ABSTRACT
BACKGROUND
Warfarin is effective in preventing thromboembolic events, but concerns exist regarding its use in patients with substance abuse.
OBJECTIVE
Identify which patients with substance abuse who receive warfarin are at risk for poor outcomes.
DESIGN
Retrospective cohort study. Diagnostic codes, lab values, and other factors were examined to identify risk of adverse outcomes.
PATIENTS
Veterans AffaiRs Study to Improve Anticoagulation (VARIA) database of 103,897 patients receiving warfarin across 100 sites.
MAIN MEASURES
Outcomes included percent time in therapeutic range (TTR), a measure of anticoagulation control, and major hemorrhagic events by ICD-9 codes.
RESULTS
Nonusers had a higher mean TTR (62 %) than those abusing alcohol (53 %), drugs (50 %), or both (44 %, p < 0.001). Among alcohol abusers, an increasing ratio of the serum hepatic transaminases aspartate aminotransferase/alanine aminotransferase (AST:ALT) correlated with inferior anticoagulation control; normal AST:ALT ≤ 1.5 predicted relatively modest decline in TTR (54 %, p < 0.001), while elevated ratios (AST:ALT 1.50–2.0 and > 2.0) predicted progressively poorer anticoagulation control (49 % and 44 %, p < 0.001 compared to nonusers). Age-adjusted hazard ratio for major hemorrhage was 1.93 in drug and 1.37 in alcohol abuse (p < 0.001 compared to nonusers), and remained significant after also controlling for anticoagulation control and other bleeding risk factors (1.69 p < 0.001 and 1.22 p = 0.003). Among alcohol abusers, elevated AST:ALT >2.0 corresponded to more than three times the hemorrhages (HR 3.02, p < 0.001 compared to nonusers), while a normal ratio AST:ALT ≤ 1.5 predicted a rate similar to nonusers (HR 1.19, p < 0.05).
CONCLUSIONS
Anticoagulation control is particularly poor in patients with substance abuse. Major hemorrhages are more common in both alcohol and drug users. Among alcohol abusers, the ratio of AST/ALT holds promise for identifying those at highest risk for adverse events.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-013-2453-x) contains supplementary material, which is available to authorized users.
KEY WORDS: anticoagulation, alcohol abuse, drug abuse
INTRODUCTION
Substance abuse is extremely common, with a lifetime prevalence in the US population of 7.7 % for drug and 17.8 % for alcohol abuse.1,2 Clinicians confront a dilemma when faced with the need for anticoagulation in patients with substance abuse disorders. They may choose not to prescribe anticoagulation, realizing that the patient will be at risk for thromboembolic events. Or clinicians may prescribe, without knowing the likelihood of successful anticoagulation control or risk of hemorrhagic events. Concerns of clinicians include pharmacologic interaction, comorbid conditions, falls, compliance, and implications of addiction and chaotic lifestyle.3,4 Alcohol may be associated with increased antithrombotic effect of warfarin by protein-binding interactions and decreased metabolism through the cytochrome P450 system.4 Furthermore, the newer direct thrombin inhibitors and factor Xa inhibitors do not offer likely alternatives because trials of the agents excluded substance abusers.5–8 Managing patients with substance abuse who require anticoagulation is an area lacking guiding evidence.
The severity of abuse has been historically difficult to measure, and studies have used a variety of quantitative and qualitative data to address this issue. The DSM-IV uses criteria (such as failure to fulfill obligations, legal problems, tolerance, withdrawal) to define abuse and dependence,9 but clinicians may not record such distinctions, making subtleties of diagnoses less helpful. Attempts to use laboratory values to identify the severity of alcohol abuse are limited; a recent study found no utility of gamma-glutamyl transpeptidase (GGT), aspartate aminotransferase/alanine aminotransferase, (AST:ALT) ratio, mean corpuscular volume (MCV) of erythrocytes, or apolipoproteins to predict unhealthy drinking.10
Therefore, this study addresses the following three questions: First, what clinically useful indicators, such as specific substance abuse diagnosis and common laboratory values, exist among patients with substance abuse to predict poor control with anticoagulation pharmacotherapy? Second, do these clinical indicators also predict rates of major hemorrhagic events? Finally, assuming patients with substance abuse have more major hemorrhagic events, does controlling for anticoagulation control eliminate the difference? We expected to find higher rates of major hemorrhage in patients with substance abuse diagnoses than others, and that more severe substance abuse would be associated with even higher rates. Answering these questions will help clinicians predict which patients with substance abuse disorders may safely receive warfarin and in which patients it is best avoided.
