Abstract
AIM: To clarify the association between Helicobacter pylori (H. pylori) infection and the risk of esophageal carcinoma through a meta-analysis of published data.
METHODS: Studies which reported the association between H. pylori infection and esophageal cancer published up to June 2013 were included. The odds ratios (ORs) and corresponding 95%CIs of H. pylori infection on esophageal cancer with respect to health control groups were evaluated. Data were extracted independently by two investigators and discrepancies were resolved by discussion with a third investigator. The statistical software, STATA (version 12.0), was applied to investigate heterogeneity among individual studies and to summarize the studies. A meta-analysis was performed using a fixed-effect or random-effect method, depending on the absence or presence of significant heterogeneity.
RESULTS: No significant association between H. pylori infection and esophageal squamous cell carcinoma (ESCC) risk was found in the pooled overall population (OR = 0.97, 95%CI: 0.76-1.24). However, significant associations between H. pylori infection and ESCC risk were found in Eastern subjects (OR = 0.66, 95%CI: 0.43-0.89). Similarly, cytotoxin-associated gene-A (CagA) positive strains of infection may decrease the risk of ESCC in Eastern subjects (OR = 0.77, 95%CI: 0.65-0.92), however, these associations were not statistically significant in Western subjects (OR = 1.26, 95%CI: 0.97-1.63). For esophageal adenocarcinoma (EAC) the summary OR for H. pylori infection and CagA positive strains of infection were 0.59 (95%CI: 0.51-0.68) and 0.56 (95%CI: 0.45-0.70), respectively.
CONCLUSION: H. pylori infection is associated with a decreased risk of ESCC in Eastern populations and a decreased risk of EAC in the overall population.
Keywords: Helicobacter pylori, Esophageal carcinoma, Cancer risk, Meta-analysis
Core tip: Based on this meta-analysis, we found that Helicobacter pylori infection may have a protective effect against esophageal squamous cell carcinoma in Eastern populations and against esophageal adenocarcinoma in the overall population.
INTRODUCTION
Esophageal cancer (EC), which mainly consists of esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), is the eighth most common cancer worldwide, with 481645 new cases in 2008, and is the sixth leading cause of cancer death, with 406533 deaths[1]. Despite advances in the molecular mechanism of carcinogenesis, the etiology of this malignancy remains unclear. A better understanding of the influencing factors and underlying mechanisms involved in EC development and progression will provide appropriate targets for the development of effective strategies for the prevention of this prevalent malignancy.
Helicobacter pylori (H. pylori) is a helical-shaped Gram-negative bacterium and has been identified as the major causative agent of various benign and malignant gastrointestinal tract diseases[2]. A study showed that cytotoxin-associated gene-A (CagA)-positive strains conferred a greater risk than CagA-negative strains[3]. Islami et al[4] carried out an excellent meta-analysis and reported an inverse association between CagA-positive H. pylori colonization and the risk of EAC, but not ESCC. However, recent studies reported inconclusive results, and some showed the reverse relationship between H. pylori and ESCC[5,6]. Therefore, an updated meta-analysis was performed which included all eligible studies to evaluate the association between H. pylori infection and EC risk.
MATERIALS AND METHODS
Search strategy
To identify all articles that examined the association between H. pylori infection and esophageal carcinoma, we conducted a literature search in the PubMed databases up to April 2013 using the following MeSH terms and keywords: “Helicobacter pylori” [MeSH] OR (Campylobacter pylori) OR (H. pylori) OR (H. pylori) AND (“Esophageal Neoplasms” [MeSH] OR (Cancer of Esophagus) OR (Cancer of the Esophagus) OR (Esophageal Cancer) OR (Esophagus Cancer) OR (Esophagus Neoplasm) OR (Neoplasms, Esophageal). Additional studies were identified by a hand search from references of original studies or review articles on this topic. Two authors reviewed the search results to reduce the possibility of missing relevant published papers. Where data were missing, we contacted the authors for the relevant information. Eligible studies included in this meta-analysis had to meet the following criteria: (1) Articles should clearly describe studies on the association between H. pylori infection and esophageal cancer risk; and (2) The esophageal cancer diagnoses and the sources of cases and controls should be stated. The literature excluded in this study was mainly due to the following reasons: lacking a normal control group, reviews, the research design being not scientific and reasonable, and including repeated data. A total of 27 publications met the eligibility criteria and were included in the present study[5-31].
Data extraction
The following data from each article were extracted: authors, year of publication, country of participants, study design, source of controls, number of controls and of cases, H. pylori detection method, and H. pylori infection status. The data were extracted and registered in two databases independently by two investigators (Zheng QQ and Wang FL) who were blind to journal names, institutions and funding grants. Any discrepancy between these two investigators was resolved by a third investigator (Xie FJ), who participated in the discussion and made the ultimate decision. Equivocal or missed data were excluded in order to unify the formation of the information[11,23]. One article was used as a partial adjusted value, due to crude data[10], and 2 articles that were included totally or partially in another article[32,33].
Statistical analysis
The dichotomous data on H. pylori positive results in the EC group and control group were summarized. OR and 95%CI of OR were calculated to assess the association between H. pylori infection and EC risk. If the H. pylori data were not shown in the article, the OR and 95%CI: value were extracted. An analysis of the heterogeneity of the studies was performed using the χ2-based Q test. A P value less than 0.05 was considered significant for heterogeneity. If the studies were shown to be homogeneous with P > 0.05 for the Q-statistics, the summary of OR was calculated by a fixed-effects model (the Mantel-Haenszel method), otherwise, the random-effects model (the DerSimonian and Laird method) was used. The potential publication bias was assessed graphically using Begg’s test and funnel plots. All analyses were performed with STATA software (version 12.0; Stata Corp LP, College Station, TX, United States), using two-sided P values.
