Abstract
AIM: To evaluate the clinical outcomes of radiation therapy (RT) for early-stage gastric mucosa-associated lymphoid tissue lymphoma (MALToma).
METHODS: The records of 64 patients treated between 1998 and 2011 were analyzed retrospectively. For Helicobacter pylori (H. pylori)-positive patients (n = 31), chemotherapy or H. pylori eradication therapy was the initial treatment. In patients with failure after H. pylori eradication, RT was performed. For H. pylori-negative patients (n = 33), chemotherapy or RT was the first-line treatment. The median RT dose was 36 Gy. The target volume included the entire stomach and the perigastric lymph node area.
RESULTS: All of the patients completed RT without interruption and showed complete remission on endoscopic biopsy after treatment. Over a median follow-up period of 39 mo, the 5-year local control rate was 89%. Salvage therapy was successful in all relapsed patients. Secondary malignancies developed in three patients. The 5-year overall survival rate was 94%. No patient presented symptoms of moderate-to-severe treatment-related toxicities during or after RT.
CONCLUSION: Radiotherapy results in favorable clinical outcomes in patients with early-stage gastric MALToma who experience failure of H. pylori eradication therapy and those who are H. pylori negative.
Keywords: Gastric mucosa-associated lymphoid tissue lymphoma, Radiation therapy, Treatment response
Core tip: Radiation therapy is an effective salvage treatment for patients with gastric mucosa-associated lymphoid tissue lymphoma (MALToma) who experience failure of Helicobacter pylori (H. pylori) eradication therapy. For patients with H. pylori-negative gastric MALToma, radiation therapy is recommended as the initial treatment. The risk of treatment-related toxicities and secondary malignancies is acceptable.
INTRODUCTION
Mucosa-associated lymphoid tissue lymphoma (MALToma) was first described by Isaacson in 1983[1]. The World Health Organization (WHO) and the Revised European American Lymphoma Classification (REAL) later categorized it as an independent entity[2,3].
The stomach is the most common extranodal site of MALToma (50%-70% of all cases), and Helicobacter pylori (H. pylori) infection is the most important risk factor[4]. In numerous studies, H. pylori eradication therapy had excellent outcomes for gastric MALToma with a reported complete remission (CR) rate of 70%-80%[4-6]. As a result, H. pylori eradication therapy is now employed as the sole first-line treatment[7]. For H. pylori-negative patients, radiation therapy (RT) has recently been accepted as a preferred treatment modality. The role of surgery has declined gradually because of the high incidence of postoperative morbidity and mortality[8].
MALToma is characteristically a localized disease[9-11], and it is very sensitive to radiation. Accordingly, MALToma at extranodal sites, such as the orbital adnexa and the parotid and thyroid glands, has been treated with a moderate dose (25-40 Gy) of RT as an initial treatment[12-17]. Retrospective studies have largely confirmed the effectiveness of RT for gastric MALToma[18-27]. However, the low case numbers of previous studies render the evidence of the effectiveness of RT insufficient.
In this study, we investigated the clinical outcomes and associated adverse effects of RT in patients with early-stage gastric MALToma who were unresponsive to H. pylori eradication therapy and in H. pylori-negative patients.
MATERIALS AND METHODS
Between November 1998 and March 2011, 71 patients who were diagnosed with localized gastric MALToma received RT at Samsung Medical Center. Among them, 7 patients were excluded from this study; 5 patients were excluded because they also had small foci of diffuse large B-cell lymphoma. Two patients were immediately lost to follow up after the completion of RT. We retrospectively analyzed the remaining 64 patients. These patients had stage I or II1 MALToma according to the Lugano staging system[28]. All of the patients were histologically diagnosed with gastric MALToma by endoscopic biopsy. Subsequently, patients underwent a systemic staging work-up, including laboratory tests, such as blood cell counts, biochemical profile and lactate dehydrogenase, computed tomography (CT), positron-emission tomography (PET) or bone marrow biopsy.