METHODS
Database
The Veterans AffaiRs Study to Improve Anticoagulation (VARIA) database included 103,897 patients receiving oral anticoagulation with warfarin from 100 sites of care in the Veterans Health Administration (VA) between October 2006 and September 2008.11 Patients were considered to be receiving warfarin and thus included only when they were in possession of warfarin as dispensed by a VA pharmacy, or when they had international normalized ratio (INR) values recorded within the VA at least every 6 weeks, as this value is used by clinicians to adjust warfarin dosing. Values obtained during hospitalizations were excluded to minimize effect of acute illness, reversal for procedures, or alternative forms of anticoagulation. For this study, patients were excluded during their first 6 months on warfarin; thus, this is a database of experienced warfarin users. Patients with valvular heart disease as the primary indication for anticoagulation pharmacotherapy were excluded due to varying goal INR ranges. This study was approved by the institutional review board (IRB) of the Bedford VA Medical Center.
Definition of Substance Abuse
Patients were defined as having substance abuse disorders if at least one of the ICD-9 codes for substance abuse was recorded in VA inpatient or outpatient data during 10/1/06–9/30/08 (Online Appendix A).
Independent Variables: Risk Predictors
The independent clinical variables were the specific alcohol-related and drug-related diagnosis. We combined drug-related diagnoses, as preliminary analyses revealed little difference in anticoagulation control among specific drug-related ICD-9 codes (Online Appendix A). Though ICD-9 codes for cannabis abuse were part of the original definition of substance abuse,11 we eliminated these from further analyses, as cannabis use had little effect on either time in therapeutic range (TTR) or hemorrhagic events (Online Appendix B). Likewise, the specific ICD-9 code used to describe alcohol abuse offered little additional predictive value, so we combined all such codes into a single category. We also examined the ability of the AUDIT-C, an annual alcohol screening tool collected on many VA patients,12 to identify patients with alcohol abuse. AUDIT-C scores did not predict anticoagulation control or hemorrhage, either in patients with ICD-9 codes for alcohol abuse or those without. Therefore, we did not retain these data (Online Appendix C). For both drug and alcohol abuse, the specific ICD-9 code, frequency of coding, inpatient vs outpatient coding had little relationship to TTR and therefore were combined into categories of drug abuse only, alcohol abuse only, or both (data not shown).
Additionally, we examined the following putative markers as predictors of TTR and major hemorrhage among patients with substance abuse. Independent variables included the worst recorded and mean outpatient serum albumin, and bilirubin, ALT, AST, and ratio of AST:ALT (when both values obtained on the same day). These labs are readily available and often used by clinicians, despite the poor sensitivity and specificity of these labs to identify and evaluate the extent of substance abuse.10 Albumin is often low in patients with poor nutritional status as well as liver disease. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are transaminases associated with metabolic processes of liver parenchymal cells and are often elevated in alcohol abuse, and a ratio of AST:ALT > 2.0 is suggestive of alcohol abuse.13
Dependent Variable: Anticoagulation Control
The percentage of time within therapeutic range (TTR) was calculated for each patient with Rosendaal’s method, which uses linear interpolation to assign an INR value to each day between two successive observed INR values.14 We then calculated the percentage of time during which the interpolated INR values lie between 2 and 3 (between 0 % and 100 %).14
Dependent Variable: Major Hemorrhagic Events
The algorithm for identifying major hemorrhage was based in part on the definition of the International Society of Thrombosis and Haemostasis (ISTH).15 ICD-9 codes were used to identify candidate events that occurred while the patients were undergoing anticoagulation using both VA and Medicare data.16 Events were retained if they occurred while a patient was receiving warfarin, as described above, and fulfilled at least one of four conditions: 1) Associated with death within 30 days; 2) Associated with blood transfusion; 3) Associated with bleeding into a critical anatomic site; or 4) Cited as the main reason for a hospitalization.16 Approximately 16 % of candidate events were retained as “major hemorrhages” by this definition, although this percentage was much higher for events likely to be severe (e.g. diverticula of colon, 46 %). Patients were censored after the first bleeding event; therefore, all analyses were time to first hemorrhagic event. For analyses of major hemorrhage, patients with Medicare Advantage were excluded due to concern for missing data, as these patients do not generate itemized bills to Medicare for each service rendered.
