Abstract
Coronary artery disease is the leading cause of death in Americans. After myocardial infarction, significant ventricular damage persists despite timely reperfusion and pharmacological management. Treatment is limited, as current modalities do not cure this damage. In the past decade, stem cell therapy has emerged as a promising therapeutic solution to restore myocardial function. Clinical trials have demonstrated safety and beneficial effects in patients suffering from acute myocardial infarction, heart failure, and dilated cardiomyopathy. These benefits include improved ventricular function, increased ejection fraction, and decreased infarct size. Mechanisms of therapy are still not clearly understood. However, it is believed that paracrine factors, including stromal cell-derived factor-1, contribute significantly to stem cell benefits. The purpose of this article is to provide medical professionals with an overview on stem cell therapy for the heart and to discuss potential future directions.
KEY WORDS: myocardial infarction, heart failure, ventricular function, stem cell, paracrine
Coronary artery disease (CAD) remains the top killer in the Western world, despite advancing medical technology. Annually, 935,000 Americans suffer from acute myocardial infarctions (AMI).1 Arterial obstruction causes inadequate perfusion and cardiomyocyte death. If flow is not quickly reestablished, loss of cardiomyocytes can be massive.2 Significant declines in CAD mortality rates are attributable to decreased AMI incidence coupled with improved survival from aggressive revascularization.1
AMI patients who previously might not have survived without percutaneous coronary intervention (PCI) are now living longer,1 but with considerable left ventricular dysfunction.3,4 Heart failure (HF) subsequently ensues, affecting 5.7 million Americans.1,2,4,5 Despite advanced therapies, this is expected to increase to 9.6 million by 2030.1 Left ventricular dysfunction ultimately affects contractility, worsening HF and increasing mortality.3,6 HF confers poor prognosis; half of Americans with HF will die within five years after diagnosis.1
Treatment of HF, due to ischemic or non-ischemic causes, is limited; heart transplantation is the only strategy addressing cardiomyocyte loss. Prospects remain dismal, because current treatment modalities may compensate for, but not cure, the condition.7 New approaches should alter the remodeling process, regenerate cardiomyocytes and repair infarcted myocardium.6
Historically, the heart was described as a terminally differentiated organ, incapable of regeneration. The discovery that myocardial injury induces cardiomyocyte proliferation challenged traditional belief.8 Identification of cardiac stem cells (CSC) in the adult heart activated by AMI supported the argument.8 AMI demands myocardial repair, causing resident CSC to reenter the cell cycle and circulating stem cells to move to the injury site. Early studies suggested that non-cardiac stem cells transdifferentiate into cardiomyocytes and repair damaged myocardium.9,10 In 2001, bone marrow mononuclear cells (BMC) transplanted into mice repaired myocardial damage and improved cardiac function.9 Later in 2001, autologous BMC were safely injected into a patient after AMI, reducing infarct size and increasing ejection fraction (EF).11
Preclinical studies showed that stem cell therapy benefits perfusion and ventricular function. Clinical trials demonstrated feasibility and safety with positive results.12–14 Benefits cannot be explained solely by stem cells, and are likely associated with paracrine factors released into injured tissue. This review explores emerging clinical applications of stem cell therapy as a promising approach for restoring myocardial function in heart disease.
CELL TYPES
The optimal cell types for treating heart disease continue to be debated. Potentially, no one type is ideal and can be exclusively used. It is possible that different forms of heart disease may require different cell types.
Embryonic stem cells (ESC) were considered favorable for their unlimited self-renewal and pluripotency.15 Being allogeneic, there are concerns for immunological incompatibility and risk of teratoma formation.7,16 Secondary to ethical, political and scientific challenges, no heart disease clinical trials used ESC.16 Animal studies using ESC demonstrated cardiomyocyte differentiation and improved ventricular function.17 These findings spurred development of ESC-like cells by reprogramming adult cells to become undifferentiated pluripotent cells for autologous transplantation, known as induced pluripotent stem cells (iPSC). Preclinical studies showed temporary benefits on remodeling and function, but teratoma concerns remain.15
A cell type infrequently used in heart disease is human umbilical cord-derived stem cells. These cells are abundant, easily obtained, and with lowered rejection risk.18,19 A challenge is whether these can be used as an allogeneic source or whether systems for genotyping donor cells need development to achieve wide-spread use.
Resident CSC were initially considered a prime cell choice because they differentiate into cardiomyocytes and demonstrate clonogenicity, self-renewal and cell cycle re-entry.20 They increase in numbers and migration after ischemia,2,21 and may be activated by transplanted cells.22 However, CSC are limited by their small population and reduced effects with aging.2,8 Moreover, their differentiation potential is low and inadequate to replace lost cardiomyocytes.23 Clinical translation is challenged by small numbers obtained from biopsy, necessitating prolonged expansion in culture before delivery.
Adipose-derived stem cells (ASC) are an attractive option. Obtained from subcutaneous adipose tissue, ASC are a combination of endothelial progenitor cells (EPC), hematopoietic stem cells (HSC), and mesenchymal stem cells (MSC).19 They differentiate into several cell lines, including cardiomyocytes.2
BMC are the most promising and dominate clinical studies, as they are easily obtained and cultured with differentiation capacity.19 BMC contain a mixture of HSC, EPC, MSC, and multipotent adult progenitor cells (MAPC).2 EPC encompass a cluster of cell types24 that express CD34 and CD133 markers,16 as well as growth factors that contribute to angiogenesis.2 EPC are found in small amounts and are reduced in CAD patients.16 MSC also differentiate into several cell types.11 MSC and MAPC are considered optimal for allogeneic therapy due to nonimmunogenic and anti-inflammatory characteristics25,26 Recent efforts use allogeneic BMC, with cells retrieved from healthy donors, cultured, and kept in stock. This allows for “off-the-shelf” treatment during AMI intervention.27
MODES OF DELIVERY
The best methods of stem cell delivery have not yet been determined. Peripheral intravenous infusion is an indirect method widely used in animal models with favorable outcomes.19 It is simple and noninvasive, relying on post-AMI physiological signals to target cells towards damaged tissues.28 Unfortunately, it is inefficient and impractical, as large cell numbers and infusions are necessary for sufficient amounts to reach infarct-related arteries,29 due to confinement in the microvasculature and losses to other organs.28,29
Direct intramyocardial injection during coronary artery bypass graft (CABG) surgery easily allows stem cells to be placed into the targeted zone.29 Suitable candidates include chronic ischemic HF patients, because chronically infarcted tissue does not release the necessary post-AMI physiological signals to attract and mobilize cells to the infarct zone.19,28 However, only a small amount of cells survive more than three days,30 because the microenvironment is problematic for cell survival secondary to inflammation and insufficient blood supply.2,28 This is further complicated by mechanical leakage2 and arrhythmia potential.28
Transendocardial injection is similar to the intramyocardial route, but uses a flexible catheter-based percutaneous technique across the aortic valve.29 Injecting cells in and around the infarct zone22 allows greater cell engraftment,28 while using fewer cells.22 Potential risks include myocardial perforation, AMI, and induction of ventricular arrhythmias.23,28,29
Intracoronary infusion into the infarct-related artery is the most popular in AMI trials.19 Cells are injected via a catheter into the affected artery. Retrograde stem cell loss is prevented by a short balloon inflation.19,29 Limitations include the complex cell preparation processes and reduced efficacy in CAD patients, secondary to atherosclerotic arteries,27 reducing delivery to the target myocardium.22 Temporary occlusion and decreased blood flow from the procedure increase the risk of microembolism, infarct, or restenosis.22,28
Building on the intracoronary approach is adventitial delivery. Cells are introduced using a balloon to temporarily occlude flow and a special catheter injects cells via a microneedle through the medial layer and into the adventitia of the infarct-related artery. By delivering directly into the adventitia, atherosclerosis issues are avoided.27
Ideal timing depends on cell types and microenvironment. Specific types may be more efficient in acute versus chronic injury31 and environments may necessitate specific timing of administration for best results. It is suggested that optimal timing of therapy post-AMI is after the inflammatory response diminishes, but before scar formation.31 Early administration during PCI has been proposed32 to avoid post-AMI damage and prevent additional procedures. Physiological mechanisms at this time help cells migrate,31,33 although the inflammatory microenvironment is unfavorable for cell survival.12,31 Several stem cell administrations may be necessary for adequate therapy.
