Abstract
The TSH receptor (TSHR) is a member of the glycoprotein hormone receptors, a subfamily of family A G protein-coupled receptors. The TSHR is of great importance for the growth and function of the thyroid gland. The TSHR and its endogenous ligand TSH are pivotal proteins with respect to a variety of physiological functions and malfunctions. The molecular events of TSHR regulation can be summarized as a process of signal transduction, including signal reception, conversion, and amplification. The steps during signal transduction from the extra- to the intracellular sites of the cell are not yet comprehensively understood. However, essential new insights have been achieved in recent years on the interrelated mechanisms at the extracellular region, the transmembrane domain, and intracellular components. This review contains a critical summary of available knowledge of the molecular mechanisms of signal transduction at the TSHR, for example, the key amino acids involved in hormone binding or in the structural conformational changes that lead to G protein activation or signaling regulation. Aspects of TSHR oligomerization, signaling promiscuity, signaling selectivity, phenotypes of genetic variations, and potential extrathyroidal receptor activity are also considered, because these are relevant to an understanding of the overall function of the TSHR, including physiological, pathophysiological, and pharmacological perspectives. Directions for future research are discussed.
-
Introduction
Naturally occurring mutations in the TSHR gene as molecular cause of thyroid diseases
General implications for the process of signal transduction
-
Signal Transduction at the TSHR
Signal induction and regulation at the extracellular region
Signaling activity regulation at the transmembrane region
Intracellular G protein coupling and activation
-
New and Controversial Aspects of TSHR Signaling
Oligomerization
Different mechanisms of signal pathway regulation
Extrathyroidal TSHR expression and function
Potential future directions for TSHR research
I. Introduction
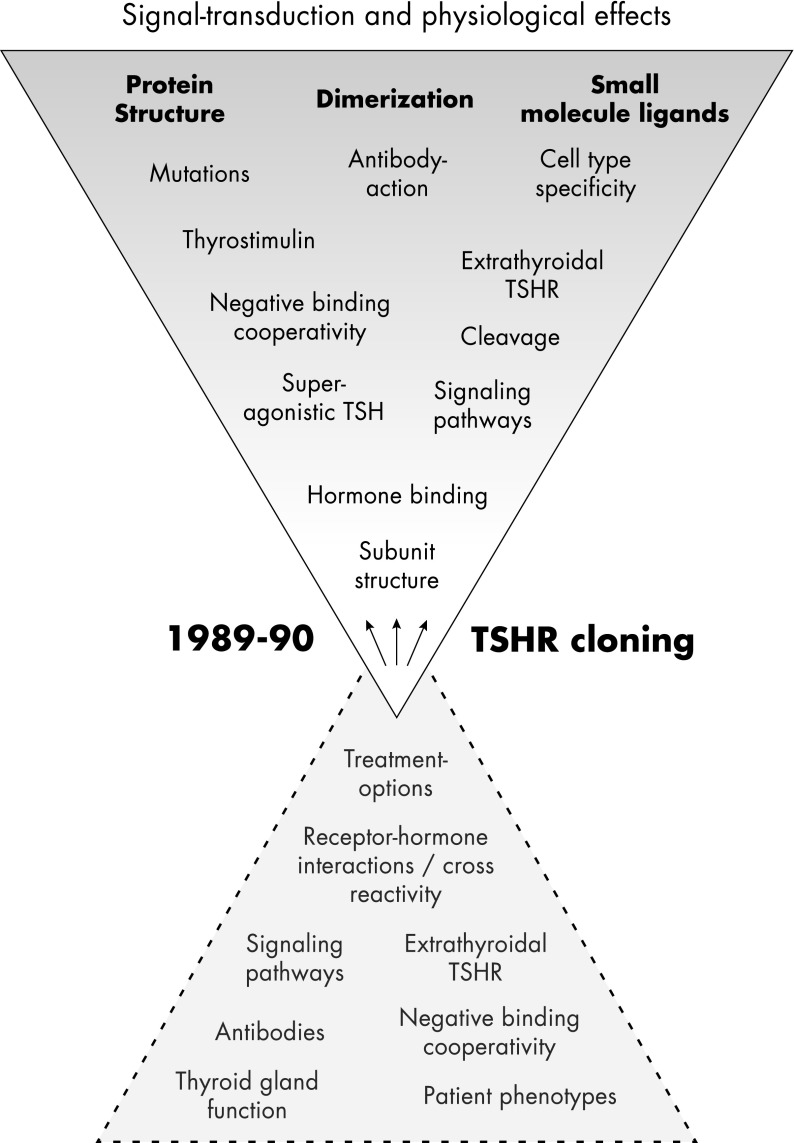
The receptors for TSH (TSHR), for FSH (FSHR), and for LH and choriogonadotropin (CG) (LHCGR), are glycoprotein hormone (GPH) receptors (GPHRs), a subfamily of family A G protein-coupling receptors (GPCRs) (1). The TSHR can activate different G protein subtypes (2–5) and signaling pathways (6, 7) and is essential for thyroid growth and function (8, 9). Between 1989 and 1990, several groups succeeded in cloning the dog and human TSHR (10–14). During the last decades, TSHR-related research has developed exponentially, including investigation of molecular properties and physiological aspects (Figure 1). Aside from fundamental questions on TSHR functions and mechanisms, many new fields have arisen, such as the involvement of the TSHR in photoperiodic regulation in several nonhuman species (15–17) and extrathyroidal TSHR expression (18–22). Unanswered questions include details of the relation between receptor oligomerization and activation (23), the interrelation between negative cooperativity (24–27) and dimerization (27), the physiological significance of specific signaling pathways (reviewed in Ref. 7) and the importance of cleavage at the extracellular region (reviewed in Ref. 28). Answers to these questions might also help to define genotype-phenotype correlations for TSHR dysfunctions (reviewed in Ref. 29) that have not yet been deciphered. Furthermore, small drug-like molecules that activate or inhibit TSHR signaling are under continuous development (reviewed in Ref. 30), together with the characterization of their potential binding sites and induced signaling mechanisms (31–34). Effects of antibodies against the TSHR are of enormous pathophysiological relevance. TSHR antibodies have been extensively studied, and the tremendous amount of data has been excellently reviewed (35–43). Therefore, this topic is beyond the scope of this review. Our focus is the discussion of knowledge concerning structure-function relationships for the entire TSHR from the extra- to the intracellular site (hormone binding plus transmembrane processes plus G protein coupling) and the description of signaling mechanisms related to the TSHR. We contemplate physiological aspects and reflect critically our current knowledge about the TSHR by implementation of general findings with respect to GPCR signaling because the TSHR must be seen in light of its function as a GPCR with common and very unique properties. Our aim was to summarize the association between TSHR properties and its diverse functionalities to support an understanding of the TSHR in its entirety.
Figure 1.
Principal topics of TSHR research. After cloning of the TSHR, scientific and medical investigation has developed exponentially, with the aim of characterizing the signal transduction process and the physiological impact of the TSHR. Many fields have been also rediscovered, but important basic questions still await answers, e.g., the relation between hormone binding and dimerization, activity regulation, or the physiological relevance of basal signaling activity. The genotype-phenotype relationship of TSHR dysfunctions cannot yet be clearly discriminated. The in vivo occurrence or physiological relevance of extracellular cleavage is still unknown, as are the tissue- and cell-specific functions and molecular characteristics of TSHR species, compared with experimental in vitro conditions. Finally, extrathyroidal TSHR expression is well supported, but the physiological impact in humans is still unknown.
A. Naturally occurring mutations in the TSHR gene as molecular cause of thyroid diseases
The formation of stimulating autoantibodies targeting the TSHR is the cause of autoimmune hyperthyroidism in patients with Graves' disease, and TSHR-blocking autoantibodies can be detected in patients suffering from autoimmune hypothyroidism. Properties and function of antibodies is of high importance and is, therefore, well investigated and already reviewed comprehensively (35–43). In consequence, this topic will not be reviewed here, and we will only summarize previous and recent insights into genetic variations of the TSHR gene as a molecular cause of hypo- or hyperfunctioning of the thyroid gland (29, 38, 42, 44–48). Moreover, genetic TSHR variations are one of the most important tools to explore and to understand structure-function relationships in view of the (patho-)physiological significance. The TSHR is the most important regulator of thyroid functions (9). Therefore, it was expected after cloning of the TSHR that disease-causing mutations in the gene will be detected. This was indeed the case with the identification of the first somatic constitutively activating TSHR mutations in 1993 (Asp619Gly and Ala623Ile) related to toxic thyroid nodules (49).
1. Constitutively activating mutations (gain-of-function)
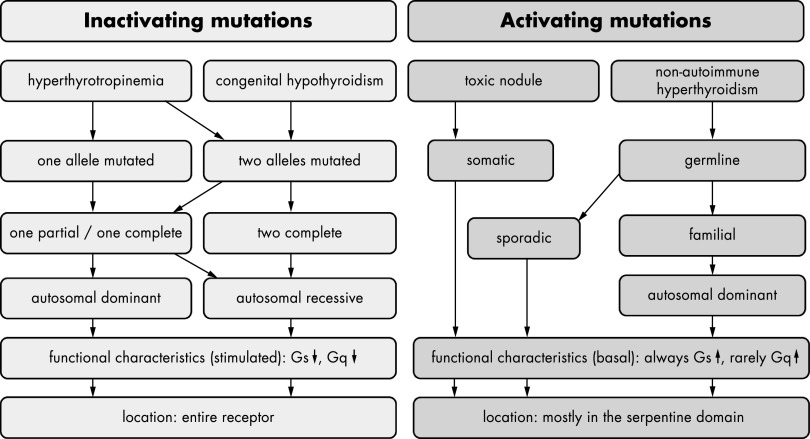
Constitutively activating mutations (CAMs) result in toxic thyroid nodules if they are somatic and in nonautoimmune hyperthyroidism if they are germline mutations (Figure 2) (50, 51). Shortly after identification of somatic TSHR mutations in toxic thyroid nodules, germline mutations were found in familial and sporadic cases (reviewed in Ref. 52). All of these mutations constitutively activate the Gs/adenylyl cyclase signaling pathway, and few of them also activate the Gq/11 phospholipase Cβ pathway (eg, Gly431Ser is the only germline mutation with constitutive Gq/11-mediated signaling activity). For germline mutations, the mode of inheritance in familial cases is autosomal dominant. So far, there is no obvious correlation between the severity of the disease and the level of constitutive activity of the mutated receptor (53). Analysis of the functional characteristics of mutant receptors in COS cells and the clinical course of individual patients suggest that sporadic mutations have a stronger activating effect than hereditary mutations (54). Moreover, a recent study has demonstrated that mutations identified both in toxic thyroid nodules and in sporadic cases show higher levels of constitutive activity (55). Due to the severe phenotypes, it is likely that newborns with neo-mutations might not survive if not treated efficiently. On the contrary, the available pedigrees suggest that the milder phenotype of patients with hereditary mutations has only a limited effect on reproductive fitness.
Figure 2.
Characteristics of inactivating and activating TSHR mutations. Phenotypic and functional implications of naturally occurring inactivating mutations are indicated in light gray. Such mutations were identified in patients suffering from hyperthyrotropinemia or congenital hypothyroidism. In the case of hyperthyrotropinemia, these mutations are either located on one (dominant inheritance) or on both alleles (recessive inheritance). In patients suffering from congenital hypothyroidism, mutations are located on both alleles. The mutations leading to congenital hypothyroidism (mostly investigated in heterologous overexpressing systems) are either complete loss-of-function mutations on both alleles or one complete loss-of-function mutation combined with a partial loss-of-function mutation. This combination of functional properties was also found in patients suffering from hyperthyrotropinemia; however, in that condition, the mutations are mostly partial loss-of-function mutations. The term “functional characteristics” refers to signaling properties after TSH simulation that resulted in a reduction of TSH-stimulated functionality. Inactivating mutations have been found in all receptor parts. Phenotypic and functional properties of activating mutations are indicated in dark gray. These mutations could occur either as somatic or as germline mutations. Germline mutations either are sporadic or were found as familial cases and were inherited in an autosomal dominant manner. Activating mutations are always characterized by an enhanced basal Gs signaling; a few mutations also show enhanced Gq signaling.
Besides activating TSHR mutations, also substitutions leading to inactivity of the TSHR are known and can either cause hperthyreotropinemia or congenital hypothyroidism. In 1995, the first inactivating TSHR mutations were identified (56).
2. Inactivating mutations (loss-of-function)
Mutations that impair the function of TSHR can either result in the mild phenotype of TSH elevation with normal thyroid hormone levels (hyperthyrotropinemia) or can manifest as congenital hypothyroidism with hypoplasia of the thyroid gland (29, 45, 51, 54). The mode of inheritance of inactivating mutations can be either recessive or dominant for hyperthyrotropinemia. Dominant-negative effects of few inactivating TSHR mutations were evidenced (eg, Cys41Ser, Leu467Pro, and Cys600Arg) (57). Based on current knowledge, congenital hypothyroidism due to TSHR mutations is inherited recessively (Figure 2).
Although the TSHR seems to be a suitable candidate gene for congenital hypothyroidism and hypoplasia of the thyroid gland (58), mutations in this group of patients have been identified in only a few cases. TSHR mutations were identified in only 2% of patients with congenital hypothyroidism and thyroid hypoplasia in a cohort of patients in Berlin, Germany (our own unpublished data). In contrast, most inactivating TSHR mutations were identified in patients with hyperthyrotropinemia, eg, with a frequency of 11.8% of heterozygous inactivating mutations in an Italian population (59). The mode of inheritance here could be autosomal recessive if two mutations on different alleles lead to the phenotype (Figure 2). In this case, the transmitting parents are healthy. However, in few cases, only one mutated TSHR allele is responsible for the phenotype (autosomal dominant) (57).
Inactivating mutations have been identified over the entire TSHR structure and are mostly missense substitutions. Phenotype-genotype correlations have been difficult to define because, rarely, similar mutations are found in patients with hyperthyrotropinemia and congenital hypothyroidism (Figure 2). One explanation for this might be that most of the functional data were obtained from studies in heterologous overexpressing systems. For some partial loss-of-function mutations, the defect could be more severe at physiologically relevant expression levels (60). Thus, only the occurrence of 2 complete loss-of-function mutations on both alleles results in congenital hypothyroidism. Recently, clinical and functional aspects of inactivating TSHR mutations as well as potential genotype-phenotype correlations were reviewed, and based on this, a comprehensive clinical work-up to decipher in which patients mutational screening is useful was recommended (61).
However, the patient phenotypes are directly linked with mutation-induced molecular changes of the receptor protein. Inevitably, this raises the questions of what defines a normal TSHR function and what the structural prerequisites are. Only with this knowledge is it possible to understand deregulated TSHR signaling and associated effects on thyroid function.
B. General implications for the process of signal transduction
The molecular function of TSHR and that of all other GPCRs can be defined as the reception, conversion, and transduction of information in a sequence of distinguishable and causally related molecular events.
First of all and in most cases (62), a ligand contacts the receptor by binding to specific amino acids. This initial event occurs at predestinated local receptor regions, and the mode of binding and induced effects are dependent on ligand-properties and specific intermolecular interactions to the receptor (63, 64). Ligands can affect the receptor generally as agonists (activation), antagonists (signaling inhibition), inverse agonists (impairment of basal [constitutive] signaling activity in addition to inhibition of agonist-induced signaling), or as modulators (tuning the effect of a second ligand up or down) (65). The endogenous ligand/receptor binding region is the orthosteric binding site in contrast to allosteric binding sites for non-natural ligands (66).
Second, ligand binding induces conformational changes at the receptor that can be understood in case of agonists as receptor activation. These conformational changes are characterized by individual features for each pair of GPCR and ligand (67, 68), despite general aspects of helical rearrangements for the GPCR families (69). The structural shifts might comprise in diverse combinations and to different extents the N-terminal tail, extracellular loops (ECLs), the transmembrane helices, and the intracellular receptor parts (70).
Third, particular intracellular amino acids contribute to configure specific contacts between the receptor and G protein (71, 72).
Fourth, these interactions lead subsequently to changes of the G protein structure and to activation of downstream effectors (73–77) as a prerequisite for activation of secondary transmitter systems (75, 78).
Each of these intermediate events is characterized by specific participating proteins, with a central role for the receptor. Importantly, the so-called signal is defined as an initial event of receptor activation induced by receptor-specific triggers. In the case of the TSHR, this initial step can be stimulated by a wide variety of different factors, including hormone binding (endogenous ligand), tryptic clipping, antibodies, mutations, or small drug-like molecules. Furthermore, in this process, the signal or information is amplified (79) and the entire process of signal transduction is accompanied by a conversion of information.
To obtain a deeper understanding of TSHR signal transduction, a general and comparative view on other GPCRs is essential. Within almost any physiological process, GPCRs are involved in a complex system of interrelations (80). The relevance of GPCRs is due to their role as signal transducers and regulators (81, 82). These receptors are activated by a huge variety of different ligands, including small peptides, nucleotides, ions, amines, or even large GPHs, and they activate various signaling pathways (83). On the basis of specific conserved amino acid motifs, GPCRs can be subdivided into several families (84, 85). They share a common structural architecture of an extracellular N-terminal region, 7 transmembrane helices (TMHs), 3 connecting intracellular loops (ICLs), 3 ECLs, and an intracellular C-terminal tail (70). The ECLs, TMHs, and ICLs constitute the membrane-spanning serpentine domain (SD). This has been confirmed for family A GPCRs by the available crystal structures (reviewed in Refs. 64, 86, and 87). The capability to activate G proteins means that the GPCRs share a common readout of their diverse signal transduction processes (reviewed in Ref. 73). These receptors are involved in nearly all physiological processes and in the action of more than 50% of approved drugs (where the target is known) (88–91). Many human diseases are linked to GPCR function and malfunction, including cancer, viral infections, inflammation, infertility, and metabolic and neurological disorders (44, 47, 48, 92, 93). Thus, structural information and mechanistic insights for GPCRs are of the greatest medical, pharmacological, and biological interest (94).
Especially in recent decades (reviewed in Ref. 95), great progress has been made in the crystallization of GPCRs (86). Crystal structures are useful to improve pharmacological approaches (89, 96–98). As a result of advanced experimental methods (99), many GPCR crystal structures have been reported, including rhodopsin variants and dopamine, chemokine, adenosine, and acetylcholine receptors (reviewed in Ref. 100). Of specific note, not only GPCR structures with bound antagonistic molecules have been elucidated but also structures of complexes with agonists or with G proteins, such as the β2-adrenergic receptor (ADRB2)/Gs complex (77, 101). In addition, a constitutively active rhodopsin structure (102) coupled with a G protein fragment (103) has been crystallized, and other activated GPCR structures are also available (reviewed in Ref. 69). These structures are important to analyze and to compare intermediate states of activation, including basally active conformations or constitutive activation by mutations that can also occur at the TSHR.
Signal transduction within the membrane region of GPCRs (63) generally takes place by restructuring the positions of transmembrane helices relative to each other (104). Most of the endogenous and synthetic ligands of family A GPCRs bind within the transmembrane domain close to the second ECL (ECL2) (105, 106). This is in contrast to other GPCR families (and the GPHRs), and it has been proposed that this circumstance might be the basis for the evolutionary success of family A GPCRs (107), reflected by the large number of members compared with the more structurally complex GPCRs of other families with large ligand-binding N termini (108). Using the pocket-like crevice between the TMHs and ECLs for both ligand recognition and signal transduction might be an evolutionary advantage, because in this way, the ligand directly affects the membrane-spanning part of the receptor that is sensitive for activation of the G protein. Second, receptor mutations in this ligand binding region might potentially lead to recognition and binding of new ligands or modulate signaling induced by the particular ligand. The family A GPCRs, therefore, should adapt, by variation, more effectively to different ligands (109) in a certain physiological context.
Of specific note, recent studies at several GPCRs, such as the α2A-adrenergic receptor (110), the P2Y1 (111, 112), or muscarinic receptor subtypes (113–115), revealed that GPCR signaling properties (agonist binding and signaling capacity) can be voltage-dependent and -regulated (reviewed in Refs. 116–118). A systematic investigation of GPCRs concerning this regulation mechanism would be of future interest.
Much experimental data suggest that a global toggle switching mechanism takes place during ligand-induced activation, whereby a vertical see-saw movement of TMH6 occurs around a pivot (119, 120). In consequence, spatial rearrangement of the TMHs is observed, to the greatest extent between TMHs 5, 6, and 7 (121–123). This structural rearrangement is supported by amino acids acting as microswitches (71, 124, 125). In addition, contacts between ECL2 and the extracellular extensions of the helices have been proposed to participate as regulators during activation (126–132). Different GPCR conformations are related to different signaling activity states (70, 93, 133).
Unfortunately, the only structural information for the TSHR is provided by the reported leucine-rich repeat (LRR) domain (LRRD)/antibody complexes (134, 135). But several experimental studies, combined with bioinformatics approaches, have made it possible to understand the processes occurring at the TSHR in detail. Insights from other GPCRs can then be applied.
II. Signal Transduction at the TSHR
A. Signal induction and regulation at the extracellular region
1. Hormone-receptor interactions
Thyrotropin binds to the extracellular N-terminal LRRD that exhibits a typical and specific structural fold (136), which was shown in the published TSHR LRRD crystal structures (134, 135). In the concave site of the scythe blade-like–shaped LRRD (137), a complementary pattern of amino acid side-chain properties is responsible for hormone recognition and binding (reviewed in Ref. 138). So far, such a GPHR/GPH complex has been crystallized only for the FSHR LRRD/FSH (139, 140). TSH binding to a purified TSHR LRRD has not been detected so far (141), but to a more extended extracellular region (142). Due to the high homology between GPHRs and GPHs, these crystallized FSHR structures offered a solid basis for the simulation of first basic (143, 144) and then advanced (145) TSHR/TSH complex models to assist analyses and comparison of hormone binding (138, 144, 146). The assumption of comparability is supported by a naturally occurring mutation (147), natural species variants (148), and site-directed TSHR mutations (149) in the LRRD that lead to hypersensitivity (promiscuity) for CG binding at the TSHR LRRD. In these cases, the bound CG initiates signal transduction at the TSHR, which suggests important similarities in GPH-LRRD orientation and the activation mechanisms, despite a few differences in GPHR-subtype–specific interactions (146).
The hormone interacts with the side chains of LRRs 2–9 (138), of 11 complete LRRs constituting the LRRD (140). The previously reported GPHR LRRD crystal structures comprised 9 full repeats and a 10th β-strand and were different in their C-terminal structures (reviewed in Ref. 150). On the basis of sequence analyses, homology modeling, and mutagenesis studies, it was speculated that the LRRD might be enlarged by additional repeats (137, 151). A newly determined FSHR/FSH crystal structure with a nearly complete extracellular region (140) indeed shows that the LRRD continues through specific amino acids of cysteine-box 2 (Cb-2, TSHR amino acids from Cys283–Cys301, Figure 3) with 11 repeats (C-terminal LRRD end at Asn288). Of note, only the 11th repeat contains a helical structure fragment that had been postulated previously for each repeat at the convex side of GPHR LRRDs on the basis of crystal structures of other known LRRDs (reviewed in Ref. 150). In addition, a few amino acids around Cys398 at the C-terminal extracellular region are arranged as a short β-strand parallel to β-strand 11 of the LRRD.
