United States) and compared with a standard curve of known concentrations of FITC-dextran diluted in rat plasma.
Immunofluorescence
After deparaffinization, the intestine sections were rehydrated and incubated in citrate buffer (Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) for heat-induced antigen retrieval. After three washes with PBS, sections were incubated with 3% bovine serum albumin (BSA) (Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) for 30 min to block nonspecific binding sites. The sections were then incubated in the ZO-1 antibody (1:100; Life Technologies, Gaithersburg, MD, United States) at 4 °C overnight. The following day, after washing with PBS three times, they were treated with Alexa Fluor 488 secondary goat anti-rabbit antibody in 1% BSA for 1 h at room temperature. Prolong Fade (Antifade Mounting Medium, Beyotime Institute of Biotechnology, Beijing, China) was added on placement of cover slips. Images were viewed using the Olympus fluorescence microscope (BX51-DP71) with exposure-matched settings.
ZO-1 expression
The harvested gut tissues were placed in 1 mL lysis buffer (50 mmol/L Tris-HCl, pH 7.4; 150 mmol/L NaCl; 1% NP-40; 0.1% sodium dodecyl sulphate), then homogenized and centrifuged at 12000 × g for 10 min. Following centrifugation, the supernatant was collected and analyzed for protein concentration. Protein concentrations were determined using a protein assay kit (Applygen Technologies Inc, Beijing, China). Total protein (100 μg) was loaded onto a sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel and run at 120 volts for 2 h. After electrophoresis, the protein was transferred to a polyvinylidene difluoride membrane (PVDF; Applygen Technologies Inc, Beijing, China) and blocked for 2 h in TBST (50 mmol/L Tris; 150 mmol/L NaCl; 0.05% Tween 20) containing 5% milk (Applygen Technologies Inc, Beijing, China). The membrane was then incubated with the primary antibodies against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:5000; Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China), and ZO-1 (1:500; Life Technologies, Gaithersburg, MD, United States) at 4 °C overnight. After 3 washes in TBST, the membrane was incubated with corresponding secondary antibodies conjugated to horseradish peroxidase at room temperature for 30 min and chemiluminescence was detected using SuperECL Plus (Applygen Technologies Inc, Beijing, China). Films were developed using a standard photographic procedure. Quantitative analysis of detected bands was carried out by densitometer scanning (ImageJ).
Statistical analysis
Data were analyzed using a commercial statistical software package (SPSS statistics 17.0). Continuous variables were expressed as mean ± SEM. Statistical significance of differences between groups was determined using one way analysis of variance (ANOVA) followed by Dunnett’s test and SNK-q for multiple comparisons. If some variables were abnormally distributed, the Kruskal-Wallis H test was used. Significance was declared for P values < 0.05.
RESULTS
Effect of EA ST36 on plasma and intestinal cytokine levels
Figure 2 illustrates the effect of EA ST36 on TNF-α (Figure 2A, B), IL-6 (Figure 2C, D) and IL-10 (Figure 2E, F) levels in plasma and intestine in rats with DFR after fatal hemorrhagic shock. Hemorrhage and DFR induced pronounced rises in the concentrations of TNF-α, IL-6 and IL-10 in plasma and intestine homogenates. EA at ST36 reduced TNF-α and IL-6 levels in both plasma and intestine homogenates after blood loss and DFR, while vagotomy or intraperitoneal injection of α-BGT before EA at ST36 reversed its anti-inflammatory effects. In contrast, EA at ST36 did not influence the increases in plasma and intestinal IL-10 during hemorrhagic shock and DFR. These evidences suggested that EA at ST36 attenuated the release of TNF-α and IL-6, but did not suppress IL-10 level in plasma and intestine.
Figure 2.
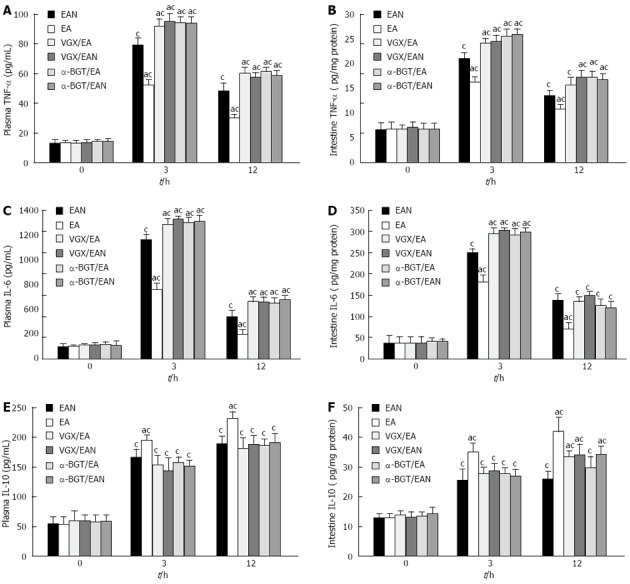
Tumor necrosis factor-α, interleukin-6 and interleukin-10 levels in plasma and intestine at 0, 3 and 12 h after blood loss. Blood samples and intestine were obtained at 0, 3 and 12 h after blood loss. Data are expressed as mean ± SD (3-5 animals per group at each time point). aP < 0.05 vs EAN group; cP < 0.05 vs 0 h in the same group. EA: Electroacupuncture; VGX: Vagotomy; α-BGT: α-bungarotoxin; TNF: Tumor necrosis factor; IL: Interleukin.