Covariates: Risk Factors for Bleeding
Additionally in the bleeding analysis, we controlled for other factors that have been validated as part of the HAS-BLED score for predicting hemorrhagic events in patients undergoing anticoagulation for atrial fibrillation.17 HAS-BLED incorporates predictors of bleeding risk, including hypertension, chronic liver disease, chronic kidney disease, substance abuse, age, labile INR control, history of stroke, history of bleeding, and use of antiplatelet medications.17 For the study, hypertension, liver disease, and history of stroke were obtained using ICD-9 codes. Age was also included, and chronic kidney disease was defined as having an eGFR below 40 by MDRD equation.18 We included TTR as a covariate for this analysis to capture labile INR control. History of bleeding and use of antiplatelet agents were not available, but we controlled for the remainder of the HAS-BLED variables and sought to better define the predictors surrounding substance abuse.
Statistical Analyses
We examined the ability of laboratory values to predict TTR, in addition to the substance abuse diagnosis itself. The laboratory values were examined first individually, and then in combination. Similarly, the ability of these laboratory values to predict major hemorrhage was also examined. To determine the proportion of hemorrhagic risk attributable to poor anticoagulation control as compared to substance abuse itself, we controlled for age in all analyses and additionally TTR and HAS-BLED risk factors in some analysis. We modeled age and TTR as categories rather than continuous variables. For bivariate tests we used ANOVA or chi-square, as appropriate; and for multivariate analyses, linear regression (for the TTR) or Cox Proportional Hazards Model (for major hemorrhage). All analyses were performed using SAS, version 9.1.
RESULTS
Characteristics of Patients with Substance Abuse
Of the 103,897 patients receiving warfarin, 6,781 (6.5 %) had a substance abuse disorder as defined by a diagnosis of any drug-related or alcohol-related ICD-9 codes (Appendix A). The baseline characteristics of the patients with and without alcohol or drug abuse are summarized in Table 1. ICD-9 codes were used to identify comorbid conditions and indications for anticoagulation. Patients with a substance abuse disorder were younger than those without (62.0 vs. 71.2 years, p < 0.001). Non-Hispanic Blacks were more likely to use drugs than non-Hispanic Whites. Patients using drugs, but not those abusing only alcohol, were less likely to be receiving warfarin for atrial fibrillation. Liver disease was much more common in patients with alcohol abuse as compared to those without (9.2 % vs. 0.7 %, p < 0.001). Remarkably, though the substance abusers were much younger than the general population, they had slightly higher rates of chronic obstructive pulmonary disease (COPD), epilepsy, and congestive heart failure (CHF). Not surprisingly, patients with substance abuse had much higher rates of mental health disorders.
Table 1.