MECHANISMS
The mechanism of therapy is not clearly understood. Some claim that transplanted cells differentiate into new cardiomyocytes, replacing necrotic cells.9 Others suggest that transplanted cells fuse with existing cardiomyocytes.34 Low engraftment and survival of transplanted cells,16 in addition to limited differentiation, imply that observed improvements in outcomes cannot solely be due to regeneration.35 Further, some effects are noted within one day, argued as a timeframe too brief for genuine regeneration.16,32
Improvements are mostly attributed to the effects of paracrine factors released from cells.26,36,37 Promptly after transplantation into injured myocardium, stem cells express a variety of paracrine secretions, including cytokines, chemokines, and growth factors.7 These appear to contribute to cardiac repair;36 possibly through neo-vascularization, angiogenesis,36 less inflammation,32,36 smaller infarct size,32,36,38 and decreased fibrosis.36 Paracrine secretions contribute to enhanced cardiomyocyte survival by decreasing apoptosis,32,36,38 while increasing cell proliferation23 and mobilizing other stem cells to the infarct zone.36 Moreover, paracrine activity encourages activation and migration of resident CSC36,38 and may stimulate differentiation.38
Migration of cells to damaged areas after AMI is known as homing.2,8,9,11,21 Successful homing permits better engraftment and survival.11,21 This is regulated by the release of stem cell homing factors, which assist in directing cells.33 One receiving considerable attention is stromal cell-derived factor-1 (SDF-1). Rapid ischemia up-regulates SDF-1 expression,29,39 which binds to its receptor, CXC chemokine receptor type 4 (CXCR4), expressed on the surface of BMC.29 Together, SDF-1 and CXCR4 are crucial in cell recruitment and homing.33,40–42 SDF-1 regulates cell trafficking,39 increases angiogenesis and cell survival,40 and improves ventricular function.41 SDF-1 is not naturally released in chronic ischemic myopthy,33,37 although homing can be established if paracrine factors are released at a time remote from AMI.33
SDF-1 is expressed immediately post-AMI and declines after 4–7 days; a time when cells are considered most responsive to SDF-1.33,41 CXCR4 is expressed 1–2 days after AMI, peaking on day 4.42 Both cell responsiveness and CXCR4 expression occur when SDF-1 is decreasing. Such dyssynchrony explains why the heart has limited ability to repair itself. This has led to efforts to alter the timing of SDF-1 or CXCR4 expressions.41 Moreover, it has been demonstrated that injection of SDF-1 alone provides similar benefit.33
ENGRAFTMENT AND SURVIVAL
Poor cell survival and incorporation into native tissue exists despite cell type or delivery. Cell death results from the ischemic environment into which cells are engrafted.28 Inflammation and diminished vascular supply causes many cells to die within seven days after transplantation.28,31 Mechanical leakage is unique to the heart because contractions squeeze out cells. Cell escape occurs when injected cells are no longer at the intended site of injury and instead are in extracardiac organs.28
There is increased interest in cell preconditioning and modification to alleviate these issues. Preconditioning involves shock, hypoxia, ischemia, and medications for the purpose of improving cell resistance to adverse stimuli. Cells can be modified to release factors to increase engraftment and survival.28
CLINICAL OUTCOMES
Acute Myocardial Infarction
There have been numerous AMI trials (Table 1).27,43–65 Many demonstrated improvement in EF,27,44–52,55,56,59,61 volumes,27,43–45,47,48,50–52,59 wall motion,43–45,47–49 and infarct size43–45,47,54,61 when compared with conventional treatment. REPAIR-AMI showed decreased mortality, recurrent AMI, and HF re-hospitalizations,51 with maintained improvement at two years.52 REPAIR-AMI and TOPCARE-AMI confirmed that patients with decreased baseline EF showed more improvement.44,45,51,52 Some trials did not show significant results,57,60,63–65 while others demonstrated some benefits without EF changes.43,53,54,62
Table 1.