RESULTS
Eligible studies
Twenty-seven eligible studies on H. pylori infection and esophageal cancer were identified through the literature search and selection based on the inclusion and exclusion criteria (Figure 1). The year of publication for these studies ranged from 1991 to 2012. There were 18 studies on Western (Finland, Germany and Ireland) populations and 9 studies on Eastern (Iran and China) populations. With respect to study type, 17 studies were population-based, 10 studies were hospital-based and one study did not specify. Adjusted ORs with corresponding 95%CIs were reported in 17 studies. The selected study characteristics are summarized in Table 1.
Figure 1.
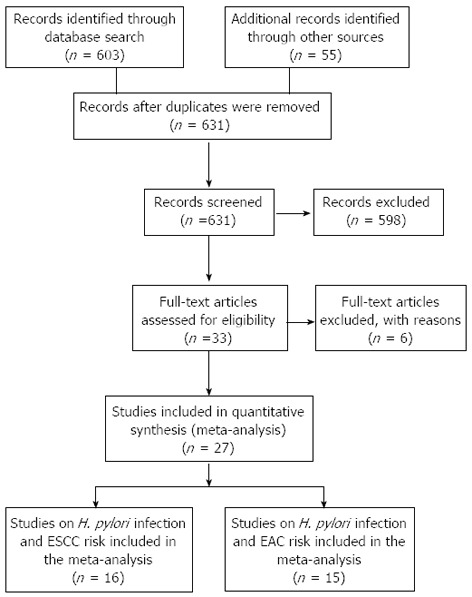
Flow diagram of study identification. ESCC: Esophageal squamous cell carcinoma; EAC: Esophageal adenocarcinoma; H. pylori: Helicobacter pylori.
Table 1.
Characteristics of literatures included in the meta-analysis
| Ref. | Study area | Year | Study type | HP Dm | Hp+ definition1 |
ESCC |
EAC |
Matched/adjusted3 | Control | ||||||
|
Case |
Control |
Case |
Control |
||||||||||||
| Hp+ | CagA+ | Hp+ | CagA+ | Hp+ | CagA+ | Hp+ | CagA+ | ||||||||
| Murphy et al[7] | Finnish | 2012 | Pop | S | HpSe+ | 64/82 | 35/82 | 63/82 | 36/82 | - | - | - | - | Yes/yes | Matched with age, smoking and Alcohol consumption, and date of blood draw to controls, all of cases and controls were male smokers |
| Khoshbaten et al[9] | Iran | 2011 | Pop | S | HpSe+ | 58/100 | 28/100 | 83/100 | 36/100 | - | - | - | - | Yes/no | Sex-matched and age-matched health individuals, any clinical evidence of gastrointestinal symptoms were excluded |
| Venerito et al[8] | Germany | 2011 | Clin | S, H, U | His+, HpSe+, CagA+ or U+ | 53/75 | 42/75 | 53/75 | 40/75 | - | - | - | - | Yes/no | Sex and age-matched individuals with upper GI symptoms but no malignancy of the upper GI tract |
| Whiteman et al[11] | Australia | 2010 | Pop | S | HpSe+ | 54/208 | - | 302/1316 | - | 35/260 | - | 302/1316 | - | Yes/yes | Randomly selected from the same areas, matched to each stratum of age and state |
| Cook et al[10] | Finnish | 2010 | Pop | S | HpSe+ | 64/78 | 35/78 | 71/91 | 42/91 | - | - | - | - | Yes/yes | Matched to case for age, years of smoking, cigarettes per day, or body mass index |
| Wu et al[5] | Taiwan | 2009 | Pop | S | HpSe+ | 112/317 | 91/317 | 563/1103 | 268/700 | - | - | - | - | No/yes | One part of control is matched by gender and age, but another part wasn't matched |
| Hu et al[6] | Taiwan | 2009 | Pop | S | HpSe+ | 66/180 | - | 102/194 | - | - | - | - | - | Yes/yes | Healthy and cancer-free individuals, matched to age, sex and ethnicity |
| Früh et al[12] | Canada | 2008 | Clin | S | CagA + or VacA+ | - | - | - | - | 36/100 | 29/100 | 43/101 | 30/101 | Yes/yes | Healthy GRERD-free, non-blood-related family member and friends of other cancer/surgical patients |
| Derakhshan et al[24] | Iran | 2008 | Clin | S | HpSe+ | - | - | - | - | 9/19 | - | 28/38 | - | Yes/yes | Dyspeptic patients with no peptic ulcer or tumor in their endoscopy |
| Anderson et al[25] | Ireland | 2008 | Pop | S | HpSe+ | - | - | - | - | 55/123 | 57/123 | 157/253 | 150/253 | Yes/yes | Randomly selected population-based controls, frequency matched to EAC cases for age and sex |
| Löfdahl et al[23] | Sweden | 2008 | Pop | S | HpSe+ or CagA+ | - | - | - | - | 130/230 | - | 304/499 | - | Yes/no | Random selected from the population register, frequency matched for age and sex |
| Anandasabapathy et al[26] | United States | 2007 | Clin | H | His+ | - | - | - | - | 4/25 | - | 10/30 | - | No/no | Barrett’s patients with no dysplasia |
| Iijima et al[27] | Japan | 2007 | Clin | S, H, U | HpSe+, His+ or U+ | 60/73 | - | 56/73 | - | - | - | - | - | Yes/yes | Endoscoped patients with no localized lesion, matched to cases for age and sex |
| Kamangar et al[14] | China | 2007 | Pop | S | HpSe+ | 231/335 | 178/335 | 662/992 | 552/992 | - | - | - | - | No/yes | Randomly selected from the entire baseline participants in the stdy cohort |