Baseline Characteristics of Patients with and without Alcohol and Drug Abuse, Among Patients Receiving More than 6 Months of Oral Anticoagulation Therapy from the VA. Number and (%) Given. Total n = 103,897
| Variable | No drugs or alcohol (reference) | Alcohol only | Drugs only | Both drugs and alcohol |
|---|---|---|---|---|
| Total, N and column % | 97,116 (93.5) | 4,988 (4.8) | 842 (0.8) | 951 (0.9) |
| Sex | ||||
| Female | 1,882 (1.9) | 51 (1.0) | 29 (3.4) | 14 (1.5) |
| Male | 95,234 (98.1) | 4,937 (99) | 813 (96.6) | 937 (98.5) |
| Age (mean) | 71.3 | 63.8 | 59.2 | 54.9 |
| Race/ethnicity | ||||
| Non-Hispanic White | 83,260 (85.7) | 4,163 (83.5) | 509 (60.5) | 549 (57.7) |
| Non-Hispanic Black | 8,465 (8.7) | 497 (10.0) | 268 (31.8) | 342 (36.0) |
| Hispanic | 3,013 (3.1) | 160 (3.2) | 31 (3.7) | 25 (2.6) |
| Asian | 378 (0.4) | 7 (0.1) | 4 (0.5) | 3 (0.3) |
| Native American | 411 (0.4) | 24 (0.5) | 9 (1.1) | 5 (0.5) |
| Other/unknown | 1,589 (1.6) | 137 (2.7) | 21 (2.5) | 27 (2.8) |
| Primary indication for warfarin* | ||||
| Atrial fibrillation | 63,109 (65.0) | 2,930 (58.7) | 344 (40.9) | 373 (39.2) |
| Venous thromboembolism | 25,701 (26.5) | 1,710 (34.3) | 427 (50.7) | 512 (53.8) |
| All others combined | 8,306 (8.6) | 348 (7.0) | 71 (8.4) | 66 (6.9) |
| Physical comorbid conditions | ||||
| Cancer (newly diagnosed) | 6,480 (6.7) | 420 (8.4) | 75 (8.9) | 58 (6.1) |
| Chronic kidney disease (GFR < 40) | 13,804 (14.2) | 526 (10.5) | 154 (18.3) | 97 (10.2) |
| Chronic liver disease | 689 (0.7) | 461 (9.2) | 17 (2.0) | 61 (6.4) |
| Chronic lung disease | 27,721 (28.5) | 1,894 (38.0) | 353 (41.9) | 343 (36.1) |
| Coronary artery disease | 49,695 (51.2) | 2,083 (41.8) | 397 (47.1) | 368 (38.7) |
| Diabetes | 39,297 (40.5) | 1,592 (31.9) | 323 (38.4) | 295 (31.0)† |
| Epilepsy | 2,577 (2.7) | 213 (4.3) | 51 (6.1) | 62 (6.5) |
| Heart failure | 31,279 (32.2) | 1,804 (36.2) | 333 (39.5) | 311 (32.7) |
| Mental comorbid conditions | ||||
| Bipolar disorder | 1,771 (1.8) | 304 (6.1) | 92 (10.9) | 192 (20.2) |
| Dementia | 5,181 (5.3) | 235 (4.7) | 53 (6.3) | 22 (2.3) |
| Major depression | 19,302 (19.9) | 1,989 (39.9) | 449 (53.3) | 638 (67.1) |
*Patients with valvular disease were excluded due to varying goal ranges of INR.
Anticoagulation Control Among Patients with Substance Abuse
Patients with any substance abuse had a mean TTR that was lower compared to patients without substance abuse, as shown in Table 2. Specifically, as compared to patients without substance abuse (mean TTR of 62 %), those with alcohol abuse alone had an average TTR of 53 %, drug abuse alone 50 %, and combination of both 44 % (p < 0.001).
Table 2.
Anticoagulation Control (Percent Time in Therapeutic Range, or TTR) in Subcategories of Substance Abuse Among Patients Receiving Anticoagulation Therapy from the VA