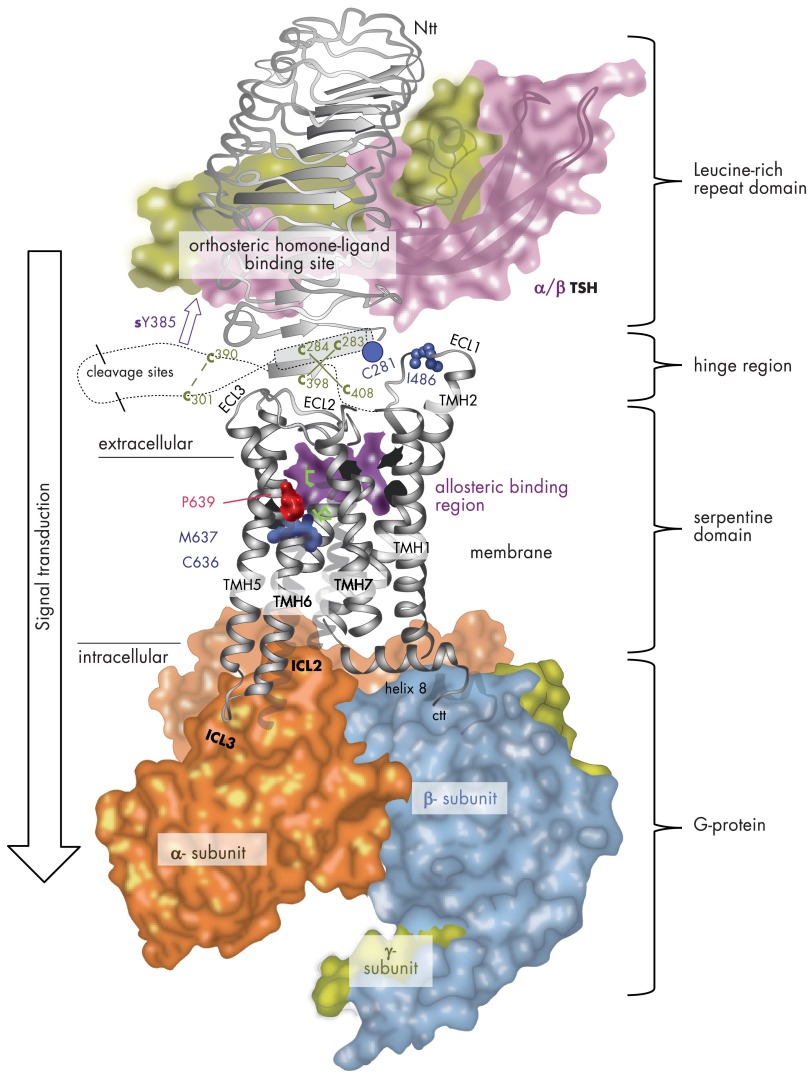
Figure 3.
TSHR homology model with putative spatial orientations and interactions between receptor components, the hormone and G protein. This combined TSHR model highlights causal relationships between functional and structural data. This includes hormone and G protein binding at an activated TSHR monomer as well as mechanisms in the transmembrane region related to the activation process. GPHR crystal structures are available only for the LRRD and hinge region (Jul 2012: PDB entry codes 3G04, 2XWT, and 4AY0). The structure and binding of bTSH (backbone-ribbon and translucent, colored surface) at the LRRD (orthosteric binding site) is based on the assumption of similarity with the crystallized FSHR LRRD/FSH complexes. The hormone interacts with specific side chains at LRRs 2–9. The newly determined homologous FSHR extracellular region (LRRD and hinge region)/FSH crystal structures have shown 11 complete repeats at the extracellular region (140). It has been observed that the LRRD continues through specific amino acids of cysteine-box 2 (amino acids from Cys283 to Cys301) with a small helix conformation (cylinder) at repeat 11. A few important amino acids of cysteine-box 3 (amino acids Cys390 to Cys408) close to TMH1 are arranged as an additional β-strand, parallel to the LRRD. Six cysteines are disulfide bridged to each other between cysteine-box 2 (Cb-2) and Cb-3. In conclusion, the unit of 11 repeats and the C terminus of the extracellular region are located in close proximity as an interface to the transmembrane region. It is noteworthy that CAMs were identified at these receptor domains, e.g., at Ser281 (blue dot). Several amino acids with an impact on hormone binding and signaling have been detected between positions 289 and 410, including the sulfated tyrosine 385 (sTyr385, magenta arrow). Details of interaction between this residue and the hormone are now represented in the recent FSHR/FSH crystal structure (140) and also confirm that the hinge region is a central region for signal transduction from the extracellular site to further receptor components. However, the precise orientation of the LRRD/hormone, hinge region complex, and the SD to each other is not known yet (just exemplarily here). Newly identified naturally occurring side-chain substitutions, e.g., at wild-type position Pro639 (TMH6, red atom spheres, inactivating mutation) or Cys636 (TMH6, blue atom spheres) and Ile486 (ECL1, blue atom spheres), that lead to TSHR inactivation (red) or activation (blue) are mapped to the transmembrane-spanning SD. Mutations at Cys636, Met637, and Pro639 have provided details into TSHR because an activity switch in this conserved family A GPCR motif is localized at TMH6. The potential allosteric binding pocket for drug-like small-molecule ligands (417) between the helices and ECL2 (magenta) is highlighted by an inner pocket surface image (violet translucent surface). Finally, the activated SD is characterized by the capacity to activate G proteins. Orientation of the activated heterotrimeric Gs protein (backbones of α-, β-, and γ- subunits with translucent colored surface) at the active-state conformation of TSHR (white backbone ribbon) is in accordance with recently published data for G protein activation at the TSHR and the crystal structures of other GPCRs (101, 289, 324).
It is well known that the region between the LRRD and TMH1 is important for hormone binding but also for signal transduction (152, 153). This so-called hinge region, cleavage domain, C-terminal cysteine-rich region, or signaling and specificity domain was subdivided into subregions on the basis of the occurrence of 2 flanking cysteine-rich fragments (Figure 3) that are connected to each other by disulfide bridges (140). These cysteine boxes are of great importance for the signaling regulation of TSHR, as indicated by the occurrence of CAMs at specific positions in these fragments (reviewed in Ref. 150). These mutations (Figure 4) activate signal transduction at the receptor independent from external stimulation (ligand independent). The 3 cysteines at the C terminus of the extended LRRD and the N terminus of the hinge region are fixed closely by disulfide bridges to 3 cysteines at the C terminus of the extracellular region close to TMH1, which locates the bound hormone obligately close to the SD (Figure 3).
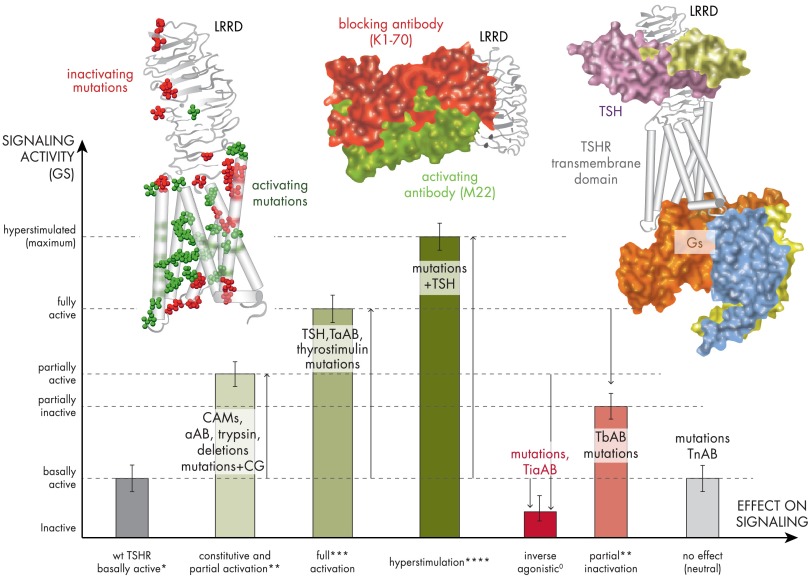
Figure 4.
Scheme of TSHR activity states related to diverse effectors. This graphic visualizes the spectrum of putative signaling activity states (active, green; inactive, red) of the TSHR that are related to different structural features and effectors. The magnitudes of the bars are relative to each other and do not reflect exact data values. The TSHR wild type (wt) exhibits a permanent (basal) signaling activity (Gs activation) that is indicated by an asterisk (*) at the x-axis. CAMs, activating antibodies, treatment with trypsin, and specific mutations in combination with an increased CG affinity lead to an enhanced but partial signaling activity (**). TSH, thyrostimulin, or some thyroid-stimulating antibodies (TsABs) are able to activate the TSHR fully (***), but few cases are reported where an activation of more than 100% was observed (****). In contrast to receptor activation, specific mutations and thyroid-blocking antibodies (TbAB) reduce the maximum TSHR activation. Both blocking antibodies and mutations can have different mechanisms of inactivation such as suppression of the intrinsic signaling capacity (TSH independent), inhibition of hormone binding, or signaling activation (G protein). A specific case is inverse agonism (also called silencing) caused by mutations or antibodies (TiaAB). Here the activity is suppressed below the basal level of the wild type or lowered in case of a constitutively activated TSHR. To complete the potential scenarios, also neutral effects are known, where mutations or intermolecular effectors (neutral antibodies [TnAB]) show no primary effect on signaling. The picture on the left shows a structural TSHR model with mapped positions of naturally occurring activating (green atom spheres) and inactivating (red atom spheres) mutations. Activating mutations do support the active-state conformation of the TSHR. The picture in the middle shows crystal structures of activating (green surface) and inactivating (red surface) antibodies bound to the LRRD that were solved previously (134, 135). They are distinguished concerning their binding mode at the LRRD. The picture on the right shows a completed TSHR model with bound TSH (green surface) and bound Gs (subunit surfaces are differently colored). The comparison of structural insights of all 3 exemplary models (left, middle, and right) reveals why specific mutations or antibodies modulate particular steps of the signal transduction process at the TSHR in diverse modes (e.g., blocking of TSH binding, inhibition of G protein activation, and hampered signal transduction at the TSHR structure).
Several amino acids between TSHR positions 289 and 410 with an impact on hormone binding and signaling have been detected by mutagenesis. A sulfated tyrosine (TSHR: Tyr385) was found to have a prominent role at the TSHR for hormone binding (152, 154), but also in other GPHR subtypes (140, 155, 156). This is additionally supported by site-directed mutagenesis studies on chimeric GPHRs (156, 157). The recent FSHR/FSH structure (140) represents a nearly complete extracellular region and provides evidence that the hinge region contains a second hormone binding site at this sulfated tyrosine (FSHR: Tyr335). The negatively charged Asp334 in FSHR adjacent to Tyr335 contributes to the hormone/receptor interactions. The homologous GPHRs are probably characterized by structural differences at this hormone-binding–sensitive hinge region because the sulfated and binding-relevant tyrosine is common as well as a negatively charged amino acid in close proximity (Asp386 in TSHR), but they are not localized at exactly equivalent positions in TSHR/LHR and FSHR (155).
Moreover, bovine TSH contains positively charged residues at the α-subunit that are known to increase hormone binding properties compared with hormone variants without these positive charges at corresponding positions such as human FSH (hFSH) and hTSH (158). The recently published advanced TSHR model (145) based on the FSHR/FSH structure suggests complementary interactions between these 4 positively charged bovine TSH (bTSH) side chains and experimentally identified negatively charged TSHR residues like Glu297, Glu303, and Asp382 at the N- and C-terminal hinge region, respectively (143, 151, 152, 154, 159). Most likely, these interactions mediate superagonistic effects of bTSH compared with hTSH.
In each case, the hormone binds to the LRRD and the hinge region and induces structural shifts by these interactions. The signal is transduced to the SD via a specific molecular switch close to TMH1 that is constituted by particular fragments of both C termini of the LRRD (the α-helix at repeat 11) and of the hinge region (145, 151). Interestingly, comparative studies showed that the binding sites for TSH and TSHR antibodies with different biological activities overlap in the TSHR LRRD. Stimulating antibodies interact with both the N and C terminus of the TSHR LRRD, but blocking antibodies do not contact the LRRD C terminus (160, 161). The new TSHR model with the hinge region revealed that activating antibodies, in contrast to inactivating antibodies, might directly activate a suggested intramolecular switch close to TMH1 without interactions with the second hormone binding site at the hinge region (145).
What else can we learn for GPHRs from the new FSHR/FSH crystal structure (140)? It is known that GPHRs form higher-order complexes (162) and that the extracellular region participates in oligomerization (27). The newly solved FSHR/FSH complex structure is a trimeric receptor/ligand complex, whereas the previous FSHR/FSH crystal structure with a shorter LRRD and without the hinge region (139) is a dimeric LRRD/hormone complex. Unfortunately, both dimeric and trimeric FSHR structures do not overlap in interactions between their monomers. Moreover, the significance of contacting amino acids in the dimer could not be confirmed by additional experimental studies (163). Therefore, a definitive constellation for oligomeric contact points at the extracellular site of the GPHRs cannot be proposed, and we will still have to rely on artificial arrangements based on implemented structural components that need to be refined by experimental support.
Furthermore, the new FSHR crystal structure supports the hypotheses that the hinge region is probably not a self-folding domain. The specific fold and orientation is more probably determined by intra- and intermolecular interactions, as was also recently suggested by protein expression studies at the TSHR (151) and was indicated by the previous observation that CAMs in the ECLs do not activate receptors without the extracellular region (164).
bTSH (mostly used for in vitro studies due to its higher affinity), hTSH, and recombinant hTSH (rhTSH) probably interact differently in detail with the TSHR hinge region (143, 159). These differences are caused by diverse interactions between complementary charged amino acids of hormone subtypes and receptors (143). Positively charged amino acids of TSH, with the exception of primate TSH (165), interact with specific negatively charged residues at the targeted receptor (159) or potentially from a second receptor molecule, which leads, however, to the increased affinity of nonprimate TSH versus primate TSH (reviewed in Ref. 166) and can also be transferred to other hormones like CG by directed amino acid substitutions (reviewed in Refs. 158 and 167). Molecular and structural details of these complementary interactions are now suggested by advanced TSHR homology models (145) based on the new FSHR/FSH crystal structure (140).
Differences in signaling have also been found to be related to hormone glycosylation. TSH and other GPHs are heterodimeric glycosylated proteins, made up of noncovalently bound subunits (α and β). The TSH subunits contain 2 (TSH α-subunit) or 1 (TSH β-subunit) asparagine-linked (N-linked) oligosaccharides (166). It was reported that the N-linked oligosaccharide chains have a minor role in the binding of GPHs to the receptors but are crucial for their bioactivity. GPHs lacking N-linked oligosaccharides act as antagonists (168). Furthermore, it was shown that the 2 dominant G protein-mediated intracellular signaling pathways, cAMP formation and inositol phosphate (IP) release, are activated by different hTSH glycosylation variants to different degrees (169). This aspect might be relevant for the comparison between pituitary hTSH and rhTSH, because glycosylation of rhTSH differs from that of the endogenous protein in both the extent and type of glycosylation (170, 171).
The finding that heterodimeric GPH variants express different effects on signaling has led to approaches to design hormone subunit derivatives based on modification of the subunit structure: fused subunits (172–176), monomeric subunits (177, 178), or tetrameric forms (178, 179), all of which can bind and interact with the receptor. The functional impact ranged from agonistic to antagonistic effects. However, it is not known where and how these designed variants interact with the receptor, especially with respect to the fact that the structural conformations of hormone subunit variants differ from those of the heterodimeric wild-type hormone, as evident in the comparison of the elucidated structures of GPH α-subunits (180, 181) and heterodimeric GPH structures (139, 140, 182–184).
It is known that single hormone subunits occur under physiological and pathophysiological conditions (24, 185–191). Cross-reactions between hormone subunit variants, dimeric hormones, and receptors have been reported (190–192). In addition, the subunits of thyrostimulin, the second endogenous TSHR ligand (193), are known to be expressed separately in different tissues (194), which might indicate that single hormone subunits modulate the targeted receptor (195). The subunits of thyrostimulin (193, 196, 197) were found to be distributed in the central nervous system, stomach, pancreas, testis, and other reproductive tissues of different animals (198, 199). Thyrostimulin was reported to be approximately 4-fold more potent than TSH in stimulation of cAMP accumulation at the TSHR, whereas no activation of the homologous FSHR or LHR was observed (200). The β-subunit of thyrostimulin alone activated the hTSHR, but at a 100-fold higher concentration than the thyrostimulin heterodimer (200). Knockout of the β-subunit of thyrostimulin causes transient hypothyroxinemia in juvenile mice, indicating an effect on the hypothalamus-pituitary-thyroid axis (201) and a role in development, because no effect could be found in adult mice.
Moreover, it is conceivable that more than 1 ligand might bind simultaneously to the extracellular region of the TSHR and other GPHRs. Evidence for distinct receptor surfaces for ligand contact in the extracellular TSHR region was provided by kinetic studies of antibody binding (202). These authors suggested that full activation of the receptor might require binding of 2 or more different antibody molecules to 1 TSHR at different binding sites. In several previous experiments (in the 1970s and 1980s), 2 different binding sites for hormone molecules were proposed, also on the basis of kinetic studies (195, 203). Such a mechanism is supported by experiments on the FSHR. A specific receptor fragment antibody and hFSH do not interfere with each other's simultaneous binding to a purified and functional extracellular region of the hFSHR (204).
Despite this detailed knowledge, the question still remains how the extracellular components transduce the signal to the SD and how these main determinants interact with each other. The described negative cooperative effects on hormone binding have not been clarified but may be important in this context (24–27).
2. Previous and new insights into signaling activity regulation at extracellular components
It is postulated that intramolecular interactions between the extracellular region and the SD are essential for switching from basal to fully activated GPHR conformations.
On the basis of site-directed mutagenesis at particular amino acids and insights from deletions leading to receptor activation, it is commonly concluded that the extracellular hinge regions of GPHRs are necessary for stabilization of a signaling-competent basal receptor conformation (164, 204–213). Constitutively active GPHR variants can be designed via mutations in the hinge region, too (see also the freely accessible Web resource and data collection for GPHRs) (214–216), whereas modifications at the LRRD do not lead to receptor activation. Therefore, it is thought that the hinge region is of central importance in receptor activation (151, 164, 204, 211, 217), including signal transduction via an extracellular intramolecular signal transmitter followed by activation of an intramolecular agonistic unit (151). A key role for this intramolecular mechanism was first assigned to TSHR amino acid Ser281 in cysteine-box 2 (corresponds to hLHR Ser277, hFSHR Ser273) that was identified by occurrence of a pathogenic activating mutation and was studied in detail by site-directed mutagenesis (218–221). This serine (Figure 3) is near amino acids at cysteine-box 3 because of disulfide-bridged cysteines between these 2 portions (156, 222), as is confirmed in the recently published FSH/FSHR crystal structure (140). Not surprisingly, activating single–side-chain substitutions were found at this C-terminal hinge region (137) as well as pathogenic activating single-point mutants (223) or deletions (224, 225).
Deletions of the extracellular portion leading to partial activation of the TSHR and FSHR encouraged the suggestion of an extracellular tethered inverse agonist that switches to an internal agonist during receptor activation (164, 204, 205, 226). The existence of such an intramolecular agonist is further supported by identification of several partially inactivating mutations (151). These wild-type amino acids are probably mandatory for the stabilization of an active conformation after signal induction. Of note, TSHR inactivation by mutations in the hinge region is almost always only partial, and partial constitutive activity by single mutations in cysteine-boxes 2 and 3 is also observed. A potential explanation for this phenomenon might be that full activation needs multiple inductions of different extracellular trigger points, which also implies a complex interrelation or interface between the SD and the extracellular region. In accordance with this, an in vitro study suggested that the SD might affect alterations in the conformation of the adjacent hinge region and therefore influence ligand binding affinity at the extracellular region (227).
Important indications for such a structural-functional interplay or cooperation between the extracellular region and the SD have been provided by pathogenic mutations and site-directed mutagenesis in the transmembrane helices, leading to hormone binding promiscuity at the FSHR (228, 229). Specific CAMs in transmembrane helices alter hormone selectivity at the extracellular site. These FSHR variants are able to bind FSH, but in contrast to the wild type, also TSH and CG. Although this result is unique for the FSHR and cannot be induced similarly at the TSHR or LHR, one potential explanation for this observed effect might be a structural shift caused by mutation-induced activation in the transmembrane region that is transduced backward to the extracellular region and leads to a loss of selectivity for hormone binding. The finding that precise interactions of the hinge region with the SD are functionally linked with ligand binding properties was further supported by experiments on chimeric TSHR constructs (157). TSHR with substituted FSHR or LHR hinge regions revealed decreased bTSH binding compared with the wild type, but hormone binding was restored by simultaneous mutation-induced constitutive activation at different spatial regions, such as the ECLs or TMHs, which again implicates modulated hormone binding properties in activated receptors (157).
Recently, evidence has been presented for the relationship between conformational changes associated with activation of the transmembrane domain and allosteric behavior on ligand binding of GPHR dimers (constituted by 2 monomers, also termed protomers) (162). It was shown that strong constitutively active mutants lost nearly all negative binding cooperativity in a dimer (hormone binding to 1 protomer within the homodimer decreases the affinity of the other) and that each protomer binds with equal affinity. The signal resulting from hormone binding to the extracellular region of one protomer is transduced downward to its membrane-spanning SD before affecting the corresponding region of the other protomer. This probably causes transmission of an upward message, lowering the binding affinity of the extracellular region of the second protomer. In consequence, constitutively active GPHRs lose negative cooperative allosteric regulation in direct relation with their constitutive signaling activity and structural conformation, which supports a model in which the transmembrane domain tunes the affinity and selectivity of the interaction between the hormone and the extracellular region (162). This emphasizes the close relation between structural (activity-related conformation and constellation-like dimers) and functional (ligand binding, intramolecular signaling regulation, and G protein activation) properties.
3. Does extracellular cleavage influence function?
In contrast to other family A GPCRs and the homologous FSHR and LHR, TSHR has the unique property of having 2 extracellular cleavage sites in the hinge region (Figure 3). Several groups have explored the exact localization of the cleavage sites (230–235), the processing protease (236, 237), and the potential physiological importance of cleavage (28, 238–241). Cleavage leads to release of 50 amino acids (the so-called C-peptide, or cleavable peptide, between positions 317 and 366) from the receptor (231) and to separation of the TSHR into a 2-subunit structure (234, 242). Cleavage is a prerequisite for shedding the extracellular subunit (also termed the A-subunit) from the membrane-anchored SD (reviews in Refs. 7, 42 and 43), termed the B-subunit. It has long been discussed whether TSHR occurs as a single-strand protein or in a 2-subunit structure in vivo (243), but the situation is still unknown despite a few insights from in vitro experiments (244). Unfortunately, also the role of cleavage under physiological conditions and the functional consequences for TSHR signaling are not yet understood (reviewed in Ref. 28). It is considered that the shed A-subunit might play a role in the maturation of thyroid-stimulating autoantibodies (245, 246) (reviewed in Refs. 41 and 43).