EA ST36 decreased intestinal injury
Histological evaluation of intestinal injury was performed at 12 h after blood loss. Sections of distal ileum from animals in EAN group showed villous tip necrosis, blunting, and sloughing of villi (Figure 3A). EA at ST36, which was applied immediately after blood loss, significantly attenuated the mucosal damage (Figure 3B). In contrast, when abdominal vagotomy or intraperitoneal injection of α-BGT was performed and as such the intact neurenteric axis was interrupted, EA at both ST36 and non-channel acupoints failed to prevent the histologic changes induced by hemorrhagic shock in the gut (Figure 3C-F). These data demonstrated that an intact vagus nerve is required for the biological effect of EA at ST36 in protecting against gut injury.
Figure 3.
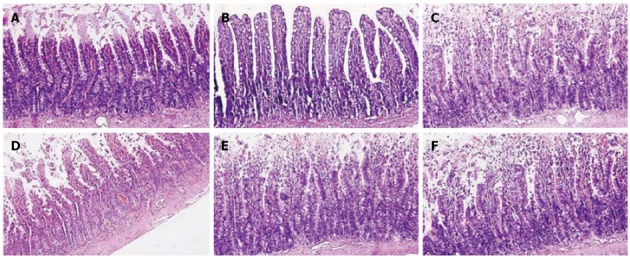
Intestinal histology at 12 h after blood loss. Electroacupuncture (EA) at ST36 protected against intestinal injury after hemorrhagic shock and delayed fluid replacement, whereas EA at ST36 after vagotomy or injection of α-bungarotoxin eliminated such protection. Sections of distal ileum were harvested at 12 h after blood loss and stained with hematoxylin and eosin. All images are taken at × 200 magnification with black bar = 5 μm (3-5 animals per group at 12 h after blood loss).
Gut injury scores were measured in all 6 groups (Figure 4). Animals in EAN group had an average injury score that was significantly higher than in EA group (3.78 ± 0.59 vs 1.85 ± 0.33, P < 0.05). Abdominal vagotomy or intraperitoneal injection of α-BGT before EA at ST36 eliminated the protective effects of EA at ST36 and resulted in similar scores as animals in VGX/EAN, α-BGT/EAN and EAN groups (3.9 ± 0.53, 4.1 ± 0.29 vs 3.78 ± 0.59; P > 0.05, respectively).
Figure 4.
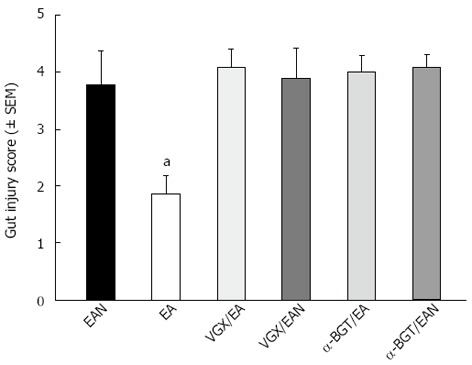
Gut injury scores at 12 h after blood loss. Gut injury was scored by a pathologist blinded to the experimental groups on a scale of 0-4, (as described in Materials and Methods). aP < 0.05 vs EAN group, (3-5 animals per group at 12 h after blood loss). EA: Electroacupuncture; VGX: Vagotomy; α-BGT: α-bungarotoxin.
EA at ST36 lowered intestinal permeability
The intestinal permeability was evaluated in an in vivo assay using FITC-Dextran (Figure 5). Animals in EA group had a significantly lower level of plasma FITC-Dextran when compared with EAN group (856.95 ± 90.65 ng/mL vs 2305.62 ± 278.32 ng/mL, P < 0.05). However, when abdominal vagotomy or α-BGT injection was performed before EA at ST36, the intestinal permeability was indistinguishable from animals in EAN group, and animals in VGX/EAN or α-BGT/EAN group also showed no protection in reducing intestinal permeability compared with animals in EAN group (2249.87 ± 294.17 ng/mL and 2400.15 ± 203.15 ng/mL vs 2305.62 ± 278.32 ng/mL).
Figure 5.
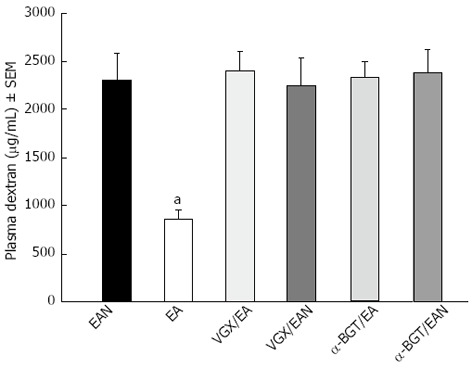
Intestinal permeability to 4-kDa fluorescein isothiocyanate-dextran at 12 h after blood loss. Electroacupuncture (EA) at ST36 protected the intestine from an increase in permeability after hemorrhagic shock and delayed fluid replacement, whereas EA at ST36 after vagotomy or injection of α-bungarotoxin eliminated such protection. aP < 0.05 vs EAN group, (3-5 animals per group at 12 h after blood loss). EA: Electroacupuncture; VGX: Vagotomy; α-BGT: α-bungarotoxin
EA ST36 prevented loss and redistribution of ZO-1
The tight junction protein, ZO-1, undergoes protein expression alterations in response to hemorrhagic shock. Exposure-matched fluorescent intensity correlated to the amount of ZO-1 protein expression after immunostaining (Figure 6). After blood loss, animals in EAN group showed a loss and redistribution in ZO-1 expression evidenced by a low fluorescent intensity at the cell periphery (Figure 6A). Animals in EA group (Figure 6B) showed preservation of the robust structure of ZO-1. In contrast, animals treated with EA at ST36 or non-channel acupoints after abdominal vagotomy, no protection was afforded to the intestinal mucosa, evidenced by the easy interruption and partial disappearance of ZO-1 staining in the periphery of villous epithelial cells (Figure 6C, D). And EA at ST36 or non-channel acupoints after α-BGT injection also offered no protection against ZO-1 disruption (Figure 6E, F).