| Variable | Number of patients | Mean % TTR (95 % CI) |
|---|---|---|
| No alcohol or drugs (reference) | 97,116 | 62.3 (62.1–62.4) |
| Alcohol only | 4,988 | 53.0 (52.4–53.6)* |
| Drugs only | 842 | 50.2 (48.6–51.7)* |
| Both alcohol and drugs | 951 | 44.0 (42.5–45.5)* |
| Alcohol Status and AST:ALT ratio | ||
| No Alcohol (reference) | 97,958 | 62.2 (62.0–62.3) |
| Alcohol and AST:ALT ≤ 1.5 | 3,708 | 54.2 (53.5–54.9)* |
| Alcohol and AST:ALT > 1.5–2.0 | 1,298 | 49.4 (48.2–50.6)* |
| Alcohol and AST:ALT > 2.0 | 933 | 44.1 (42.6–45.5)* |
*p < 0.001, compared to the reference category
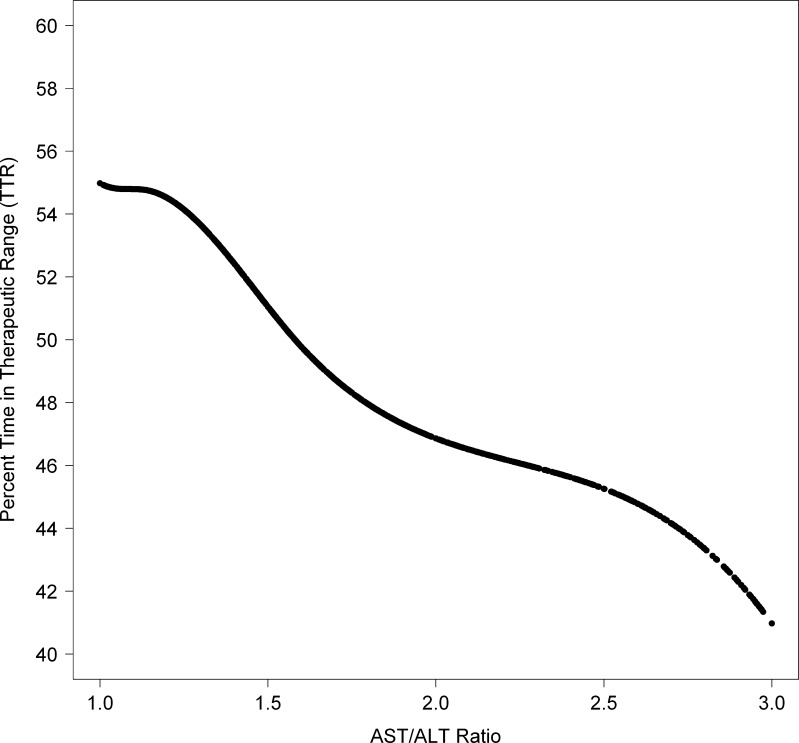
We examined the relationship of potential clinical indicators of poor outcomes in patients with substance abuse, with a goal of finding a marker for more severe decrements in TTR and hemorrhagic risk. Several factors had some ability to predict TTR, including levels of bilirubin, AST, ALT, and albumin (data not shown). However, the most striking finding was among alcohol abusers with increasing ratios of AST:ALT. Those with AST:ALT ratios greater than 2.0 had exceptionally poor TTR (44 %), while alcohol abusers with a normal ratio of ≤ 1.5 had only a modestly decreased TTR (54 %) compared to nonusers. Increasing ratios from 1.0 to 3.0 displayed a near linear decrease in TTR; this continuous predictor and continuous outcome is depicted by a cubic smoothing spline in Fig. 1.
Figure 1.
Among patients receiving oral anticoagulation pharmacotherapy from the VA with alcohol abuse, patient-level percent time in therapeutic range (TTR) vs. AST:ALT ratio, plotted using a cubic smoothing spline.34
Hemorrhagic Events among Patients with Substance Abuse
Patients with any sort of substance abuse had increased incidence of major hemorrhage (Table 3). We observed 142,348 patient years among 84,492 patients, with a total of 4,525 major hemorrhagic events. Age-adjusted hazard ratios (HR) for major hemorrhage were 1.93 (p < 0.001) in patients with drug abuse and 1.37 (p < 0.001) with alcohol. Among patients abusing alcohol, an increasing AST:ALT ratio was associated with more hemorrhagic events. We modeled the AST:ALT ratio as a time-varying covariate, allowing AST:ALT to vary as often as every 30 days. An AST:ALT ratio of > 2.0 had an age adjusted hazard ratio of 3.02 compared to nonusers (p < 0.001), while those with a normal ratio had a HR of 1.19 compared to nonusers (p < 0.05). Controlling for anticoagulation control and other bleeding risk factors, in addition to age, attenuated these effects somewhat, but the increase in hemorrhagic events among patients with an abnormal AST:ALT ratio remained present and statistically significant (Table 3).
Table 3.