Treatment before RT
Of the 64 patients, 40 patients received treatment related to gastric MALToma before RT. H. pylori eradication therapy consisting of a 1-2 wk course of amoxicillin (1000 mg twice daily), clarithromycin (500 mg twice daily) and a proton pump inhibitor (omeprazole or esomeprazole) followed by maintenance of the proton pump inhibitor was performed in 31 patients. Among the patients treated with H. pylori eradication therapy, 3 had no evidence of H. pylori infection.
Chemotherapy consisting of either a combination of cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) every 3 wk or cyclophosphamide, vincristine and prednisolone (CVP) every 3 wk was performed in 15 patients until 2002. Among them, 6 patients received both H. pylori eradication therapy and chemotherapy (Figure 1).
Figure 1.
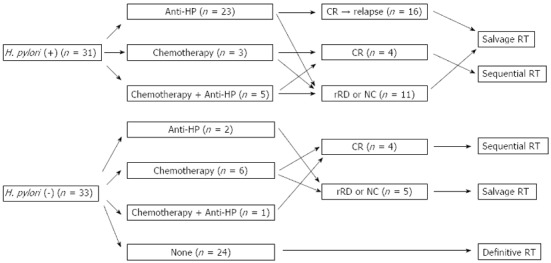
Initial treatment before radiation therapy. H. pylori: Helicobacter pylori; Anti-HP: anti-Helicobacter pylori therapy; CR: Complete remission; rRD: Residual responding disease; NC: No change; RT: Radiation therapy.
Twenty-four patients without H. pylori infection did not receive any treatment before RT.
Radiation therapy
All patients underwent simulation using fluoroscopy or a CT simulator. Before simulation, the patients were trained to breathe in a regular and shallow cycle to maintain a constant stomach motion.
The clinical target volume (CTV) was the entire stomach plus the perigastric lymph nodes for stage I gastric MALToma. The CTV for stage II1 patients was determined as the CTV for stage I gastric MALToma plus a generous margin extending from the involved lymph nodes. To cover the perigastric lymph nodes, we included a 2-cm margin from the outline of stomach wall, which was observed on fluoroscopy after the ingestion of a barium suspension. The planned target volume (PTV) was individualized, considering setup error and stomach movement. CT planning was conducted for 39 patients following the introduction of the CT simulator. The entire stomach was defined at every respiratory stage in 4-dimensional CT slices. After the internal target volume (ITV) was defined, the CTV was set as the ITV plus a 1-cm margin to cover the perigastric lymph nodes. The PTV was the CTV plus 1 cm, and the planning geometry consisted of 2 to 4 coplanar or non-coplanar beams using high-photon energy (10-15 MV).
The daily fraction size was either 1.8 Gy or 2.0 Gy. The total radiation dose ranged from 30 Gy to 44 Gy, with a median of 36 Gy.
Treatment response
We assessed the treatment response histologically using endoscopic biopsy after the completion of RT. The first post-RT endoscopic examination was typically performed 1-2 mo after RT. The next endoscopic biopsies were performed every 3 to 6 mo in the first two or three years and annually thereafter. CT findings were not considered to evaluate the treatment response. The response criteria were based on the GELA (Groupe d’Etude des Lymphomes de l’Adulte) histologic grading system[29].
Statistical analysis
The end points were overall survival (OS) and local control (LC). Time was calculated from the initiation of RT to the event of interest. LC was defined as the time interval to relapse, which was confirmed by biopsy. OS was defined as the period from diagnosis to the date of last follow up or death. We calculated the LC and OS rates using the Kaplan-Meier method. All analyses were performed using IBM SPSS Statics version 19.0 (SPSS Inc., Chicago, IL).
RESULTS
Patient characteristics
Patient characteristics are summarized in Table 1. The median age was 49 years, and 53 patients (83%) were stage I. The gastric body was the site most commonly involved, and H. pylori infection was detected in 31 patients (48%). As an initial therapy, 31 patients (48%) received H. pylori eradication therapy, and 24 patients (38%) received RT.
Table 1.