| Simán et al[13] | Sweden | 2007 | Pop | S | HpSe+ | 15/37 | 24/37 | 68/129 | 82/129 | 4/12 | 6/12 | 24/47 | 32/47 | Yes/yes | Randomly selected from the study cohorted, matched with age, sex, and date enrollment |
| Wang et al[28] | China | 2006 | Pop | S | HpSe+ | ?/107 | - | ?/107 | - | - | - | - | - | Yes/yes | Neighborhood controls, randomly selected, and matched to cases for age and gender |
| Wu et al[15] | Taiwan | 2005 | Pop | S | HpSe+ | 28/127 | - | 74/171 | - | - | - | - | - | No/yes | Randomly selected from the same community |
| de Martel et al[29] | United States | 2005 | Pop | S | HpSe+ | - | - | - | - | 19/51 | 9/18 | 74/150 | 44/71 | Yes/yes | Randomly selected from the study cohorted, matched with age, sex, and date enrollment race, and study site |
| Ye et al[16] | Sweden | 2004 | Pop | S | HpSe+ | 32/85 | 63/85 | 198/499 | 293/499 | 18/97 | 42/97 | 198/499 | 293/499 | Yes/yes | Randomly selected population-based controls, frequency matched to EAC cases for age and sex |
| 2Wang et al[30] | China | 2003 | Pop | S | HpSe+ | 33/63 | - | 145/310 | - | - | - | - | - | Yes/no | Healthy subjects with no difference in age and gender |
| Wu et al[22] | United States | 2003 | Pop | S | HpSe+ | - | - | - | - | 49/80 | 18/80 | 230/356 | 106/356 | Yes/yes | Matched to cases for age, sex, neighborhood of residence, and race |
| El Omar et al[21] | United States | 2003 | Pop | S | HpSe+ | 31/53 | 7/26 | 84/210 | 46/224 | 35/108 | 5/68 | 84/210 | 46/224 | Yes/no | Matched to cases for age, sex, and study center |
| Weston et al[20] | United States | 2000 | Clin | H | His+ | - | - | - | - | 3/20 | - | 96/217 | - | No/no | Patients with GERD symptoms but no Barrett’s esophagus |
| Vieth et al[19] | Germany | 2000 | Clin | H | His+ | - | - | - | - | 66/138 | - | 468/712 | - | No/no | Patients with non-ulcer dyspepsia and no endoscopic signs of GERD |
| Peek et al[18] | United States | 1999 | Clin | S, H | HpSe+ or His+ | - | - | - | - | 11/30 | 3/30 | 20/48 | 25/48 | No/no | Patients endoscoped for reasons other Than GERD or Barrett’s |
| Oberg et al[31] | United States | 1999 | Clin | H | His+ | - | - | - | - | 5/37 | - | 32/229 | - | No/no | Patients with foregut symptoms and benign diseases |
| Talley et al[17] | United States | 1991 | Clin | S | HpSe+ | 20/41 | - | 96/252 | - | - | - | - | - | No/no | Asymptomatic volunteers and patients with benign esophageal, lung, or culoskeletal disorders |
Helicobacter pylori (H. pylori) positivity definition;
Because the article showed that there was no difference in age and gender between cases and health controls, this study was analyzed as a matched population study;
Individual-matching of controls to cases for age and gender/reporting adjusted ORs for the association between H. pylori and cancer. Clin: Clinical based; Pop: Population based; NR: Not reported; Hp Dm: Hpdetection method; S: Serology; H: Histology; U: Rapid urease test; His+: Positive histological examination of tissue samples; HpSe+: Sero-positivity for antibodies to whole-cell; VacA+: Sero-positivity for antibodies to VacA; CagA+: Sero-positivity for antibodies to cytotoxin-associated gene-A; U+: Positive rapid urease test; GERD: Gastroesophageal reflux disease; ESCC: Esophageal squamous cell carcinoma; EAC: Esophageal adenocarcinoma; GI: Gastrointestinal.
Test of heterogeneity
We analyzed the heterogeneity of all 16 studies on ESCC and the fifteen studies on EAC, respectively. For H. pylori infection in the ESCC risk study, the Q statistic was significant (P < 0.01) and the I2 statistic showed a high variation (I2 = 74.5%) among the study results, thus a random-effect model was used for further analysis (Figure 2A and Table 2). In the EAC study, no significant heterogeneity was observed in the overall comparison (I2 = 29.9%, Pheterogeneity = 0.131), and a fixed-effect model was used to calculate the overall ORs (Figure 2B and Table 2).
Figure 2.
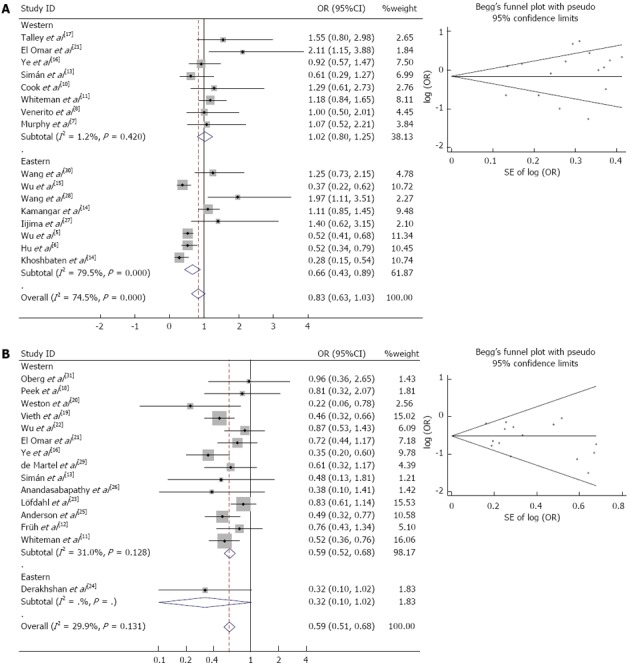
Forest plot and Begg’s funnel plot of the association between Helicobacter pylori infection and esophageal carcinoma. Studies are sorted in order of publication year. A: Esophageal squamous cell carcinoma (random-effect model); B: Esophageal adenocarcinoma (fixed-effect model).