In vitro, cleavage is prevented by deletion of the C-peptide (position 317–366) that inhibits cleavage at site 1 (231), in combination with triple substitution of TSHR amino acids 367Gly-Gln-Glu369 to corresponding residues of the LHR (motif Asn-Glu-Thr) at cleavage site 2 (232). It is important to note that the synthetic uncleavable TSHR is already without the C-peptide like a cleaved TSHR, and is structurally 50 amino acids shorter than the wild type. Furthermore, the in vitro C-peptide–deleted TSHR is a single-chain protein, in contrast to a cleaved TSHR in vivo. These facts must be considered with caution in the interpretation of functional studies based on this construct (247) because the uncleavable receptor (without the C-peptide) is neither fully comparable with an uncleaved wild-type TSHR (the C-peptide is present) nor with a cleaved wild-type TSHR (without the C-peptide, but it is not a single-chain protein) and, therefore, simulates a situation that does not occur in vivo. However, it is an appropriate tool to study and to compare a shorter uncleavable single-chain TSHR with a shorter and cleaved TSHR.
It has been reported that none of the in vitro-designed modifications at the C-peptide has caused differences in ligand binding or basal (ligand-independent) cAMP accumulation. In contrast, whereas complete C-peptide deletion is without effect on basal TSHR cAMP accumulation (238), deletion of a C-peptide fragment (residues 339–367) enhances ligand-independent TSHR cAMP signaling (213). Furthermore, trypsin treatment of TSHR destroys the C-peptide fragment between residues 354 and 359, which is associated with partial activation of the TSHR (248), whereas the target of tryptic action is probably located at positively charged amino acids between positions 290 and 293 (226). These findings cannot be explained in detail yet.
Moreover, it was postulated in a previous study that the occurrence of TSHR cleavage might depend on cell-cell contacts, because almost complete cleavage was observed in confluent cells as in thyroid tissue, whereas in sparse cells (cultured thyrocytes and nonthyroid cells), most of the TSHR was observed in an uncleaved form (247). Activation of Gq was found to be reduced in cultured cells with uncleaved TSHR (247). These findings would support the idea that cleavage should be of relevance for induction of both Gs- and Gq-mediated signaling pathways, which has to be tested further.
4. The ECLs as signal transducers and modulators
Taken together, these findings generally support the idea of a complex interplay between the N-terminal extracellular region and the SD, which is confirmed by other detailed studies (210, 226, 227, 249, 250) and especially emphasized by findings that CAMs in the ECLs of the TSHR are functional only in the presence of the hinge region (164). In addition, it has been examined whether a combination of activating single mutations at the ECLs might cause increased receptor activation by additive or cooperative effects (251). Indeed, a combination of CAMs at the ECLs results in higher signaling activity than in single mutations. Such an important role for the ECLs is not unique to the TSHR and has been found for many other GPCRs (130, 132, 252, 253).
For the TSHR ECLs (not for the FSHR nor the LHR), naturally occurring activating mutations in the ECLs have been reported several times in ECL2/Ile568Thr (255), Val (256), Phe (225, 257), and Glu575Lys (258) and in ECL3/Asn650Tyr (259) and Val656Phe (260) as well as naturally occurring inactivating mutations in ECL1/Trp488Arg (261), Gln489His (262) and in ECL3/Leu653Val (263). Recently, a new germline TSHR mutation (Ile486Asn) located in the ECL1 was identified, and its characterization supports the relevance of extracellular structures in TSHR signaling (264). Three somatic mutations at this position 486 have been found previously in toxic thyroid adenomas: Ile486Phe (255), Ile486Met (255), and Ile486Asn (265). Functional characterization of mutations Ile486Phe and Ile486Met demonstrated constitutive activation of both Gs- and Gq-mediated signaling. In contrast, the new germline Ile486Asn TSHR variant and the somatic Ile486Asn mutation are not constitutively active for Gq-mediated signaling. In addition, a germline mutation (Ala485Val) at the adjacent position 485 has been reported (266). This mutation also reveals constitutive activation of the Gs-mediated pathway, whereas the basal level of inositol-3-phosphate formation remained unchanged. Stimulation of this mutated TSHR with high concentrations of bTSH also failed to activate the Gq pathway. In conclusion, mutations at the ECL1 can be selective in their functional impact (signaling pathway selectivity), and this loop is, in addition to ECL2 (128), another key player for TSHR signal transduction (208, 251).
Finally, the ECLs cooperatively mediate signals to the transmembrane helices, and this raises the question as to how signal transduction in the transmembrane region is organized.
B. Signaling activity regulation at the transmembrane region
1. Determinants of signal transport and regulation at the transmembrane helices
One of the essential steps for signal transduction in the transmembrane region occurs between helices 5 and 6 (267–271). Most available data for TSHR TMH5 and TMH6 were based on studies of pathogenic mutations in this region (reviewed in Ref. 29). Recent systematic mutagenesis studies on the modifications of biophysical features of the TMH5-TMH6 interface have provided more comprehensive data (272). First of all, the TSHR has an alanine (Ala593) instead of the proline at TMH5 that is conserved among family A GPCRs at this position (unified family A GPCR position number 5.50) (273). Therefore, the TSHR TMH5 most probably has a regular transmembrane α-helix conformation, in contrast to most other family A GPCRs, which have a proline-induced kinked helix conformation, as is visible in several crystal structures (reviewed in Ref. 274). Recently, this assumption for the TSHR has been strongly supported by a new GPCR crystal structure. The sphingosine 1-phosphate receptor 1 (pdb entry code 3V2W) has no proline at this position comparable to the TSHR and, indeed, the crystallized sphingosine 1-phosphate receptor TMH5 shows a regular helix conformation without a kink (275). Proline distorts the ideal helix geometry because of steric conflicts with the preceding residue and the loss of a backbone H-bond. These proline-induced kinks in membrane proteins are functionally important, because they create weak points to facilitate movements required for receptor activation (276, 277). It has been speculated that the lack of a kinked TMH5 could be related to the altered functional properties of such receptors, eg, the occurrence of basal signaling activity (reviewed in Ref. 119). The TSHR Ala593 was recently replaced by site-directed mutagenesis to proline and amino acids with larger side chains to provide insight into the functionality of this position (272). The mutants Ala593Gly and Ala593Pro showed increased basal signaling activity, which indeed indicates the functional importance of the orientation and adjustment of TMH5 in relation to other helices. Furthermore, the pathogenic CAM Ala593Asn (278) and the inactivating mutation Ala593Val (279) have been reported in TSHR. The functional characterization of the naturally occurring inactivating mutant Ala593Val have shown decreased cell surface expression, decreased basal signaling (cAMP) activity, and diminished capacity for ligand-induced signaling. This impeded signaling capacity is probably induced by altered interaction with the counterpart amino acid Val509 at TMH3 (280).
The TSHR also shows another specific difference in the amino acid constitution compared with other family A GPCRs. Methionine 637 is located at TMH6, whereas in around 80% of the family A GPCRs, a tryptophan at the corresponding position can be found (281). This tryptophan has been suggested to move slightly during activation (TMH6 rotation) and stabilizes the active GPCR conformation by forming new interactions to TMH5 (reviewed in Ref. 120). The significance of this tryptophan in ligand-induced signal transduction has also been reported for rhodopsin and other GPCRs (282–284). Methionine 637 in TSHR might undergo comparable movement toward TMH5 during activation and interacts in the active state with aromatic residues in helices 5 and 6 (eg, Phe594 and Phe634). However, introduction of an aromatic and bulky tryptophan to TSHR at position 637 instead of methionine cannot be tolerated spatially and leads to repulsion by surrounding amino acids, thus causing constitutive receptor activation (32). These data lead to the conclusion that the TSHR-specific methionine is involved in the stabilization of the activated conformation.
Furthermore, the Met637 is part of the TSHR motif 636Cys-Met-Ala-Pro639, which is slightly different from other family A GPCRs, with a highly conserved Cys-Trp-(Leu)-Pro motif at TMH6 (Figure 3). It should be noted that 3 new naturally occurring TSHR mutations have been reported in this motif. The mutation Pro639Leu was identified in 2 siblings with congenital hypothyroidism; the second mutation (Cys636Arg) was found in a patient suffering from nonautoimmune hyperthyroidism, and the third heterozygous Cys636Trp mutation was identified in a patient with weak signs of hyperthyroidism. The underlying phenotypes of the patients have been recently described, and the mechanisms of these mutations were explored by in vitro studies of side-chain variations and characterization of their effects on TSHR signaling (285, 286). In the inactive TSHR conformation, cysteine 636 is probably embedded in a tightly packed hydrophobic cage constituted by amino acids at TMH7 (Figure 3). In contrast to the hydrophobic small cysteine side chain's interaction with residues in TMH7, the bulky tryptophan is sterically repelled by TMH7 and thus shifted toward TMH3 in the inner region of the helical bundle, causing receptor activation (286). In addition, larger side-chain variations at this position lead to partially or completely abolished capacity for bTSH-induced signaling. This suppression is due to blockade of the flexibility of helix movement. In consequence, these data indicate that Cys636 has a dual role in the regulation of activity states, comparable to that described for Met637.
The TMH6 of TSHR is generally a hot spot for naturally occurring mutations, but only for those mutations leading to constitutive activation (see also GPHR information resource at http://www.ssfa-gphr.de) (215, 216). In 1998, the disease-causing gain-of-function mutant Pro639Ser for hTSHR was described (287), and a loss-of-function mutation at the corresponding FSHR TMH6 proline (Pro587His) was reported (288). The TSHR proline 639 plays a role in helix distortion, as described above for prolines at GPCR TMH5. It is the ability to establish differential packing interactions for the helix segments rather than the structural properties of the proline kink itself that emerges as the key factor for the distortion (276, 277). This suggested role of prolines in transmembrane helices is confirmed by testing different TSHR Pro639 mutants; nearly all mutants designed in vitro are constitutively active, except the disease-causing inactivating mutation Pro639Leu. Mutations of Pro639 to alanine, glycine, or serine with small side chains showed only moderate decreases in TSH-induced Gs- and Gq-mediated signaling. The wild-type–like cell surface expression and constitutive receptor activation of these mutants with maintained signaling capacity indicate that the helical conformation is still kinked in this mutant TSHR variant, which is able to transduce defined helix movements for hormone-induced signal transduction. In contrast, the Pro639Leu mutation, with a large branched hydrophobic side chain, impaired TSHR signaling completely, although cell surface expression was comparable to the wild type. These findings indicate that there is a tightly packed and conserved amino acid interaction cluster that does not tolerate substitution with larger amino acid side chains.
Of note, a specifically designed TSHR variant in TMH6 with a glycine substitution at position 639 and the insertion of an additional glycine (Pro639Gly_insGly at position 640) was reported, together with the investigation of the Pro639Leu mutant (285). This variant was tested for mimicry of a helix kink caused by 2 glycines that should probably maintain structural flexibility. Surprisingly, this mutant activates TSHR to a high degree for cAMP accumulation, but in contrast to other Pro639 mutants, it also showed constitutive activity for IP accumulation. Furthermore, this mutant lost the capacity for further stimulation by bTSH, which might be caused by modification of helical interfaces and side-chain interactions between TMH6 relative to TMH3-TMH5-TMH7 or it might be that this mutation induces a fully active receptor conformation that cannot be stimulated further.
2. Characteristics of signal transduction are intrinsically encoded at the transmembrane domain structure
It is confirmed that the basal state of the TSHR is constrained by polar contacts between TMH2, -3, -6, and -7. Amino acids Asp460 (TMH2), Asp633 (TMH6), and Asn674 (TMH7) in these helices interact with each other through hydrophilic contacts, and several CAMs have been identified by mutagenesis studies or by naturally occurring mutations at these positions (270, 271). Signal induction (by ligands or mutation), therefore, might release such constraints for activation-related distinct movement of TMH6 (Figure 3) or they must stabilize TMH5 and TMH6 in the active conformation. As a result, the intracellular end of TMH6 moves outward, as observed in the active GPCR crystal structures (101, 289, 290). Whereas TMH6 is probably involved in stabilization of both the inactive and active states, TMH5 stabilizes the active-state conformation in interplay with TMH6. In consequence, the recently described mutations at TMH5-TMH6, previously published data of the TSHR, and new insights from GPCR crystal structures support a subdivision of the transmembrane part into 2 functional regions with different preferences: 2) the inactive state is constrained by a polar core set between TMH2, -3, -6, and -7 and 2) the interface between TMH5 and TMH6 (plus TMH3) stabilizes the active state predominantly by hydrophobic interactions (272).
In addition, the preference for G protein coupling and activation is also influenced by specific amino acids at the TMHs (81, 82), especially in the evolutionarily conserved binding pocket for small ligands close to the ECL2 (105, 106, 291). It is assumed that specific conformational differences in the transmembrane domain (induced by different artificial ligands at different residues) support preferences for a certain G protein subtype (292). In conclusion, different signaling cascades are induced at 1 receptor by diverse ligands, as it has been reported for the LHR (293). Such parameters determining signaling are also known for the TSHR, as described in Section III.B.
However, comparison between different GPCR crystal structures has shown not only that the active state is stabilized by intramolecular interactions or ligand binding but also that intracellular coupling of an effector protein is necessary to maintain an active conformation (69, 290, 295–297). Agonistic ligands were found to bind into their binding pockets, but without an intracellularly interacting effector (eg, a G protein), these GPCR structures are not kept in an active conformation (101, 290). For the TSHR, this is of great importance, because this receptor expresses a permanent ligand-independent (basal) signaling activity (cAMP accumulation) and is activated by numerous mutations located in nearly all regions of the SD (Figure 4). As is the case for Gs activation by the TSHR, around 20% of all GPCRs are speculated to permanently activate G proteins in the basal state (93, 298–300). The ligand-independent activity of GPCRs is responsible for the maintenance of a basal signaling tone that is of (patho-)physiological relevance in case of absence of the endogenous ligand and is of significance, therefore, also for pharmacological research (301–303). But how does the G protein bind to the TSHR and why is this receptor not stringently selective for one subtype?
C. Intracellular G protein coupling and activation
1. G protein binding and selectivity at GPCRs
GPCRs interact and activate heterotrimeric guanine nucleotide-binding proteins (G proteins) on the intracellular side, which plays a crucial role in signal transduction toward second messenger cascades (reviewed in Refs. 73 and 304). Several different signal transducers in addition to G proteins are known, eg, arrestins (75, 78). GPCR-mediated G protein activation is characterized by structural shifts inside and between the G protein subunits to each other, followed by exchange of GDP for GTP in the α-subunit and (partial) separation of the Gα- from the Gβγ-subunits. This opens up interfaces on the G protein subunits to further contact partners, such as phosphodiesterases (75).
There have been 3 proposals as to how selectivity determination and coupling of G proteins may be linked. First, different conformational states of a receptor may be selective for a specific G protein subtype, because extracellular mutations and diverse ligands can cause different G protein subtype preferences for a single receptor (81, 292, 305, 306). In support of this, the crystal structure of the M3 muscarinic acetylcholine receptor was published in 2012 (307), and these authors compared structural features of all reported GPCR crystal structures to identify potential differences in the intracellular spatial properties between GPCRs with specificity for Gi, Gq, or Gs. They correlated the interhelical distances of particular structures to each other and indeed found clustering of GPCRs with different G protein specificities. These clusters support a relationship (predisposition) between the spatial distances of the helices to each other that favored interaction to a certain G protein subtype. Second, there may be selective intracellular residues that are responsible for G protein subtype-specific interactions (308–311). And third, G protein preference may be determined by the set and availability of cell-specific G protein subtype (82).
2. Prerequisites and determinants of TSHR G protein coupling at the ICLs
Activation of the Gs/adenylyl cyclase pathway is of greatest importance for the TSHR (9), and additionally, it has been suggested and supported by pathogenic mutations and mice studies that activation of the Gq pathway is also important for thyroid growth and thyroid hormone synthesis (59, 263, 286, 312). Furthermore, it has been shown that some TSHR antibodies might be able to influence primarily Gq effectors and induce cell proliferation (313). G protein coupling generally occurs at the ICLs and the intracellular transitions between the transmembrane helices and loops (314, 315). Mutagenesis studies of the intracellular TSHR region were performed in the early 1990s (316–320). Through multiple substitutions, these studies provided the first hints that sensitivity, but also selectivity for G protein activation, is determined in the ICLs and C-terminal region. Further intensive mutagenesis studies at the TSHR and naturally occurring mutations have led to the identification of amino acid side chains involved in G protein coupling at this receptor (219, 261, 321–325). These studies suggest that parts of the ICLs and also helix 8 establish direct side-chain contacts with the α-subunits of both Gs and Gq. Based on these data, molecular models of a putative TSHR/G protein complex were generated (322, 324), and the recent insights can be described as follows.
a. Intracellular loop 1. Alanine mutations of Ile438 and Ser442 in ICL1 and Arg450 at the transition to TMH2 decreased both Gs- and Gq-mediated signaling, whereas Leu440Ala, Thr441Ala, and His443Ala selectively impaired IP generation (324). Of particular interest is Arg450 at the transition between ICL1 and TMH2, where several cases of pathogenic naturally occurring mutations were reported (326–329). It is suggested that Arg450 directly interacts with the C-terminal α5-helix of Gα and therefore participates in (selective) G protein coupling and activation. Directed side-chain substitutions at this amino acid position likely modify the receptor/G protein interplay and thus signaling parameters (324). In summary, the ICL1 and adjacent TMH regions must be considered as determinants for G protein coupling and activation.
b. Intracellular loop 2. Specific regions of ICL2 contribute to G protein activation in the TSHR (322, 325). Within the ICL2 of the TSHR, amino acids Met527, Arg528, and Asp530 appeared to be critical for both Gs and Gq activation, whereas alanine mutations of Ile523, Phe525, and Leu529 selectively impaired Gq-mediated signaling. Alanine mutations of Met527, Asp530, and Arg531 caused impaired basal cAMP accumulation. Chimeric mutagenesis studies between the TSHR and the LHR support this observation (330). This study revealed that a single point mutation in the ICL2 of the TSHR with the corresponding LHR amino acid led to decreased basal signaling activity, as was observed for the less active LHR. Such mutations are termed silencing mutations, inverse agonistic mutations, or constitutively inactivating mutations (331) (Figure 4). For the TSHR, it was proposed that the basally activated conformation can facilitate a higher capacity for induced signaling by ligands or activating mutations, which might be a prerequisite for promiscuous G protein recognition (331).
c. Intracellular loop 3. In addition, the intracellular transitions between TMH5-ICL3 and ICL3-TMH6 are known to be involved in G protein activation. Single substitutions of residues at TMH5/ICL3 (Tyr601, Tyr605, and Val608) and at ICL3/TMH6 (Lys618, Lys621, and Ile622) are selectively important for Gq activation (267, 322). Mutations at Asp617 and Asp619 lead to constitutive receptor activation for the Gs-mediated pathway (322, 332–334). It is likely that these mutations cause modulation of intramolecular stabilizing interactions important for switching to an active conformation (335). However, these mutation-based studies of the TSHR have shown that interactions and binding modes between receptor and Gs or Gq partially overlap, whereas completely inactivating mutations were found only for the receptor/Gq-heterotrimer complex.
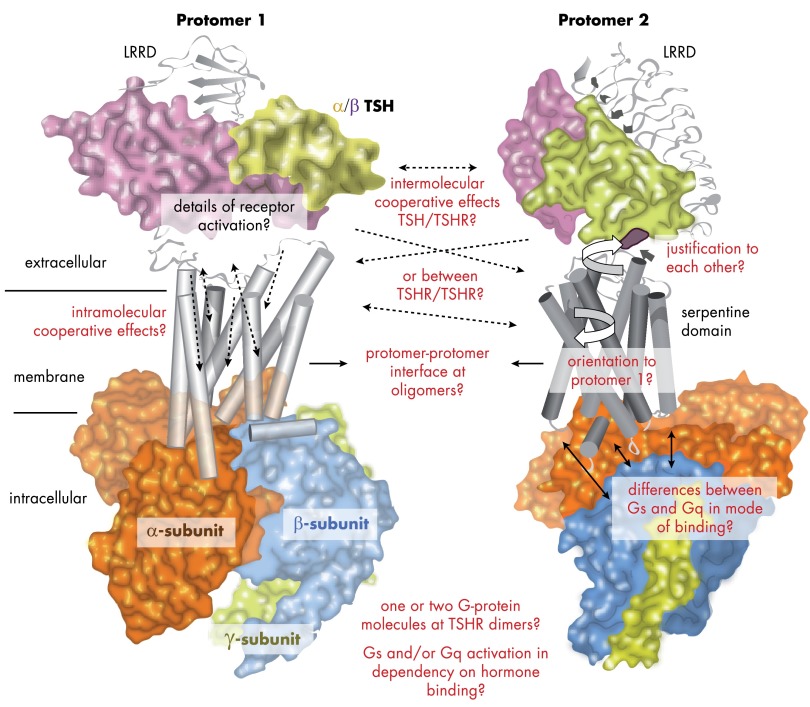
Beside these mechanisms and determinants for regulated G protein coupling and activation in the cytoplasmic region, a new contributing signaling parameter for the TSHR has been recently identified: TSH binding to dimeric TSHR (336). Dimerization is of major importance for the TSHR because it has an impact on several functional properties (27, 337).
III. New and Controversial Aspects of TSHR Signaling
A. Oligomerization
1. Oligomerization of GPCRs
Organization of GPCRs as dimers or oligomers has been demonstrated for many GPCRs, even in native tissue (338–340), and is an important topic under structural and functional aspects. Dimerization is a general term to describe a GPCR-x/GPCR-x (homodimer) or GPCR-x/GPCR-y (heterodimer) constellation. Oligomerization is used for dimeric, tetrameric, or higher-order complexes between GPCR protomers (protomers are alternatively termed monomers). It is well documented that dimerization can have a major influence on signaling properties of interacting protomers as in ligand binding (341, 342), G protein coupling selectivity, and signal transduction mechanisms (reviewed in Refs. 339 and 343) or cell surface expression (344). Allosteric effects of one protomer on the other (one GPCR by interaction modifies the functional capacity of the interacting GPCR) have been observed (345). Direct structural cross talk (346) should modulate mutually GPCR signaling properties (347), and intersubunit rearrangement during receptor activation has been reported (348). It is noteworthy that oligomerization dramatically widens the spectrum of potential functional specifications or the fine-tuning of physiological functions (349–351) and is also of relevance for pathological situations (352–354).
The functional and structural prerequisites for a description or definition of an oligomeric receptor state have not yet been clearly defined. A GPCR-GPCR interaction could be characterized by the following parameters: 1) physical interactions (side-chain interactions, but varying in number and/or properties of interactions), 2) direct mutual functional modulation, and 3) specific spatial distance (span) to each other. An increasing number of known GPCR heterodimers and experimental observations lead to the hypothesis that heterodimerization is not dependent on close evolutionary relationships between these receptors. Strikingly, a relevant biological function must be indicative for the significance of homo- and heterodimeric constellations and contact interfaces, which also implicates the possibility of functionally neutral (but biophysically [spatial] detectable interaction) oligomeric GPCRs (or GPCR–non-GPCR interactions [heteromers]) constellations.
Several GPCR-GPCR interfaces were reported to be located at the region of ICL2-TMH4 (348, 355–358). TMH1 and TMH5–6 were also found to be sensitive to dimer arrangement in several of these studies (359–361) and particular amino acids at TMH4–5 are evidenced as a dimer-interface (362), or the extracellular N-terminal region could be involved (363). Therefore, it must be assumed that the constituting interfaces and types of interactions can differ or they are flexible (for a comprehensive overview of available experiments and literature see also the “The G Protein Coupled Receptor-Oligomerization Knowledge Base Project” (364).