Patient characteristics n (%)
| Characteristic | No. of patients (n = 64) |
| Gender | |
| Male | 38 (59) |
| Female | 26 (41) |
| Age (yr) | |
| Median, 49 | |
| Range, 24-75 | |
| Lugano staging system | |
| I | 53 (83) |
| II1 | 11 (17) |
| Disease location | |
| Cardia | 2 (3) |
| Fundus | 5 (8) |
| Body | 35 (55) |
| Antrum | 7 (11) |
| Diffuse | 15 (23) |
| H. pylori infection | |
| Negative | 33 (52) |
| Positive | 31 (48) |
| Prior treatment | |
| H. pylori eradication | 25 (39) |
| Chemotherapy | 9 (14) |
| Combined | 6 (9) |
| None | 24 (38) |
| Response to prior treatment | |
| Complete histologic remission (CR) | 17 |
| Responding residual disease (rRD) | 11 |
| No change (NC) | 12 |
| Not evaluable | 24 |
| Radiation dose | Median, 36 Gy |
| ≥ 40 Gy | 18 (28) |
| 36 Gy | 38 (59) |
| 30 Gy | 8 (13) |
| RT technique | |
| AP/PA | 25 (39) |
| 3-D conformal | 39 (61) |
H. pylori: Helicobacter pylori; RT: Radiation therapy; AP/PA: Anteroposterior/posteroanterior.
Seventeen of 40 patients (43%) achieved CR with prior treatment before RT. Nine patients who achieved CR with H. pylori eradication therapy relapsed after a median disease-free interval of 19 mo (range: 9 to 56 mo). CR after chemotherapy was achieved in 5 of 9 patients. The combination of H. pylori eradication therapy and chemotherapy was successful in 3 patients. Those who achieved CR after chemotherapy received RT as sequential therapy. The remaining 23 patients had persistent disease at the time of referral for salvage RT (Figure 1).
The median interval between the initiation of first treatment and the initiation of RT was 8 mo (range, 1 to 40 mo).
RT response
All patients showed a histological CR at the post-treatment endoscopic biopsy. Three patients had not achieved CR at the first post-treatment endoscopic biopsy, but they had achieved a histological CR at a subsequent evaluation 4 to 5 mo after RT without any further treatment.
Survival
Over a median follow-up period of 39 mo (range, 9-131 mo), MALToma relapsed in 5 patients (Table 2), and the 5-year LC of all patients was 89% (Figure 2A). Two patients (cases 4 and 5 in Table 2) received H. pylori eradication therapy due to positive conversion of H. pylori at relapse and finally gained secondary CR. The other three patients (cases 1, 2 and 3) who were not H. pylori infected at relapse did not receive any treatment, and MALToma was not detected on subsequent endoscopy.
Table 2.
Patients with local recurrence after achieving complete remission with radiation therapy
| Initial H. pylori | Initial Tx before RT | DFI (mo) | H. pylori at relapse | Tx after relapse | Final response | |
| Case 1 | + | Anti-HP | 14 | - | Observation | CR |
| Case 2 | - | None | 11 | - | Observation | CR |
| Case 3 | - | None | 26 | - | Observation | CR |
| Case 4 | - | None | 20 | + | Anti-HP | CR |
| Case 5 | - | None | 38 | + | Anti-HP | CR |
H. pylori: Helicobacter pylori; anti-HP: anti-H. pylori antibiotics; CR: Complete remission; DFI: Disease-free interval; Tx: Treatment.
Figure 2.
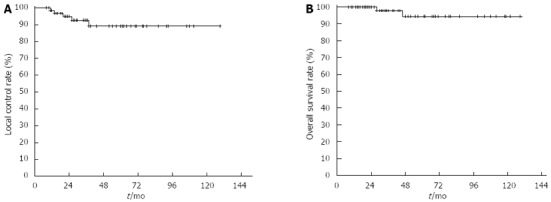
Local control rate (A) and overall survival rate (B) for all patients.
At the study end point, 62 patients remained alive. One patient died due to relapse following the development of malignant lymphoma involving the mesenteric lymph nodes and bone marrow at 29 mo after RT. Another patient died of intercurrent disease 47 mo after RT. The 5-year overall survival rate was 94% (Figure 2B).