Acute Myocardial Infarction (AMI) Major Clinical Trials
| Study | Patients (treated/control) | Cell Type | Route | Time Post-AMI | Imaging Technique | Follow-Up (months) | Outcomes in Treated Group |
|---|---|---|---|---|---|---|---|
| Strauer43 | 10/10 | BMC | IC | 5–9 days | LV angiogram, DSE | 3 | Improved infarct size, volumes & wall motion, safety outcomes, No difference in EF |
| TOPCARE-AMI44,45 | 29 vs. 30 | BMC vs. EPC | IC | < 5 days | LV angiogram, Cardiac MRI | 4 & 12 | Improved EF by 8 %, infarct size, volumes & wall motion, + safety outcomes |
| BOOST46 | 30/30 | BMC | IC | 4–8 days | Cardiac MRI | 6 & 18 | Transient improved EF by 6.7 %, + safety outcomes |
| Chen47 | 34/35 | MSC | IC | 18 days | LV angiogram, Echo | 6 | Improved EF by 18 %, infarct size, wall motion & LVEDV, + safety outcomes |
| Fernandez-Aviles48 | 20/13 | BMC | IC | 12–20 days | Cardiac MRI, LV angiogram | 6 | Improved EF by 5.8 %, volumes & wall motion, + safety outcomes |
| Bartunek49 | 19/16 | BMC (CD133+) | IC | 10–12 days | LV angiogram, SPECT | 4 | Improved EF by 7 % & wall motion |
| Ruan50 | 9/11 | BMC | IC | < 7 days | Echo | 6 | Improved EF by 6 %, volumes & + safety outcomes |
| REPAIR-AMI51,52 | 102/102 | BMC | IC | 3–7 days | LV angiogram, Cardiac MRI | 4, 12 & 24 | Improved EF by 5.5 %, volumes & mortality, + safety outcomes |
| ASTAMI53 | 47/50 | BMC | IC | 4–7 days | SPECT, Echo, Cardiac MRI | 12 | + safety outcomes, no difference in EF |
| Janssens54 | 33/34 | BMC | IC | < 1 day | Cardiac MRI | 4 | Improved infarct size, No difference in EF, + safety outcomes |
| Fincell55 | 40/40 | BMC | IC | 2–6 days | LV angiogram, IVUS, Echo, | 6 | Improved EF by 7 %, + safety outcomes |
| Krause56 | 20/0 | BMC | Trans-endocardial | 10.5 days | Echo, EMM, LV angiogram | 6 & 12 | Improved electromechanical parameters, Improved EF by 6.8 %, + safety outcomes |
| REGENT57 | 80 vs. 80/40 | BMC vs. CD34+CXCR4+ | IC | 7 days | LV angiogram, Cardiac MRI | 6 | No difference in EF or volumes |
| MYSTAR58 | 60 | BMC | IM vs. IC | 3–6 weeks vs. 3–4 months | LV angiogram | 9–12 | Improved EF but no significant difference between groups |
| Hare59 | 53 | MSC (allogeneic) | IV | 1–10 days | Echo, Cardiac MRI | 12 | Improved EF by 5.2 % & volumes, Decreased arrhythmias, + safety outcomes |
| LateTIME60 | 58/29 | BMC | IC | 2–3 weeks | Cardiac MRI | 6 | No difference in EF or volumes |
| Penn27 | 19/6 | MAPC (allogeneic) | Adventitial | 2–5 days | Echo | 4 | Significant improved EF by 12.6 % & volumes, + safety outcomes |
| APOLLO61 | 9/4 | ASC | IC | < 1 day | Cardiac MRI, SPECT | 6 | Improved EF by 4 %, scar formation, & perfusion defect, + safety outcomes |
| Osiris62 | 110/110 | MSC (allogeneic) | IV | < 7 days | Cardiac MRI | 6 | Decreased hypertrophy, arrhythmias & re-hospitalizations, + safety outcomes (No mention of EF) |
| TIME63,64 | 43/24 vs. 36/17 | BMC | IC | 3 vs. 7 days | Cardiac MRI | 6 | No difference in EF or effect on LV function |
| SWISS-AMI65 | 59 vs. 49/60 | BMC | IC | 1 week vs. 4 weeks | Cardiac MRI | Ongoing | No effect on LV function at 4 months |
BMC bone marrow-derived cells; IC intracoronary; LV left ventricular; DSE dobutamine stress echocardiogram; EF ejection fraction; EPC endothelial progenitor cells; MRI magnetic resonance imaging; + positive; MSC mesenchymal stem cells; Echo echocardiogram; LVEDV left ventricular end-diastolic volume; SPECT single photon emission computed tomography; IVUS intravascular ultrasound; EMM electromechanical mapping; CXCR4 CXC chemokine receptor type-4; IM intramuscular; IV intravenous; MAPC multipotent adult progenitor cells; ASC adipose-derived stem cells
Heart Failure
Additionally, there have been multiple HF trials (Table 2).66–86 Many demonstrated benefits in ventricular function noted by increased EF,68–73,75,79,82,83 improved functional class,75,78,80,82,83,86 reduced infarct size,70,72,80,82–84 decreased mortality,79 and acceptable safety outcomes.66–69,75,78,80,82–84,86 SCIPIO was the first trial using autologous CSC in HF and showed improvement in EF, infarct size, viable tissue, and HF scores.82 Two-year follow-up of the treated group showed an EF even higher than at 1-year follow-up without adverse effects.83 STAR-Heart, the largest HF study, demonstrated improved ventricular function with significantly decreased mortality at 5 years.79
Table 2.
Heart Failure (HF) Major Clinical Trials
| Study | Patients (treated/control) | Cell Type | Route | Time Post-MI | Imaging Technique | Follow-Up (months) | Outcomes in Treated Group |
|---|---|---|---|---|---|---|---|
| Tse66 | 8/0 | BMC | Transendocardial | Not reported | SPECT | 3 | Decreased angina, + safety outcomes, No change in EF |
| Fuchs67 | 10/0 | BMC | Transendocardial | Not reported | SPECT | 3 | Decreased angina, + safety outcomes, No change in EF |
| Perin68 | 14/7 | BMC | Transendocardial | Not reported | Echo, SPECT | 4 | Improved EF by 9 % & volumes, + safety outcomes |
| Stamm69 | 12/0 | BMC enriched for CD133+ | IM with CABG | 1–4 months | SPECT | 10 | Improved EF by 9 %, Improved perfusion, + safety outcomes |
| Erbs70 | 13/13 | G-CSF mobilized EPC | IC | >7 months | Cardiac MRI | 3 | Improved EF by 14 % & infarct size |
| Patel71 | 10/10 | BMC enriched for CD34+ | IM with CABG | Not reported | Echo, SPECT, LV angiogram | 6 | Improved EF by 16 % |
| IACT72 | 18/18 | BMC | IC | 5 months–8.5 years | LV angiogram | 3 | Improved EF by 15 % & infarct size |
| TOPCARE-CHD73 | 28 vs. 24/23 | BMC vs. EPC | IC | > 3 months | LV angiogram | 3 | Improved EF by 2.9 % in BMC group |
| Hendrikx74 | 10/10 | BMC | IM with CABG | 2–12 months | Cardiac MRI | 4 | Improved contractile function, No difference in EF |
| PROTECT-CAD75 | 19/9 | BMC | Transendocardial | Not reported | Cardiac MRI, SPECT | 6 | Improved EF by 5.4 %, Improved functional class, + safety outcomes |
| Ang76 | 21 vs. 21/20 | BMC | IM vs. IC | > 6 weeks | DSE, Cardiac MRI | 6 | No difference in EF or scar size |
| Yao77 | 24/23 | BMC | IC | > 4 months | Echo, Cardiac MRI, SPECT | 6 | Improved diastolic function, No change in EF |
| CAuSMIC78 | 12/11 | SMB | Transendocardial | > 1 month | Echo, Questionnaire | 12 | Improved viability & functional class, + safety outcomes (EF not studied) |
| STAR-Heart79 | 191/200 | BMC | IC | 5–11 years | LV angiogram | 5 years | Improved EF by 7.4 %, Decreased mortality |
| PRECISE80 | 21/6 | ASC | Transendocardial | Not reported | MRI, SPECT, Echo | 18 | Improved infarct size & functional capacity, + safety outcomes, No increase in EF |
| ACT-3481 | 167/0 | CD34+ | IM | Not reported | ETT, SPECT, Questionnaire | 12 | Improved angina frequency & exercise tolerance (EF not studied) |
| SCIPIO82,83 | 20/13 | CSC | IC | Not reported | Echo, Cardiac MRI, Questionnaire | Ongoing | Improved EF by 8.1 % at 1 year & 12.9 % at 2 years, Decreased scar size, Improved functional class, + safety outcomes |
| CADUCEUS84 | 17/8 | CDC | IC | 1.5–3 months | Cardiac MRI | 6 | Improved scar size and contractility, + safety outcomes, No difference in EF |
| FOCUS-CCTRN85 | 61/31 | BMC | Transendocardial | Not reported | SPECT | Ongoing | No difference in LVESV at 6 months (EF was not a pre-specified endpoint) |
| POSEIDON86 | 15 vs. 15 | MSC Allogeneic vs. Autologous | Transendocardial | 0.2–32 years | Cardiac CT | 13 | + safety outcomes, Improved functional class & ventricular remodeling, No change in EF |
HF heart failure; BMC bone marrow-derived cells; SPECT single photon emission computed tomography; + positive; EF ejection fraction; Echo echocardiogram; IM intramuscular; CABG coronary artery bypass graft; G-CSF granulocyte colony stimulating factor; EPC endothelial progenitor cells; IC intracoronary; MRI Magnetic Resonance Imaging; LV left ventricular; DSE dobutamine stress echocardiogram; SMB skeletal myoblasts; ASC adipose-derived stem cells; ETT exercise tolerance testing; CSC cardiac stem cells; CDC cardiosphere-derived cells; MSC mesenchymal stem cells; CT computed tomography
Some trials did not show significant results,76,85 while others demonstrated benefits, but no effect on EF.66,67,74,77,80,84,86 A highly anticipated trial, FOCUS-CCTRN, assessed BMC via transendocardial injection in chronic HF. However, results indicated no significant change in endpoints.85
Dilated Cardiomyopathy
There are few trials on dilated cardiomyopathy (DCM) (Table 3).87,88 These two studies used the same cell type and delivery method. Both demonstrated improved EF.87,88
Table 3.