Table 2.
Meta-analysis of the Helicobacter pylori infection on the risk of esophageal squamous cell carcinoma and esophageal adenocarcinoma
| Studies | P1 | I2,2 | Overall OR (95%CI) | |
| Esophageal squamous cell carcinoma | ||||
| Case/control (1961/5704) | ||||
| All studies | 16 | < 0.01 | 74.50% | 0.83 (0.63, 1.03) |
| Population-based studies | 14 | < 0.01 | 76.00% | 0.79 (0.59, 1.00) |
| Clinical-based studies | 2 | 0.86 | < 0.01 | 1.49 (0.66, 2.31) |
| Eastern studies | 8 | < 0.01 | 79.50% | 0.66 (0.43, 0.89) |
| Western studies | 8 | 0.42 | 1.20% | 1.02 (0.80, 1.25)3 |
| Studies with matched controls | 11 | < 0.01 | 71.80% | 0.90 (0.61, 1.20) |
| Studies without matched controls | 5 | < 0.01 | 82.90% | 0.79 (0.46, 1.12) |
| Hp+ only definition as HpSe+ | 14 | < 0.01 | 76.80% | 0.81 (0.60, 1.02) |
| Adjusted results | 11 | < 0.01 | 80.50% | 0.84 (0.56, 1.12) |
| CagA+ vs Hp- | 9 | 0.03 | 52.00% | 0.97 (0.76, 1.24) |
| Eastern study | 3 | 0.22 | 35.00% | 0.77 (0.65, 0.92)3 |
| Western studies | 6 | 0.39 | 3.60% | 1.26 (0.97, 1.63)3 |
| Esophageal adenocarcinoma | ||||
| Case/control (1330/4705) | ||||
| All studies | 15 | 0.131 | 29.9 | 0.59 (0.51, 0.68) |
| Population-based studies | 8 | 0.106 | 40.9 | 0.62 (0.52, 0.73) |
| Clinical-based studies | 7 | 0.319 | 14.5 | 0.53 (0.40, 0.68) |
| Eastern study | 1 | - | - | - |
| Western studies | 14 | 0.128 | 31 | 0.60 (0.52, 0.68) |
| Studies with matched controls | 10 | 0.139 | 33.6 | 0.62 (0.53, 0.72) |
| Studies without matched controls | 5 | 0.333 | 12.7 | 0.49 (0.36, 0.66) |
| Hp+ definition as HpSe+ | 8 | 0.299 | 16.6 | 0.55 (0.45, 0.66) |
| Hp+ definition as His+ | 4 | 0.334 | 11.7 | 0.46 (0.33, 0.64) |
| Adjusted results | 8 | 0.200 | 28.6 | 0.51 (0.40, 0.61) |
| CagA+ vs Hp- | 8 | 0.11 | 39.9 | 0.56 (0.45, 0.70) |
| Eastern study | 0 | - | - | - |
| Western studies | 8 | 0.11 | 39.9 | 0.56 (0.45, 0.70) |
1P value for Q statistical in random effects model; 2Higgins I2 statistic for heterogeneity in random effects model;
The overall value synthesized by fixed effects model. HpSe+: Sero-positivity for antibodies to whole-cell; CagA+: Sero-positivity for antibodies to cytotoxin-associated gene-A.
H. pylori infection and ESCC risk
The association between H. pylori infection and ESCC risk is shown in Figure 2A and Table 2. With the exception of the clinical-based Western studies, CagA+ strains in the Eastern and Western studies did not show obvious heterogeneity calculated using the fixed-effect model. The remaining results were significantly heterogeneous (P < 0.01) calculated using the random-effect model.
In the random-effects model, no statistically significant factor influenced the risk of ESCC in the presence of H. pylori infection (OR = 0.83, 95%CI: 0.63-1.03). When population-based studies were analyzed alone, the combined OR for the association between H. pylori infection and ESCC risk was 2.86 (95%CI: 1.60-5.11). When clinical-based studies were analyzed alone, the combined OR for H. pylori infection was 1.49 (95%CI: 0.66-2.31). When stratified by study location, there was a statistically significant decrease in ESCC risk in the Eastern population (OR = 0.66, 95%CI: 0.43-0.89), however, we did not find a significant association in the Western population (OR = 1.02, 95%CI: 0.80-1.24). In the sub-group analyses of “Hp+ only definition as HpSe+”, “studies with matched controls”, “studies without matched controls” no significant correlation between H. pylori infection and ESCC risk was found.
As studies have indicated that individuals infected with CagA-positive H. pylori strains have a higher risk of developing peptic ulcers and gastric cancer compared to those harboring CagA-negative H. pylori strains[34,35], the association between CagA+ stains and ESCC was also evaluated (Figure 3A). The overall OR was 0.97 (95%CI: 0.76-1.24) and showed high heterogeneity among the studies (I2 = 52.0%, Pheterogeneity = 0.03). This high heterogeneity may be caused partly by regional or ethnic differences, as heterogeneity values may weaken during location subgroup analysis. Similarly, CagA+ strains of infection may decrease the risk of ESCC in Eastern subjects (OR = 0.77, 95%CI: 0.65-0.92), but not in Western subjects (OR = 1.26, 95%CI: 0.97-1.63) or the overall population (OR = 0.90, 95%CI: 0.78-1.05).
Figure 3.