Treatment-related toxicities and secondary malignancies
All patients completed RT without interruptions due to treatment-related toxicities. Some patients experienced mild nausea and epigastric soreness, but these symptoms were managed with anti-emetics or antacids. None of the patients complained of gastrointestinal bleeding or perforations. Neither renal nor hepatic toxicities developed.
One patient with H. pylori infection was diagnosed with metachronous gastric cancer 21 mo after RT. He underwent subtotal gastrectomy with Billroth type I reconstruction. On pathologic examination, tubular adenocarcinoma was confined to the mucosa, and metastasis to the regional lymph nodes was not found.
Transformation into malignant lymphoma occurred in two patients at intervals of 26 and 31 mo following the initiation of RT. The sites involved were the mesenteric lymph node and the supraclavicular lymph node, respectively. These patients received 6 cycles of salvage chemotherapy with the CHOP regimen. One patient died of rapid disease progression 6 mo later. The other patient survived in a cured state.
DISCUSSION
Currently, H. pylori eradication therapy is the only established treatment for low-grade gastric MALToma in H. pylori-positive patients[7]. The treatment modality that should be selected for patients who do not respond to H. pylori eradication therapy is currently controversial. Recently, many small retrospective studies and a pooled-data analysis[19,21,22,26,30] have demonstrated that RT has excellent clinical outcomes and feasibility. As a result, RT has become a preferred non-invasive local treatment modality. The results of our present study also support the effectiveness of RT for these patients.
The application of salvage RT in this setting depends to a high degree on defining the time at which H. pylori eradication failed. Most studies with salvage RT for gastric MALToma have not defined the time to H. pylori eradication therapy failure. Our institution also did not have consensus regarding the failure of H. pylori eradication therapy. The range of the interval between the initiation of prior treatment and the timing of RT initiation was variable and not homogeneous. Previous studies have demonstrated that 80%-90% of patients may achieve a histological CR within one year after H. pylori eradication therapy[6,20]. The European Society of Medical Oncology has proposed that at least 12 mo be allowed before initiating another treatment in patients who achieve clinical and endoscopic remission in addition to H. pylori eradication, albeit with persistent lymphoma at the histological level[7].
In addition, reference data for the evaluation of RT response are lacking. In this study, 3 patients did not show CR at the first post-treatment endoscopy, which was performed 1 to 2 mo after RT, but they had achieved CR at the next endoscopy, which was performed 4 to 5 mo later. We therefore suggest that a minimum follow-up period of 6 mo is needed to evaluate the effects of RT.
For H. pylori-negative patients, H. pylori eradication therapy can be attempted as an initial treatment because of the possibility of a false-negative test or low bacteria counts. However, the response rate has been reported to vary (0%-83%)[21,27,31]. In contrast, several small retrospective studies reported a response rate of 100% for RT as the initial treatment for H. pylori-negative patients with MALToma[18,21,25,27]. In these previous studies, CR was confirmed in all patients at the post-treatment endoscopic biopsy after RT. In the absence of randomized trial data due to the rarity of disease, we cannot directly compare H. pylori eradication therapy with RT as an initial treatment for H. pylori-negative patients. However, a survey of the literature suggests that RT shows superior performance compared to H. pylori eradication therapy in the initial treatment of H. pylori-negative patients. Recent evidence indicates that the t(11;18)(q21;q21) translocation in gastric MALToma predicts resistance to H. pylori eradication therapy[32]. This same translocation has been detected in nearly half of patients with H. pylori-negative gastric MALToma[33]. Therefore, in these subgroups, RT may be an appropriate treatment. As additional data become available, routine testing for the t(11;18)(q21;q21) translocation at the time of MALToma diagnosis may become a useful guide for selecting the initial treatment.