Dilated Cardiomyopathy (DCM) Major Clinical Trials
| Study | Patients (treated/control) | Cell Type | Delivery | Imaging Technique | Follow-Up (months) | Outcomes in Treated Group |
|---|---|---|---|---|---|---|
| ABCD87 | 24/20 | BMC | IC | LV angiogram | 6 | Improved EF by 5.4 % & ESV, Improved functional class |
| TOPCARE-DCM88 | 33/0 | BMC | IC | LV angiogram | 3 | Improved EF by 2.2 % |
BMC bone marrow-derived cells; IC intracoronary; LV left ventricular; EF ejection fraction; ESV end-systolic volumes
Cell Type Comparisons
ASC had favorable results in APOLLO and PRECISE. Both trials demonstrated the efficacy of ASC in AMI and left ventricular dysfunction in settings of marked myocardial ischemia.61,80 Landmark, large-scale trials using BMC showed benefits in neovascularization, ventricular function and remodeling.12–14 The ACT-34 study demonstrated that EPC mobilization, isolation and injection significantly improved recurrent angina.81
One-year results from the largest trial using allogeneic MSC post-AMI showed ventricular benefit, with significant decreases in HF and re-hospitalizations in the treated group.62 POSEIDON was the first trial to compare safety and efficacy of allogeneic and autologous MSC in HF. Results indicated ventricular improvement, but no significant change in EF in either group. The allogeneic group demonstrated acceptable safety outcomes.86
Cell Delivery Comparisons
Intravenous delivery has been shown to be safe in AMI with fewer arrhythmias and improved ventricular function.59,62 Intracoronary delivery has been used in AMI,43–55,58,61 HF,70,72,73,77,79,82–84 and DCM87,88 trials with safety and efficacy demonstrated. The majority showed improved EF in the treated groups.44–52,55,61,70,72,73,79,82,83,87,88 Intramyocardial delivery was studied mostly in HF trials, with benefits and safety noted.69,71,74,81 Results were mixed in terms of EF improvement. Transendocardial delivery was demonstrated as safe and beneficial after AMI56 with mixed results in the highlighted HF trials, as only two showed improved EF.68,75 While others demonstrated no effect on EF, there were still benefits.66,67,78,80,85,86 Using allogeneic multipotent cells via adventitial delivery, Penn et al.27 demonstrated a significant EF increase compared to results witnessed in other trials. There were no adverse effects or signs of infarction.
Timing Comparisons
Multiple trials showed positive results when administration is within 1 week of an AMI.27,44–46,50,51,55,59,61,62 However, few studies have primarily assessed optimal timing. LateTIME showed negative results at 2–3 weeks.60 MYSTAR showed short-term increased EF, but no significant differences between treatment at 3–6 weeks and 3–4 months.58 TIME, comparing BMC delivered on day 3 and day 7 after AMI, aimed to determine optimal timing.63 Results showed no significant recovery benefit on ventricular function in either timing group.64 SWISS-AMI assessed BMC delivery at 1 week versus 3–4 weeks after AMI. Although not yet published, announced results revealed no improvement in ventricular function in either timing group compared with a control group at 4 months.65
Benefits and Safety
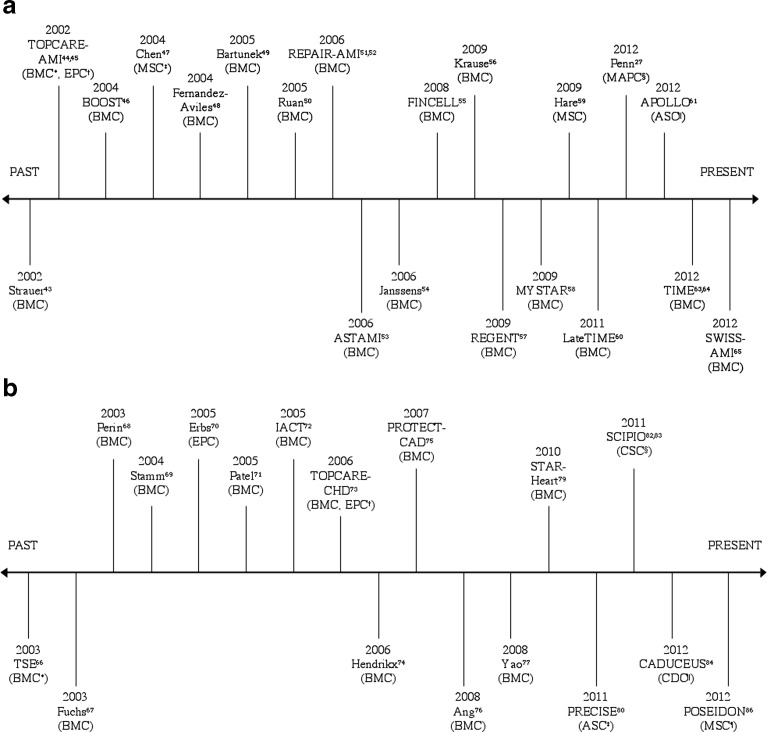
Tables 1, 2 and 3 show that various cell types have been shown to improve cardiac function. There were less re-infarctions, death, or HF hospitalizations in cell treated groups.13 The EF increase is modest, sometimes transient, and less than expected compared to animal models (Figs. 1a and b).12–14 Inconsistent results are attributable to the lack of standardization among trials in cell types used, dosages, delivery methods, timing, and follow-up.7 Lastly, measured endpoints vary between studies.