Time to Event Analysis of Any Major Hemorrhage in Patients with and without Alcohol and/or Drug Abuse Receiving Oral Anticoagulation in the VA Cohort. (n = 4,525 Major Bleeding Events in 86,492 Patients)
| Variable | Number of patients | Number of events | Total patient-years of follow-up | Event per 100 patient-years | Hazard ratio adjusted for age only (95 % CI) | Hazard ratio adjusted for bleeding risk factors* (95 % CI) |
|---|---|---|---|---|---|---|
| No alcohol or drugs | 80,337 | 4,206 | 132,560 | 3.2 | – | – |
| Alcohol only | 4,482 | 232 | 7,224 | 3.2 | 1.37 (1.20–1.57)** | 1.23 (1.07–1.41)‡ |
| Drugs only | 777 | 48 | 1,219 | 3.9 | 1.93 (1.45–2.58)** | 1.59 (1.19–2.12)‡ |
| Both alcohol and drugs | 896 | 39 | 1,442 | 2.7 | 1.61 (1.17–2.22)‡ | 1.27 (0.92–1.75) |
| No alcohol | 81,114 | 4,254 | 133,780 | 3.2 | – | – |
| Alcohol and AST:ALT ≤1.5† | 3,382 | 125 | 5,549 | 2.3 | 1.19 (1.03–1.38)‡ | 1.08 (0.93–1.26) |
| Alcohol and AST:ALT >1.5–2.0† | 1,153 | 76 | 1,835 | 4.1 | 1.88 (1.42–2.48)** | 1.53 (1.15–2.03)‡ |
| Alcohol and AST:ALT >2.0† | 843 | 70 | 1,282 | 5.5 | 3.02 (2.13–4.28)** | 2.31 (1.63–3.29)** |
*Controls for hypertension, renal disease, liver disease, age, sex, race, TTR, history of stroke by ICD-9 code.
†The AST:ALT ratio was analyzed as a time-varying covariate and was allowed to change as often as every 30 days, depending on when it was re-measured.
‡P < 0.05
**P < 0.001
DISCUSSION
Using data from the VA, we looked for predictors of anticoagulation control and major hemorrhagic events among substance-abusing patients receiving warfarin. Patients with substance abuse disorders had poorer anticoagulation control and elevated hazard of major hemorrhage. Additionally, in patients abusing alcohol, an elevated AST:ALT ratio identified patients with especially poor anticoagulation control and outcomes. This finding may have considerable clinical utility, by offering a simple, readily available indicator of a patient’s likelihood to succeed on warfarin. While a ratio of AST:ALT of 2.0 is often used to identify alcohol pattern liver damage,13 our study shows poorer outcomes increase substantially beginning at a ratio of 1.5. Another important finding is that while controlling for TTR somewhat attenuated the impact of substance abuse on rates of hemorrhage, it did not fully explain the association between abuse and hemorrhage. This suggests that the excess bleeding risk is not entirely attributable to poor anticoagulation control; hence, improving anticoagulation control should not be the only clinical intervention in these patients.
This study is consistent with earlier findings that substance abuse is an important risk factor for major hemorrhage.17 Risk of hemorrhage is a major concern for physicians contemplating prescribing warfarin. Drug and alcohol abuse have been incorporated into the HAS-BLED score,17 a validated scoring system used to predict hemorrhagic events in patients undergoing anticoagulation for atrial fibrillation. Many studies have shown that warfarin is underutilized and its risks are over-estimated by physicians.19–22 In particular, warfarin is underutilized in patients with atrial fibrillation with co-morbid substance abuse,23 but evidence to support this decision has been sparse.24, 25 A recent systematic review found a statistically significant increase in hemorrhages in alcohol abusers in less than half of the studies and reported overall strength of the evidence as low.25
Poorer anticoagulation control in substance-abusing patients is concerning, since improved control is associated with fewer adverse events.26–30 The reasons for poorer anticoagulation control among substance abusers are not fully understood, but likely involve pharmacology as well as compliance. While the specific interactions of warfarin and street drugs is not known, alcohol enhances the antithrombotic effect of warfarin through protein-binding interactions and decreased metabolism of warfarin through the cytochrome P450 system.4 Notably, the poorer anticoagulation control in patients with substance abuse does not completely explain their increased rate of hemorrhagic events. Even after controlling for age and TTR, substance abusers had more hemorrhages, which may reflect a lifestyle of addiction, risky living environments, more frequent falls, underlying alcohol-related gastritis and varices leading to a propensity for bleed, or other factors. Therefore, clinicians might emphasize substance abuse intervention in addition to medically managing other modifiable bleeding risk factors.