The 5-year LC rate in this study was 89%, which is comparable to those reported by previous studies[13,17,18]. Until 6 mo after the completion of RT, all patients showed histological CR on follow-up endoscopic biopsy. However, 5 patients developed relapse at disease-free intervals of 11 to 38 mo. Three patients achieved histological CR on the subsequent evaluation without any further treatment, which could be due to false-positive results at the initial examinations. The other two patients, who were initially H. pylori negative, developed recurrence coincident with H. pylori infection, and they were successfully treated with H. pylori eradication therapy as a salvage treatment. This example may indicate an important role for H. pylori infection in the mechanism of the local recurrence of gastric MALToma.
The major concern regarding stomach irradiation is the risk of perforation and bleeding. A collective review places this risk at 4% or less[34]. Additionally, the risk may be much lower in early-stage and low-grade disease, given the lower radiation doses and target volumes. Another concern is renal toxicity. In the past, the irradiated target volume was the entire abdomen followed by a additional dose to the entire stomach and the perigastric lymph nodes[24]. With increasing knowledge of the spread pattern of low-grade gastric MALToma, the target volume has been reduced to the stomach plus the regional lymphatics[11]. Local RT with the use of three-dimensional conformal or intensity-modulated techniques has the particular advantage of reducing the radiation dose to the kidneys, particularly on the left side[19,35]. Our study, in agreement with numerous other studies, demonstrated no gastric perforation or bleeding and no renal toxicity attributable to RT.
Whether secondary malignancies such as gastric cancer develop years after irradiation is still controversial. Reports of secondary malignancies within the RT field in the treatment of gastric MALToma appear to be rare[13,21,23]. In this study, secondary gastric cancer occurred in one case. For secondary gastric cancer, gastric MALToma itself could be an additional risk factor because of the common pathogenesis, i.e., H. pylori infection, irrespective of the treatment modality[34,36-38]. However, the effects of irradiation cannot be neglected. Because the latent period from irradiation to the development of secondary malignancies can reach a few decades and nearly all patients with gastric MALToma are expected to be long-term survivors in a cured state, close, long-term follow up is required to observe the development of radiation-induced secondary cancer.
Malignant transformation in MALToma is uncommon. The transformation rates reported in some studies ranged from 3% to 19% and did not apparently depend on the site involved[10,14,17]. In the present study, transformation developed in 2 patients (3.1%) with a disease-free interval of 26 to 31 mo, which is consistent with other published reports (ranging from 6 to 116 mo)[14,17]. Following malignant transformation, the mortality rate is higher than that for MALT lymphoma.
In conclusion, the results of the present study further support the use of RT for patients with MALToma who experience failure of H. pylori eradication therapy and for H. pylori-negative patients. The rationale of treating such patients with RT can be summarized as follows. First, gastric MALToma tends to be a localized disease. Second, MALToma is a radio-sensitive tumor. Third, RT has the advantage of stomach preservation. Lastly, RT has low treatment-related morbidity. Although local relapse developed in some cases, all cases were salvaged successfully. Ongoing risks for secondary malignancy or malignant transformation warrant regular long-term follow-up with systemic evaluation and testing for H. pylori infection.
COMMENTS
Background
Gastric mucosa-associated lymphoid tissue lymphoma (MALToma) is highly associated with Helicobacter pylori (H. pylori) infection. Therefore, H. pylori eradication therapy is the standard treatment. However, in clinical practice, persistent disease or recurrence after H. pylori eradication therapy are often found. Additionally, a substantial number of gastric MALToma patients are not H. pylori positive. Radiation therapy (RT) has been shown to have good clinical outcomes and feasibility for the treatment of gastric MALToma in these patients in many small retrospective studies.
Research frontiers
Some reports from single institutions have shown that RT is effective for patients with gastric MALToma that is resistant to H. pylori eradication therapy or that recurs following an initial clinical response. Additionally, in other small reports, patients with H. pylori-negative gastric MALToma and low response rates to H. pylori eradication therapy were treated successfully with RT.
Innovations and breakthroughs
This study is one of the largest single-institution experiences to report the clinical outcomes of gastric MALToma treated with RT. RT has an excellent role as either a salvage or definitive treatment for gastric MALToma. In particular, for H. pylori-negative gastric MALToma patients, in whom the t(11;18)(q21;q21) translocation is frequently found, RT should be applied as the initial treatment because this translocation tends to be resistant to H. pylori eradication therapy. With aid of the three-dimensional conformal technique, fatal toxicities related to radiation can be minimized.