Figure 1.
a Timeline of Major Positive and Negative Acute Myocardial Infarction Clinical Trials. b Timeline of Major Positive and Negative Heart Failure Clinical Trials. Trials above horizontal line represent positive trials that resulted in increased ejection fraction in treatment group over control group. Trials below horizontal line represent negative trials that resulted in unchanged or no difference in ejection fraction between treatment and control groups. Each trial is identified with the specific cell type used. * = Bone Marrow Derived Cells; † = Endothelial Progenitor Cells; ‡ = Mesenchymal Stem Cells; § = Multipotent Adult Progenitor Cells; || = Adipose Derived Stem Cells.
Stem cell therapy has been reasonably safe. Inflammation, tumor formation, arrhythmias, and restenosis were not increased in the trials in which they were measured.12–14 Use of adult allogeneic and autologous cells is considered to be without ethical concerns.29 The only known contraindications for use of autologous cells include chronic infectious diseases, malignant solid tumors, and diseases of the bone marrow and stem cells.11
IMPLICATIONS FOR PRACTICE
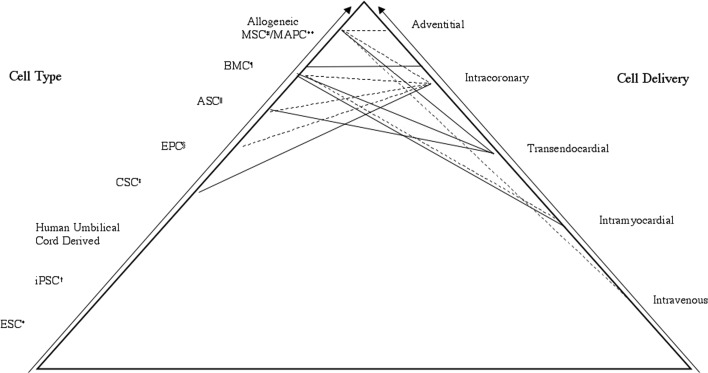
Figure 2 displays lines connecting cell types and delivery methods showing combinations used in major trials to date. Arguably, progression up the pyramid reveals the more promising and useful approaches most likely to be applicable in practice. Clinicians should convey to patients that although stem cell therapy is novel, trials demonstrate benefits supplementing conventional treatment. It should be emphasized that therapy appears to be safe and without ethical concerns. Patients should be advised that optimal cell type and delivery have not yet been determined so there are a variety of different methods. Additional research and study participants are needed; primary care providers are essential in identifying and referring patients who may be suitable candidates.
Figure 2.
Clinical Approaches Displaying Cell Types and Deliveries. Lines connecting cell type and delivery method show which combinations have been used in major trials to date. Dashed lines represent acute myocardial infarction trials. Solid lines represent heart failure trials. Progression up the pyramid reveals the more promising and useful clinical approaches. * = Embryonic Stem Cells; † = induced Pluripotent Stem Cells; ‡ = Cardiac Stem Cells; § = Endothelial Progenitor Cells; || = Adipose-derived Stem Cells; ¶ = Bone Marrow Derived Cells; # = Mesenchymal Stem Cells; ** = Multipotent Adult Progenitor Cells
FUTURE RESEARCH
Questions remain unanswered regarding optimal cell type, dosing, timing, and delivery. Future studies will focus on these areas. Ultimately, standardization of variables and procedure protocols will be necessary for adequate comparison. More effective treatment development will focus on better understanding of cellular therapy mechanisms. Increasing knowledge of engraftment and paracrine involvement will lead to advanced therapies that increase cell survival. Future therapy may deliver certain paracrine proteins instead of cells. Use of biomaterials and new imaging techniques will become increasingly important.
Selected ongoing AMI, HF, and DCM trials are listed in Table 4.89–101 A trial with great potential is BAMI, delivering autologous BMC via intracoronary infusion 5 days post-AMI. This aims to determine whether there are mortality benefits to stem cell therapy shortly after AMI. It will be the largest trial using stem cells post-AMI, involving over 3,000 patients from 11 countries.93 REGEN-IHD focuses on addressing the optimal delivery by comparing three different routes in HF.97 STOP-HF uses endomyocardial injection of a DNA plasmid encoding SDF-1 to recruit stem cells to peri-infarct regions to improve ventricular function in HF patients.99
Table 4.
Major Ongoing Clinical Trials
| Trial | Condition | Cell Type | Route | Time Post-MI | Outcomes |
|---|---|---|---|---|---|
| REGEN-AMI89 | AMI | BMC | IC | < 6 h | Recruiting—Assessing safety & efficacy in anterior AMI |
| Allogeneic MPCs after AMI90 | AMI | MPC (Allogeneic) | Transendocardial | 2–10 days | Recruiting—Assessing safety & efficacy |
| Prochymal after AMI91 | AMI | MPC (Allogeneic) | IV | < 7 days | Ongoing—Assessing LVESV |
| ADVANCE92 | AMI | ASC | IC | > 1 day | Recruiting—Assessing safety & efficacy |
| BAMI93 | AMI | BMC | IC | < 5 days | Not yet recruiting—Assessing safety and mortality reduction |
| ALLSTAR94 | AMI | CDC (Allogeneic) | IC | 1–12 months | Recruiting—Assessing safety and efficacy |
| REVITALIZE95 | HF | MSC | IC | Not specified | Ongoing—Assessing safety & feasibility |
| PERFECT96 | HF | BMC CD133+ | Transendocardial with CABG | Not specified | Recruiting—Assessing efficacy |
| REGEN-IHD97 | HF | BMC | G-CSF mobilization vs. IC vs. Transendocardial | Not specified | Ongoing—Comparing three different delivery routes |
| IMPACT-CABG98 | HF | CD133+ | Transendocardial with CABG | Not specified | Recruiting—Assessing efficacy |
| STOP-HF99 | HF | JVS-100 | Endomyocardial | Not specified | Ongoing—Assessing safety and efficacy |
| REGENERATE-DCM100 | DCM | BMC + G-CSF | IC | Not applicable | Recruiting—Assessing efficacy & safety |
| Long-Term Evaluation of Patients Receiving BMC Administration for Heart Disease101 | AMI, HF, DCM | BMC | IC | Not specified | Recruiting—Assessing long-term effects up to 10 years after transplantation |
AMI acute myocardial infarction; BMC bone marrow-derived cells; IC intracoronary; MPC mesenchymal precursor cells; IV intravenous; LVESV left ventricular end-systolic volumes; ASC adipose-derived stem cells; CDC cardiosphere-derived cells; HF heart failure; MSC mesenchymal stem cells; CABG coronary artery bypass graft; G-CSF granulocyte colony stimulating factor; DCM dilated cardiomyopathy
SUMMARY
Stem cell therapy is an exciting and revolutionary treatment for heart disease. Numerous clinical trials demonstrated improved ventricular function with positive safety outcomes. Although modest, benefits noted after cell transplantation have surpassed those with conventional treatment. If the next decade brings as much significant advancement as this past one, cell therapy may realistically become standard treatment for heart disease (Table 5).