It is of specific note that GPCRs might exist in a monomer/dimer/oligomer equilibrium (365, 366). However, GPCR oligomerization does not exclude functional GPCR monomers (367, 368). So far, just a few examples of GPCR oligomer dissociation into monomers are known (357, 359, 369, 370), but it will be of high interest for additional studies to compare the functionality of monomeric and oligomeric GPCRs.
In summary, oligomerization is a promising new field for GPCR-related research and pharmacological GPCR modifications (343, 371) including diversification and increased selectivity of receptor/ligand-mediated effects. Heterodimerization may also be considered under aspects of side effects induced by drugs, possibly by exerting effects on interacting GPCR partners (371).
2. Oligomerization of the TSHR
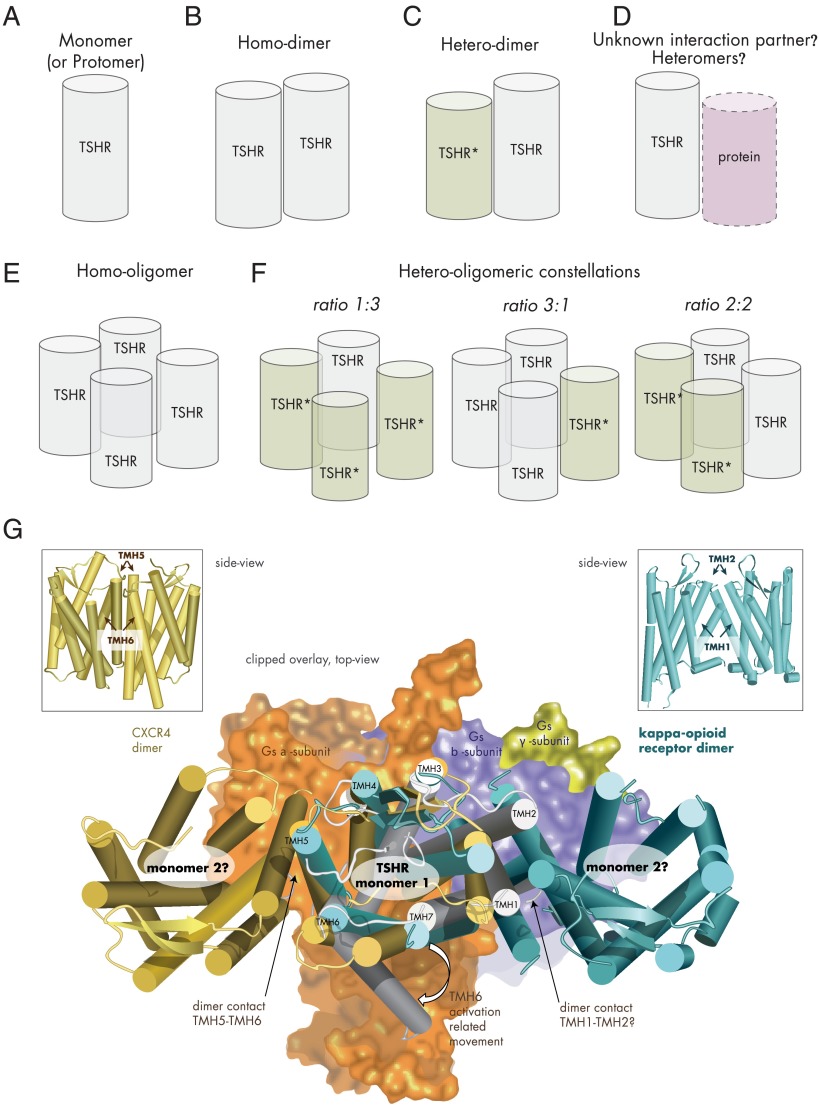
The initial evidence for TSHR oligomerization was provided by studies with antibodies (372). With a fluorescence-based technology (Förster/fluorescence resonance energy transfer, FRET), TSHR dimerization was then shown after expression in Chinese hamster ovary (CHO) cells, in which TSHR is expressed at a more physiologically relevant density (373). TSHR di- and oligomerization (Figure 5) occurs early in the endoplasmic reticulum and is suggested to be obligatory for proper receptor expression (337). Studies of TSHR oligomer properties with respect to basal signaling activity and hormone binding capacity (compared ligand/receptor stoichiometries) indicated that TSHR probably forms higher orders of homomers rather than dimers (162).
Figure 5.
Potential TSHR constellations. A, TSHR as monomer (schematic cylinder indicates the entire receptor). B, Two monomers interacting as TSHR homodimer. C, Dimerization of wild-type and mutant TSHR (TSHR*), which can result in a dominant-negative effect for heterozygous inactivating mutations (57). D, Potentially, the TSHR might be able also to interact with as yet unknown membrane-spanning proteins. E, The TSHR may also form homo-oligomers with different interfaces between the monomers. F, Assuming tetrameric (or higher-order) TSHR oligomers (373, 418), complexes with different monomer ratios could be formed from mutated variants and wild-type receptor: 1) wild type receptors only, 2) mutant receptors only, 3) 1 wild-type and 3 mutant receptors, 4) 2 wild-type and 2 mutant receptors or 3 wild-type and 1 mutant receptor. G, The crystallized complex between the ADRB2 and the heterotrimeric Gs protein pointed to significant structural differences between the inactive and active GPCR conformations (101). Especially, TMH6 is moved toward the membrane during G protein activation (active TSHR model [Figure 3], gray background, arrow at TMH6). Combining a monomeric active-state TSHR model coupled with Gs (subunits as surface), based on the crystallized ADRB2/Gs complex (101), with dimeric GPCR structures (CXCR4, dark-yellow) (388), and the κ-opioid receptor (light blue) (389) by structural overlay makes it obvious that in putative TSHR monomer-monomer complexes according to these crystal structure arrangements, coupling of 2 large activated bulky G protein molecules simultaneously at each receptor monomer would be sterically hindered, which would lead to asymmetric G protein binding.
Dominant-negative effects for a few mutations have been shown for partially inactivating TSHR mutations. These mutations (Cys41Ser, Leu467Pro, and Cys600Arg) were identified in patients with autosomal dominant inheritance of TSH resistance. Here, TSHR di- or oligomerization presents a molecular explanation as to why these TSHR mutations exhibit a phenotypic effect even in the heterozygous state of an inactivating mutation (374).
The hyt/hyt mouse is a hypothyroid mouse model because of the Pro556Leu mutation (375, 376). It was suggested that the TSHR mutant Pro556Leu has a dominant-negative effect on TSHR wild type by impairing oligomer to monomer dissociation, which should decrease TSH responsiveness and also induces hypothyroidism in the heterozygous Tshr hyt mice (377). This mutation is localized in TMH4 at the junction with ECL2, and this region is probably not accessible for TSH, which binds exclusively at the extracellular region. Therefore, it was postulated that disturbed interactions between the extracellular and transmembrane regions cause the observed impaired ligand binding properties (375). However, the level of cell surface expression significantly influences the signaling properties of TSHR (378), which is a relevant factor for altered signal transduction properties likely also for this murine TSHR proline mutant. Interestingly, the single–amino-acid variation Gly558Arg in the chicken TSHR (in hTSHR Gly559), very close to Pro556, was found to be potentially correlated with the domestication of the chicken (379). Their detailed functional impact on TSHR still awaits characterization as well as the physiological relevance of this mutant. In conclusion, TSHR variants with deficiency in cell surface expression, signaling, or ligand binding could be defective in their functional dimeric constellation and different TSHR variant constellations (Figure 5, A–F) should have consequences for diverse signaling properties in vivo and in vitro.
It is an open issue as to whether TSH influences dimer formation (27, 380). On one hand, it was proposed that oligomeric TSHR rapidly dissociates into active monomers upon TSH binding (381). It was suggested that a constitutively active TSHR dimer or monomer is naturally inhibited by the formation of higher-order complexes (380). On the other hand, in exhaustive studies on the TSHR and other GPHRs, dimerization was found not to be affected by agonist (hormone and antibodies) binding and action. Functional complementation studies indicate that TSHR dimers function as a single unit (27).
In accordance with this, it was investigated whether heterozygous CAMs might influence dimeric TSHR arrangements and naturally activating mutations in TMH6 have been studied by coexpression experiments with wild-type TSHR (285). It was observed that these activating mutations do not change the TSHR dimerization state. This implies that constitutive activation of the TSHR specifically at TMH6 does not interfere with TSHR dimerization. This finding was generalized for CAMs distributed over the entire TSHR structure. None of the tested CAMs, eg, in the ECL1 or the TMH3, -5 or -7, lead to TSHR oligomer separation into protomers, whereas differences in the geometry of the complexes have been suggested (162). Moreover, comparable findings have been reported for the homologous LHR. Oligomeric LHR complexes are constitutively formed early in the endoplasmic reticulum; they are expressed on the cell surface membrane, and LHR oligomers are not affected by CAMs or by hormone interaction (382).
Detailed characterization of TSHR di- or oligomerization pointed to the transmembrane SD as a main determinant for intermolecular receptor-receptor interplay and indicated that the extracellular receptor region might modulate this constellation. The demonstrated dimerization of the coexpressed SD with the full-length TSHR supports the dominant influence of transmembrane contacts between receptor monomers (23, 27). The first intermolecular communication between GPHR monomers related to transmembrane fragments was provided by studies on the LHR in 1997 (383). Recent advanced investigation of determinants mediating negative ligand binding cooperativity for the GPHRs has shown that the SD is responsible for such effects and that the initial signal from one protomer is transported horizontally from one protomer to the other (162). This also clearly supports the SD as a key contact between TSHR protomers in an oligomeric form.
It is noteworthy that the first reported dimeric FSHR LRRD/FSH crystal structure (139, 384, 385) suggested specific extracellular contacts between monomers by interactions at the convex LRRD site. This postulated contact was investigated at the TSHR, and the authors claimed that mutation of the conserved tyrosine residue (TSHR Tyr116) of the LRR3 totally abrogated ectodomain multimers (386), which would confirm the crystal structure-based hypotheses. However, direct investigation of this specific tyrosine (FSHR Tyr110) in relation to the FSHR oligomeric state failed to confirm any impact of this residue on dimerization (163). In support of this, no such contact interface is observable in the new trimeric FSHR/FSH structure (387), whereas the extracellular region and the SD are shown to have an impact in the FSHR dimer (163).
Moreover, after combining a monomeric active-state TSHR model coupled with Gs based on the crystallized ADRB2/Gs complex (101) with recent dimeric GPCR structures CXCR4 (388) and the κ-opioid receptor (389), it becomes obvious that at these putative monomer-monomer arrangements (suggested by the crystallized GPCR dimers), coupling and activation of 2 large G protein molecules simultaneously at each receptor monomer should be sterically hindered (Figure 5G). These specific structural GPCR arrangements with interfaces as in TMH1/2 or TMH5/6 would imply a receptor/G protein ratio of 2:1 rather than of 2:2. It will be of future interest to unravel the specific dimeric/oligomeric constellations of TSHR in the inactive or active states (Figure 6).
Figure 6.
Questions that shall be answered by additional studies on TSHR structure and function visualized at models of signaling complexes. As pointed out throughout this review, specific questions regarding the mechanisms of TSHR signaling are still awaiting answers. These topics are related to complex situations, i.e., binding of the G protein subtypes, the arrangement and justification of receptor components relative to each other, and the aspects of a dimeric or oligomeric state of the TSHR. So far, it is unknown how receptor components like the hinge region and the SD interrelate to each other or how they influence their function mutually. The same is true for the potential interaction interface between 2 receptor molecules. Strikingly, these missing data hamper a detailed determination of components that transfer known synergistic effects. The signal transduction process at the TSHR leads to intracellular activation of signaling pathways. Therefore, molecular events will be better understood if we will be able to answer the following questions. Why does the TSHR express a permanent basal signaling activity? Do 1 or 2 G protein molecules bind at a receptor dimer? Is there a relation between monomeric and dimeric constellation and G protein selectivity? Answers to these questions will allow a better explanation of pathogenic constellations on the molecular level of TSHR activation.
In conclusion, oligomerization is a common feature of GPCRs and has also been demonstrated for the TSHR. Oligomerization of TSHR (and homologous GPHRs) has consequences for functional features like expression, ligand binding, signal induction, and conversion. This has now been demonstrated clearly under several aspects:
Pathogenic constellations such as dominant-negative effects by mutations (57) are related to TSHR oligomerization, which complicates linear genotype-phenotype relations and the comparison between in vitro and in vivo studies.
The negative cooperative effect on hormone binding (24–26, 27) is linked to oligomerized GPHR constellations (27), which has been studied and described in detail (162) (see also Section II.A.2). The mechanism is based on allosteric communication between receptor protomers and might hold true also for the evolutionarily linked and structurally similar leucine-rich repeat containing G protein coupled receptors (390, 391).
A recent study (336) led to the observation that 2 TSH molecules bound at a TSHR homodimer are necessary to activate both effectors Gs (cAMP) and Gq (IP).
Furthermore, a trans-activation (in contrast to so-called cis-activation) mechanism by GPHRs was investigated with different coexpressed receptor constructs (27, 383, 392). In trans-activation (also termed intermolecular cooperation) (393), a hormone molecule binds to a receptor (or receptor fragment) and induces activation of a different receptor monomer from the directly targeted one.
For the FSHR, it was reported that intermolecular receptor cooperation (trans-activation) can also induce selective signaling by activation of either Gs or Gq but not both (394). Studies of the mouse LHR demonstrated that transgenic mice coexpressing binding- and signaling-inhibited LHR variants can reconstitute nearly normal LHR function likely through functional complementation (trans-activation) between the receptor variants (393). Of note, the concept of trans-activation mechanisms in vivo by specific LHR constructs is still under controversial discussion (396).
B. Different mechanisms of signaling pathway regulation
The above information revealed one important general aspect of TSHR signaling, the capacity for multiple or promiscuous signaling. Although Gs-mediated cAMP accumulation is the most prominent and important pathway, Gq-related IP generation has also been found to be of physiological importance (59, 263, 286, 312). Which facts are in support of this assumption and which determinants are involved in signaling regulation and specificity?
The mode of TSH binding at TSHR oligomers has been described as a determinant of dual signaling capacity. TSHR dimers with only 1 bound TSH molecule selectively activate only cAMP accumulation (Gs) but not Gq (336).
Hormone properties are directly linked with induction of promiscuous or selective pathways. Replacing an alanine (position 36 in the α-subunit of TSH, with numbering starting after the signal peptide) with a different type of side-chain property (negatively charged) has been reported to reduce thyroid growth promotion but not cAMP stimulation (158, 397).
In accordance with this, differences in signaling induced by the hormones were also found to be related to the glycosylation pattern (169). The 2 dominant intracellular signaling pathways (cAMP accumulation and IP release) are activated to different degrees by diverse hTSH glycosylation variants and diverse antibodies acting as TSHR ligands have been reported to modulate specific signaling pathways differentially (398).
In the extracellular region of the TSHR, 6 glycosylation motifs are localized (399–401). It is suggested that glycosylation of at least 4 sites appears necessary for expression of the fully functional TSHR and that N-linked glycosylation of Asn77 and Asn113 might play the most critical role in the expression of a biologically active receptor on the cell surface.
In line with these reports of endogenous ligands at the TSHR, stimulation of the FSHR or LHR by small allosterically bound agonistic molecules induces selective activation of signaling pathways (293, 402). These ligands interact within the transmembrane region of those receptors, as is also shown for the TSHR (Figure 3) (31–33, 403). It is well known for GPCRs that different types of agonists that bind to the transmembrane region can trigger diverse intracellular conformations and signaling pathways, as shown for β2-adrenergic receptor (292). In conclusion, the transmembrane receptor amino acids also regulate signal transduction of information for multiple or selective intracellular signal conduction.
By combining particular CAMs at different TMHs of the TSHR, a significance of specific helices for induction of diverse signaling pathways (Gs and/or Gq) was found (404).
Furthermore, several single point mutations in the ECLs, the SD, and in the ICLs have been shown to selectively increase or decrease TSHR signal transduction. For example, the naturally occurring loss-of-function mutation Leu653Val in ECL 3 (263) leads to selective impairment of IP but not of cAMP accumulation after TSH treatment. At the ICLs, amino acids such as Phe525 were found by side-chain substitution to interfere with Gq but not Gs activation (325).
In several previous studies, it was investigated how sodium ions might modulate the signaling capacities of GPHRs. The LHR and TSHR show differential sensitivity to low NaCl concentrations, and it was suggested that the unliganded TSHR is less constrained by sodium than the LHR (405). Moreover, differences between GPHR signaling and sodium concentrations were confirmed and specified by comparison between TSHR, LHR, and FSHR (406). It is noteworthy that the highly conserved aspartate at TMH2 (position 2.50, according to the Ballesteros and Weinstein [273] family A GPCR numbering scheme) has shown experimentally to be involved in sodium binding in the LHR in the 1990s, with differential effects on the affinity for the gonadotropins LH and CG (407). These interactions between sodium ions/receptors and regulating effects on ligand actions may be of general importance for GPCRs. Details of those interactions at the transmembrane region (TMH2 and TMH3) are now provided by a high-resolution GPCR crystal structure where these ions and participating water molecules are visible (408).
Differences in signaling properties, such as signaling capacity or G protein selectivity, have been observed between TSHR variants with (Gs and Gq activation by TSH) or without (Gs but no Gq activation by TSH) basal activity (331).
Finally, the cellular context influences self-organization and the functional properties of TSHR (247, 409, 410). Different types of cells can be very diverse in their G protein pool, their specific protein expression levels, or cell organization (eg, polarity).
C. Extrathyroidal TSHR expression and function
It has been reported that TSHR is also expressed outside the thyroid gland, including the lymphocytes, brain, thymus, testicles, ovaries, kidneys, adipose tissue, heart, and bones (mRNA and protein) (reviewed in Refs. 20 and 381). The detailed function of the TSHR in these tissues is still under discussion and investigation. Recently, TSHR has been shown to have a significant influence on photoperiodic control in the reproduction cycle of different species (15–17, 379, 411), which is allocated in the pars tuberalis. This implies that TSH and/or TSHR have a direct impact on reproductive characteristics via neuroendocrine regulation. In accordance, it might be of significance that mutations in the TSHR can have an influence on reproductive activity (379). Furthermore, activation of the TSHR is reported to be related to the development of ophthalmopathy (21, 412, 413). Finally, early reports conclude that TSH is a dominating lipolytic hormone during the neonatal life period (22). In conclusion, it should be emphasized that, hypothetically, each pharmacological intervention that targets the TSHR directly (254, 414, 415) might also lead to undesired effects of the drug on systems other than the thyroid gland. The question of whether such alternative mechanisms of the TSHR are also linked to TSH binding is still open as well; hormone variants or hormone subunits might participate in other tissues (193, 194, 196, 197, 199). However, humans with complete loss of TSHR function (or patients with athyreosis) treated with thyroid hormone do not show obvious signs of any disease.
D. Potential future directions for TSHR research
In the authors' opinion, the following questions for TSHR research are of specific and general interest. The mechanism of constitutive activation by deletions in the hinge region is worthy of further study (164, 208), because it cannot be observed by similar approaches at corresponding regions of the LHR, pointing to significant differences between these 2 GPHR subtypes (210, 211). It is also of interest to investigate why complete C-peptide deletion is without effect on basal TSHR cAMP accumulation (238), but deletion of a C-peptide fragment (residues 339–367) enhances ligand-independent TSHR cAMP signaling (213). Moreover, interactions between the extracellular region and the SD must be deciphered in detail and also in combination with properties of receptor oligomerization (protomer interactions). Thus, an analysis of the effects of intermolecular interactions (23, 27, 158, 383, 392, 393) and communications between receptor protomers or specific receptor components (24–27, 162, 164, 210) would be desirable (Figure 6). The role of TSHR in extrathyroidal tissues is an important issue for clarification, and finally, the development and improvement of low–molecular-weight ligands concerning selectivity and affinity will be of high priority. Comprehensive answers to these questions would help to complete the puzzle of how the TSHR and GPHR signal is transduced from the extra- to the intracellular site of the cell and how the TSHR can be modulated under controlled conditions.
Acknowledgments
We are very thankful to Gilbert Vassart (Institut de Recherche Interdisciplinaire en Biologie Humaine et Moléculaire, Faculty of Medicine, Université Libre de Bruxelles, Bruxelles, Belgium) for the stimulating scientific discussion and his important suggestions to improve this review. We are grateful for the important corrections made by Catherine L. Sargent.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG), projects BI 893/6-2, BI893/5-1, and KL 2334/21, -2, and by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (Z01DK011006).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADRB2
- β2-adrenergic receptor
- bTSH
- bovine TSH
- CAM
- constitutively activating mutation
- CG
- choriogonadotropin
- ECL
- extracellular loop
- FSHR
- FSH receptor
- GPCR
- G protein-coupling receptor
- GPH
- glycoprotein hormone
- GPHR
- glycoprotein hormone receptor
- hFSH
- human FSH
- ICL
- intracellular loop
- IP
- inositol phosphate
- LHCGR
- LH and CG receptor
- LRR
- leucine-rich repeat
- LRRD
- LRR domain
- rhTSH
- recombinant hTSH
- SD
- serpentine domain
- TMH
- transmembrane helix
- TSHR
- TSH receptor.