Applications
RT is the preferred salvage treatment modality for H. pylori infection-associated gastric MALToma patients who are unresponsive to H. pylori eradication therapy. Additionally, it can be an effective initial treatment for gastric MALToma patients without H. pylori infection.
Terminology
MALToma is a B cell-originating cancer in the marginal zone of the MALT, which is the diffuse system of small areas of lymphoid tissue found in various sites of the body.
Peer review
This paper is well witten. It is acceptable for publication.
Footnotes
P- Reviewers Bayrahtar Y, Freeman HJ, Hess A S- Editor Wen LL L- Editor A E- Editor Zhang DN
References
- 1.Isaacson P, Wright DH. Extranodal malignant lymphoma arising from mucosa-associated lymphoid tissue. Cancer. 1984;53:2515–2524. doi: 10.1002/1097-0142(19840601)53:11<2515::aid-cncr2820531125>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 2.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 3.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 4.Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 5.Bayerdörffer E, Neubauer A, Rudolph B, Thiede C, Lehn N, Eidt S, Stolte M. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet. 1995;345:1591–1594. doi: 10.1016/s0140-6736(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 6.Wündisch T, Thiede C, Morgner A, Dempfle A, Günther A, Liu H, Ye H, Du MQ, Kim TD, Bayerdörffer E, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol. 2005;23:8018–8024. doi: 10.1200/JCO.2005.02.3903. [DOI] [PubMed] [Google Scholar]
- 7.Zucca E, Dreyling M. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v175–v176. doi: 10.1093/annonc/mdq182. [DOI] [PubMed] [Google Scholar]
- 8.Yoon SS, Coit DG, Portlock CS, Karpeh MS. The diminishing role of surgery in the treatment of gastric lymphoma. Ann Surg. 2004;240:28–37. doi: 10.1097/01.sla.0000129356.81281.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montalbán C, Castrillo JM, Abraira V, Serrano M, Bellas C, Piris MA, Carrion R, Cruz MA, Laraña JG, Menarguez J. Gastric B-cell mucosa-associated lymphoid tissue (MALT) lymphoma. Clinicopathological study and evaluation of the prognostic factors in 143 patients. Ann Oncol. 1995;6:355–362. doi: 10.1093/oxfordjournals.annonc.a059184. [DOI] [PubMed] [Google Scholar]
- 10.Thieblemont C, Bastion Y, Berger F, Rieux C, Salles G, Dumontet C, Felman P, Coiffier B. Mucosa-associated lymphoid tissue gastrointestinal and nongastrointestinal lymphoma behavior: analysis of 108 patients. J Clin Oncol. 1997;15:1624–1630. doi: 10.1200/JCO.1997.15.4.1624. [DOI] [PubMed] [Google Scholar]
- 11.Park W, Chang SK, Yang WI, Ko YH, Huh SJ, Ahn YC, Suh CO. Rationale for radiotherapy as a treatment modality in gastric mucosa-associated lymphoid tissue lymphoma. Int J Radiat Oncol Biol Phys. 2004;58:1480–1486. doi: 10.1016/j.ijrobp.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Hitchcock S, Ng AK, Fisher DC, Silver B, Bernardo MP, Dorfman DM, Mauch PM. Treatment outcome of mucosa-associated lymphoid tissue/marginal zone non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2002;52:1058–1066. doi: 10.1016/s0360-3016(01)02714-6. [DOI] [PubMed] [Google Scholar]
- 13.Tsang RW, Gospodarowicz MK, Pintilie M, Wells W, Hodgson DC, Sun A, Crump M, Patterson BJ. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol. 2003;21:4157–4164. doi: 10.1200/JCO.2003.06.085. [DOI] [PubMed] [Google Scholar]