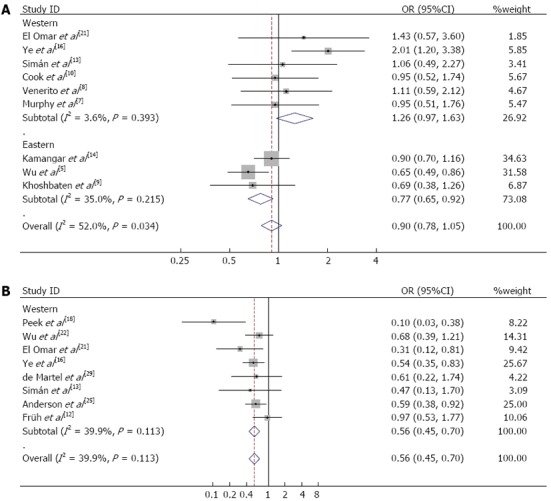
Meta-analysis with a fixed-effect model for the association between cytotoxin-associated gene-A-positive Helicobacter pylori infection and esophageal cancer. A: Esophageal squamous cell carcinoma (random-effect model); B: Esophageal adenocarcinoma (fixed-effect model).
H. pylori infection reduced the risk of EAC
Forest plot analyses are shown in Figure 2B. The overall positive rate of H. pylori infection in EAC was 35.96% (479/1332), which was significantly lower than that in normal controls 44.00% (2070/4705; OR = 0.71, 95%CI: 0.63-0.81). Quantitative meta-analyses showed that, compared with the control group, the combined OR of EAC in the presence of H. pylori infection was 0.59 (95%CI: 0.51-0.68). Table 2 also shows the results of subgroup analyses. The summary OR (95%CI) for clinical-based and population-based studies were 0.62 (95%CI: 0.52-0.73) and 0.53 (95%CI: 0.40-0.68), respectively. In the sub-group analyses of “Western studies”, “studies with matched controls”, “studies without matched controls”, “Hp+ definition as HpSe+” and “adjusted results”, H. pylori infection was also inversely associated with EAC risk. Furthermore, compared with the Hp-population, CagA+ strains of H. pylori also played a protective role in EAC carcinogenesis (OR = 0.56, 95%CI: 0.45-0.70, Figure 3B). However, only one study focused on the Eastern population and the OR was 0.32 (95%CI: 0.10-1.02).
Sensitivity analysis and publication bias
Sensitivity analysis was performed to assess the influence of each individual study on the pooled ORs by omitting a single study each time, and no substantial change in the corresponding pooled OR (data not shown) was observed. Begg’s funnel plot and Egger’s test were performed to assess publication bias. Begg’s funnel plots were symmetrical (Figure 2), and the P values for ESCC and EAC were 0.753 and 0.621, respectively. The statistical results still did not show publication bias using Egger’s test, and the P values for ESCC and EAC were 0.424 and 0.371, respectively. Therefore, there was no significant publication bias in the eligible studies.
DISCUSSION
In the present study, we collected all available, published studies and performed a meta-analysis to examine the association between H. pylori infection and the risk of esophageal cancer. Twenty-seven studies were critically reviewed to clarify the controversial results from previous reports. Our meta-analysis showed that H. pylori infection significantly decreased the risk of EAC in Western populations. In terms of ESCC risk, no significant association was found when the Eastern and Western populations were pooled. In the stratified analysis of study location, no significant association between H. pylori infection and ESCC risk in Western subjects was found. However, we observed a significant association between H. pylori infection and decreased risk of ESCC in East Asian populations.
There are several explanations for this phenomenon. There are fundamental differences in the carcinogenesis pathways between ESCC and EAC. Possible risk factors for ESCC include cigarette smoking, alcohol consumption, hot-temperature food, low intake of vegetables, salty food, pickled vegetables, nutrient deficiency, chronic mucosal irritation and a family history of cancer[36,37], while EAC is closely related to Barrett’s esophagus[38,39]. Genetic differences between ethnic groups may also induce diverse effects. For example, Umar et al[40] conducted a meta-analysis which showed that the PLCE1 polymorphism conferred significant risk for gastric and esophageal tumors in Asians (Chinese), but not in Caucasians. In Eastern populations, the incidence rates of EAC are generally higher in urban areas, where diet and lifestyle are similar to those in Western counties. Therefore, nutritional intake and lifestyle combined with H. pylori infection may have parallel effects in Eastern and Western populations[41,42]. In contrast, ESCC patients were mainly found in areas of Eastern developing countries, where nutrient absence and hot beverage intake are more universal than in Western populations. These different factors may influence the protective effect of H. pylori infection. Genetic factors, tumor biological characteristics and their complicated interactions with environmental factors may modulate risk in ESCC.
Our study also showed that H. pylori infection is a strong protective factor against EAC, which is highly consistent with previous reports[4,43]. The underlying mechanism whereby H. pylori infection protects the esophagus has not been fully elucidated. H. pylori infection-related gastritis may result in lower gastric acid secretion[44]. Hypoacidity induced by atrophic gastritis has been proffered as one reason for this inverse association with EC. H. pylori infection reduced ghrelin synthesis in infected persons, which induced early satiety thereby preventing obesity and rapid gastric emptying, thus reducing the likelihood of gastroesophageal reflux, which may explain this protective effect[45].
Two other meta-analyses have summarized the relationship between H. pylori infection and EC risk[4,43]. The advantages of our meta-analysis are as follows: Compared with the previous two meta-analyses, the present study was much larger, with more than twice as many cancer cases as the earlier studies. In addition, several subgroup analyses were conducted to identify potential sources of heterogeneity. Secondly, according to our selection criteria, all the studies in our meta-analysis had acceptable quality and the cases and controls were collated from all included studies, which significantly increased the statistical power. Thirdly, our study suggested that H. pylori infection decreased the risk of ESCC. This study should be repeated which could be beneficial in detecting novel mechanisms to reduce the risk of EC. We also found that our study had several limitations. Heterogeneity for the ORs in ESCC was observed among the studies. This heterogeneity may be due to various factors, such as diversity in the population characteristics, differences in the number of cases and controls, H. pylori detection methods and study design. However, heterogeneity was eliminated in the Western population after stratifying by ethnicity. The variables used to adjust these values were not consistent across the studies, which may limit the reliability of the data. Too few studies were identified to allow for subgroup analysis by covariates. Subgroup analyses regarding other confounding factors such as age and gender were conducted in the present study, but did not reduce the heterogeneity in the Eastern population. Only one study focused on the relationship between H. pylori infection and EAC risk in Eastern subjects (OR = 0.32, 95%CI: 0.10-1.02) which was not statistically significant (P = 0.05). Further studies are required to confirm the protective role of H. pylori.