Table 5.
Abbreviations in Order They Appear in Text
| CAD | Coronary artery disease |
| AMI | Acute myocardial infarction |
| PCI | Percutaneous coronary intervention |
| HF | Heart failure |
| EF | Ejection fraction |
| CSC | Cardiac stem cells |
| BMC | Bone marrow mononuclear cells |
| ESC | Embryonic stem cells |
| iPSC | Induced pluripotent stem cells |
| ASC | Adipose-derived stem cells |
| EPC | Endothelial progenitor cells |
| HSC | Hematopoietic progenitor stem cells |
| MSC | Mesenchymal stem cells |
| MAPC | Multipotent adult progenitor cells |
| CABG | Coronary artery bypass graft |
| SDF-1 | Stromal cell-derived factor-1 |
| CXCR4 | CXC chemokine receptor type 4 |
| DCM | Dilated cardiomyopathy |
Acknowledgements
There are no contributors, funders, or prior presentations for this manuscript.
Conflicts of Interest
Shannon Puliafico and Dr. Silver do not have any conflicts of interests. Dr. Penn is named as an inventor on patent applications submitted by The Cleveland Clinic Foundation for the use of SDF-1 to prevent and treat tissue injury. He is the founder and CMO of Juventas Therapeutics, Inc., which has licensed the use of these patents for the commercial development of SDF-1 to prevent and treat tissue injury.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jezierska-Wozniak K, Mystkowska D, Tutas A, Jurkowski MK. Stem cells as therapy for cardiac disease-a review. Folia Histochem Cytobiol. 2011;49:13–25. doi: 10.5603/fhc.2011.0004. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72. doi: 10.1161/01.CIR.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 4.Guidry UC, Evans JC, Larson MG, Wilson PW, Murabito JM, Levy D. Temporal trends in event rates after Q-wave myocardial infarction: the Framingham Heart Study. Circulation. 1999;100:2054–9. doi: 10.1161/01.CIR.100.20.2054. [DOI] [PubMed] [Google Scholar]
- 5.Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53:13–20. doi: 10.1016/j.jacc.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 6.Rosenstrauch D, Poglajen G, Zidar N, Gregoric ID. Stem cell therapy for ischemic heart failure. Tex Heart Inst J. 2005;32:339–47. [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn A, O’Brien T. Stem cell therapy for cardiac disease. Expert Opin Biol Ther. 2011;11:177–87. doi: 10.1517/14712598.2011.543894. [DOI] [PubMed] [Google Scholar]
- 8.Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–7. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 9.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 10.Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res. 2003;92:139–50. doi: 10.1161/01.RES.0000053618.86362.DF. [DOI] [PubMed] [Google Scholar]
- 11.Strauer BE, Schannwell CM, Brehm M. Therapeutic potentials of stem cells in cardiac diseases. Minerva Cardioangiol. 2009;57:249–67. [PubMed] [Google Scholar]
- 12.Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 13.Lipinski MJ, Biondi-Zoccai GG, Abbate A, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–7. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 14.Kuswardhani RA, Soejitno A. Bone marrow-derived stem cells as an adjunctive treatment for acute myocardial infarction: a systematic review and meta-analysis. Acta Med Indones. 2011;43:168–77. [PubMed] [Google Scholar]
- 15.Shiba Y, Hauch KD, Laflamme MA. Cardiac applications for human pluripotent stem cells. Curr Pharm Des. 2009;15:2791–806. doi: 10.2174/138161209788923804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joggerst SJ, Hatzopoulos AK. Stem cell therapy for cardiac repair: benefits and barriers. Expert Rev Mol Med. 2009;11:1–19. doi: 10.1017/S1462399409001124. [DOI] [PubMed] [Google Scholar]
- 17.Singla DK, Hacker TA, Ma L, et al. Transplantation of embryonic stem cells intro the infarcted mouse heart: formation of multiple cell types. J Mol Cell Cardiol. 2006;40:195–200. doi: 10.1016/j.yjmcc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Wu KH, Zhou B, Yu CT, et al. Therapeutic potential of human umbilical cord derived stem cells in a rat myocardial infarction model. Ann Thorac Surg. 2007;83:1491–8. doi: 10.1016/j.athoracsur.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 19.Mozid AM, Arnous S, Sammut EC, Mathur A. Stem cell therapy for heart disease. Br Med Bull. 2011;98:143–59. doi: 10.1093/bmb/ldr014. [DOI] [PubMed] [Google Scholar]
- 20.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/S0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 21.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei HM, Wong P, Hsu LF, Shim W. Human bone marrow-derived adult stem cells for post-myocardial infarction cardiac repair: current status and future directions. Singapore Med J. 2009;50:935–42. [PubMed] [Google Scholar]
- 23.Krause K, Schneider C, Kuck KH, Jaquet K. Review: stem cell therapy in cardiovascular disorders. Cardiovas Ther. 2010;28:e101–10. doi: 10.1111/j.1755-5922.2010.00208.x. [DOI] [PubMed] [Google Scholar]
- 24.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–16. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 25.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102:11474–9. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van’t Hof W, Mal N, Huang Y, et al. Direct delivery of syngeneic and allogeneic large-scale expanded multipotent adult progenitor cells improves cardiac function after myocardial infarct. Cytotherapy. 2007;9:477–87. doi: 10.1080/14653240701452065. [DOI] [PubMed] [Google Scholar]
- 27.Penn MS, Ellis S, Gandhi S, et al. Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: Phase I clinical study. Circ Res. 2012;110:304–11. doi: 10.1161/CIRCRESAHA.111.253427. [DOI] [PubMed] [Google Scholar]
- 28.Wu KH, Mo XM, Han ZC, Zhou B. Stem cell engraftment and survival in the ischemic heart. Ann Thorac Surg. 2011;92:1917–25. doi: 10.1016/j.athoracsur.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Strauer BE, Steinhoff G. 10 years of intracoronary and intramyocardial bone marrow stem cell therapy of the heart from the methodological origin to clinical practice. J Am Coll Cardiol. 2011;58:1095–104. doi: 10.1016/j.jacc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki K, Murtuza B, Beauchamp JR, et al. Dynamics and mediators of acute graft attrition after myoblast transplantation to the heart. FASEB J. 2004;18:1153–5. doi: 10.1096/fj.03-1308fje. [DOI] [PubMed] [Google Scholar]