Figure 6.
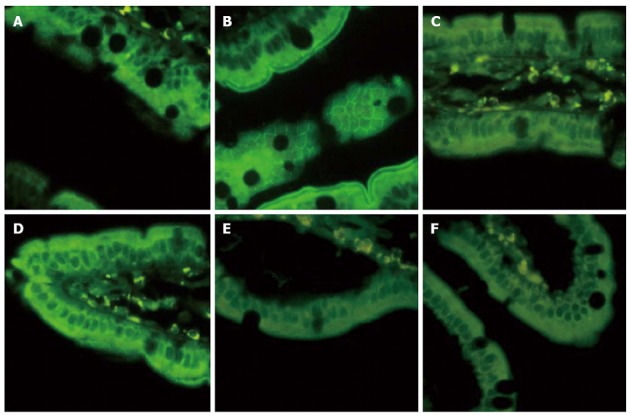
Intestinal ZO-1 immunofluorescent staining at 12 h after blood loss. Animals in EAN group showed a low fluorescent intensity at the cell periphery after hemorrhagic shock, and electroacupuncture (EA) at ST36 showed preservation of the robust structure of ZO-1 staining, whereas after vagotomy or injection of α-bungarotoxin, it eliminated such protection. All images are taken at × 400 magnification with black bar = 5 μm. (3-5 animals per group at 12 h after blood loss, size bar = 2 μm).
These results were confirmed by Western blotting for the ZO-1 protein in intestinal tissue lysates (Figure 7). When compared with the average relative band density of animals in EAN group, animals treated with EA at ST36 had significantly higher ZO-1 expression (P < 0.05). In contrast, intestinal ZO-1 protein levels were significantly decreased in animals receiving abdominal vagotomy or α-BGT injection before EA at ST36. ZO-1 protein levels in VGX/EAN or α-BGT/EAN group receiving EA at non-channel acupoints after vagotomy or α-BGT injection were not significantly different from that in EAN group.
Figure 7.
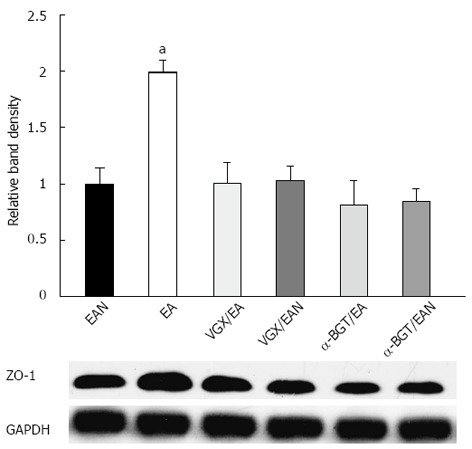
Intestinal ZO-1 protein expression at 12 h after blood loss. Intestinal extracts were obtained from animals at 12 h after blood loss for measurement of ZO-1 protein expression using Western blotting. Representative Western blotting for the ZO-1 protein is shown with its corresponding glyceraldehyde 3-phosphate dehydrogenase loading control to demonstrate equal protein load in all lanes. Electroacupuncture at ST36 resulted in preservation of protein expression. Significant reduction in ZO-1 expression was seen in all the other groups. aP < 0.05 vs EAN group, (3-5 animals per group at 12h after blood loss). EA: Electroacupuncture; VGX: Vagotomy; α-BGT: α-bungarotoxin; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
EA ST36 lowered plasma ALT, CK-MB and Cr
There was a significant decrease in ALT, CK-MB and Cr in the EA group compared with EAN group (P < 0.05; Table 1). However, there was no significant difference of ALT, CK-MB, BUN and Cr among the EAN, VGX/EA, VGX/EAN, α-BGT/EA and α-BGT/EAN groups.
Table 1.