In conclusion, despite these limitations, our meta-analysis indicated that H. pylori infection may contribute to the decreased risk of EAC in the overall population and of ESCC in the Eastern population. To confirm our findings, further well-designed studies with large sample size and standardized laboratory methods in diverse ethnic populations should be performed to validate this association. The potential molecular mechanism of these protective effects should also be clarified to reduce the high morbidity caused by this malignancy.
COMMENTS
Background
Esophageal cancer is one of the most deadly malignancies. Many studies have explored the association between Helicobacter pylori (H. pylori) infection and esophageal cancer risk. However, the results were inconclusive and even controversial. Therefore, it is necessary to perform a meta-analysis in order to obtain a more precise evaluation of the relationship between H. pylori infection and esophageal cancer risk.
Research frontiers
H. pylori has been identified as a pathogen in gastric cancers. To date, there have been many case-control studies on the association between H. pylori infection and esophageal cancer risk, but few meta-analyses have been conducted on this topic.
Innovations and breakthroughs
This meta-analysis indicated that H. pylori infection might play a protective in esophageal squamous cell carcinoma (ESCC) risk in Eastern populations and in esophageal adenocarcinoma (EAC) risk in the overall population. Further studies are required to confirm these findings.
Applications
H. pylori infection is inversely associated with ESCC risk in Eastern populations and with EAC risk in the overall population. This meta-analysis provided a structured and systematic integration of information on the etiology of esophageal cancer, and the results may provide valuable information for researchers and clinicians.
Terminology
In cytotoxin-associated gene-A (CagA) positive strains of H. pylori the genome contains the cag pathogenicity island. This island includes approximately 31 putative genes, including CagA- the gene that encodes the CagA protein strains that translocate the CagA protein into host cells and are significantly more likely to cause gastric cancer and other gastric diseases than CagA-negative strains.
Peer review
These researchers performed a meta-analysis to clarify the association between H. pylori infection and development of esophageal carcinoma. The results are very important and create other questions in mind that lead to further studies.
Footnotes
Supported by China Postdoctoral Science Foundation, No. 2012M521189; Zhejiang Provincial Postdoctoral Science Foundation, No. Bsh1202064; National Natural Science Foundation of China, No. 81172081; Zhejiang Provincial Natural Science Foundation, No. LY13H160024; and Wu Jieping Medical Foundation, No. 2011, 3206750.11059 and 11091
P- Reviewers Day AS, Fakheri H, Franceschi F, Iera E, Jadallah KA S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Handa O, Naito Y, Yoshikawa T. Redox biology and gastric carcinogenesis: the role of Helicobacter pylori. Redox Rep. 2011;16:1–7. doi: 10.1179/174329211X12968219310756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 4.Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila) 2008;1:329–338. doi: 10.1158/1940-6207.CAPR-08-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu IC, Wu DC, Yu FJ, Wang JY, Kuo CH, Yang SF, Wang CL, Wu MT. Association between Helicobacter pylori seropositivity and digestive tract cancers. World J Gastroenterol. 2009;15:5465–5471. doi: 10.3748/wjg.15.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu HM, Kuo CH, Lee CH, Wu IC, Lee KW, Lee JM, Goan YG, Chou SH, Kao EL, Wu MT, et al. Polymorphism in COX-2 modifies the inverse association between Helicobacter pylori seropositivity and esophageal squamous cell carcinoma risk in Taiwan: a case control study. BMC Gastroenterol. 2009;9:37. doi: 10.1186/1471-230X-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy G, Kamangar F, Albanes D, Stanczyk FZ, Weinstein SJ, Taylor PR, Virtamo J, Abnet CC, Dawsey SM, Freedman ND. Serum ghrelin is inversely associated with risk of subsequent oesophageal squamous cell carcinoma. Gut. 2012;61:1533–1537. doi: 10.1136/gutjnl-2011-300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venerito M, Kohrs S, Wex T, Adolf D, Kuester D, Schubert D, Peitz U, Mönkemüller K, Malfertheiner P. Helicobacter pylori infection and fundic gastric atrophy are not associated with esophageal squamous cell carcinoma: a case-control study. Eur J Gastroenterol Hepatol. 2011;23:859–864. doi: 10.1097/MEG.0b013e3283496469. [DOI] [PubMed] [Google Scholar]
- 9.Khoshbaten M, Zadimani A, Bonyadi MR, Mohammadzadeh M, Gachkar L, Pourhoseingholi MA. Helicobacter pylori infection reduces the risk of esophageal squamous cell carcinoma: a case-control study in iran. Asian Pac J Cancer Prev. 2011;12:149–151. [PubMed] [Google Scholar]