- 31.ter Horst KW. Stem cell therapy for myocardial infarction: are we missing time? Cardiology. 2010;117:1–10. doi: 10.1159/000318840. [DOI] [PubMed] [Google Scholar]
- 32.Kupatt C, Hinkel R, Lamparter M, et al. Retroinfusion of embryonic endothelial progenitor cells attenuates ischemia-reperfusion injury in pigs: role of phosphatidylinositol 3-kinase/AKT Kinase. Circulation. 2005;112:I-117–22. doi: 10.1161/CIRCULATIONAHA.104.524801. [DOI] [PubMed] [Google Scholar]
- 33.Askari AT, Unzek S, Popvic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 34.Nygren J, Jovinge S, Breitbach M, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 35.Penn MS, Mayorga ME. Searching for understanding with the cellular lining of life. Circ Res. 2010;106:1554–6. doi: 10.1161/CIRCRESAHA.110.221499. [DOI] [PubMed] [Google Scholar]
- 36.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penn MS, Dong F, Klein S, Mayora ME. Stem cells for myocardial regeneration. Clin Pharm Ther. 2011;90:499–501. doi: 10.1038/clpt.2011.196. [DOI] [PubMed] [Google Scholar]
- 38.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–21. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 39.Ceradini DJ, Kulkarni AR, Callagan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi J, Kusano KF, Mauo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endoethlial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8. doi: 10.1161/01.CIR.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 41.Penn MS. Importance of the SDF-1: CXCR4 axis in myocardial repair. Circ Res. 2009;104:1133–5. doi: 10.1161/CIRCRESAHA.109.198929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M, Mal N, Kiedrowski M, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 43.Strauer BE, Brehm M, Zeus T, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–8. doi: 10.1161/01.CIR.0000034046.87607.1C. [DOI] [PubMed] [Google Scholar]
- 44.Assmus B, Schachinger V, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009–17. doi: 10.1161/01.CIR.0000043246.74879.CD. [DOI] [PubMed] [Google Scholar]
- 45.Schachinger V, Assmus B, Britten MB, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–9. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 46.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 47.Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. American Journal of Cardiology. 2004;94:92–5. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez-Aviles F, San Roman JA, Garcia-Frade J, et al. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004;95:742–8. doi: 10.1161/01.RES.0000144798.54040.ed. [DOI] [PubMed] [Google Scholar]
- 49.Bartunek J, Vanderheyden M, Vandekerckhove B, et al. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction. Circulation. 2005;112:I-178–83. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 50.Ruan W, Pan CZ, Huang GQ, Li YL, Ge JB, Shu XH. Assessment of left ventricular segmental function after autologous bone marrow stem cells transplantation in patients with acute myocardial infarction by tissue tracking and strain imaging. Chin Med J (Engl) 2005;118:1175–81. [PubMed] [Google Scholar]
- 51.Schachinger V, Erbs S, Elasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 52.Assmus B, Rolf A, Erbs S, et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010;3:89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- 53.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Eng J Med. 2006;355:1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 54.Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–21. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 55.Huikuri HV, Kervinen K, Niemala M, et al. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart J. 2008;29:2723–32. doi: 10.1093/eurheartj/ehn436. [DOI] [PubMed] [Google Scholar]
- 56.Krause K, Jaquet K, Schneider C, et al. Percutaneous intramyocardial stem cell injection in patients with acute myocardial infarction: first-in-man study. Heart. 2009;95:1145–52. doi: 10.1136/hrt.2008.155077. [DOI] [PubMed] [Google Scholar]
- 57.Tendera M, Wojakowski W, Ruzytto W, et al. Intracoronary infusion of bone marrow-derived selected CD34 + CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009;30:1313–21. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- 58.Gyongyosi M, Lang I, Dettke M, et al. Combined delivery approach of bone marrow mononuclear stem cells early and late after myocardial infarction: the MYSTAR prospective, randomised study. Nat Clin Pract Cardiovasc Med. 2009;6:70–81. doi: 10.1038/ncpcardio1388. [DOI] [PubMed] [Google Scholar]
- 59.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (Prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Traverse JH, Henry TD, Ellis SG, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTime randomized trial. JAMA. 2011;306:2110–9. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Houtgraaf JH, den Dekker WK, van Dalen BM, et al. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:539–40. doi: 10.1016/j.jacc.2011.09.065. [DOI] [PubMed] [Google Scholar]
- 62.Osiris Therapeutics. Prochymal significantly reduces hypertrophy, arrhythmia and progression to heart failure in patients suffering a heart attack. July 2 2012. Available at: http://www.osiris.com/pdf/2012-07-02%20Interim%20Cardiac%20Results.pdf Accessed April 21, 2013.
- 63.Traverse JH, Henry TD, Vaughan DE, et al. Rationale and design for TIME: a phase II, randomized, double-blind, placebo-controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells following acute myocardial infarction. Am Heart J. 2009;158:356–63. doi: 10.1016/j.ahj.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Traverse JH, Henry TD, Pepine CJ, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;1–10. [DOI] [PMC free article] [PubMed]
- 65.Surder D. Intracoronary infusion of BM-MNC early or late after AMI- 4 months results of the SWISS-AMI trial: 2012 Scientific sessions of the AHA- late breaking trials. November 2012. Available at: http://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/@scon/documents/downloadable/ucm_446442.pdf Accessed April 21, 2013.