References
- 1. Vassart G, Pardo L, Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci. 2004;29:119–126 [DOI] [PubMed] [Google Scholar]
- 2. Allgeier A, Offermanns S, Van Sande J, Spicher K, Schultz G, Dumont JE. The human thyrotropin receptor activates G-proteins Gs and Gq/11. J Biol Chem. 1994;269:13733–13735 [PubMed] [Google Scholar]
- 3. Laugwitz KL, Allgeier A, Offermanns S, Spicher K, Van Sande J, Dumont JE, Schultz G. The human thyrotropin receptor: a heptahelical receptor capable of stimulating members of all four G protein families. Proc Natl Acad Sci U S A. 1996;93:116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laurent E, Mockel J, Van Sande J, Graff I, Dumont JE. Dual activation by thyrotropin of the phospholipase C and cyclic AMP cascades in human thyroid. Mol Cell Endocrinol. 1987;52:273–278 [DOI] [PubMed] [Google Scholar]
- 5. Van Sande J, Raspé E, Perret J, et al. Thyrotropin activates both the cyclic AMP and the PIP2 cascades in CHO cells expressing the human cDNA of TSH receptor. Mol Cell Endocrinol. 1990;74:R1–R6 [DOI] [PubMed] [Google Scholar]
- 6. Büch TR, Biebermann H, Kalwa H, et al. G13-dependent activation of MAPK by thyrotropin. J Biol Chem. 2008;283:20330–20341 [DOI] [PubMed] [Google Scholar]
- 7. Latif R, Morshed SA, Zaidi M, Davies TF. The thyroid-stimulating hormone receptor: impact of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on multimerization, cleavage, and signaling. Endocrinol Metab Clin North Am. 2009;38:319–341, viii [DOI] [PubMed] [Google Scholar]
- 8. Postiglione MP, Parlato R, Rodriguez-Mallon A, et al. Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland. Proc Natl Acad Sci U S A. 2002;99:15462–15467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vassart G, Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992;13:596–611 [DOI] [PubMed] [Google Scholar]
- 10. Libert F, Lefort A, Gerard C, et al. Cloning, sequencing and expression of the human thyrotropin (TSH) receptor: evidence for binding of autoantibodies. Biochem Biophys Res Commun. 1989;165:1250–1255 [DOI] [PubMed] [Google Scholar]
- 11. Nagayama Y, Kaufman KD, Seto P, Rapoport B. Molecular cloning, sequence and functional expression of the cDNA for the human thyrotropin receptor. Biochem Biophys Res Commun. 1989;165:1184–1190 [DOI] [PubMed] [Google Scholar]
- 12. Parmentier M, Libert F, Maenhaut C, et al. Molecular cloning of the thyrotropin receptor. Science 1989;246:1620–1622 [DOI] [PubMed] [Google Scholar]
- 13. Frazier AL, Robbins LS, Stork PJ, Sprengel R, Segaloff DL, Cone RD. Isolation of TSH and LH/CG receptor cDNAs from human thyroid: regulation by tissue specific splicing. Mol Endocrinol. 1990;4:1264–1276 [DOI] [PubMed] [Google Scholar]
- 14. Misrahi M, Loosfelt H, Atger M, Sar S, Guiochon-Mantel A, Milgrom E. Cloning, sequencing and expression of human TSH receptor. Biochem Biophys Res Commun. 1990;166:394–403 [DOI] [PubMed] [Google Scholar]
- 15. Hanon EA, Lincoln GA, Fustin JM, et al. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr Biol. 2008;18:1147–1152 [DOI] [PubMed] [Google Scholar]
- 16. Hanon EA, Routledge K, Dardente H, Masson-Pévet M, Morgan PJ, Hazlerigg DG. Effect of photoperiod on the thyroid-stimulating hormone neuroendocrine system in the European hamster (Cricetus cricetus). J Neuroendocrinol. 2010;22:51–55 [DOI] [PubMed] [Google Scholar]
- 17. Ono H, Hoshino Y, Yasuo S, et al. Involvement of thyrotropin in photoperiodic signal transduction in mice. Proc Natl Acad Sci U S A. 2008;105:18238–18242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bodó E, Kany B, Gáspár E, et al. Thyroid-stimulating hormone, a novel, locally produced modulator of human epidermal functions, is regulated by thyrotropin-releasing hormone and thyroid hormones. Endocrinology. 2010;151:1633–1642 [DOI] [PubMed] [Google Scholar]
- 19. Cianfarani F, Baldini E, Cavalli A, et al. TSH receptor and thyroid-specific gene expression in human skin. J Invest Dermatol. 2010;130:93–101 [DOI] [PubMed] [Google Scholar]
- 20. de Lloyd A, Bursell J, Gregory JW, Rees DA, Ludgate M. TSH receptor activation and body composition. J Endocrinol. 2010;204:13–20 [DOI] [PubMed] [Google Scholar]
- 21. Fernando R, Atkins S, Raychaudhuri N, et al. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci U S A. 2012;109:7427–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marcus C, Ehrén H, Bolme P, Arner P. Regulation of lipolysis during the neonatal period. Importance of thyrotropin. J Clin Invest. 1988;82:1793–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Latif R, Michalek K, Davies TF. Subunit interactions influence TSHR multimerization. Mol Endocrinol. 2010;24:2009–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bolonkin D, Tate RL, Luber JH, Kohn LD, Winand RJ. Experimental exophthalmos. Binding of thyrotropin and an exophthalmogenic factor derived from thyrotropin to retro-orbital tissue plasma membranes. J Biol Chem. 1975;250:6516–6521 [PubMed] [Google Scholar]
- 25. Chazenbalk GD, Kakinuma A, Jaume JC, McLachlan SM, Rapoport B. Evidence for negative cooperativity among human thyrotropin receptors overexpressed in mammalian cells. Endocrinology. 1996;137:4586–4591 [DOI] [PubMed] [Google Scholar]
- 26. Tate RL, Schwartz HI, Holmes JM, Kohn LD. Thyrotropin receptors in thyroid plasma membranes. Characteristics of thyrotropin binding and solubilization of thyrotropin receptor activity by tryptic digestion. J Biol Chem. 1975;250:6509–6515 [PubMed] [Google Scholar]
- 27. Urizar E, Montanelli L, Loy T, et al. Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J. 2005;24:1954–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vassart G, Costagliola S. A physiological role for the posttranslational cleavage of the thyrotropin receptor? Endocrinology. 2004;145:1–3 [DOI] [PubMed] [Google Scholar]
- 29. Biebermann H, Winkler F, Kleinau G. Genetic defects, thyroid growth and malfunctions of the TSHR in pediatric patients. Front Biosci. 2010;15:913–933 [DOI] [PubMed] [Google Scholar]
- 30. Neumann S, Raaka BM, Gershengorn MC. Human TSH receptor ligands as pharmacological probes with potential clinical application. Expert Rev Endocrinol Metab. 2009;4:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haas AK, Kleinau G, Hoyer I, et al. Mutations that silence constitutive signaling activity in the allosteric ligand-binding site of the thyrotropin receptor. Cell Mol Life Sci. 2011;68:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kleinau G, Haas AK, Neumann S, et al. Signaling-sensitive amino acids surround the allosteric ligand binding site of the thyrotropin receptor. FASEB J. 2010;24:2347–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moore S, Jaeschke H, Kleinau G, et al. Evaluation of small-molecule modulators of the luteinizing hormone/choriogonadotropin and thyroid stimulating hormone receptors: structure-activity relationships and selective binding patterns. J Med Chem. 2006;49:3888–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neumann S, Huang W, Titus S, et al. Small-molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice. Proc Natl Acad Sci U S A. 2009;106:12471–12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Akamizu T. Antithyrotropin receptor antibody: an update. Thyroid. 2001;11:1123–1134 [DOI] [PubMed] [Google Scholar]
- 36. Bahn RS. Autoimmunity and Graves' disease. Clin Pharmacol Ther. 2012;91:577–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin receptor expression in Graves' orbital adipose/connective tissues: potential autoantigen in Graves' ophthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002 [DOI] [PubMed] [Google Scholar]
- 38. Davies TF, Ando T, Lin RY, Tomer Y, Latif R. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest. 2005;115:1972–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kohn LD, Harii N. Thyrotropin receptor autoantibodies (TSHRAbs): epitopes, origins and clinical significance. Autoimmunity. 2003;36:331–337 [DOI] [PubMed] [Google Scholar]
- 40. Ludgate M. Animal models of Graves' disease. Eur J Endocrinol. 2000;142:1–8 [DOI] [PubMed] [Google Scholar]
- 41. Michalek K, Morshed SA, Latif R, Davies TF. TSH receptor autoantibodies. Autoimmun Rev. 2009;9:113–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev. 1998;19:673–716 [DOI] [PubMed] [Google Scholar]
- 43. Rapoport B, McLachlan SM. The thyrotropin receptor in Graves' disease. Thyroid. 2007;17:911–922 [DOI] [PubMed] [Google Scholar]
- 44. García-Jiménez C, Santisteban P. TSH signalling and cancer. Arq Bras Endocrinol Metabol. 2007;51:654–671 [DOI] [PubMed] [Google Scholar]
- 45. Persani L, Calebiro D, Cordella D, et al. Genetics and phenomics of hypothyroidism due to TSH resistance. Mol Cell Endocrinol. 2010;322:72–82 [DOI] [PubMed] [Google Scholar]
- 46. Rodien P, Ho SC, Vlaeminck V, Vassart G, Costagliola S. Activating mutations of TSH receptor. Ann Endocrinol (Paris). 2003;64:12–16 [PubMed] [Google Scholar]
- 47. Schöneberg T, Schulz A, Biebermann H, Hermsdorf T, Römpler H, Sangkuhl K. Mutant G-protein-coupled receptors as a cause of human diseases. Pharmacol Ther. 2004;104:173–206 [DOI] [PubMed] [Google Scholar]
- 48. Vassart G, Costagliola S. G protein-coupled receptors: mutations and endocrine diseases. Nat Rev Endocrinol. 2011;7:362–372 [DOI] [PubMed] [Google Scholar]
- 49. Parma J, Duprez L, Van Sande J, et al. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature. 1993;365:649–651 [DOI] [PubMed] [Google Scholar]
- 50. Biebermann H, Schöneberg T, Krude H, Gudermann T, Grüters A. Constitutively activating TSH-receptor mutations as a molecular cause of non-autoimmune hyperthyroidism in childhood. Langenbecks Arch Surg. 2000;385:390–392 [DOI] [PubMed] [Google Scholar]
- 51. Gruters A, Krude H, Biebermann H. Molecular genetic defects in congenital hypothyroidism. Eur J Endocrinol. 2004;151(Suppl 3):U39–U44 [DOI] [PubMed] [Google Scholar]
- 52. Hébrant A, van Staveren WC, Maenhaut C, Dumont JE, Leclère J. Genetic hyperthyroidism: hyperthyroidism due to activating TSHR mutations. Eur J Endocrinol. 2011;164:1–9 [DOI] [PubMed] [Google Scholar]
- 53. Fuhrer D, Lewis MD, Alkhafaji F, et al. Biological activity of activating thyroid-stimulating hormone receptor mutants depends on the cellular context. Endocrinology. 2003;144:4018–4030 [DOI] [PubMed] [Google Scholar]
- 54. Corvilain B, Van Sande J, Dumont JE, Vassart G. Somatic and germline mutations of the TSH receptor and thyroid diseases. Clin Endocrinol (Oxf). 2001;55:143–158 [PubMed] [Google Scholar]
- 55. Lueblinghoff J, Eszlinger M, Jaeschke H, et al. Shared sporadic and somatic thyrotropin receptor mutations display more active in vitro activities than familial thyrotropin receptor mutations. Thyroid. 2011;21:221–229 [DOI] [PubMed] [Google Scholar]
- 56. Sunthornthepvarakui T, Gottschalk ME, Hayashi Y, Refetoff S. Brief report: resistance to thyrotropin caused by mutations in the thyrotropin-receptor gene. N Engl J Med. 1995;332:155–160 [DOI] [PubMed] [Google Scholar]
- 57. Calebiro D, de Filippis T, Lucchi S, et al. Intracellular entrapment of wild-type TSH receptor by oligomerization with mutants linked to dominant TSH resistance. Hum Mol Genet. 2005;14:2991–3002 [DOI] [PubMed] [Google Scholar]
- 58. Krude H, Biebermann H, Gopel W, Gruters A. The gene for the thyrotropin receptor (TSHR) as a candidate gene for congenital hypothyroidism with thyroid dysgenesis. Exp Clin Endocrinol Diabetes. 1996;104(Suppl 4):117–120 [DOI] [PubMed] [Google Scholar]
- 59. Calebiro D, Gelmini G, Cordella D, et al. Frequent TSH receptor genetic alterations with variable signaling impairment in a large series of children with nonautoimmune isolated hyperthyrotropinemia. J Clin Endocrinol Metab. 2012;97:E156–E160 [DOI] [PubMed] [Google Scholar]
- 60. Biebermann H, Schöneberg T, Krude H, Schultz G, Gudermann T, Grüters A. Mutations of the human thyrotropin receptor gene causing thyroid hypoplasia and persistent congenital hypothyroidism. J Clin Endocrinol Metab. 1997;82:3471–3480 [DOI] [PubMed] [Google Scholar]
- 61. Cassio A, Nicoletti A, Rizzello A, Zazzetta E, Bal M, Baldazzi L. Current loss-of-function mutations in the thyrotropin receptor gene: when to investigate, clinical effects, and treatment. J Clin Res Pediatr Endocrinol. 2013;5(Suppl 1):29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Eilers M, Hornak V, Smith SO, Konopka JB. Comparison of class A and D G protein-coupled receptors: common features in structure and activation. Biochemistry. 2005;44:8959–8975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hanson MA, Stevens RC. Discovery of new GPCR biology: one receptor structure at a time. Structure. 2009;17:8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54:323–374 [DOI] [PubMed] [Google Scholar]
- 66. Mohr K, Schmitz J, Schrage R, Tränkle C, Holzgrabe U. Molecular alliance-from orthosteric and allosteric ligands to dualsteric/bitopic agonists at G protein coupled receptors. Angew Chem Int Ed Engl. 2013;52:508–516 [DOI] [PubMed] [Google Scholar]
- 67. Limbird LE. The receptor concept: a continuing evolution. Mol Interv. 2004;4:326–336 [DOI] [PubMed] [Google Scholar]
- 68. Gether U, Asmar F, Meinild AK, Rasmussen SG. Structural basis for activation of G-protein-coupled receptors. Pharmacol Toxicol. 2002;91:304–312 [DOI] [PubMed] [Google Scholar]
- 69. Lebon G, Warne T, Tate CG. Agonist-bound structures of G protein-coupled receptors. Curr Opin Struct Biol. 2012;22:482–490 [DOI] [PubMed] [Google Scholar]
- 70. Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:397–406 [DOI] [PubMed] [Google Scholar]
- 71. Ahuja S, Smith SO. Multiple switches in G protein-coupled receptor activation. Trends Pharmacol Sci. 2009;30:494–502 [DOI] [PubMed] [Google Scholar]
- 72. Salon JA, Lodowski DT, Palczewski K. The significance of G protein-coupled receptor crystallography for drug discovery. Pharmacol Rev. 2011;63:901–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71 [DOI] [PubMed] [Google Scholar]
- 74. Oldham WM, Van Eps N, Preininger AM, Hubbell WL, Hamm HE. Mechanism of the receptor-catalyzed activation of heterotrimeric G proteins. Nat Struct Mol Biol. 2006;13:772–777 [DOI] [PubMed] [Google Scholar]
- 75. Smrcka AV. G protein βγ subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci. 2008;65:2191–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Van Eps N, Preininger AM, Alexander N, et al. Interaction of a G protein with an activated receptor opens the interdomain interface in the α subunit. Proc Natl Acad Sci U S A. 2011;108:9420–9424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chung KY, Rasmussen SG, Liu T, et al. Conformational changes in the G protein Gs induced by the β2 adrenergic receptor. Nature. 2011;477:611–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sun Y, McGarrigle D, Huang XY. When a G protein-coupled receptor does not couple to a G protein. Mol Biosyst. 2007;3:849–854 [DOI] [PubMed] [Google Scholar]
- 79. Angelova K, Felline A, Lee M, Patel M, Puett D, Fanelli F. Conserved amino acids participate in the structure networks deputed to intramolecular communication in the lutropin receptor. Cell Mol Life Sci. 2011;68:1227–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fanelli F, De Benedetti PG. Update 1 of: computational modeling approaches to structure-function analysis of G protein-coupled receptors. Chem Rev 2011;111:PR438–PR535 [DOI] [PubMed] [Google Scholar]
- 81. Raymond JR. Multiple mechanisms of receptor-G protein signaling specificity. Am J Physiol. 1995;269:F141–F158 [DOI] [PubMed] [Google Scholar]
- 82. Wess J. Molecular basis of receptor/G-protein-coupling selectivity. Pharmacol Ther. 1998;80:231–264 [DOI] [PubMed] [Google Scholar]
- 83. Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther. 2004;103:21–80 [DOI] [PubMed] [Google Scholar]
- 84. Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272 [DOI] [PubMed] [Google Scholar]
- 85. Fredriksson R, Schioth HB. The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol Pharmacol. 2005;67:1414–1425 [DOI] [PubMed] [Google Scholar]
- 86. Kobilka B, Schertler GF. New G-protein-coupled receptor crystal structures: insights and limitations. Trends Pharmacol Sci. 2008;29:79–83 [DOI] [PubMed] [Google Scholar]
- 87. Lodowski DT, Angel TE, Palczewski K. Comparative analysis of GPCR crystal structures. Photochem Photobiol. 2009;85:425–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730 [DOI] [PubMed] [Google Scholar]
- 89. Mason JS, Bortolato A, Congreve M, Marshall FH. New insights from structural biology into the druggability of G protein-coupled receptors. Trends Pharmacol Sci. 2012;33:249–260 [DOI] [PubMed] [Google Scholar]
- 90. Schlyer S, Horuk R. I want a new drug: G-protein-coupled receptors in drug development. Drug Discov Today. 2006;11:481–493 [DOI] [PubMed] [Google Scholar]
- 91. Tyndall JD, Sandilya R. GPCR agonists and antagonists in the clinic. Med Chem. 2005;1:405–421 [DOI] [PubMed] [Google Scholar]
- 92. Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94 [DOI] [PubMed] [Google Scholar]
- 93. Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:381–416 [DOI] [PubMed] [Google Scholar]
- 94. Katritch V, Cherezov V, Stevens RC. Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol Sci. 2012;33:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Costanzi S, Siegel J, Tikhonova IG, Jacobson KA. Rhodopsin and the others: a historical perspective on structural studies of G protein-coupled receptors. Curr Pharm Des. 2009;15:3994–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shoichet BK, Kobilka BK. Structure-based drug screening for G-protein-coupled receptors. Trends Pharmacol Sci. 2012;33:268–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Carlsson J, Coleman RG, Setola V, et al. Ligand discovery from a dopamine D3 receptor homology model and crystal structure. Nat Chem Biol. 2011;7:769–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kontoyianni M, Liu Z. Structure-based design in the GPCR target space. Curr Med Chem. 2012;19:544–556 [DOI] [PubMed] [Google Scholar]
- 99. Tate CG, Schertler GF. Engineering G protein-coupled receptors to facilitate their structure determination. Curr Opin Struct Biol. 2009;19:386–395 [DOI] [PubMed] [Google Scholar]
- 100. Zhao Q, Wu BL. Ice breaking in GPCR structural biology. Acta Pharmacol Sin. 2012;33:324–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rasmussen SG, DeVree BT, Zou Y, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Deupi X, Edwards P, Singhal A, et al. Stabilized G protein binding site in the structure of constitutively active metarhodopsin-II. Proc Natl Acad Sci U S A. 2012;109:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Standfuss J, Edwards PC, D'Antona A, et al. The structural basis of agonist-induced activation in constitutively active rhodopsin. Nature. 2011;471:656–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Trzaskowski B, Latek D, Yuan S, Ghoshdastider U, Debinski A, Filipek S. Action of molecular switches in GPCRs: theoretical and experimental studies. Curr Med Chem. 2012;19:1090–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Surgand JS, Rodrigo J, Kellenberger E, Rognan D. A chemogenomic analysis of the transmembrane binding cavity of human G-protein-coupled receptors. Proteins. 2006;62:509–538 [DOI] [PubMed] [Google Scholar]
- 106. Wichard JD, Ter Laak A, Krause G, Heinrich N, Kuhne R, Kleinau G. Chemogenomic analysis of G-protein coupled receptors and their ligands deciphers locks and keys governing diverse aspects of signalling. PLoS One. 2011;6:e16811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Strotmann R, Schröck K, Böselt I, Stäubert C, Russ A, Schöneberg T. Evolution of GPCR: change and continuity. Mol Cell Endocrinol. 2011;331:170–178 [DOI] [PubMed] [Google Scholar]
- 108. Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357 [DOI] [PubMed] [Google Scholar]
- 109. Lin H, Sassano MF, Roth BL, Shoichet BK. A pharmacological organization of G protein-coupled receptors. Nat Methods. 2013;10:140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rinne A, Birk A, Bunemann M. Voltage regulates adrenergic receptor function. Proc Natl Acad Sci U S A. 2013;110:1536–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gurung IS, Martinez-Pinna J, Mahaut-Smith MP. Novel consequences of voltage-dependence to G-protein-coupled P2Y1 receptors. Br J Pharmacol. 2008;154:882–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Martinez-Pinna J, Gurung IS, Vial C, et al. Direct voltage control of signaling via P2Y1 and other Gαq-coupled receptors. J Biol Chem. 2005;280:1490–1498 [DOI] [PubMed] [Google Scholar]
- 113. Ben-Chaim Y, Chanda B, Dascal N, Bezanilla F, Parnas I, Parnas H. Movement of ‘gating charge’ is coupled to ligand binding in a G-protein-coupled receptor. Nature. 2006;444:106–109 [DOI] [PubMed] [Google Scholar]
- 114. Dekel N, Priest MF, Parnas H, Parnas I, Bezanilla F. Depolarization induces a conformational change in the binding site region of the M2 muscarinic receptor. Proc Natl Acad Sci U S A. 2012;109:285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Navarro-Polanco RA, Moreno Galindo EG, Ferrer-Villada T, et al. Conformational changes in the M2 muscarinic receptor induced by membrane voltage and agonist binding. J Physiol. 2011;589:1741–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kupchik YM, Barchad-Avitzur O, Wess J, Ben-Chaim Y, Parnas I, Parnas H. A novel fast mechanism for GPCR-mediated signal transduction: control of neurotransmitter release. J Cell Biol. 2011;192:137–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mahaut-Smith MP, Martinez-Pinna J, Gurung IS. A role for membrane potential in regulating GPCRs? Trends Pharmacol Sci. 2008;29:421–429 [DOI] [PubMed] [Google Scholar]
- 118. Zohar A, Dekel N, Rubinsky B, Parnas H. New mechanism for voltage induced charge movement revealed in GPCRs: theory and experiments. PLoS One. 2010;5:e8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Schwartz TW, Frimurer TM, Holst B, Rosenkilde MM, Elling CE. Molecular mechanism of 7TM receptor activation: a global toggle switch model. Annu Rev Pharmacol Toxicol. 2006;46:481–519 [DOI] [PubMed] [Google Scholar]
- 120. Smit MJ, Vischer HF, Bakker RA, et al. Pharmacogenomic and structural analysis of constitutive G protein-coupled receptor activity. Annu Rev Pharmacol Toxicol. 2007;47:53–87 [DOI] [PubMed] [Google Scholar]