- 10.Cook MB, Dawsey SM, Diaw L, Blaser MJ, Perez-Perez GI, Abnet CC, Taylor PR, Albanes D, Virtamo J, Kamangar F. Serum pepsinogens and Helicobacter pylori in relation to the risk of esophageal squamous cell carcinoma in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2010;19:1966–1975. doi: 10.1158/1055-9965.EPI-10-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whiteman DC, Parmar P, Fahey P, Moore SP, Stark M, Zhao ZZ, Montgomery GW, Green AC, Hayward NK, Webb PM. Association of Helicobacter pylori infection with reduced risk for esophageal cancer is independent of environmental and genetic modifiers. Gastroenterology. 2010;139:73–83; quiz e11-2. doi: 10.1053/j.gastro.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Früh M, Zhou W, Zhai R, Su L, Heist RS, Wain JC, Nishioka NS, Lynch TJ, Shepherd FA, Christiani DC, et al. Polymorphisms of inflammatory and metalloproteinase genes, Helicobacter pylori infection and the risk of oesophageal adenocarcinoma. Br J Cancer. 2008;98:689–692. doi: 10.1038/sj.bjc.6604234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simán JH, Engstrand L, Berglund G, Forsgren A, Florén CH. Helicobacter pylori and CagA seropositivity and its association with gastric and oesophageal carcinoma. Scand J Gastroenterol. 2007;42:933–940. doi: 10.1080/00365520601173863. [DOI] [PubMed] [Google Scholar]
- 14.Kamangar F, Qiao YL, Blaser MJ, Sun XD, Katki H, Fan JH, Perez-Perez GI, Abnet CC, Zhao P, Mark SD, et al. Helicobacter pylori and oesophageal and gastric cancers in a prospective study in China. Br J Cancer. 2007;96:172–176. doi: 10.1038/sj.bjc.6603517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu DC, Wu IC, Lee JM, Hsu HK, Kao EL, Chou SH, Wu MT. Helicobacter pylori infection: a protective factor for esophageal squamous cell carcinoma in a Taiwanese population. Am J Gastroenterol. 2005;100:588–593. doi: 10.1111/j.1572-0241.2005.40623.x. [DOI] [PubMed] [Google Scholar]
- 16.Ye W, Held M, Lagergren J, Engstrand L, Blot WJ, McLaughlin JK, Nyrén O. Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia. J Natl Cancer Inst. 2004;96:388–396. doi: 10.1093/jnci/djh057. [DOI] [PubMed] [Google Scholar]
- 17.Talley NJ, Zinsmeister AR, Weaver A, DiMagno EP, Carpenter HA, Perez-Perez GI, Blaser MJ. Gastric adenocarcinoma and Helicobacter pylori infection. J Natl Cancer Inst. 1991;83:1734–1739. doi: 10.1093/jnci/83.23.1734. [DOI] [PubMed] [Google Scholar]
- 18.Peek RM, Vaezi MF, Falk GW, Goldblum JR, Perez-Perez GI, Richter JE, Blaser MJ. Role of Helicobacter pylori cagA(+) strains and specific host immune responses on the development of premalignant and malignant lesions in the gastric cardia. Int J Cancer. 1999;82:520–524. doi: 10.1002/(sici)1097-0215(19990812)82:4<520::aid-ijc9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Vieth M, Masoud B, Meining A, Stolte M. Helicobacter pylori infection: protection against Barrett’s mucosa and neoplasia? Digestion. 2000;62:225–231. doi: 10.1159/000007820. [DOI] [PubMed] [Google Scholar]
- 20.Weston AP, Badr AS, Topalovski M, Cherian R, Dixon A, Hassanein RS. Prospective evaluation of the prevalence of gastric Helicobacter pylori infection in patients with GERD, Barrett’s esophagus, Barrett’s dysplasia, and Barrett’s adenocarcinoma. Am J Gastroenterol. 2000;95:387–394. doi: 10.1111/j.1572-0241.2000.01758.x. [DOI] [PubMed] [Google Scholar]
- 21.El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 22.Wu AH, Crabtree JE, Bernstein L, Hawtin P, Cockburn M, Tseng CC, Forman D. Role of Helicobacter pylori CagA+ strains and risk of adenocarcinoma of the stomach and esophagus. Int J Cancer. 2003;103:815–821. doi: 10.1002/ijc.10887. [DOI] [PubMed] [Google Scholar]
- 23.Löfdahl HE, Lu Y, Lagergren J. Sex-specific risk factor profile in oesophageal adenocarcinoma. Br J Cancer. 2008;99:1506–1510. doi: 10.1038/sj.bjc.6604701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derakhshan MH, Malekzadeh R, Watabe H, Yazdanbod A, Fyfe V, Kazemi A, Rakhshani N, Didevar R, Sotoudeh M, Zolfeghari AA, et al. Combination of gastric atrophy, reflux symptoms and histological subtype indicates two distinct aetiologies of gastric cardia cancer. Gut. 2008;57:298–305. doi: 10.1136/gut.2007.137364. [DOI] [PubMed] [Google Scholar]
- 25.Anderson LA, Murphy SJ, Johnston BT, Watson RG, Ferguson HR, Bamford KB, Ghazy A, McCarron P, McGuigan J, Reynolds JV, et al. Relationship between Helicobacter pylori infection and gastric atrophy and the stages of the oesophageal inflammation, metaplasia, adenocarcinoma sequence: results from the FINBAR case-control study. Gut. 2008;57:734–739. doi: 10.1136/gut.2007.132662. [DOI] [PubMed] [Google Scholar]
- 26.Anandasabapathy S, Jhamb J, Davila M, Wei C, Morris J, Bresalier R. Clinical and endoscopic factors predict higher pathologic grades of Barrett dysplasia. Cancer. 2007;109:668–674. doi: 10.1002/cncr.22451. [DOI] [PubMed] [Google Scholar]