Alanine aminotransferase, MB isoenzyme of creatine kinase and creatinine of rats of all groups after hemorrhagic shock with delayed fluid replacement
| Variables | EAN | EA | VGX/EA | VGX/EAN | α-BGT/EA | α-BGT/EAN |
| ALT (μ/L) | ||||||
| 0 h | 34.0 ± 3.4 | 33.2 ± 1.3 | 35.9 ± 2.9 | 36.1 ± 1.2 | 32.7 ± 5.4 | 32.8 ± 4.1 |
| 3 h | 76.7 ± 10.1c | 46.6 ± 5.7ac | 78.6 ± 9.7c | 79.5 ± 11.2c | 81.2 ± 8. 5c | 81.4 ± 9.2c |
| 12 h | 63.0 ± 7.9c | 38.1 ± 7.1ac | 65.1 ± 10.3c | 67.5 ± 9.7c | 62.4 ± 11.6c | 63.7 ± 12.3c |
| CK-MB (μ/L) | ||||||
| 0 h | 112 ± 21 | 110 ± 17 | 113 ± 18 | 114 ± 21 | 112 ± 15 | 119 ± 22 |
| 3 h | 468 ± 32c | 232 ± 25ac | 459 ± 37c | 469 ± 33c | 477 ± 32c | 469 ± 27c |
| 12 h | 365 ± 49c | 184 ± 32ac | 333 ± 42c | 351 ± 47c | 378 ± 48c | 398 ± 51c |
| Cr (μmol/L) | ||||||
| 0 h | 23.8 ± 3.3 | 23.1 ± 4.5 | 23.4 ± 4.4 | 24.1 ± 2.7 | 23.4 ± 3.6 | 23.2 ± 3.7 |
| 3 h | 59.2 ± 9.2c | 41.5 ± 3.3ac | 58.9 ± 8.4c | 62.1 ± 9.5c | 64.8 ± 10.7c | 65.7 ± 11.2c |
| 12 h | 48.1 ± 5.3c | 30.2 ± 4.2ac | 48.7 ± 4.6c | 48.9 ± 3.6c | 48.2 ± 5.2c | 48.5 ± 4.5c |
P < 0.05 vs EAN group;
P < 0.05 vs 0 h in the same group, (3-5 animals per group at each time point after blood loss). ALT: Alanine aminotransferase; CK-MB: MB isoenzyme of creatine kinase; Cr: Creatinine; EA: Electroacupuncture; VGX: Vagotomy; α-BGT: α-bungarotoxin.
EA at ST36 improved the blood pressure and raised the survival rate
MAP in each group was significantly lowered after blood loss. MAP at time 0 was only 35%-45% of that before blood loss; after 3 h, MAP in each group increased to different degrees. At 3 h and 12 h after hemorrhage, the EA group displayed higher MAP levels than the EAN group (P < 0.05) (Figure 8) while the VGX/EA, VGX/EAN, α-BGT/EA and α-BGT/EAN groups had low MAP levels similar to the EAN group.
Figure 8.
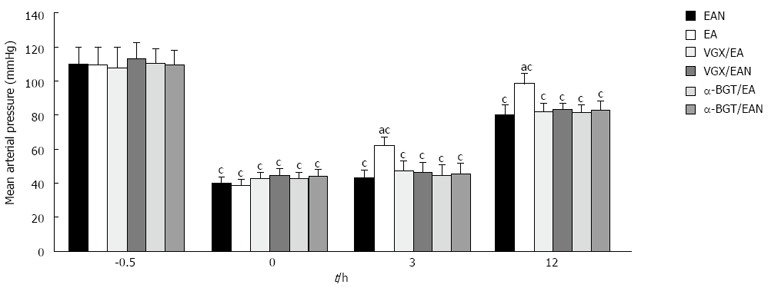
Effect of electroacupuncture ST36 on mean arterial pressure in rats after hemorrhagic shock with delayed fluid replacement. aP < 0.05 vs EAN group; cP < 0.05 vs 0 h in the same group, (3-5 animals per group at 12 h after blood loss). EA: Electroacupuncture; VGX: Vagotomy; α-BGT: α-bungarotoxin.
As shown in our previous study[2], at 12 h after blood loss, the survival rate of the EA group was significantly higher than that of the other groups (P < 0.05). Ten (83.3%) of 12 rats in the EA group were alive 12 h after blood loss. In contrast, only 5 (41.7%) of 12 rats in the EAN group survived after being subjected to hemorrhagic shock treated with EA at non-channel acupoints and DFR. When rats subjected to hemorrhagic shock were treated with EA at ST36 after abdominal vagotomy or α-BGT injection, 6 (50%) of 12 rats in VGX/EA group, 5 (41.7%) in α-BGT/EA group, 5 (41.7%) in VGX/EAN group and 5 (41.7%) in α-BGT/EAN group survived 12 h after blood loss.
DISCUSSION
The most important novel findings from this study are that when delayed resuscitation is inevitable during emergency situations such as hemorrhagic shock in war without sufficient fluids, EA at ST36 can be performed and it can successfully attenuate systemic inflammation, decrease gut injury and permeability and improve blood pressure and outcomes, which is consistent with preserved intestinal barrier function after hemorrhage and delayed fluid resuscitation. Based on our previous studies from this lab[2,24], these results have provided further evidence for a role of acupuncture in an emergency treatment after shock and trauma.
It is regarded that the intestinal tract is one of the earlier organs involved in ischemia-reperfusion injury after hemorrhagic shock. In order to maintain the blood supply of the vital organs during hemorrhagic shock, the intestinal blood flow sharply reduced, and dysfunction of the intestinal mucosal barrier occurs. The ischemia of the small intestine leads rapidly to an impairment of mucosa barrier function, and thus the earliest restitution of the mesenteric blood flow is essential. However, in a very harsh environment with a lack of decent medical support, such as accident and war, immediate resuscitation is sometimes unavailable, the delayed fluid resuscitation occurs, threatening the life of those with extensive injury or hemorrhagic shock. It has also been recognized that a delay in such replenishment could sometimes be fatal due to complications subsequent to delayed resuscitation of hypovolemic shock. One strategy for reducing such a hazard is to try to supplement liquid with sufficient electrolytes by mouth until intravenous infusion fluids are available[25]. In recent years, we have been working on the alternative methods to treat the complications caused by delayed fluid resuscitation. We have found that oral resuscitation is an effective way to partly replace immediate resuscitation when the intravenous infusion is not available[1]. More recently, we have demonstrated that EA at ST36 can significantly improve the survival rate and blood pressure after fatal hemorrhagic shock in rats[2].