- 66.Tse HF, Kwong YL, Chan JK, Lo G, Ho CL, Lau CP. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003;361:47–9. doi: 10.1016/S0140-6736(03)12111-3. [DOI] [PubMed] [Google Scholar]
- 67.Fuchs S, Satler LF, Kornowski R, et al. Catheter-based autologous bone marrow myocardial injection in no-option patients with advanced coronary artery disease. J Am Coll Cardiol. 2003;41:1721–4. doi: 10.1016/S0735-1097(03)00328-0. [DOI] [PubMed] [Google Scholar]
- 68.Perin EC, Dohmann HF, Borojevic R, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 69.Stamm C, Kleine HD, Westphal B, et al. CABG and bone marrow stem cell transplantation after myocardial infarction. Thorac Cardiovasc Surg. 2004;52:152–8. doi: 10.1055/s-2004-817981. [DOI] [PubMed] [Google Scholar]
- 70.Erbs S, Linke A, Adams V, et al. Transplantation of blood-derived progenitor cells after recanalization of chronic coronary artery occlusion. Circ Res. 2005;97:756–62. doi: 10.1161/01.RES.0000185811.71306.8b. [DOI] [PubMed] [Google Scholar]
- 71.Patel AN, Geffner L, Vina RF, et al. Surgical treatment for congestive heart failure with autologous adult stem cell transplantation: a prospective randomized study. J Thoarc Cardiovasc Surg. 2005;130:1631–8. doi: 10.1016/j.jtcvs.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 72.Strauer BE, Brehm M, Zeus T, et al. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: The IACT Study. J Am Coll Cardiol. 2005;46:1651–8. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 73.Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Eng J Med. 2006;355:1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 74.Hendrikx M, Hensen K, Clijsters C, et al. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial. Circulation. 2006;114:I-101–7. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- 75.Tse HF, Thambar S, Kwong YL, et al. Prospective randomized trial of direct endomyocardial implantation of bone marrow cells for treatment of severe coronary artery diseases (PROTECT-CAD trial) Eur Heart J. 2007;28:2998–3005. doi: 10.1093/eurheartj/ehm485. [DOI] [PubMed] [Google Scholar]
- 76.Ang KL, Chin D, Leyva F, et al. Randomized controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium. Nat Clin Pract Cardiovasc Med. 2008;5:663–70. doi: 10.1038/ncpcardio1321. [DOI] [PubMed] [Google Scholar]
- 77.Yao K, Huang R, Qian J, et al. Administration of intracoronary bone marrow mononuclear cells on chronic myocardial infarction improves diastolic function. Heart. 2008;94:1147–53. doi: 10.1136/hrt.2007.137919. [DOI] [PubMed] [Google Scholar]
- 78.Dib N, Dinsmore J, Lababidi Z, et al. One-year follow-up of feasibility and safety of the first U.S., randomized controlled study using 3-dimensional guided catheter-based delivery of autologous skeletal myoblasts for ischemic cardiomyopathy (CAuSMIC study) J Am Coll Cardiol Intv. 2009;2:9–16. doi: 10.1016/j.jcin.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 79.Strauer BE, Yousef M, Schannwell CM. The acute and long-term effects of intracoronary Stem cell Transplantation in 191 patients with chronic heARt failure: the STAR-heart study. Eur J Heart Fail. 2010;12:721–9. doi: 10.1093/eurjhf/hfq095. [DOI] [PubMed] [Google Scholar]
- 80.Gutierrez E, Sanz-Ruiz R, Alvarez EV, et al. General overview of the seventh international symposium on stem cell therapy and cardiovascular innovations. J Cardiovasc Transl Res. 2011;4:115–20. doi: 10.1007/s12265-010-9247-x. [DOI] [PubMed] [Google Scholar]
- 81.Losordo DW, Henry TD, Davidson C, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–36. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Bolli R, Chugh A, D’Amario D, et al. Effect of cardiac stem cells in patients with ischemic cardiomyopathy: Interim results of the SCIPIO Trial up to 2 years after therapy [abstract]. Late-breaking clinical trials: Cell-based therapies for myocardial regeneration. November 2012. Available at http://newsroom.heart.org/pr/aha/document/LBCT05-05-SCIPIO-abstract.pdf Accessed April 21, 2013.
- 84.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perin EC, Willerson JT, Pepine CJ, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The FOCUS-CCTRN Trial. JAMA. 2012;307:1717–26. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON Randomized Trial. JAMA. 2012;1–11. [DOI] [PMC free article] [PubMed]
- 87.Seth S, Narang R, Bhargava B, et al. Percutaneous intracoronary cellular cardiomyoplasty for nonischemic cardiomyopathy: clinical and histopathological results: the first-in-man ABCD (Autologous Bone Marrow Cells in Dilated Cardiomyopathy) Trial. J Am Coll Cardiol. 2006;48:2350–1. doi: 10.1016/j.jacc.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 88.Fischer-Rasokat U, Assmus B, Seeger FH, et al. A pilot trial to assess potential effects of selective intracoronary bone marrow-derived progenitor cell infusion in patients with nonischemic dilated cardiomyopathy: final 1-year results of the transplantation of progenitor cells and functional regeneration enhancement pilot trial in patients with nonischemic dilated cardiomyopathy. Circulation. 2009;2:417–23. doi: 10.1161/CIRCHEARTFAILURE.109.855023. [DOI] [PubMed] [Google Scholar]
- 89.Barts & The London NHS Trust. Bone marrow derived adult stem cells for acute anterior myocardial infarction (REGEN-AMI). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2013 April 21]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00765453?term=nct00765453&rank=1 NLM Identifier: NCT00765453.
- 90.Angioblast Systems. Safety study of allogeneic mesenchymal precursor cells (MPCs) in subjects with recent acute myocardial infarction. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2013 April 21]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00555828?term=nct00555828&rank=1 NLM Identifier: NCT00555828.
- 91.Osiris Therapeutics. Prochymal (human adult stem cells) intravenous infusion following acute myocardial infarction (AMI). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2013 April 21]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00877903?term=nct00877903&rank=1 NLM Identifier: NCT00877903.
- 92.Cytori Therapeutics. Safety and efficacy of ADRCs delivered via the intracoronary route in the treatment of patients with ST-elevation acute myocardial infarction (ADVANCE). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2013 April 21]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01216995?term=nct01216995&rank=1 NLM Identifier: NCT01216995.
- 93.Barts & The London NHS Trust. BAMI. The effect of intracoronary reinfusion of bone marrow-derived mononuclear cells (BM-MNC) on all cause mortality in acute myocardial infarction. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2013 April 21]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01569178?term=BAMI+trial&rank=1 NLM Identifier: NCT01569178.
- 94.Capricor Inc. Allogeneic heart stem cells to achieve myocardial regeneration (ALLSTAR). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2013 April 21]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01458405?term=nct01458405&rank=1 NLM Identifier: NCT01458405.
- 95.Cedars-Sinai Medical Center. Randomized evaluation of intracoronary transplantation of bone marrow stem cells in myocardial infarction (REVITALIZE). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2013 April 21]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00874354?term=nct00874354&rank=1 NLM Identifier: NCT00874354.
- 96.Miltenyi Biotec GmbH. Intramyocardial transplantation of bone marrow stem cells in addition to coronary artery bypass graft (CABG) surgery (PERFECT). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2013 April 21]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00950274?term=nct00950274&rank=1 NLM Identifier: NCT00950274.
- 97.Barts & The London NHS Trust. Bone marrow derived adult stem cells for chronic heart failure (REGEN-IHD). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2013 April 21]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00747708?term=nct00747708&rank=1 NLM Identifier: NCT00747708.
- 98.Centre hospitalier de l’Universite de Montreal (CHUM). IMPACT-CABG Trial: Implantation of autologous CD133+ stem cells in patients undergoing CABG. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2013 April 21]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01033617?term=nct01033617&rank=1 NLM Identifier: NCT01033617.
- 99.Juventas Therapeutics, Inc. Study to evaluate the safety and efficacy of JVS-100 administered to adults with ischemic heart failure. (STOP-HF). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2013 April 21]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01643590?term=stop-hf&rank=2 NLM Identifier: NCT01643590.
- 100.Barts & The London NHS Trust. Bone marrow derived adult stem cells for dilated cardiomyopathy (REGENERATE-DCM). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2013 April 21]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01302171?term=nct01302171&rank=1 NLM Identifier: NCT01302171.
- 101.Johann Wolfgang Goethe University Hospitals. Long-term evaluation of patients receiving bone marrow-derived cell administration for heart disease (BMC registry). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2013 April 21]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00962364?term=nct00962364&rank=1 NLM Identifier: NCT00962364.