- 121. Scheerer P, Heck M, Goede A, Park JH, Choe HW, Ernst OP, Hofmann KP, Hildebrand PW. Structural and kinetic modeling of an activating helix switch in the rhodopsin-transducin interface. Proc Natl Acad Sci U S A. 2009;106:10660–10665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Scheerer P, Park JH, Hildebrand PW, et al. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502 [DOI] [PubMed] [Google Scholar]
- 123. Schertler GF. Signal transduction: the rhodopsin story continued. Nature. 2008;453:292–293 [DOI] [PubMed] [Google Scholar]
- 124. Hofmann KP, Scheerer P, Hildebrand PW, et al. A G protein-coupled receptor at work: the rhodopsin model. Trends Biochem Sci. 2009;34:540–552 [DOI] [PubMed] [Google Scholar]
- 125. Nygaard R, Frimurer TM, Holst B, Rosenkilde MM, Schwartz TW. Ligand binding and micro-switches in 7TM receptor structures. Trends Pharmacol Sci. 2009;30:249–259 [DOI] [PubMed] [Google Scholar]
- 126. Ahuja S, Hornak V, Yan EC, et al. Helix movement is coupled to displacement of the second extracellular loop in rhodopsin activation. Nat Struct Mol Biol. 2009;16:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bokoch MP, Zou Y, Rasmussen SG, et al. Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature. 2010;463:108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kleinau G, Claus M, Jaeschke H, et al. Contacts between extracellular loop two and transmembrane helix six determine basal activity of the thyroid-stimulating hormone receptor. J Biol Chem. 2007;282:518–525 [DOI] [PubMed] [Google Scholar]
- 129. Massotte D, Kieffer BL. The second extracellular loop: a damper for G protein-coupled receptors? Nat Struct Mol Biol. 2005;12:287–288 [DOI] [PubMed] [Google Scholar]
- 130. Peeters MC, van Westen GJ, Li Q, IJzerman AP. Importance of the extracellular loops in G protein-coupled receptors for ligand recognition and receptor activation. Trends Pharmacol Sci. 2011;32:35–42 [DOI] [PubMed] [Google Scholar]
- 131. Wheatley M, Simms J, Hawtin SR, et al. Extracellular loops and ligand binding to a subfamily of Family A G-protein-coupled receptors. Biochem Soc Trans. 2007;35:717–720 [DOI] [PubMed] [Google Scholar]
- 132. Wheatley M, Wootten D, Conner MT, et al. Lifting the lid on GPCRs: the role of extracellular loops. Br J Pharmacol. 2012;165:1688–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Deupi X, Standfuss J. Structural insights into agonist-induced activation of G-protein-coupled receptors. Curr Opin Struct Biol. 2011;21:541–551 [DOI] [PubMed] [Google Scholar]
- 134. Sanders J, Chirgadze DY, Sanders P, et al. Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid. 2007;17:395–410 [DOI] [PubMed] [Google Scholar]
- 135. Sanders P, Young S, Sanders J, et al. Crystal structure of the TSH receptor (TSHR) bound to a blocking-type TSHR autoantibody. J Mol Endocrinol. 2011;46:81–99 [DOI] [PubMed] [Google Scholar]
- 136. Enkhbayar P, Kamiya M, Osaki M, Matsumoto T, Matsushima N. Structural principles of leucine-rich repeat (LRR) proteins. Proteins. 2004;54:394–403 [DOI] [PubMed] [Google Scholar]
- 137. Kleinau G, Jäschke H, Neumann S, Lättig J, Paschke R, Krause G. Identification of a novel epitope in the thyroid-stimulating hormone receptor ectodomain acting as intramolecular signaling interface. J Biol Chem. 2004;279:51590–51600 [DOI] [PubMed] [Google Scholar]
- 138. Caltabiano G, Campillo M, De Leener A, Smits G, Vassart G, Costagliola S, Pardo L. The specificity of binding of glycoprotein hormones to their receptors. Cell Mol Life Sci. 2008;65:2484–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005;433:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jiang X, Liu H, Chen X, et al. Structure of follicle-stimulating hormone in complex with the entire ectodomain of its receptor. Proc Natl Acad Sci U S A. 2012;109(31):12491–12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chazenbalk GD, Jaume JC, McLachlan SM, Rapoport B. Engineering the human thyrotropin receptor ectodomain from a non-secreted form to a secreted, highly immunoreactive glycoprotein that neutralizes autoantibodies in Graves' patients' sera. J Biol Chem. 1997;272:18959–18965 [DOI] [PubMed] [Google Scholar]
- 142. Cornelis S, Uttenweiler-Joseph S, Panneels V, Vassart G, Costagliola S. Purification and characterization of a soluble bioactive amino-terminal extracellular domain of the human thyrotropin receptor. Biochemistry. 2001;40:9860–9869 [DOI] [PubMed] [Google Scholar]
- 143. Mueller S, Kleinau G, Jaeschke H, Paschke R, Krause G. Extended hormone binding site of the human thyroid stimulating hormone receptor: distinctive acidic residues in the hinge region are involved in bovine thyroid stimulating hormone binding and receptor activation. J Biol Chem. 2008;283:18048–18055 [DOI] [PubMed] [Google Scholar]
- 144. Núñez Miguel R, Sanders J, Chirgadze DY, Blundell TL, Furmaniak J, Rees Smith B. FSH and TSH binding to their respective receptors: similarities, differences and implication for glycoprotein hormone specificity. J Mol Endocrinol. 2008;41:145–164 [DOI] [PubMed] [Google Scholar]
- 145. Krause G, Kreuchwig A, Kleinau G. Extended and structurally supported insights into extracellular hormone binding, signal transduction and organization of the thyrotropin receptor. PLoS One. 2012;7:e52920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Angelova K, de Jonge H, Granneman JC, Puett D, Bogerd J. Functional differences of invariant and highly conserved residues in the extracellular domain of the glycoprotein hormone receptors. J Biol Chem. 2010;285:34813–34827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rodien P, Brémont C, Sanson ML, et al. Familial gestational hyperthyroidism caused by a mutant thyrotropin receptor hypersensitive to human chorionic gonadotropin. N Engl J Med. 1998;339:1823–1826 [DOI] [PubMed] [Google Scholar]
- 148. Royer J, Lefevre-Minisini A, Caltabiano G, et al. The cloned equine thyrotropin receptor is hypersensitive to human chorionic gonadotropin: identification of three residues in the extracellular domain involved in ligand specificity. Endocrinology. 2008;149:5088–5096 [DOI] [PubMed] [Google Scholar]
- 149. Smits G, Campillo M, Govaerts C, et al. Glycoprotein hormone receptors: determinants in leucine-rich repeats responsible for ligand specificity. EMBO J. 2003;22:2692–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kleinau G, Krause G. Thyrotropin and homologous glycoprotein hormone receptors: structural and functional aspects of extracellular signaling mechanisms. Endocr Rev. 2009;30:133–151 [DOI] [PubMed] [Google Scholar]
- 151. Kleinau G, Mueller S, Jaeschke H, et al. Defining structural and functional dimensions of the extracellular thyrotropin receptor region. J Biol Chem. 2011;286:22622–22631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kosugi S, Ban T, Akamizu T, Kohn LD. Site-directed mutagenesis of a portion of the extracellular domain of the rat thyrotropin receptor important in autoimmune thyroid disease and nonhomologous with gonadotropin receptors. Relationship of functional and immunogenic domains. J Biol Chem. 1991;266:19413–19418 [PubMed] [Google Scholar]
- 153. Nagayama Y, Wadsworth HL, Chazenbalk GD, Russo D, Seto P, Rapoport B. Thyrotropin-luteinizing hormone/chorionic gonadotropin receptor extracellular domain chimeras as probes for thyrotropin receptor function. Proc Natl Acad Sci U S A. 1991;88:902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Costagliola S, Panneels V, Bonomi M, Koch J, Many MC, Smits G, Vassart G. Tyrosine sulfation is required for agonist recognition by glycoprotein hormone receptors. EMBO J. 2002;21:504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Bonomi M, Busnelli M, Persani L, Vassart G, Costagliola S. Structural differences in the hinge region of the glycoprotein hormone receptors: evidence from the sulfated tyrosine residues. Mol Endocrinol. 2006;20:3351–3363 [DOI] [PubMed] [Google Scholar]
- 156. Bruysters M, Verhoef-Post M, Themmen AP. Asp330 and Tyr331 in the C-terminal cysteine-rich region of the luteinizing hormone receptor are key residues in hormone-induced receptor activation. J Biol Chem. 2008;283:25821–25828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Jaeschke H, Schaarschmidt J, Günther R, Mueller S. The hinge region of the TSH receptor stabilizes ligand binding and determines different signaling profiles of human and bovine TSH. Endocrinology. 2011;152:3986–3996 [DOI] [PubMed] [Google Scholar]
- 158. Grossmann M, Weintraub BD, Szkudlinski MW. Novel insights into the molecular mechanisms of human thyrotropin action: structural, physiological, and therapeutic implications for the glycoprotein hormone family. Endocr Rev. 1997;18:476–501 [DOI] [PubMed] [Google Scholar]
- 159. Mueller S, Kleinau G, Szkudlinski MW, Jaeschke H, Krause G, Paschke R. The superagonistic activity of bovine thyroid-stimulating hormone (TSH) and the human TR1401 TSH analog is determined by specific amino acids in the hinge region of the human TSH receptor. J Biol Chem. 2009;284:16317–16324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Núñez Miguel R, Sanders J, Chirgadze DY, Furmaniak J, Rees Smith B. Thyroid stimulating autoantibody M22 mimics TSH binding to the TSH receptor leucine rich domain: a comparative structural study of protein-protein interactions. J Mol Endocrinol. 2009;42:381–395 [DOI] [PubMed] [Google Scholar]
- 161. Núñez Miguel R, Sanders J, Sanders P, et al. Similarities and differences in interactions of thyroid stimulating and blocking autoantibodies with the TSH receptor. J Mol Endocrinol. 2012;49:137–151 [DOI] [PubMed] [Google Scholar]
- 162. Zoenen M, Urizar E, Swillens S, Vassart G, Costagliola S. Evidence for activity-regulated hormone-binding cooperativity across glycoprotein hormone receptor homomers. Nat Commun. 2012;3:1007. [DOI] [PubMed] [Google Scholar]
- 163. Guan R, Wu X, Feng X, Zhang M, Hébert TE, Segaloff DL. Structural determinants underlying constitutive dimerization of unoccupied human follitropin receptors. Cell Signal. 2010;22:247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Vlaeminck-Guillem V, Ho SC, Rodien P, Vassart G, Costagliola S. Activation of the cAMP pathway by the TSH receptor involves switching of the ectodomain from a tethered inverse agonist to an agonist. Mol Endocrinol. 2002;16:736–746 [DOI] [PubMed] [Google Scholar]
- 165. Szkudlinski MW, Grossmann M, Weintraub BD. Structure-function studies of human TSH: new advances in design of glycoprotein hormone analogs. Trends Endocrinol Metab. 1996;7:277–286 [DOI] [PubMed] [Google Scholar]
- 166. Szkudlinski MW, Fremont V, Ronin C, Weintraub BD. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol Rev. 2002;82:473–502 [DOI] [PubMed] [Google Scholar]
- 167. Szkudlinski MW, Teh NG, Grossmann M, Tropea JE, Weintraub BD. Engineering human glycoprotein hormone superactive analogues. Nat Biotechnol. 1996;14:1257–1263 [DOI] [PubMed] [Google Scholar]
- 168. Fares F. The role of O-linked and N-linked oligosaccharides on the structure-function of glycoprotein hormones: development of agonists and antagonists. Biochim Biophys Acta. 2006;1760:560–567 [DOI] [PubMed] [Google Scholar]
- 169. Schaaf L, Leiprecht A, Saji M, Hübner U, Usadel KH, Kohn LD. Glycosylation variants of human TSH selectively activate signal transduction pathways. Mol Cell Endocrinol. 1997;132:185–194 [DOI] [PubMed] [Google Scholar]
- 170. Cole ES, Lee K, Lauziere K, et al. Recombinant human thyroid stimulating hormone: development of a biotechnology product for detection of metastatic lesions of thyroid carcinoma. Biotechnology (N Y). 1993;11:1014–1024 [DOI] [PubMed] [Google Scholar]
- 171. Thotakura NR, Desai RK, Bates LG, Cole ES, Pratt BM, Weintraub BD. Biological activity and metabolic clearance of a recombinant human thyrotropin produced in Chinese hamster ovary cells. Endocrinology. 1991;128:341–348 [DOI] [PubMed] [Google Scholar]
- 172. Azzam N, Bar-Shalom R, Fares F. Conversion of TSH heterodimer to a single polypeptide chain increases bioactivity and longevity. Endocrinology. 2012;153:954–960 [DOI] [PubMed] [Google Scholar]
- 173. Azzam N, Bar-Shalom R, Kraiem Z, Fares F. Human thyrotropin (TSH) variants designed by site-directed mutagenesis block TSH activity in vitro and in vivo. Endocrinology. 2005;146:2845–2850 [DOI] [PubMed] [Google Scholar]
- 174. Fares FA, Yamabe S, Ben-Menahem D, Pixley M, Hsueh AJ, Boime I. Conversion of thyrotropin heterodimer to a biologically active single-chain. Endocrinology. 1998;139:2459–2464 [DOI] [PubMed] [Google Scholar]
- 175. Rayalam S, Eizenstat LD, Davis RR, Hoenig M, Ferguson DC. Expression and purification of feline thyrotropin (fTSH): immunological detection and bioactivity of heterodimeric and yoked glycoproteins. Domest Anim Endocrinol. 2006;30:185–202 [DOI] [PubMed] [Google Scholar]
- 176. Rayalam S, Eizenstat LD, Hoenig M, Ferguson DC. Cloning and sequencing of feline thyrotropin (fTSH): heterodimeric and yoked constructs. Domest Anim Endocrinol. 2006;30:203–217 [DOI] [PubMed] [Google Scholar]
- 177. Angelova K, Fremont V, Jain R, et al. Human α-subunit analogs act as partial agonists to the thyroid-stimulating hormone receptor: differential effects of free and yoked subunits. Endocrine. 2004;24:25–31 [DOI] [PubMed] [Google Scholar]
- 178. Garcia-Campayo V, Kumar TR, Boime I. Thyrotropin, follitropin, and chorionic gonadotropin expressed as a single multifunctional unit reveal remarkable permissiveness in receptor-ligand interactions. Endocrinology. 2002;143:3773–3778 [DOI] [PubMed] [Google Scholar]
- 179. Garcia-Campayo V, Boime I, Ma X, Daphna-Iken D, Kumar TR. A single-chain tetradomain glycoprotein hormone analog elicits multiple hormone activities in vivo. Biol Reprod. 2005;72:301–308 [DOI] [PubMed] [Google Scholar]
- 180. Erbel PJ, Karimi-Nejad Y, De Beer T, Boelens R, Kamerling JP, Vliegenthart JF. Solution structure of the α-subunit of human chorionic gonadotropin. Eur J Biochem. 1999;260:490–498 [DOI] [PubMed] [Google Scholar]
- 181. Erbel PJ, Karimi-Nejad Y, van Kuik JA, Boelens R, Kamerling JP, Vliegenthart JF. Effects of the N-linked glycans on the 3D structure of the free α-subunit of human chorionic gonadotropin. Biochemistry. 2000;39:6012–6021 [DOI] [PubMed] [Google Scholar]
- 182. Fox KM, Dias JA, Van Roey P. Three-dimensional structure of human follicle-stimulating hormone. Mol Endocrinol. 2001;15:378–389 [DOI] [PubMed] [Google Scholar]
- 183. Lapthorn AJ, Harris DC, Littlejohn A, et al. Crystal structure of human chorionic gonadotropin. Nature. 1994;369:455–461 [DOI] [PubMed] [Google Scholar]
- 184. Tegoni M, Spinelli S, Verhoeyen M, Davis P, Cambillau C. Crystal structure of a ternary complex between human chorionic gonadotropin (hCG) and two Fv fragments specific for the α and β-subunits. J Mol Biol. 1999;289:1375–1385 [DOI] [PubMed] [Google Scholar]
- 185. Faglia G, Beck-Peccoz P, Ballabio M, Nava C. Excess of β-subunit of thyrotropin (TSH) in patients with idiopathic central hypothyroidism due to the secretion of TSH with reduced biological activity. J Clin Endocrinol Metab. 1983;56:908–914 [DOI] [PubMed] [Google Scholar]
- 186. Kourides IA, Ridgway EC, Weintraub BD, Bigos ST, Gershengorn MC, Maloof F. Thyrotropin-induced hyperthyroidism: use of α and β subunit levels to identify patients with pituitary tumors. J Clin Endocrinol Metab. 1977;45:534–543 [DOI] [PubMed] [Google Scholar]
- 187. Kourides IA, Weintraub BD, Ridgway EC, Maloof F. Pituitary secretion of free α and β subunit of human thyrotropin in patients with thyroid disorders. J Clin Endocrinol Metab. 1975;40:872–885 [DOI] [PubMed] [Google Scholar]
- 188. Lavoie HB, Marsh EE, Hall JE. Absence of apparent circadian rhythms of gonadotropins and free α-subunit in postmenopausal women: evidence for distinct regulation relative to other hormonal rhythms. J Biol Rhythms. 2006;21:58–67 [DOI] [PubMed] [Google Scholar]
- 189. Nisula BC, Morgan FJ, Canfield RE. Evidence that chorionic gonadotropin has intrinsic thyrotropic activity. Biochem Biophys Res Commun. 1974;59:86–91 [DOI] [PubMed] [Google Scholar]
- 190. Williams JF, Davies TF, Catt KJ, Pierce JG. Receptor-binding activity of highly purified bovine luteinizing hormone and thyrotropin, and their subunits. Endocrinology. 1980;106:1353–1359 [DOI] [PubMed] [Google Scholar]
- 191. Wolff J, Winand RJ, Kohn LD. The contribution of subunits of thyroid stimulating hormone to the binding and biological activity of thyrotropin. Proc Natl Acad Sci U S A. 1974;71:3460–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Yoshimura M, Hershman JM. Thyrotropic action of human chorionic gonadotropin. Thyroid. 1995;5:425–434 [DOI] [PubMed] [Google Scholar]
- 193. Hsu SY, Nakabayashi K, Bhalla A. Evolution of glycoprotein hormone subunit genes in bilateral metazoa: identification of two novel human glycoprotein hormone subunit family genes, GPA2 and GPB5. Mol Endocrinol. 2002;16:1538–1551 [DOI] [PubMed] [Google Scholar]
- 194. Dos Santos S, Bardet C, Bertrand S, Escriva H, Habert D, Querat B. Distinct expression patterns of glycoprotein hormone-α2 and -β5 in a basal chordate suggest independent developmental functions. Endocrinology. 2009;150:3815–3822 [DOI] [PubMed] [Google Scholar]
- 195. Lefort GP, Amr S, Carayon P, Nisula BC. Relevance of the low and high affinity thyrotropin-binding sites of human thyroid membranes to the stimulation of adenylate cyclase. Endocrinology. 1984;114:1005–1011 [DOI] [PubMed] [Google Scholar]
- 196. Park JI, Semyonov J, Chang CL, Hsu SY. Conservation of the heterodimeric glycoprotein hormone subunit family proteins and the LGR signaling system from nematodes to humans. Endocrine. 2005;26:267–276 [DOI] [PubMed] [Google Scholar]
- 197. Sellami A, Agricola HJ, Veenstra JA. Neuroendocrine cells in Drosophila melanogaster producing GPA2/GPB5, a hormone with homology to LH, FSH and TSH. Gen Comp Endocrinol. 2011;170:582–588 [DOI] [PubMed] [Google Scholar]
- 198. Li C, Hirooka Y, Habu S, Takagi J, Gotoh M, Nogimori T. Distribution of thyrostimulin in the rat: an immunohistochemical study. Endocr Regul. 2004;38:131–142 [PubMed] [Google Scholar]
- 199. Sun SC, Hsu PJ, Wu FJ, Li SH, Lu CH, Luo CW. Thyrostimulin, but not thyroid-stimulating hormone (TSH), acts as a paracrine regulator to activate the TSH receptor in mammalian ovary. J Biol Chem. 2010;285:3758–3765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Okada SL, Ellsworth JL, Durnam DM, et al. A glycoprotein hormone expressed in corticotrophs exhibits unique binding properties on thyroid-stimulating hormone receptor. Mol Endocrinol. 2006;20:414–425 [DOI] [PubMed] [Google Scholar]
- 201. van Zeijl CJ, Surovtseva OV, Wiersinga WM, Boelen A, Fliers E. Transient hypothyroxinemia in juvenile glycoprotein hormone subunit B5 knock-out mice. Mol Cell Endocrinol. 2010;321:231–238 [DOI] [PubMed] [Google Scholar]
- 202. Van Sande J, Costa MJ, Massart C, et al. Kinetics of thyrotropin-stimulating hormone (TSH) and thyroid-stimulating antibody binding and action on the TSH receptor in intact TSH receptor-expressing CHO cells. J Clin Endocrinol Metab. 2003;88:5366–5374 [DOI] [PubMed] [Google Scholar]
- 203. Saltiel AR, Thomas CG, Jr, Nayfeh SN. Thyrotropin binding to porcine thyroid plasma membranes: kinetic and thermodynamic analyses. Mol Cell Endocrinol. 1982;28:299–312 [DOI] [PubMed] [Google Scholar]
- 204. Agrawal G, Dighe RR. Critical involvement of the hinge region of the follicle-stimulating hormone receptor in the activation of the receptor. J Biol Chem. 2009;284:2636–2647 [DOI] [PubMed] [Google Scholar]
- 205. Ho SC, Goh SS, Su Q, Khoo DH. Cysteine 390 mutation of the TSH receptor modulates its ectodomain as an inverse agonist on the serpentine domain with decrease in basal constitutive activity. Mol Cell Endocrinol. 2005;245:158–168 [DOI] [PubMed] [Google Scholar]
- 206. Ho SC, Van Sande J, Lefort A, Vassart G, Costagliola S. Effects of mutations involving the highly conserved S281HCC motif in the extracellular domain of the thyrotropin (TSH) receptor on TSH binding and constitutive activity. Endocrinology. 2001;142:2760–2767 [DOI] [PubMed] [Google Scholar]
- 207. Jaquette J, Segaloff DL. Constitutive activation of the LH receptor is associated with an alteration in the conformation of the ectodomain. Mol Cell Endocrinol. 2002;194:211–215 [DOI] [PubMed] [Google Scholar]
- 208. Mizutori Y, Chen CR, McLachlan SM, Rapoport B. The thyrotropin receptor hinge region is not simply a scaffold for the leucine-rich domain but contributes to ligand binding and signal transduction. Mol Endocrinol. 2008;22:1171–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Nakabayashi K, Kudo M, Hsueh AJ, Maruo T. Activation of the luteinizing hormone receptor in the extracellular domain. Mol Cell Endocrinol. 2003;202:139–144 [DOI] [PubMed] [Google Scholar]
- 210. Nurwakagari P, Breit A, Hess C, Salman-Livny H, Ben-Menahem D, Gudermann T. A conformational contribution of the luteinizing hormone-receptor ectodomain to receptor activation. J Mol Endocrinol. 2007;38:259–275 [DOI] [PubMed] [Google Scholar]
- 211. Sangkuhl K, Schulz A, Schultz G, Schöneberg T. Structural requirements for mutational lutropin/choriogonadotropin receptor activation. J Biol Chem. 2002;277:47748–47755 [DOI] [PubMed] [Google Scholar]
- 212. Zhang M, Tong KP, Fremont V, et al. The extracellular domain suppresses constitutive activity of the transmembrane domain of the human TSH receptor: implications for hormone-receptor interaction and antagonist design. Endocrinology. 2000;141:3514–3517 [DOI] [PubMed] [Google Scholar]
- 213. Zhang ML, Sugawa H, Kosugi S, Mori T. Constitutive activation of the thyrotropin receptor by deletion of a portion of the extracellular domain. Biochem Biophys Res Commun. 1995;211:205–210 [DOI] [PubMed] [Google Scholar]
- 214. Kleinau G, Brehm M, Wiedemann U, Labudde D, Leser U, Krause G. Implications for molecular mechanisms of glycoprotein hormone receptors using a new sequence-structure-function analysis resource. Mol Endocrinol. 2007;21:574–580 [DOI] [PubMed] [Google Scholar]
- 215. Kleinau G, Kreuchwig A, Worth CL, Krause G. An interactive web-tool for molecular analyses links naturally occurring mutation data with three-dimensional structures of the rhodopsin-like glycoprotein hormone receptors. Hum Mutat. 2010;31:E1519–E1525 [DOI] [PubMed] [Google Scholar]