In our study, patients with alcohol abuse and normal AST:ALT ratio had a similar risk of hemorrhagic events as nonusers. Physicians then may find that the benefits of oral anticoagulation likely outweigh the risks in such patients. Conversely, patients with an AST:ALT >1.5, and especially >2.0, had considerably poorer outcomes, both in terms of anticoagulation control and hemorrhagic events. While our study is not equipped to inform the entire risk-benefit calculation, it may assist the clinician to identify patients at risk for poor anticoagulation control and bleeding complications.
Some limitations should be noted. First, the study population of veterans was heavily male and may not reflect a general population of substance abusers. Second, the study cohort included only patients who had completed at least 6 months of warfarin, and may not apply to patients undergoing shorter courses or initiating therapy. This may select for patients who already demonstrated stability and those receiving warfarin for more serious indications. Third, our study does not consider patients with valvular heart disease due to variations in target therapeutic range. Fourth, we assessed major hemorrhagic events using automated data rather than chart review, although our algorithm is the result of careful development.16 Fifth, while we show that controlling for anticoagulation control only partially impacts bleeding risk, we do not distinguish if the INR is supratherapeutic at the time of the bleeding event. Sixth, while we control for many known risk factors for hemorrhages while on warfarin, we are unable to account for previous hemorrhagic events or antiplatelet pharmacotherapy. Finally, we considered neither strokes nor recurrent venous thromboembolic events, as these outcomes are difficult to assess from automated data alone.31–33 Future studies could collect more detailed information, particularly regarding the extent of substance abuse. Furthermore, comparing outcomes such as thromboembolic events in this population of both those who receive and do not receive warfarin therapy, would help clinicians balance the risks and benefits of therapy. While the present study provides helpful information regarding the risks of anticoagulation in patients with substance abuse, clearly the risks of foregoing such therapy must also be considered.
In conclusion, we examined a large database of VA patients receiving anticoagulation with warfarin for varied indications. Patients with substance abuse had notably poor anticoagulation control and elevated risk for major hemorrhage, even after controlling for covariates. Among patients with alcohol abuse, an elevated AST:ALT ratio holds promise for separating patients with elevated risk of hemorrhage from those whose risk may not differ meaningfully from the overall population.
Electronic supplementary material
(DOCX 26 kb)
Acknowledgements
Contributors
No further contributors.
Funding
This study was supported by a grant from VA Health Services Research and Development (IIR-10-374). Dr. Rose is supported by a career development award from VA Health Services Research and Development (CDA-08-017). The sponsor had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, and approval of the manuscript.
Disclaimer
The opinions expressed in this manuscript do not necessarily represent the official views of the Department of Veterans Affairs.
Guarantor
Dr. Rose is the guarantor of the entire manuscript.
Presentations
No prior presentations.
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
REFERENCES
- 1.Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64:566–76. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- 2.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 3.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition) Chest. 2008;133:160S–98S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 4.Havrda DE, Mai T, Chonlahan J. Enhanced antithrombotic effect of warfarin associated with low-dose alcohol consumption. Pharmacotherapy. 2005;25:303–7. doi: 10.1592/phco.25.2.303.56955. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 6.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 365:981–92. [DOI] [PubMed]