- 27.Iijima K, Koike T, Abe Y, Inomata Y, Sekine H, Imatani A, Nakaya N, Ohara S, Shimosegawa T. Extensive gastric atrophy: an increased risk factor for superficial esophageal squamous cell carcinoma in Japan. Am J Gastroenterol. 2007;102:1603–1609. doi: 10.1111/j.1572-0241.2007.01257.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Tang L, Sun G, Tang Y, Xie Y, Wang S, Hu X, Gao W, Cox SB, Wang JS. Etiological study of esophageal squamous cell carcinoma in an endemic region: a population-based case control study in Huaian, China. BMC Cancer. 2006;6:287. doi: 10.1186/1471-2407-6-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Martel C, Llosa AE, Farr SM, Friedman GD, Vogelman JH, Orentreich N, Corley DA, Parsonnet J. Helicobacter pylori infection and the risk of development of esophageal adenocarcinoma. J Infect Dis. 2005;191:761–767. doi: 10.1086/427659. [DOI] [PubMed] [Google Scholar]
- 30.Wang KX, Wang XF, Peng JL, Cui YB, Wang J, Li CP. Detection of serum anti-Helicobacter pylori immunoglobulin G in patients with different digestive malignant tumors. World J Gastroenterol. 2003;9:2501–2504. doi: 10.3748/wjg.v9.i11.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberg S, Peters JH, Nigro JJ, Theisen J, Hagen JA, DeMeester SR, Bremner CG, DeMeester TR. Helicobacter pylori is not associated with the manifestations of gastroesophageal reflux disease. Arch Surg. 1999;134:722–726. doi: 10.1001/archsurg.134.7.722. [DOI] [PubMed] [Google Scholar]
- 32.Tran B, Lucas R, Kimlin M, Whiteman D, Neale R. Association between ambient ultraviolet radiation and risk of esophageal cancer. Am J Gastroenterol. 2012;107:1803–1813. doi: 10.1038/ajg.2012.329. [DOI] [PubMed] [Google Scholar]
- 33.Lin SW, Fan JH, Dawsey SM, Taylor PR, Qiao YL, Abnet CC. Serum thyroglobulin, a biomarker for iodine deficiency, is not associated with increased risk of upper gastrointestinal cancers in a large Chinese cohort. Int J Cancer. 2011;129:2284–2289. doi: 10.1002/ijc.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arents NL, van Zwet AA, Thijs JC, Kooistra-Smid AM, van Slochteren KR, Degener JE, Kleibeuker JH, van Doorn LJ. The importance of vacA, cagA, and iceA genotypes of Helicobacter pylori infection in peptic ulcer disease and gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:2603–2608. doi: 10.1111/j.1572-0241.2001.04104.x. [DOI] [PubMed] [Google Scholar]
- 35.Epplein M, Zheng W, Xiang YB, Peek RM, Li H, Correa P, Gao J, Michel A, Pawlita M, Cai Q, et al. Prospective study of Helicobacter pylori biomarkers for gastric cancer risk among Chinese men. Cancer Epidemiol Biomarkers Prev. 2012;21:2185–2192. doi: 10.1158/1055-9965.EPI-12-0792-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palladino-Davis AG, Mendez BM, Fisichella PM, Davis CS. Dietary habits and esophageal cancer. Dis Esophagus. 2013:Epub ahead of print. doi: 10.1111/dote.12097. [DOI] [PubMed] [Google Scholar]
- 37.Yokokawa Y, Ohta S, Hou J, Zhang XL, Li SS, Ping YM, Nakajima T. Ecological study on the risks of esophageal cancer in Ci-Xian, China: the importance of nutritional status and the use of well water. Int J Cancer. 1999;83:620–624. doi: 10.1002/(sici)1097-0215(19991126)83:5<620::aid-ijc9>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 38.Yu X, Zhang T, Zhang H, Hu A, Hu Y, Guo W, Wang Y. Comparison of lifestyle and living environment among high risk immigrant and low risk host residents: implications for esophageal cancer etiology. Asian Pac J Cancer Prev. 2010;11:1827–1831. [PubMed] [Google Scholar]
- 39.Matsuhisa T, Tsukui T. Relation between reflux of bile acids into the stomach and gastric mucosal atrophy, intestinal metaplasia in biopsy specimens. J Clin Biochem Nutr. 2012;50:217–221. doi: 10.3164/jcbn.11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umar M, Upadhyay R, Mittal B. PLCE1 rs2274223 A>G polymorphism and cancer risk: a meta-analysis. Tumour Biol. 2013:Epub ahead of print. doi: 10.1007/s13277-013-0932-7. [DOI] [PubMed] [Google Scholar]
- 41.Choi Y, Song S, Song Y, Lee JE. Consumption of red and processed meat and esophageal cancer risk: meta-analysis. World J Gastroenterol. 2013;19:1020–1029. doi: 10.3748/wjg.v19.i7.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bosetti C, La Vecchia C, Talamini R, Simonato L, Zambon P, Negri E, Trichopoulos D, Lagiou P, Bardini R, Franceschi S. Food groups and risk of squamous cell esophageal cancer in northern Italy. Int J Cancer. 2000;87:289–294. [PubMed] [Google Scholar]
- 43.Zhuo X, Zhang Y, Wang Y, Zhuo W, Zhu Y, Zhang X. Helicobacter pylori infection and oesophageal cancer risk: association studies via evidence-based meta-analyses. Clin Oncol (R Coll Radiol) 2008;20:757–762. doi: 10.1016/j.clon.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Koike T, Ohara S, Inomata Y, Abe Y, Iijima K, Shimosegawa T. The prevalence of Helicobacter pylori infection and the status of gastric acid secretion in patients with gastroesophageal junction adenocarcinoma in Japan. Inflammopharmacology. 2007;15:61–64. doi: 10.1007/s10787-006-1549-x. [DOI] [PubMed] [Google Scholar]
- 45.Sýkora J, Malán A, Záhlava J, Varvarská J, Stozĭcký F, Siala K, Schwarz J. Gastric emptying of solids in children with H. pylori-positive and H. pylori-negative non-ulcer dyspepsia. J Pediatr Gastroenterol Nutr. 2004;39:246–252. doi: 10.1097/00005176-200409000-00004. [DOI] [PubMed] [Google Scholar]