The gut barrier has been found to be seriously damaged at the early phase of hemorrhagic shock[26]. The protective effect of traditional Chinese medicine against intestinal and gastric mucosal injury after hemorrhagic shock in rats has been investigated[27,28]. Increasing evidences suggest that the effect of EA at ST36 for gastrointestinal disorders may involve vagal reflex. The dorsal vagal complex (DVC) consists of the nucleus of the solitary tract (NTS), which receives primary visceral afferent information, and the dorsal motor nucleus of the vagus (DMV), which contains the efferent vagal neurons innervating visceral organs. Therefore, DVC plays an important role in regulating visceral functions. A previous study has demonstrated that there is a commonality of central nervous system (CNS) cell groups in brain controlling ST36 point, including DMV and NTS[29]. EA at ST36 generated an increased c-Fos expression in the neurons of DMV[30] and promoted the gastric myoelectric activity, which was regulated by the vagus, and substance P (SP) which is widely present in DVC and involved in the excitatory effects[31]. It has been proved that EA at ST36 can increase the efferent activity of the vagal nerve[32]. Taken together, these data suggest that EA at ST36 is relevant to vagus nerve and it has beneficial effects in intestinal barrier function. In this set of experiments, we stimulated ST36 points after hemorrhagic shock, established its efficacy, and compared its effects with EA at ST36 or non-channel acupoints after abdominal vagotomy or α-BGT injection. We demonstrated that EA at ST36 is effective in preventing intestinal barrier breakdown after hemorrhagic shock with delayed resuscitation. We also showed, as a proof of concept, that its biological effect is dependent on an intact vagus nerve.
Increasing evidence points to extensive cross-talk between intestinal barrier breakdown and cytokine overproduction. The gut has been shown to be a source of inflammatory cytokine with capability of priming neutrophils after ischemia injury or hemorrhagic shock[6,33]. These cytokines originating from the gut may then exacerbate the systemic inflammatory response and potentially lead to a further damage to gut permeability[7,34]. It has also been demonstrated that IL-6 is essential for the development of gut barrier dysfunction after hemorrhagic shock[35]. IL-10 has also been proved to play a pivotal role in regulating proinflammatory cytokine release following trauma-hemorrhage[36]. In our previous studies, we demonstrated that EA at ST36 can alleviate intestinal pro-inflammatory factors, tissue edema and insult of intestinal mucosa[37], significantly protect against tumor necrosis factor-α induced-multiple organ dysfunction in rats with sepsis[38], and promote gastric emptying in rats with a 40% blood volume loss[39]. EA at ST36 has been shown to have a regulatory effect on TNF-α level in rats[40]. This study also demonstrated that acupuncture at ST36 significantly attenuated the expression of pro-inflammatory cytokine TNF-α and IL-6 levels in plasma and intestinal homogenate, but failed to suppress the anti-inflammatory cytokine IL-10 levels in plasma and intestine. These data suggests the anti-inflammatory potential of the use of ST36 acupuncture against hemorrhagic shock. Non-channel acupoints acupuncturing, vagotomy or α-BGT injection before EA at ST36 showed no effects in reducing the production of TNF-α and IL-6, which suggested that anti-inflammatory effect of EA at ST36 may be exerted through the vagal nerves, and their integrity is essential.
In our previous study, we demonstrated that EA at ST36 can effectively protect organ function[24], improve the early survival rate, increase the intestinal tissue diamine oxidase activity and alleviate intestinal ischemia in rats with DFR after hemorrhagic shock[2], but its mechanism is unknown. This study demonstrated that this protective effect of EA at ST36 may be related to its protection of intestinal barrier function. In this study, EA at ST36 effectively prevented histologic injury of the gut mucosa, reduced permeability of the distal ileum to 4-kDa FITC-dextran and maintained intestinal tight junction protein expression and function. We also proved that this biological effect is dependent on an intact vagus nerve. Disrupting the neurenteric axis via surgical abdominal vagotomy would abolish the protective effect of EA at ST36.
It has been found that tight junction proteins are critical structural proteins to maintain the mucosal barrier function[41,42], and loss of gut barrier function is a major contributor to the systemic inflammatory response that ultimately leads to multiple organ failure[43]. Modulation of tight junction proteins can be effective in protecting the remote organ function in burned mice[44], but its mechanism is also unclear. In this study, EA at ST36 can also significantly improve blood pressure, parameters of organ function and survival rate, which is in accordance with decreased intestinal permeability and improved intestinal tight junction protein distribution and expression.
There are several limitations in this study. All of our measures were evaluated with the rats under anesthesia, which might be different between human and animals. We did not monitor the change of vagal nerve activity before and after EA at ST36. The experimental duration is short. Meanwhile, more in vitro researches are needed to clarify the mechanism of ZO1 protein affected by EA at ST36. Besides, the model we used in this study is subjected to withdraw about 45% of the calculated TBV and such a large volume may be lethal and cause severe damage to the animals.