- 216. Kreuchwig A, Kleinau G, Kreuchwig F, Worth CL, Krause G. Update and extension of a glycoprotein hormone receptors web application. Mol Endocrinol. 2011;25:707–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Hamidi S, Chen CR, Mizutori-Sasai Y, McLachlan SM, Rapoport B. Relationship between thyrotropin receptor hinge region proteolytic posttranslational modification and receptor physiological function. Mol Endocrinol. 2011;25:184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Duprez L, Parma J, Costagliola S, et al. Constitutive activation of the TSH receptor by spontaneous mutations affecting the N-terminal extracellular domain. FEBS Lett. 1997;409:469–474 [DOI] [PubMed] [Google Scholar]
- 219. Grüters A, Schöneberg T, Biebermann H, et al. Severe congenital hyperthyroidism caused by a germ-line neo mutation in the extracellular portion of the thyrotropin receptor. J Clin Endocrinol Metab. 1998;83:1431–1436 [DOI] [PubMed] [Google Scholar]
- 220. Kopp P, Muirhead S, Jourdain N, Gu WX, Jameson JL, Rodd C. Congenital hyperthyroidism caused by a solitary toxic adenoma harboring a novel somatic mutation (serine281→isoleucine) in the extracellular domain of the thyrotropin receptor. J Clin Invest. 1997;100:1634–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Jaeschke H, Neumann S, Kleinau G, et al. An aromatic environment in the vicinity of serine 281 is a structural requirement for thyrotropin receptor function. Endocrinology. 2006;147:1753–1760 [DOI] [PubMed] [Google Scholar]
- 222. Ho SC, Goh SS, Li S, Khoo DH, Paterson M. Effects of mutations involving cysteine residues distal to the S281HCC motif at the C-terminus on the functional characteristics of a truncated ectodomain-only thyrotropin receptor anchored on glycosylphosphatidyl-inositol. Thyroid. 2008;18:1313–1319 [DOI] [PubMed] [Google Scholar]
- 223. Fukata S, Hishinuma A, Nakatake N, Tajiri J. A Japanese family with familial nonautoimmune hyperthyroidism with a novel mutation (Asn406Ser) in extracellular domain of thyrotrophin receptor. Clin Endocrinol (Oxf). 2012;77:329–330 [DOI] [PubMed] [Google Scholar]
- 224. Nishihara E, Chen CR, Mizutori-Sasai Y, et al. Deletion of thyrotropin receptor residue Asp403 in a hyperfunctioning thyroid nodule provides insight into the role of the ectodomain in ligand-induced receptor activation. J Endocrinol Invest. 2012;35:49–53 [DOI] [PubMed] [Google Scholar]
- 225. Russo D, Costante G, Bruno R, et al. TSH receptor extracellular region mutations in thyroid functioning nodules: further evidence for the functional role of this region in the receptor activation. Endocrine. 2011;40:492–494 [DOI] [PubMed] [Google Scholar]
- 226. Chen CR, Chazenbalk GD, McLachlan SM, Rapoport B. Evidence that the C terminus of the A subunit suppresses thyrotropin receptor constitutive activity. Endocrinology. 2003;144:3821–3827 [DOI] [PubMed] [Google Scholar]
- 227. Chen CR, McLachlan SM, Rapoport B. Evidence that the thyroid-stimulating hormone (TSH) receptor transmembrane domain influences kinetics of TSH binding to the receptor ectodomain. J Biol Chem. 2011;286:6219–6224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Montanelli L, Van Durme JJ, Smits G, et al. Modulation of ligand selectivity associated with activation of the transmembrane region of the human follitropin receptor. Mol Endocrinol. 2004;18:2061–2073 [DOI] [PubMed] [Google Scholar]
- 229. Vasseur C, Rodien P, Beau I, et al. A chorionic gonadotropin-sensitive mutation in the follicle-stimulating hormone receptor as a cause of familial gestational spontaneous ovarian hyperstimulation syndrome. N Engl J Med. 2003;349:753–759 [DOI] [PubMed] [Google Scholar]
- 230. Chazenbalk GD, McLachlan SM, Chen CR, Rapoport B. Insight into thyrotropin receptor cleavage by engineering the single polypeptide chain luteinizing hormone receptor into a cleaving, two subunit receptor. Eur J Biochem. 2001;268:2261–2269 [DOI] [PubMed] [Google Scholar]
- 231. Chazenbalk GD, Tanaka K, Nagayama Y, et al. Evidence that the thyrotropin receptor ectodomain contains not one, but two, cleavage sites. Endocrinology. 1997;138:2893–2899 [DOI] [PubMed] [Google Scholar]
- 232. Kakinuma A, Chazenbalk GD, Tanaka K, et al. An N-linked glycosylation motif from the noncleaving luteinizing hormone receptor substituted for the homologous region (Gly367 to Glu369) of the thyrotropin receptor prevents cleavage at its second, downstream site. J Biol Chem. 1997;272:28296–28300 [DOI] [PubMed] [Google Scholar]
- 233. Tanaka K, Chazenbalk GD, McLachlan SM, Rapoport B. Thyrotropin receptor cleavage at site 1 does not involve a specific amino acid motif but instead depends on the presence of the unique, 50 amino acid insertion. J Biol Chem. 1998;273:1959–1963 [DOI] [PubMed] [Google Scholar]
- 234. Tanaka K, Chazenbalk GD, McLachlan SM, Rapoport B. The shed thyrotropin receptor is primarily a carboxyl terminal truncated form of the A subunit, not the entire A subunit. Mol Cell Endocrinol. 1999;150:113–119 [DOI] [PubMed] [Google Scholar]
- 235. Tanaka K, Chazenbalk GD, McLachlan SM, Rapoport B. Evidence that cleavage of the thyrotropin receptor involves a “molecular ruler” mechanism: deletion of amino acid residues 305–320 causes a spatial shift in cleavage site 1 independent of amino acid motif. Endocrinology. 2000;141:3573–3577 [DOI] [PubMed] [Google Scholar]
- 236. Couet J, Sar S, Jolivet A, Hai MT, Milgrom E, Misrahi M. Shedding of human thyrotropin receptor ectodomain. Involvement of a matrix metalloprotease. J Biol Chem. 1996;271:4545–4552 [DOI] [PubMed] [Google Scholar]
- 237. Kaczur V, Puskas LG, Nagy ZU, et al. Cleavage of the human thyrotropin receptor by ADAM10 is regulated by thyrotropin. J Mol Recognit. 2007;20:392–404 [DOI] [PubMed] [Google Scholar]
- 238. Chazenbalk GD, Tanaka K, McLachlan SM, Rapoport B. On the functional importance of thyrotropin receptor intramolecular cleavage. Endocrinology. 1999;140:4516–4520 [DOI] [PubMed] [Google Scholar]
- 239. Chen CR, Chazenbalk GD, McLachlan SM, Rapoport B. Targeted restoration of cleavage in a noncleaving thyrotropin receptor demonstrates that cleavage is insufficient to enhance ligand-independent activity. Endocrinology. 2003;144:1324–1330 [DOI] [PubMed] [Google Scholar]
- 240. Misrahi M, Milgrom E. Cleavage and shedding of the TSH receptor. Eur J Endocrinol. 1997;137:599–602 [DOI] [PubMed] [Google Scholar]
- 241. Quellari M, Desroches A, Beau I, Beaudeux E, Misrahi M. Role of cleavage and shedding in human thyrotropin receptor function and trafficking. Eur J Biochem. 2003;270:3486–3497 [DOI] [PubMed] [Google Scholar]
- 242. de Bernard S, Misrahi M, Huet JC, et al. Sequential cleavage and excision of a segment of the thyrotropin receptor ectodomain. J Biol Chem. 1999;274:101–107 [DOI] [PubMed] [Google Scholar]
- 243. Loosfelt H, Pichon C, Jolivet A, et al. Two-subunit structure of the human thyrotropin receptor. Proc Natl Acad Sci U S A. 1992;89:3765–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Chen CR, Chazenbalk GD, Wawrowsky KA, McLachlan SM, Rapoport B. Evidence that human thyroid cells express uncleaved, single-chain thyrotropin receptors on their surface. Endocrinology. 2006;147:3107–3113 [DOI] [PubMed] [Google Scholar]
- 245. Chen CR, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM. The thyrotropin receptor autoantigen in Graves disease is the culprit as well as the victim. J Clin Invest. 2003;111:1897–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Mizutori Y, Chen CR, Latrofa F, McLachlan SM, Rapoport B. Evidence that shed thyrotropin receptor A subunits drive affinity maturation of autoantibodies causing Graves' disease. J Clin Endocrinol Metab. 2009;94:927–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Vu MT, Radu A, Ghinea N. The cleavage of thyroid-stimulating hormone receptor is dependent on cell-cell contacts and regulates the hormonal stimulation of phospholipase C. J Cell Mol Med. 2009;13:2253–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Van Sande J, Massart C, Costagliola S, et al. Specific activation of the thyrotropin receptor by trypsin. Mol Cell Endocrinol. 1996;119:161–168 [DOI] [PubMed] [Google Scholar]
- 249. Karges B, Gidenne S, Aumas C, et al. Zero-length cross-linking reveals that tight interactions between the extracellular and transmembrane domains of the luteinizing hormone receptor persist during receptor activation. Mol Endocrinol. 2005;19:2086–2098 [DOI] [PubMed] [Google Scholar]
- 250. Neumann S, Claus M, Paschke R. Interactions between the extracellular domain and the extracellular loops as well as the 6th transmembrane domain are necessary for TSH receptor activation. Eur J Endocrinol. 2005;152:625–634 [DOI] [PubMed] [Google Scholar]
- 251. Kleinau G, Jaeschke H, Mueller S, et al. Evidence for cooperative signal triggering at the extracellular loops of the TSH receptor. FASEB J. 2008;22:2798–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Barwell J, Woolley MJ, Wheatley M, Conner AC, Poyner DR. The role of the extracellular loops of the CGRP receptor, a family B GPCR. Biochem Soc Trans. 2012;40:433–437 [DOI] [PubMed] [Google Scholar]
- 253. Liapakis G, Cordomí A, Pardo L. The G-protein coupled receptor family: actors with many faces. Curr Pharm Des. 2012;18:175–185 [DOI] [PubMed] [Google Scholar]
- 254. van Zeijl CJ, van Koppen CJ, Surovtseva OV, et al. Complete inhibition of rhTSH-, Graves' disease IgG-, and M22-induced cAMP production in differentiated orbital fibroblasts by a low-molecular-weight TSHR antagonist. J Clin Endocrinol Metab. 2012;97:E781–E785 [DOI] [PubMed] [Google Scholar]
- 255. Parma J, Van Sande J, Swillens S, Tonacchera M, Dumont J, Vassart G. Somatic mutations causing constitutive activity of the thyrotropin receptor are the major cause of hyperfunctioning thyroid adenomas: identification of additional mutations activating both the cyclic adenosine 3′,5′-monophosphate and inositol phosphate-Ca2+ cascades. Mol Endocrinol. 1995;9:725–733 [DOI] [PubMed] [Google Scholar]
- 256. Claus M, Maier J, Paschke R, Kujat C, Stumvoll M, Führer D. Novel thyrotropin receptor germline mutation (Ile568Val) in a Saxonian family with hereditary nonautoimmune hyperthyroidism. Thyroid. 2005;15:1089–1094 [DOI] [PubMed] [Google Scholar]
- 257. Nanba K, Usui T, Minamiguchi S, et al. Two rare TSH receptor amino acid substitutions in toxic thyroid adenomas. Endocr J. 2012;59:13–19 [DOI] [PubMed] [Google Scholar]
- 258. Nishihara E, Chen CR, Higashiyama T, et al. Subclinical nonautoimmune hyperthyroidism in a family segregates with a thyrotropin receptor mutation with weakly increased constitutive activity. Thyroid. 2010;20:1307–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Tonacchera M, Van Sande J, Cetani F, et al. Functional characteristics of three new germline mutations of the thyrotropin receptor gene causing autosomal dominant toxic thyroid hyperplasia. J Clin Endocrinol Metab. 1996;81:547–554 [DOI] [PubMed] [Google Scholar]
- 260. Führer D, Holzapfel HP, Wonerow P, Scherbaum WA, Paschke R. Somatic mutations in the thyrotropin receptor gene and not in the Gsα protein gene in 31 toxic thyroid nodules. J Clin Endocrinol Metab. 1997;82:3885–3891 [DOI] [PubMed] [Google Scholar]
- 261. Camilot M, Teofoli F, Gandini A, et al. Thyrotropin receptor gene mutations and TSH resistance: variable expressivity in the heterozygotes. Clin Endocrinol (Oxf). 2005;63:146–151 [DOI] [PubMed] [Google Scholar]
- 262. Sura-Trueba S, Aumas C, Carre A, et al. An inactivating mutation within the first extracellular loop of the thyrotropin receptor impedes normal posttranslational maturation of the extracellular domain. Endocrinology. 2009;150:1043–1050 [DOI] [PubMed] [Google Scholar]
- 263. Grasberger H, Van Sande J, Hag-Dahood Mahameed A, Tenenbaum-Rakover Y, Refetoff S. A familial thyrotropin (TSH) receptor mutation provides in vivo evidence that the inositol phosphates/Ca2+ cascade mediates TSH action on thyroid hormone synthesis. J Clin Endocrinol Metab. 2007;92:2816–2820 [DOI] [PubMed] [Google Scholar]
- 264. Biebermann H, Winkler F, Handke D, Gruters A, Krude H, Kleinau G. Molecular description of non-autoimmune hyperthyroidism at a neonate caused by a new thyrotropin receptor germline mutation. Thyroid Res. 2011;4)Suppl 1):S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Gozu HI, Bircan R, Krohn K, et al. Similar prevalence of somatic TSH receptor and Gsα mutations in toxic thyroid nodules in geographical regions with different iodine supply in Turkey. Eur J Endocrinol. 2006;155:535–545 [DOI] [PubMed] [Google Scholar]
- 266. Akcurin S, Turkkahraman D, Tysoe C, et al. A family with a novel TSH receptor activating germline mutation (p.Ala485Val). Eur J Pediatr. 2008;167:1231–1237 [DOI] [PubMed] [Google Scholar]
- 267. Biebermann H, Schöneberg T, Schulz A, et al. A conserved tyrosine residue (Y601) in transmembrane domain 5 of the human thyrotropin receptor serves as a molecular switch to determine G-protein coupling. FASEB J. 1998;12:1461–1471 [DOI] [PubMed] [Google Scholar]
- 268. Kosugi S, Mori T. Constitutive activation of the thyrotropin receptor by mutating CYS-636 in the sixth transmembrane segment. Biochem Biophys Res Commun. 1996;222:713–717 [DOI] [PubMed] [Google Scholar]
- 269. Kosugi S, Shenker A, Mori T. Constitutive activation of cyclic AMP but not phosphatidylinositol signaling caused by four mutations in the 6th transmembrane helix of the human thyrotropin receptor. FEBS Lett. 1994;356:291–294 [DOI] [PubMed] [Google Scholar]
- 270. Neumann S, Krause G, Chey S, Paschke R. A free carboxylate oxygen in the side chain of position 674 in transmembrane domain 7 is necessary for TSH receptor activation. Mol Endocrinol. 2001;15:1294–1305 [DOI] [PubMed] [Google Scholar]
- 271. Urizar E, Claeysen S, Deupí X, et al. An activation switch in the rhodopsin family of G protein-coupled receptors: the thyrotropin receptor. J Biol Chem. 2005;280:17135–17141 [DOI] [PubMed] [Google Scholar]
- 272. Kleinau G, Hoyer I, Kreuchwig A, et al. From molecular details of the interplay between transmembrane helices of the thyrotropin receptor to general aspects of signal transduction in family A G-protein-coupled receptors (GPCRs). J Biol Chem. 2011;286:25859–25871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Ballesteros JA, Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relationships in G-protein coupled receptors. Methods Neurosci. 1995;25:366–428 [Google Scholar]
- 274. Worth CL, Kleinau G, Krause G. Comparative sequence and structural analyses of G-protein-coupled receptor crystal structures and implications for molecular models. PLoS One. 2009;4:e7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Hanson MA, Roth CB, Jo E, et al. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335:851–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Reiersen H, Rees AR. The hunchback and its neighbours: proline as an environmental modulator. Trends Biochem Sci. 2001;26:679–684 [DOI] [PubMed] [Google Scholar]
- 277. Yohannan S, Faham S, Yang D, Whitelegge JP, Bowie JU. The evolution of transmembrane helix kinks and the structural diversity of G protein-coupled receptors. Proc Natl Acad Sci U S A. 2004;101:959–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Sykiotis GP, Neumann S, Georgopoulos NA, et al. Functional significance of the thyrotropin receptor germline polymorphism D727E. Biochem Biophys Res Commun. 2003;301:1051–1056 [DOI] [PubMed] [Google Scholar]
- 279. Fricke-Otto S, Pfarr N, Mühlenberg R, Pohlenz J. Mild congenital primary hypothyroidism in a Turkish family caused by a homozygous missense thyrotropin receptor (TSHR) gene mutation (A593 V). Exp Clin Endocrinol Diabetes. 2005;113:582–585 [DOI] [PubMed] [Google Scholar]
- 280. Karges B, Krause G, Homoki J, Debatin KM, de Roux N, Karges W. TSH receptor mutation V509A causes familial hyperthyroidism by release of interhelical constraints between transmembrane helices TMH3 and TMH5. J Endocrinol. 2005;186:377–385 [DOI] [PubMed] [Google Scholar]
- 281. Sum CS, Tikhonova IG, Costanzi S, Gershengorn MC. Two arginine-glutamate ionic locks near the extracellular surface of FFAR1 gate receptor activation. J Biol Chem. 2009;284:3529–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Deflorian F, Engel S, Colson AO, Raaka BM, Gershengorn MC, Costanzi S. Understanding the structural and functional differences between mouse thyrotropin-releasing hormone receptors 1 and 2. Proteins. 2008;71:783–794 [DOI] [PubMed] [Google Scholar]
- 283. Rashid M, Manivet P, Nishio H, et al. Identification of the binding sites and selectivity of sarpogrelate, a novel 5-HT2 antagonist, to human 5-HT2A, 5-HT2B and 5-HT2C receptor subtypes by molecular modeling. Life Sci. 2003;73:193–207 [DOI] [PubMed] [Google Scholar]
- 284. Tikhonova IG, Best RB, Engel S, Gershengorn MC, Hummer G, Costanzi S. Atomistic insights into rhodopsin activation from a dynamic model. J Am Chem Soc. 2008;130:10141–10149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Biebermann H, Winkler F, Handke D, et al. New pathogenic thyrotropin receptor mutations decipher differentiated activity switching at a conserved helix 6 motif of family A GPCR. J Clin Endocrinol Metab. 2012;97:E228–E232 [DOI] [PubMed] [Google Scholar]
- 286. Winkler F, Kleinau G, Tarnow P, et al. A new phenotype of nongoitrous and nonautoimmune hyperthyroidism caused by a heterozygous thyrotropin receptor mutation in transmembrane helix 6. J Clin Endocrinol Metab. 2010;95:3605–3610 [DOI] [PubMed] [Google Scholar]
- 287. Tonacchera M, Chiovato L, Pinchera A, et al. Hyperfunctioning thyroid nodules in toxic multinodular goiter share activating thyrotropin receptor mutations with solitary toxic adenoma. J Clin Endocrinol Metab. 1998;83:492–498 [DOI] [PubMed] [Google Scholar]
- 288. Kuechler A, Hauffa BP, Köninger A, et al. An unbalanced translocation unmasks a recessive mutation in the follicle-stimulating hormone receptor (FSHR) gene and causes FSH resistance. Eur J Hum Genet. 2010;18:656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–187 [DOI] [PubMed] [Google Scholar]
- 290. Rasmussen SG, Choi HJ, Fung JJ, et al. Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature. 2011;469:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Heitman LH, Kleinau G, Brussee J, Krause G, Ijzerman AP. Determination of different putative allosteric binding pockets at the lutropin receptor by using diverse drug-like low molecular weight ligands. Mol Cell Endocrinol. 2012;351:326–336 [DOI] [PubMed] [Google Scholar]
- 292. Wenzel-Seifert K, Seifert R. Molecular analysis of β2-adrenoceptor coupling to G(s)-, G(i)-, and G(q)-proteins. Mol Pharmacol. 2000;58:954–966 [DOI] [PubMed] [Google Scholar]
- 293. van Koppen CJ, Zaman GJ, Timmers CM, et al. A signaling-selective, nanomolar potent allosteric low molecular weight agonist for the human luteinizing hormone receptor. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:503–514 [DOI] [PubMed] [Google Scholar]
- 294. Gershengorn MC, Neumann S. Update in TSH receptor agonists and antagonists. J Clin Endocrinol Metab. 2012;97:4287–4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Warne T, Moukhametzianov R, Baker JG, et al. The structural basis for agonist and partial agonist action on a β1-adrenergic receptor. Nature. 2011;469:241–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Xu F, Wu H, Katritch V, et al. Structure of an agonist-bound human A2A adenosine receptor. Science. 2011;332:322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Yao XJ, Vélez Ruiz G, Whorton MR, et al. The effect of ligand efficacy on the formation and stability of a GPCR-G protein complex. Proc Natl Acad Sci U S A. 2009;106:9501–9506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Bond RA, Ijzerman AP. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci. 2006;27:92–96 [DOI] [PubMed] [Google Scholar]
- 299. Cotecchia S, Fanelli F, Costa T. Constitutively active G protein-coupled receptor mutants: implications on receptor function and drug action. Assay Drug Dev Technol. 2003;1:311–316 [DOI] [PubMed] [Google Scholar]
- 300. Parnot C, Miserey-Lenkei S, Bardin S, Corvol P, Clauser E. Lessons from constitutively active mutants of G protein-coupled receptors. Trends Endocrinol Metab. 2002;13:336–343 [DOI] [PubMed] [Google Scholar]
- 301. Kenakin T. The physiological significance of constitutive receptor activity. Trends Pharmacol Sci. 2005;26:603–605 [Google Scholar]
- 302. Schöneberg T, Schulz A, Gudermann T. The structural basis of G-protein-coupled receptor function and dysfunction in human diseases. Rev Physiol Biochem Pharmacol. 2002;144:143–227 [PubMed] [Google Scholar]
- 303. Srinivasan S, Lubrano-Berthelier C, Govaerts C, et al. Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. J Clin Invest. 2004;114:1158–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Assmann SM. Heterotrimeric and unconventional GTP binding proteins in plant cell signaling. Plant Cell. 2002;14(Suppl):S355–S373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Evans PD, Robb S, Cheek TR, Reale V, Hannan FL, Swales LS, Hall LM, Midgley JM. Agonist-specific coupling of G-protein-coupled receptors to second-messenger systems. Prog Brain Res. 1995;106:259–268 [DOI] [PubMed] [Google Scholar]
- 306. Perez DM, Karnik SS. Multiple signaling states of G-protein-coupled receptors. Pharmacol Rev. 2005;57:147–161 [DOI] [PubMed] [Google Scholar]
- 307. Kruse AC, Hu J, Pan AC, et al. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature. 2012;482:552–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Horn F, van der Wenden EM, Oliveira L, IJzerman AP, Vriend G. Receptors coupling to G proteins: is there a signal behind the sequence? Proteins. 2000;41:448–459 [DOI] [PubMed] [Google Scholar]
- 309. Hu J, Wang Y, Zhang X, Lloyd JR, Li JH, Karpiak J, Costanzi S, Wess J. Structural basis of G protein-coupled receptor-G protein interactions. Nat Chem Biol. 2010;6:541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Moller S, Vilo J, Croning MD. Prediction of the coupling specificity of G protein coupled receptors to their G proteins. Bioinformatics. 2001;17(Suppl 1):S174–S181 [DOI] [PubMed] [Google Scholar]
- 311. Wong SK. G protein selectivity is regulated by multiple intracellular regions of GPCRs. Neurosignals. 2003;12:1–12 [DOI] [PubMed] [Google Scholar]