- 7.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 8.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 9.Dawson DA, Saha TD, Grant BF. A multidimensional assessment of the validity and utility of alcohol use disorder severity as determined by item response theory models. Drug Alcohol Depend. 2010;107:31–8. doi: 10.1016/j.drugalcdep.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liangpunsakul S, Qi R, Crabb DW, Witzmann F. Relationship between alcohol drinking and aspartate aminotransferase:alanine aminotransferase (AST:ALT) ratio, mean corpuscular volume (MCV), gamma-glutamyl transpeptidase (GGT), and apolipoprotein A1 and B in the U.S. population. J Stud Alcohol Drugs. 2010;71:249–52. doi: 10.15288/jsad.2010.71.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rose AJ, Hylek EM, Ozonoff A, Ash AS, Reisman JI, Berlowitz DR. Patient characteristics associated with oral anticoagulation control: results of the Veterans AffaiRs Study to Improve Anticoagulation (VARIA) J Thromb Haemost. 2010;8:2182–91. doi: 10.1111/j.1538-7836.2010.03996.x. [DOI] [PubMed] [Google Scholar]
- 12.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 13.Hannuksela ML, Liisanantti MK, Nissinen AE, Savolainen MJ. Biochemical markers of alcoholism. Clin Chem Lab Med. 2007;45:953–61. doi: 10.1515/CCLM.2007.190. [DOI] [PubMed] [Google Scholar]
- 14.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–9. [PubMed] [Google Scholar]
- 15.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 16.Jasuja GK, Reisman JI, Miller DR, et al. Identifying Major Hemorrhage with Automated Data: Results of the Veterans Affairs Study to Improve Anticoagulation (VARIA). Thrombosis research. 2012. [DOI] [PubMed]
- 17.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173–80. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160:41–6. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 20.McCormick D, Gurwitz JH, Goldberg RJ, et al. Prevalence and quality of warfarin use for patients with atrial fibrillation in the long-term care setting. Arch Intern Med. 2001;161:2458–63. doi: 10.1001/archinte.161.20.2458. [DOI] [PubMed] [Google Scholar]
- 21.Monette J, Gurwitz JH, Rochon PA, Avorn J. Physician attitudes concerning warfarin for stroke prevention in atrial fibrillation: results of a survey of long-term care practitioners. J Am Geriatr Soc. 1997;45:1060–5. doi: 10.1111/j.1532-5415.1997.tb05967.x. [DOI] [PubMed] [Google Scholar]
- 22.Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–45 e4. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Baczek VL, Chen WT, Kluger J, Coleman CI. Predictors of warfarin use in atrial fibrillation in the United States: a systematic review and meta-analysis. BMC Fam Pract. 2012;13:5. doi: 10.1186/1471-2296-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen WT, White CM, Phung OJ, et al. Are the risk factors listed in warfarin prescribing information associated with anticoagulation-related bleeding? A systematic literature review. Int J Clin Pract. 2011;65:749–63. doi: 10.1111/j.1742-1241.2011.02694.x. [DOI] [PubMed] [Google Scholar]
- 25.Schauer DP, Moomaw CJ, Wess M, Webb T, Eckman MH. Psychosocial risk factors for adverse outcomes in patients with nonvalvular atrial fibrillation receiving warfarin. J Gen Intern Med. 2005;20:1114–9. doi: 10.1111/j.1525-1497.2005.0242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connolly SJ, Pogue J, Eikelboom J, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118:2029–37. doi: 10.1161/CIRCULATIONAHA.107.750000. [DOI] [PubMed] [Google Scholar]
- 27.van Leeuwen Y, Rosendaal FR, Cannegieter SC. Prediction of hemorrhagic and thrombotic events in patients with mechanical heart valve prostheses treated with oral anticoagulants. J Thromb Haemost. 2008;6:451–6. doi: 10.1111/j.1538-7836.2007.02874.x. [DOI] [PubMed] [Google Scholar]
- 28.Veeger NJ, Piersma-Wichers M, Tijssen JG, Hillege HL, van der Meer J. Individual time within target range in patients treated with vitamin K antagonists: main determinant of quality of anticoagulation and predictor of clinical outcome. A retrospective study of 2300 consecutive patients with venous thromboembolism. Br J Haematol. 2005;128:513–9. doi: 10.1111/j.1365-2141.2004.05348.x. [DOI] [PubMed] [Google Scholar]
- 29.Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376:975–83. doi: 10.1016/S0140-6736(10)61194-4. [DOI] [PubMed] [Google Scholar]
- 30.White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med. 2007;167:239–45. doi: 10.1001/archinte.167.3.239. [DOI] [PubMed] [Google Scholar]
- 31.Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118:253–62. doi: 10.1016/j.thromres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):154–62. doi: 10.1002/pds.2341. [DOI] [PubMed] [Google Scholar]
- 33.White RH, Garcia M, Sadeghi B, et al. Evaluation of the predictive value of ICD-9-CM coded administrative data for venous thromboembolism in the United States. Thromb Res. 2010;126:61–7. doi: 10.1016/j.thromres.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Hastie TJ, Tibshirani RJ. Generalized additive models. New York: Chapman and Hall; 1990. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 26 kb)