In summary, this study showed that EA at ST36 attenuated the release of TNF-α and IL-6 in plasma and intestine, alleviated the impairment of intestinal barrier function, and improved parameters of organ function and the early survival rate of hemorrhagic shock rats with DFR. The protective role of EA at ST36 is possibly related to an intact vagus nerve and it might exert its effects via cholinergic α7 nicotinic acetylcholine receptor.
ACKNOWLEDGMENTS
The authors thank Drs. Jiang-Yang Lu, Yi-Duo Jin, Xiu-Li Ma, Hui-Zhen Ding, Na Feng, Wen-Xia Yang, Yi Yang, Ming-Feng Jia and Dong-Mei Yang from Department of Pathology, First Hospital Affiliated to the PLA General Hospital, for their technical assistance concerning pathology in this project.
COMMENTS
Background
Fluid resuscitation for hemorrhagic/burn shock is often challenging when mass casualties occurring in austere circumstances such as in the battlefield or site of an unexpected accident or a disaster, where intravenous fluid resuscitation is difficult or even impossible. Subsequent to delayed resuscitation of hypovolemic shock, a high mortality and an increase in incidence of serious complications such as systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) may thus befall to these victims. Thus, interventions such as drug or acupuncture to prevent excessive inflammation and subsequent organ dysfunction when hemorrhagic shock prolonged due to delayed resuscitation are of great significance in controlling hemorrhagic shock without sufficient fluid infusion, especially during evacuation and transportation.
Research frontiers
The current treatment for hypovolemic shock focuses on maintaining sufficient tissue perfusion and vital organ function with early and adequate fluid replenishment. It is of great importance to seek clinically alternative therapies when delayed fluid replenishment is inevitable. Activation of the cholinergic anti-inflammatory pathway by vagus nerve stimulation has been demonstrated to inhibit inflammatory response, prolong survival and protect against the development of hypotension during lethal hemorrhagic shock. Gut, especially the intestinal barrier function, plays a key role in the development of intestinal and systemic inflammatory response and protection of organ function following hemorrhagic shock. Recently, researchers have demonstrated an expanded role for vagus nerve stimulation and the cholinergic anti-inflammatory mechanism that provides a protective effect on the gut against epithelial barrier dysfunction and alleviates inflammatory injury in intestine and remote organs. However, due to complicated manipulation and untoward side effects, including serious tissue injury, it is still difficult to apply electrical stimulation to the vagus nerve in clinical practice. Therefore, a more clinically desirable alternative therapy is needed during the resuscitative phase of trauma care. Acupuncture as one of the therapeutic maneuvers in traditional Chinese medicine (TCM) has been applied in clinics for thousands of years, and it has been found to have a bidirectional neuron-endocrine-immune system regulating effect, and antagonize the systemic inflammatory response without side effects. The authors demonstrated that electroacupuncture (EA) at Zusanli had a significant positive therapeutic effect in hemorrhagic shock rats with delayed fluid resuscitation, however, its mechanism remains unknown. The authors had also proved that EA can alleviate intestinal barrier insult and system inflammation in a rat ischemia model through activating the cholinergic anti-inflammatory pathway.
Innovations and breakthroughs
The most important novel findings from this study are that when delayed resuscitation is inevitable during emergency situations such as hemorrhagic shock occurring in the battlefield or site of an unexpected accident or a disaster without sufficient fluids, EA at ST36 can be performed and it can successfully attenuate systemic inflammation, decrease gut injury and permeability and improve blood pressure and outcomes, which is consistent with preserved intestinal barrier function after hemorrhage and delayed fluid resuscitation.
Applications
The study results provide evidences for acupuncture as an emergency intervention of anti-shock and protection of gut barrier function after severe hemorrhage and trauma.
Terminology
Cholinergic anti-inflammatory pathway: The cholinergic anti-inflammatory pathway is a neural mechanism that inhibits the expression of cytokine through the interaction of the principle vagus nerve neurotransmitter, acetylcholine, and the α7nAChR subunit located on cytokine expressing cells by stimulating the vagus nerve by either electrical or pharmacological methods. EA: EA is a modification of conventional acupuncture that stimulates acupoints with electrical current instead of manual manipulations and appears to have more consistently reproducible results in both clinical and research settings.
Peer review
The study is somewhat interesting, well designed and results seem to be very congruent among the different aspects of systemic inflammation and intestinal barrier function investigated.