- 312. Kero J, Ahmed K, Wettschureck N, et al. Thyrocyte-specific Gq/G11 deficiency impairs thyroid function and prevents goiter development. J Clin Invest. 2007;117:2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Morshed SA, Latif R, Davies TF. Characterization of thyrotropin receptor antibody-induced signaling cascades. Endocrinology. 2009;150:519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Cabrera-Vera TM, Vanhauwe J, et al. Insights into G protein structure, function, and regulation. Endocr Rev. 2003;24:765–781 [DOI] [PubMed] [Google Scholar]
- 315. Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev. 2000;21:90–113 [DOI] [PubMed] [Google Scholar]
- 316. Chazenbalk GD, Nagayama Y, Russo D, Wadsworth HL, Rapoport B. Functional analysis of the cytoplasmic domains of the human thyrotropin receptor by site-directed mutagenesis. J Biol Chem. 1990;265:20970–20975 [PubMed] [Google Scholar]
- 317. Kosugi S, Kohn LD, Akamizu T, Mori T. The middle portion in the second cytoplasmic loop of the thyrotropin receptor plays a crucial role in adenylate cyclase activation. Mol Endocrinol. 1994;8:498–509 [DOI] [PubMed] [Google Scholar]
- 318. Kosugi S, Mori T. The amino-terminal half of the cytoplasmic tail of the thyrotropin receptor is essential for full activities of receptor function. Biochem Biophys Res Commun. 1994;200:401–407 [DOI] [PubMed] [Google Scholar]
- 319. Kosugi S, Mori T. The intracellular region adjacent to plasma membrane (residues 684–692) of the thyrotropin receptor is important for phosphoinositide signaling but not for agonist-induced adenylate cyclase activation. Biochem Biophys Res Commun. 1994;199:1497–1503 [DOI] [PubMed] [Google Scholar]
- 320. Kosugi S, Mori T. The first cytoplasmic loop of the thyrotropin receptor is important for phosphoinositide signaling but not for agonist-induced adenylate cyclase activation. FEBS Lett. 1994;341:162–166 [DOI] [PubMed] [Google Scholar]
- 321. Cangul H, Morgan NV, Forman JR, et al. Novel TSHR mutations in consanguineous families with congenital nongoitrous hypothyroidism. Clin Endocrinol (Oxf). 2010;73:671–677 [DOI] [PubMed] [Google Scholar]
- 322. Claus M, Neumann S, Kleinau G, Krause G, Paschke R. Structural determinants for G-protein activation and specificity in the third intracellular loop of the thyroid-stimulating hormone receptor. JMolMed. 2006;84:943–954 [DOI] [PubMed] [Google Scholar]
- 323. de Roux N, Misrahi M, Brauner R, et al. Four families with loss of function mutations of the thyrotropin receptor. J Clin Endocrinol Metab. 1996;81:4229–4235 [DOI] [PubMed] [Google Scholar]
- 324. Kleinau G, Jaeschke H, Worth CL, et al. Principles and determinants of G-protein coupling by the rhodopsin-like thyrotropin receptor. PLoS One. 2010;5:e9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Neumann S, Krause G, Claus M, Paschke R. Structural determinants for G protein activation and selectivity in the second intracellular loop of the thyrotropin receptor. Endocrinology. 2005;146:477–485 [DOI] [PubMed] [Google Scholar]
- 326. Chang WC, Liao CY, Chen WC, et al. R450H TSH receptor mutation in congenital hypothyroidism in Taiwanese children. Clin Chim Acta. 2012;413:1004–1007 [DOI] [PubMed] [Google Scholar]
- 327. Mizuno H, Kanda K, Sugiyama Y, Imamine H, Ito T, Kato I, Togari H, Kamoda T, Onigata K. Longitudinal evaluation of patients with a homozygous R450H mutation of the TSH receptor gene. Horm Res. 2009;71:318–323 [DOI] [PubMed] [Google Scholar]
- 328. Nagashima T, Murakami M, Onigata K, et al. Novel inactivating missense mutations in the thyrotropin receptor gene in Japanese children with resistance to thyrotropin. Thyroid. 2001;11:551–559 [DOI] [PubMed] [Google Scholar]
- 329. Narumi S, Nagasaki K, Ishii T, et al. Nonclassic TSH resistance: TSHR mutation carriers with discrepantly high thyroidal iodine uptake. J Clin Endocrinol Metab. 2011;96:E1340–E1345 [DOI] [PubMed] [Google Scholar]
- 330. Feng X, Müller T, Mizrachi D, Fanelli F, Segaloff DL. An intracellular loop (IL2) residue confers different basal constitutive activities to the human lutropin receptor and human thyrotropin receptor through structural communication between IL2 and helix 6, via helix 3. Endocrinology. 2008;149:1705–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Kleinau G, Jaeschke H, Mueller S, Worth CL, Paschke R, Krause G. Molecular and structural effects of inverse agonistic mutations on signaling of the thyrotropin receptor: a basally active GPCR. Cell Mol Life Sci. 2008;65:3664–3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Claeysen S, Govaerts C, Lefort A, et al. A conserved Asn in TM7 of the thyrotropin receptor is a common requirement for activation by both mutations and its natural agonist. FEBS Lett. 2002;517:195–200 [DOI] [PubMed] [Google Scholar]
- 333. Nishihara E, Nagayama Y, Amino N, Hishinuma A, Takano T, Yoshida H, Kubota S, Fukata S, Kuma K, Miyauchi A. A novel thyrotropin receptor germline mutation (Asp617Tyr) causing hereditary hyperthyroidism. Endocr J. 2007;54:927–934 [DOI] [PubMed] [Google Scholar]
- 334. Van Sande J, Parma J, Tonacchera M, Swillens S, Dumont J, Vassart G. Somatic and germline mutations of the TSH receptor gene in thyroid diseases. J Clin Endocrinol Metab. 1995;80:2577–2585 [DOI] [PubMed] [Google Scholar]
- 335. Schulz A, Bruns K, Henklein P, et al. Requirement of specific intrahelical interactions for stabilizing the inactive conformation of glycoprotein hormone receptors. J Biol Chem. 2000;275:37860–37869 [DOI] [PubMed] [Google Scholar]
- 336. Allen MD, Neumann S, Gershengorn MC. Occupancy of both sites on the thyrotropin (TSH) receptor dimer is necessary for phosphoinositide signaling. FASEB J. 2011;25:3687–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Persani L, Calebiro D, Bonomi M. Technology Insight: modern methods to monitor protein-protein interactions reveal functional TSH receptor oligomerization. Nat Clin Pract Endocrinol Metab. 2007;3:180–190 [DOI] [PubMed] [Google Scholar]
- 338. Albizu L, Cottet M, Kralikova M, et al. Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat Chem Biol. 2010;6:587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Bouvier M. Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci. 2001;2:274–286 [DOI] [PubMed] [Google Scholar]
- 340. Rozenfeld R, Devi LA. Exploring a role for heteromerization in GPCR signalling specificity. Biochem J. 2011;433:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Levoye A, Dam J, Ayoub MA, et al. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J. 2006;25:3012–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Lohse MJ. Dimerization in GPCR mobility and signaling. Curr Opin Pharmacol. 2010;10:53–58 [DOI] [PubMed] [Google Scholar]
- 343. George SR, O'Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov. 2002;1:808–820 [DOI] [PubMed] [Google Scholar]
- 344. Uberti MA, Hague C, Oller H, Minneman KP, Hall RA. Heterodimerization with β2-adrenergic receptors promotes surface expression and functional activity of α1D-adrenergic receptors. J Pharmacol Exp Ther. 2005;313:16–23 [DOI] [PubMed] [Google Scholar]
- 345. Schelshorn D, Joly F, Mutel S, et al. Lateral allosterism in the glucagon receptor family: glucagon-like peptide 1 induces G-protein-coupled receptor heteromer formation. Mol Pharmacol. 2012;81:309–318 [DOI] [PubMed] [Google Scholar]
- 346. Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. Conformational cross-talk between α2A-adrenergic and μ-opioid receptors controls cell signaling. Nat Chem Biol. 2008;4:126–131 [DOI] [PubMed] [Google Scholar]
- 347. Rediger A, Piechowski CL, Yi CX, T, et al. Mutually opposite signal modulation by hypothalamic heterodimerization of ghrelin and melanocortin-3 receptors. J Biol Chem. 2011;286:39623–39631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Guo W, Shi L, Filizola M, Weinstein H, Javitch JA. Crosstalk in G protein-coupled receptors: changes at the transmembrane homodimer interface determine activation. Proc Natl Acad Sci U S A. 2005;102:17495–17500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Ciruela F, Vilardaga JP, Fernández-Dueñas V. Lighting up multiprotein complexes: lessons from GPCR oligomerization. Trends Biotechnol. 2010;28:407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Smith NJ, Milligan G. Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol Rev. 2010;62:701–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. White JF, Grodnitzky J, Louis JM, et al. Dimerization of the class A G protein-coupled neurotensin receptor NTS1 alters G protein interaction. Proc Natl Acad Sci U S A. 2007;104:12199–12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Tadagaki K, Jockers R, Kamal M. History and biological significance of GPCR heteromerization in the neuroendocrine system. Neuroendocrinology. 2012;95:223–231 [DOI] [PubMed] [Google Scholar]
- 353. Tadagaki K, Tudor D, Gbahou F, et al. Human cytomegalovirus-encoded UL33 and UL78 heteromerize with host CCR5 and CXCR4 impairing their HIV coreceptor activity. Blood. 2012;119:4908–4918 [DOI] [PubMed] [Google Scholar]
- 354. Tschische P, Tadagaki K, Kamal M, Jockers R, Waldhoer M. Heteromerization of human cytomegalovirus encoded chemokine receptors. Biochem Pharmacol. 2011;82:610–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Carrillo JJ, López-Giménez JF, Milligan G. Multiple interactions between transmembrane helices generate the oligomeric α1b-adrenoceptor. Mol Pharmacol. 2004;66:1123–1137 [DOI] [PubMed] [Google Scholar]
- 356. Guo W, Urizar E, Kralikova M, et al. Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J. 2008;27:2293–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Hernanz-Falcón P, Rodríguez-Frade JM, Serrano A, et al. Identification of amino acid residues crucial for chemokine receptor dimerization. Nat Immunol. 2004;5:216–223 [DOI] [PubMed] [Google Scholar]
- 358. Mancia F, Assur Z, Herman AG, Siegel R, Hendrickson WA. Ligand sensitivity in dimeric associations of the serotonin 5HT2c receptor. EMBO Rep. 2008;9:363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Hebert TE, Moffett S, Morello JP, et al. A peptide derived from a β2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem. 1996;271:16384–16392 [DOI] [PubMed] [Google Scholar]
- 360. McMillin SM, Heusel M, Liu T, Costanzi S, Wess J. Structural basis of M3 muscarinic receptor dimer/oligomer formation. J Biol Chem. 2011;286:28584–28598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Yanagawa M, Yamashita T, Shichida Y. Comparative fluorescence resonance energy transfer analysis of metabotropic glutamate receptors: implications about the dimeric arrangement and rearrangement upon ligand bindings. J Biol Chem. 2011;286:22971–22981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Gorinski N, Kowalsman N, Renner U, et al. Computational and experimental analysis of the transmembrane domain 4/5 dimerization interface of the serotonin 5-HT1A receptor. Mol Pharmacol. 2012;82:448–463 [DOI] [PubMed] [Google Scholar]
- 363. Uddin MS, Kim H, Deyo A, Naider F, Becker JM. Identification of residues involved in homodimer formation located within a β-strand region of the N-terminus of a yeast G protein-coupled receptor. J Recept Signal Transduct Res. 2012;32:65–75 [DOI] [PubMed] [Google Scholar]
- 364. Khelashvili G, Dorff K, Shan J, et al. GPCR-OKB: the G protein coupled receptor oligomer knowledge base. Bioinformatics. 2010;26:1804–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Calebiro D, Rieken F, Wagner J, et al. Single-molecule analysis of fluorescently labeled G-protein-coupled receptors reveals complexes with distinct dynamics and organization. Proc Natl Acad Sci U S A. 2013;110:743–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Lambert NA. GPCR dimers fall apart. Sci Signal 2010;3:pe12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Whorton MR, Bokoch MP, Rasmussen SG, et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci U S A. 2007;104:7682–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Whorton MR, Jastrzebska B, Park PS, et al. Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J Biol Chem. 2008;283:4387–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Moreno JL, Muguruza C, Umali A, et al. Identification of three residues essential for 5-hydroxytryptamine 2A-metabotropic glutamate 2 (5-HT2A·mGlu2) receptor heteromerization and its psychoactive behavioral function. J Biol Chem. 2012;287:44301–44319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Teichmann A, Rutz C, Kreuchwig A, Krause G, Wiesner B, Schülein R. The pseudo signal peptide of the corticotropin-releasing factor receptor type 2A prevents receptor oligomerization. J Biol Chem. 2012;287:27265–27274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Rozenfeld R, Devi LA. Receptor heteromerization and drug discovery. Trends Pharmacol Sci. 2010;31:124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Graves PN, Vlase H, Bobovnikova Y, Davies TF. Multimeric complex formation by the thyrotropin receptor in solubilized thyroid membranes. Endocrinology. 1996;137:3915–3920 [DOI] [PubMed] [Google Scholar]
- 373. Latif R, Graves P, Davies TF. Oligomerization of the human thyrotropin receptor: fluorescent protein-tagged hTSHR reveals post-translational complexes. J Biol Chem. 2001;276:45217–45224 [DOI] [PubMed] [Google Scholar]
- 374. Tenenbaum-Rakover Y, Grasberger H, Mamanasiri S, et al. Loss-of-function mutations in the thyrotropin receptor gene as a major determinant of hyperthyrotropinemia in a consanguineous community. J Clin Endocrinol Metab. 2009;94:1706–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Gu WX, Du GG, Kopp P, et al. The thyrotropin (TSH) receptor transmembrane domain mutation (Pro556-Leu) in the hypothyroid hyt/hyt mouse results in plasma membrane targeting but defective TSH binding. Endocrinology. 1995;136:3146–3153 [DOI] [PubMed] [Google Scholar]
- 376. Stein SA, Oates EL, Hall CR, et al. Identification of a point mutation in the thyrotropin receptor of the hyt/hyt hypothyroid mouse. Mol Endocrinol. 1994;8:129–138 [DOI] [PubMed] [Google Scholar]
- 377. Endo T, Kobayashi T. Dominant negative effect of mutated thyroid stimulating hormone receptor (P556L) causes hypothyroidism in C.RF-Tshr(hyt/wild) mice. PLoS One. 2012;7:e42358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Moeller LC, Kimura S, Kusakabe T, Liao XH, Van Sande J, Refetoff S. Hypothyroidism in thyroid transcription factor 1 haploinsufficiency is caused by reduced expression of the thyroid-stimulating hormone receptor. Mol Endocrinol. 2003;17:2295–2302 [DOI] [PubMed] [Google Scholar]
- 379. Rubin CJ, Zody MC, Eriksson J, et al. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature. 2010;464:587–591 [DOI] [PubMed] [Google Scholar]
- 380. Latif R, Graves P, Davies TF. Ligand-dependent inhibition of oligomerization at the human thyrotropin receptor. J Biol Chem. 2002;277:45059–45067 [DOI] [PubMed] [Google Scholar]
- 381. Davies T, Marians R, Latif R. The TSH receptor reveals itself. J Clin Invest. 2002;110:161–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Guan R, Feng X, Wu X, et al. Bioluminescence resonance energy transfer studies reveal constitutive dimerization of the human lutropin receptor and a lack of correlation between receptor activation and the propensity for dimerization. J Biol Chem. 2009;284:7483–7494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Osuga Y, Hayashi M, Kudo M, Conti M, Kobilka B, Hsueh AJ. Co-expression of defective luteinizing hormone receptor fragments partially reconstitutes ligand-induced signal generation. J Biol Chem. 1997;272:25006–25012 [DOI] [PubMed] [Google Scholar]
- 384. Fan QR, Hendrickson WA. Assembly and structural characterization of an authentic complex between human follicle stimulating hormone and a hormone-binding ectodomain of its receptor. Mol Cell Endocrinol. 2007;260–262:73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Fan QR, Hendrickson WA. Comparative structural analysis of the binding domain of follicle stimulating hormone receptor. Proteins. 2008;72:393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Latif R, Michalek K, Morshed SA, Davies TF. A tyrosine residue on the TSH receptor stabilizes multimer formation. PLoS One. 2010;5:e9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Jiang X, Liu H, Chen X, et al. Structure of follicle-stimulating hormone in complex with the entire ectodomain of its receptor. Proc Natl Acad Sci U S A. 2012;109:12491–12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Wu B, Chien EY, Mol CD, et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Wu H, Wacker D, Mileni M, et al. Structure of the human κ-opioid receptor in complex with JDTic. Nature. 2012;485:327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiol Rev. 2013;93:405–480 [DOI] [PubMed] [Google Scholar]
- 391. Svendsen AM, Vrecl M, Knudsen L, et al. Dimerization and negative cooperativity in the relaxin family peptide receptors. Ann N Y Acad Sci. 2009;1160:54–59 [DOI] [PubMed] [Google Scholar]
- 392. Jeoung M, Lee C, Ji I, Ji TH. Trans-activation, cis-activation and signal selection of gonadotropin receptors. Mol Cell Endocrinol. 2007;260–262:137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Rivero-Müller A, Chou YY, Ji I, et al. Rescue of defective G protein-coupled receptor function in vivo by intermolecular cooperation. Proc Natl Acad Sci U S A. 2010;107:2319–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Ji I, Lee C, Jeoung M, Koo Y, Sievert GA, Ji TH. Trans-activation of mutant follicle-stimulating hormone receptors selectively generates only one of two hormone signals. Mol Endocrinol. 2004;18:968–978 [DOI] [PubMed] [Google Scholar]
- 395. Kaczur V, Puskás LG, Takács M, et al. Evolution of the thyrotropin receptor: a G protein coupled receptor with an intrinsic capacity to dimerize. Mol Genet Metab. 2003;78:275–290 [DOI] [PubMed] [Google Scholar]
- 396. Zhang M, Guan R, Segaloff DL. Revisiting and questioning functional rescue between dimerized LH receptor mutants. Mol Endocrinol. 2012;26:655–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Kendall SK, Samuelson LC, Saunders TL, Wood RI, Camper SA. Targeted disruption of the pituitary glycoprotein hormone α-subunit produces hypogonadal and hypothyroid mice. Genes Dev. 1995;9:2007–2019 [DOI] [PubMed] [Google Scholar]
- 398. Morshed SA, Ando T, Latif R, Davies TF. Neutral antibodies to the TSH receptor are present in Graves' disease and regulate selective signaling cascades. Endocrinology. 2010;151:5537–5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Nagayama Y, Nishihara E, Namba H, Yamashita S, Niwa M. Identification of the sites of asparagine-linked glycosylation on the human thyrotropin receptor and studies on their role in receptor function and expression. J Pharmacol Exp Ther. 2000;295:404–409 [PubMed] [Google Scholar]
- 400. Oda Y, Sanders J, Roberts S, et al. Analysis of carbohydrate residues on recombinant human thyrotropin receptor. J Clin Endocrinol Metab. 1999;84:2119–2125 [DOI] [PubMed] [Google Scholar]
- 401. Russo D, Chazenbalk GD, Nagayama Y, Wadsworth HL, Rapoport B. Site-directed mutagenesis of the human thyrotropin receptor: role of asparagine-linked oligosaccharides in the expression of a functional receptor. Mol Endocrinol. 1991;5:29–33 [DOI] [PubMed] [Google Scholar]
- 402. Arey BJ, Yanofsky SD, Claudia Pérez M, et al. Differing pharmacological activities of thiazolidinone analogs at the FSH receptor. Biochem Biophys Res Commun. 2008;368:723–728 [DOI] [PubMed] [Google Scholar]
- 403. Jäschke H, Neumann S, Moore S, et al. A low molecular weight agonist signals by binding to the transmembrane domain of thyroid-stimulating hormone receptor (TSHR) and luteinizing hormone/chorionic gonadotropin receptor (LHCGR). J Biol Chem. 2006;281:9841–9844 [DOI] [PubMed] [Google Scholar]
- 404. Jaeschke H, Kleinau G, Sontheimer J, Mueller S, Krause G, Paschke R. Preferences of transmembrane helices for cooperative amplification of Gαs and Gαq signaling of the thyrotropin receptor. Cell Mol Life Sci. 2008;65:4028–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Cetani F, Tonacchera M, Vassart G. Differential effects of NaCl concentration on the constitutive activity of the thyrotropin and the luteinizing hormone/chorionic gonadotropin receptors. FEBS Lett. 1996;378:27–31 [DOI] [PubMed] [Google Scholar]
- 406. Angelova K, Puett D. Differential responses of an invariant region in the ectodomain of three glycoprotein hormone receptors to mutagenesis and assay conditions. Endocrine. 2002;19:147–154 [DOI] [PubMed] [Google Scholar]
- 407. Quintana J, Wang H, Ascoli M. The regulation of the binding affinity of the luteinizing hormone/choriogonadotropin receptor by sodium ions is mediated by a highly conserved aspartate located in the second transmembrane domain of G protein-coupled receptors. Mol Endocrinol. 1993;7:767–775 [DOI] [PubMed] [Google Scholar]
- 408. Liu W, Chun E, Thompson AA, et al. Structural basis for allosteric regulation of GPCRs by sodium ions. Science. 2012;337:232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Ghinea N, Baratti-Elbaz C, De Jesus-Lucas A, Milgrom E. TSH receptor interaction with the extracellular matrix: role on constitutive activity and sensitivity to hormonal stimulation. Mol Endocrinol. 2002;16:912–923 [DOI] [PubMed] [Google Scholar]
- 410. Werthmann RC, Volpe S, Lohse MJ, Calebiro D. Persistent cAMP signaling by internalized TSH receptors occurs in thyroid but not in HEK293 cells. FASEB J. 2012;26:2043–2048 [DOI] [PubMed] [Google Scholar]
- 411. Nakao N, Ono H, Yamamura T, et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008;452:317–322 [DOI] [PubMed] [Google Scholar]
- 412. Gillespie EF, Papageorgiou KI, Fernando R, et al. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J Clin Endocrinol Metab. 2012;97:E740–E746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Stan MN, Bahn RS. Risk factors for development or deterioration of Graves' ophthalmopathy. Thyroid. 2010;20:777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Neumann S, Eliseeva E, McCoy JG, et al. A new small-molecule antagonist inhibits Graves' disease antibody activation of the TSH receptor. J Clin Endocrinol Metab. 2011;96:548–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Neumann S, Pope A, Geras-Raaka E, Raaka BM, Bahn RS, Gershengorn MC. A drug-like antagonist inhibits thyrotropin receptor-mediated stimulation of cAMP production in graves' orbital fibroblasts. Thyroid. 2012;22:839–843 [DOI] [PMC free article] [PubMed] [Google Scholar]