- 14.Tsai HK, Li S, Ng AK, Silver B, Stevenson MA, Mauch PM. Role of radiation therapy in the treatment of stage I/II mucosa-associated lymphoid tissue lymphoma. Ann Oncol. 2007;18:672–678. doi: 10.1093/annonc/mdl468. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita H, Nakagawa K, Asari T, Murakami N, Igaki H, Ohtomo K. Radiotherapy for 41 patients with stages I and II MALT lymphoma: a retrospective study. Radiother Oncol. 2008;87:412–417. doi: 10.1016/j.radonc.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Tomita N, Kodaira T, Tachibana H, Nakamura T, Mizoguchi N, Takada A. Favorable outcomes of radiotherapy for early-stage mucosa-associated lymphoid tissue lymphoma. Radiother Oncol. 2009;90:231–235. doi: 10.1016/j.radonc.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Goda JS, Gospodarowicz M, Pintilie M, Wells W, Hodgson DC, Sun A, Crump M, Tsang RW. Long-term outcome in localized extranodal mucosa-associated lymphoid tissue lymphomas treated with radiotherapy. Cancer. 2010;116:3815–3824. doi: 10.1002/cncr.25226. [DOI] [PubMed] [Google Scholar]
- 18.Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol. 1998;16:1916–1921. doi: 10.1200/JCO.1998.16.5.1916. [DOI] [PubMed] [Google Scholar]
- 19.Park HC, Park W, Hahn JS, Kim CB, Lee YC, Noh JK, Suh CO. Low grade MALT lymphoma of the stomach: treatment outcome with radiotherapy alone. Yonsei Med J. 2002;43:601–606. doi: 10.3349/ymj.2002.43.5.601. [DOI] [PubMed] [Google Scholar]
- 20.Lee SK, Lee YC, Chung JB, Chon CY, Moon YM, Kang JK, Park IS, Suh CO, Yang WI. Low grade gastric mucosa associated lymphoid tissue lymphoma: treatment strategies based on 10 year follow-up. World J Gastroenterol. 2004;10:223–226. doi: 10.3748/wjg.v10.i2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akamatsu T, Mochizuki T, Okiyama Y, Matsumoto A, Miyabayashi H, Ota H. Comparison of localized gastric mucosa-associated lymphoid tissue (MALT) lymphoma with and without Helicobacter pylori infection. Helicobacter. 2006;11:86–95. doi: 10.1111/j.1523-5378.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 22.Sugimoto M, Kajimura M, Shirai N, Furuta T, Kanaoka S, Ikuma M, Sato Y, Hishida A. Outcome of radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma refractory to Helicobacter pylori eradication therapy. Intern Med. 2006;45:405–409. doi: 10.2169/internalmedicine.45.1473. [DOI] [PubMed] [Google Scholar]
- 23.Lin ML, Wirth A, Chao M, Milner AD, DiIulio J, MacManus M, Seymour JF. Radiotherapy for low-grade gastric marginal zone lymphoma: a retrospective study. Intern Med J. 2007;37:172–180. doi: 10.1111/j.1445-5994.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- 24.Vrieling C, de Jong D, Boot H, de Boer JP, Wegman F, Aleman BM. Long-term results of stomach-conserving therapy in gastric MALT lymphoma. Radiother Oncol. 2008;87:405–411. doi: 10.1016/j.radonc.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Chung SJ, Kim JS, Kim H, Kim SG, Kim CW, Jung HC, Song IS. Long-term clinical outcome of helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma is comparable to that of h. pylori-positive lymphoma. J Clin Gastroenterol. 2009;43:312–317. doi: 10.1097/MCG.0b013e31816a48f8. [DOI] [PubMed] [Google Scholar]
- 26.Gobbi PG, Corbella F, Valentino F, Bergonzi M, Sangalli C, Perfetti V, Corazza GR. Complete long-term response to radiotherapy of gastric early-stage marginal zone lymphoma resistant to both anti-Helicobacter pylori antibiotics and chemotherapy. Ann Oncol. 2009;20:465–468. doi: 10.1093/annonc/mdn668. [DOI] [PubMed] [Google Scholar]