Footnotes
Supported by The National Basic Research Program of China, 973 Program, Grant No. 2012CB518101
P- Reviewers Keszthelyi D, Kim K, Yu HP S- Editor Wen LL L- Editor A E- Editor Zhang DN
References
- 1.Hu S, Che JW, Tian YJ, Sheng ZY. Carbachol promotes gastrointestinal function during oral resuscitation of burn shock. World J Gastroenterol. 2011;17:1746–1752. doi: 10.3748/wjg.v17.i13.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi X, Zhong Y, Yao J, Hu S, Wang L, Litscher G. The influence of zusanli and nonmeridian acupuncture points on the survival rate and intestinal tissue features after fatal hemorrhagic shock in rats. Evid Based Complement Alternat Med. 2013;2013:750620. doi: 10.1155/2013/750620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron BJ, Sinert RH, Sinha AK, Buckley MC, Shaftan GW, Scalea TM. Effects of traditional versus delayed resuscitation on serum lactate and base deficit. Resuscitation. 1999;43:39–46. doi: 10.1016/s0300-9572(99)00122-7. [DOI] [PubMed] [Google Scholar]
- 4.Lee CC, Chang IJ, Yen ZS, Hsu CY, Chen SY, Su CP, Chiang WC, Chen SC, Chen WJ. Delayed fluid resuscitation in hemorrhagic shock induces proinflammatory cytokine response. Ann Emerg Med. 2007;49:37–44. doi: 10.1016/j.annemergmed.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 5.Santibanez-Gallerani AS, Barber AE, Williams SJ, ZhaoB S Y, Shires GT. Improved survival with early fluid resuscitation following hemorrhagic shock. World J Surg. 2001;25:592–597. doi: 10.1007/s002680020115. [DOI] [PubMed] [Google Scholar]
- 6.Deitch EA, Xu D, Franko L, Ayala A, Chaudry IH. Evidence favoring the role of the gut as a cytokine-generating organ in rats subjected to hemorrhagic shock. Shock. 1994;1:141–145. doi: 10.1097/00024382-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Smail N, Wang P, Chaudry IH. Increased gut permeability after hemorrhage is associated with upregulation of local and systemic IL-6. J Surg Res. 1998;79:39–46. doi: 10.1006/jsre.1998.5385. [DOI] [PubMed] [Google Scholar]
- 8.Thuijls G, de Haan JJ, Derikx JP, Daissormont I, Hadfoune M, Heineman E, Buurman WA. Intestinal cytoskeleton degradation precedes tight junction loss following hemorrhagic shock. Shock. 2009;31:164–169. doi: 10.1097/SHK.0b013e31817fc310. [DOI] [PubMed] [Google Scholar]
- 9.Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. 2005;21:177–196. doi: 10.1016/j.ccc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 12.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, Marini H, Squadrito G, Minutoli L, Bertolini A, Marini R, et al. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189–1194. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- 14.Costantini TW, Bansal V, Peterson CY, Loomis WH, Putnam JG, Rankin F, Wolf P, Eliceiri BP, Baird A, Coimbra R. Efferent vagal nerve stimulation attenuates gut barrier injury after burn: modulation of intestinal occludin expression. J Trauma. 2010;68:1349–1354; discussion 1349-1354. doi: 10.1097/TA.0b013e3181dccea0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costantini TW, Bansal V, Krzyzaniak M, Putnam JG, Peterson CY, Loomis WH, Wolf P, Baird A, Eliceiri BP, Coimbra R. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1308–G1318. doi: 10.1152/ajpgi.00156.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krzyzaniak M, Peterson C, Loomis W, Hageny AM, Wolf P, Reys L, Putnam J, Eliceiri B, Baird A, Bansal V, et al. Postinjury vagal nerve stimulation protects against intestinal epithelial barrier breakdown. J Trauma. 2011;70:1168–1175; discussion 1168-1175. doi: 10.1097/TA.0b013e318216f754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu S, Du MH, Luo HM, Wang H, Lv Y, Ma L, Lin ZL, Shi X, Gaischek I, Wang L, et al. Electroacupuncture at Zusanli (ST36) Prevents Intestinal Barrier and Remote Organ Dysfunction following Gut Ischemia through Activating the Cholinergic Anti-Inflammatory-Dependent Mechanism. Evid Based Complement Alternat Med. 2013;2013:592127. doi: 10.1155/2013/592127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72–76. [PubMed] [Google Scholar]
- 19.Gao W, Huang YX, Chen H, Song DY, Wang QL. Regulatory effects of electro-acupuncture at Zusanli on ir-SP content in rat pituitary gland and peripheral blood and their immunity. World J Gastroenterol. 2000;6:581–584. doi: 10.3748/wjg.v6.i4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Z, Müller MH, Karpitschka M, Mittler S, Kasparek MS, Renz B, Sibaev A, Glatzle J, Li Y, Kreis ME. Role of the vagus nerve on the development of postoperative ileus. Langenbecks Arch Surg. 2010;395:407–411. doi: 10.1007/s00423-010-0594-5. [DOI] [PubMed] [Google Scholar]
- 21.Marinou M, Tzartos SJ. Identification of regions involved in the binding of alpha-bungarotoxin to the human alpha7 neuronal nicotinic acetylcholine receptor using synthetic peptides. Biochem J. 2003;372:543–554. doi: 10.1042/BJ20021537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bansal V, Costantini T, Kroll L, Peterson C, Loomis W, Eliceiri B, Baird A, Wolf P, Coimbra R. Traumatic brain injury and intestinal dysfunction: uncovering the neuro-enteric axis. J Neurotrauma. 2009;26:1353–1359. doi: 10.1089/neu.2008.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kao NR, Xenocostas A, Driman DK, Rui T, Huang W, Jiao X, Martin CM. Recombinant human erythropoietin improves gut barrier function in a hemorrhagic shock and resuscitation rat model. J Trauma. 2011;71:S456–S461. doi: 10.1097/TA.0b013e318232e782. [DOI] [PubMed] [Google Scholar]