- 27.Park HS, Kim YJ, Yang WI, Suh CO, Lee YC. Treatment outcome of localized Helicobacter pylori-negative low-grade gastric MALT lymphoma. World J Gastroenterol. 2010;16:2158–2162. doi: 10.3748/wjg.v16.i17.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohatiner A, d’Amore F, Coiffier B, Crowther D, Gospodarowicz M, Isaacson P, Lister TA, Norton A, Salem P, Shipp M. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994;5:397–400. doi: 10.1093/oxfordjournals.annonc.a058869. [DOI] [PubMed] [Google Scholar]
- 29.Copie-Bergman C, Gaulard P, Lavergne-Slove A, Brousse N, Fléjou JF, Dordonne K, de Mascarel A, Wotherspoon AC. Proposal for a new histological grading system for post-treatment evaluation of gastric MALT lymphoma. Gut. 2003;52:1656. doi: 10.1136/gut.52.11.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zullo A, Hassan C, Andriani A, Cristofari F, Bassanelli C, Spinelli GP, Tomao S, Morini S. Treatment of low-grade gastric MALT-lymphoma unresponsive to Helicobacter pylori therapy: a pooled-data analysis. Med Oncol. 2010;27:291–295. doi: 10.1007/s12032-009-9207-y. [DOI] [PubMed] [Google Scholar]
- 31.Raderer M, Streubel B, Wöhrer S, Häfner M, Chott A. Successful antibiotic treatment of Helicobacter pylori negative gastric mucosa associated lymphoid tissue lymphomas. Gut. 2006;55:616–618. doi: 10.1136/gut.2005.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H, Ye H, Ruskone-Fourmestraux A, De Jong D, Pileri S, Thiede C, Lavergne A, Boot H, Caletti G, Wündisch T, et al. T(11; 18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology. 2002;122:1286–1294. doi: 10.1053/gast.2002.33047. [DOI] [PubMed] [Google Scholar]
- 33.Ye H, Liu H, Raderer M, Chott A, Ruskone-Fourmestraux A, Wotherspoon A, Dyer MJ, Chuang SS, Dogan A, Isaacson PG, et al. High incidence of t(11; 18)(q21; q21) in Helicobacter pylori-negative gastric MALT lymphoma. Blood. 2003;101:2547–2550. doi: 10.1182/blood-2002-10-3167. [DOI] [PubMed] [Google Scholar]
- 34.Schechter NR, Yahalom J. Low-grade MALT lymphoma of the stomach: a review of treatment options. Int J Radiat Oncol Biol Phys. 2000;46:1093–1103. doi: 10.1016/s0360-3016(99)00522-2. [DOI] [PubMed] [Google Scholar]
- 35.Della Biancia C, Hunt M, Furhang E, Wu E, Yahalom J. Radiation treatment planning techniques for lymphoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;62:745–751. doi: 10.1016/j.ijrobp.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 36.Copie-Bergman C, Locher C, Levy M, Chaumette MT, Haioun C, Delfau-Larue MH, Leroy K, Gaulard P, Delchier JC. Metachronous gastric MALT lymphoma and early gastric cancer: is residual lymphoma a risk factor for the development of gastric carcinoma? Ann Oncol. 2005;16:1232–1236. doi: 10.1093/annonc/mdi242. [DOI] [PubMed] [Google Scholar]
- 37.Capelle LG, de Vries AC, Looman CW, Casparie MK, Boot H, Meijer GA, Kuipers EJ. Gastric MALT lymphoma: epidemiology and high adenocarcinoma risk in a nation-wide study. Eur J Cancer. 2008;44:2470–2476. doi: 10.1016/j.ejca.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Ono S, Kato M, Takagi K, Kodaira J, Kubota K, Matsuno Y, Komatsu Y, Asaka M. Long-term treatment of localized gastric marginal zone B-cell mucosa associated lymphoid tissue lymphoma including incidence of metachronous gastric cancer. J Gastroenterol Hepatol. 2010;25:804–809. doi: 10.1111/j.1440-1746.2009.06204.x. [DOI] [PubMed] [Google Scholar]