- 24.Zhong YX, Shi X, Du MH, Yao JR. [Effects of electroacupuncture at “Zusanli” (ST 36) on hepatic ischemic injury in rats with delayed fluid replacement after hemorrhagic shock] Zhongguo Zhen Jiu. 2012;32:825–828. [PubMed] [Google Scholar]
- 25.Hu S. [The resuscitation strategies for hypovolemic shock under austere condition lacking intravenous fluid resuscitation facility] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2010;22:323–325. [PubMed] [Google Scholar]
- 26.Chang JX, Chen S, Ma LP, Jiang LY, Chen JW, Chang RM, Wen LQ, Wu W, Jiang ZP, Huang ZT. Functional and morphological changes of the gut barrier during the restitution process after hemorrhagic shock. World J Gastroenterol. 2005;11:5485–5491. doi: 10.3748/wjg.v11.i35.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hei ZQ, Huang HQ, Zhang JJ, Chen BX, Li XY. Protective effect of Astragalus membranaceus on intestinal mucosa reperfusion injury after hemorrhagic shock in rats. World J Gastroenterol. 2005;11:4986–4991. doi: 10.3748/wjg.v11.i32.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang LH, Yao CB, Gao MQ, Li HQ. Gastric mucosal injury due to hemorrhagic reperfusion and efficacy of Salvia miltiorrhizae extract F and cimetidine. World J Gastroenterol. 2005;11:2830–2833. doi: 10.3748/wjg.v11.i18.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CH, Jung HS, Lee TY, Lee SR, Yuk SW, Lee KG, Lee BH. Studies of the central neural pathways to the stomach and Zusanli (ST36) Am J Chin Med. 2001;29:211–220. doi: 10.1142/S0192415X01000241. [DOI] [PubMed] [Google Scholar]
- 30.Kim MH, Park YC, Namgung U. Acupuncture-stimulated activation of sensory neurons. J Acupunct Meridian Stud. 2012;5:148–155. doi: 10.1016/j.jams.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Liu JH, Yan J, Yi SX, Chang XR, Lin YP, Hu JM. Effects of electroacupuncture on gastric myoelectric activity and substance P in the dorsal vagal complex of rats. Neurosci Lett. 2004;356:99–102. doi: 10.1016/j.neulet.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 32.Ye XF, LI JG, Du ZH, Peng ZQ, Zhou Q, Jia BH. Effect of Electroacupuncture at “Zusanli” (ST 36) on Vagal Electrical Activity in the Rat. Zhenci Yanjiu. 2006;31:290–293. [Google Scholar]
- 33.Grotz MR, Ding J, Guo W, Huang Q, Deitch EA. Comparison of plasma cytokine levels in rats subjected to superior mesenteric artery occlusion or hemorrhagic shock. Shock. 1995;3:362–368. [PubMed] [Google Scholar]
- 34.Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg. 1996;20:411–417. doi: 10.1007/s002689900065. [DOI] [PubMed] [Google Scholar]
- 35.Yang R, Han X, Uchiyama T, Watkins SK, Yaguchi A, Delude RL, Fink MP. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:G621–G629. doi: 10.1152/ajpgi.00177.2003. [DOI] [PubMed] [Google Scholar]
- 36.Yokoyama Y, Kitchens WC, Toth B, Schwacha MG, Rue LW, Bland KI, Chaudry IH. Role of IL-10 in regulating proinflammatory cytokine release by Kupffer cells following trauma-hemorrhage. Am J Physiol Gastrointest Liver Physiol. 2004;286:G942–G946. doi: 10.1152/ajpgi.00502.2003. [DOI] [PubMed] [Google Scholar]
- 37.Hu S, Zhang LJ, Bai HY, Bao CM. [The effects of electro-acupuncturing at Zusanli point on intestinal proinflammatory factors, diamine oxidase and tissue water content in rats with sepsis] Zhongguo Weizhongbing Jijiu Yixue. 2009;21:485–487. [PubMed] [Google Scholar]
- 38.Hu S, Zhang LJ, Bai HY, Tian YJ. Effect of electro-acupuncture at Zusanli point on tumor necrosis factor-α induced-multiple organ dysfunction in rats with sepsis. Zhongguo Binglixue Zazhi. 2010;26:353–356. [Google Scholar]
- 39.Zhang LJ, Hu S, Hou JY, Zhou GY, Shi X, Sheng ZY. Effect of electro-acupuncture at Zusanli points on gastric emptying and plasma contents of NO and MTL during oral fluid resuscitation of hemorrhagic shock in rats with blood volume loss. Shijie Huaren Xiaohua Zazhi. 2009;17:395–398. [Google Scholar]
- 40.Tian L, Huang YX, Tian M, Gao W, Chang Q. Downregulation of electroacupuncture at ST36 on TNF-alpha in rats with ulcerative colitis. World J Gastroenterol. 2003;9:1028–1033. doi: 10.3748/wjg.v9.i5.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samak G, Suzuki T, Bhargava A, Rao RK. c-Jun NH2-terminal kinase-2 mediates osmotic stress-induced tight junction disruption in the intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2010;299:G572–G584. doi: 10.1152/ajpgi.00265.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strauman MC, Harper JM, Harrington SM, Boll EJ, Nataro JP. Enteroaggregative Escherichia coli disrupts epithelial cell tight junctions. Infect Immun. 2010;78:4958–4964. doi: 10.1128/IAI.00580-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krzyzaniak MJ, Peterson CY, Cheadle G, Loomis W, Wolf P, Kennedy V, Putnam JG, Bansal V, Eliceiri B, Baird A, et al. Efferent vagal nerve stimulation attenuates acute lung injury following burn: The importance of the gut-lung axis. Surgery. 2011;150:379–389. doi: 10.1016/j.surg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


