ABSTRACT
BACKGROUND
Among aging HIV-infected adults, polypharmacy and its consequences have not been well-described.
OBJECTIVE
To characterize the extent of polypharmacy and the risk of antiretroviral (ARV) drug interactions among persons of different ages.
DESIGN AND PARTICIPANTS
Cross-sectional analysis among patients within the HIV Outpatient Study (HOPS) cohort who were prescribed ARVs during 2006–2010.
MAIN MEASURES
We used the University of Liverpool HIV drug interactions database to identify ARV/non-ARV interactions with potential for clinical significance.
KEY RESULTS
Of 3,810 patients analyzed (median age 46 years, 34 % ≥ 50 years old) at midpoint of observation, 1,494 (39 %) patients were prescribed ≥ 5 non-ARV medications: 706 (54 %) of 1,312 patients ≥ 50 years old compared with 788 (32 %) of 2,498 patients < 50 years. During the five-year period, the number of patients who were prescribed at least one ARV/non-ARV combination that was contraindicated or had moderate or high evidence of interaction was 267 (7 %) and 1,267 (33 %), respectively. Variables independently associated with having been prescribed a contraindicated ARV/non-ARV combination included older age (adjusted odds ratio [aOR] per 10 years of age 1.17, 95 % CI 1.01–1.35), anxiety (aOR 1.78, 95 % CI 1.32–2.40), dyslipidemia (aOR 1.96, 95 % CI 1.28–2.99), higher daily non-ARV medication burden (aOR 1.13, 95 % CI 1.10–1.17), and having been prescribed a protease inhibitor (aOR 2.10, 95 % CI 1.59–2.76). Compared with patients < 50 years, older patients were more likely to have been prescribed an ARV/non-ARV combination that was contraindicated (unadjusted OR 1.44, 95 % CI 1.14–1.82), or had moderate or high evidence of interaction (unadjusted OR 1.29, 95 % CI 1.15–1.44).
CONCLUSIONS
A substantial percentage of patients were prescribed at least one ARV/non-ARV combination that was contraindicated or had potential for a clinically significant interaction. As HIV-infected patients age and experience multiple comorbidities, systematic reviews of current medications by providers may reduce risk of such exposures.
KEY WORDS: polypharmacy, drug interactions, HIV, aging
INTRODUCTION
Combination antiretroviral (ARV) therapy has significantly decreased morbidity and mortality in the HIV-infected population.1–4 With continued use of ARV therapy and maintenance of long-term adherence, HIV becomes a chronic and manageable condition.2,5 As persons infected with HIV live longer, the percentage of older individuals in the HIV-infected population has increased. In the United States (US) in 2009, persons aged 50 years and older accounted for 33 % of all individuals living with HIV/AIDS, nearly double the 17 % reported for 2001.6 It is estimated that by 2020, more than 50 % of persons living with HIV infection will be aged 50 years or older.7
Polypharmacy, defined as the concomitant use of multiple medications (e.g., customarily five or more medications), has been associated with increasing age8,9 and with an increased risk of adverse drug reactions, increased hospitalizations, poor adherence, inappropriate drugs, falls and fractures, and drug–drug interactions.10–15 The risk for drug–drug interactions may be particularly increased among the aging population of HIV-infected adults due to treatments for multiple comorbidities in this population16–19 as well as the concomitant use of ARV therapy. Among the antiretroviral classes, non-nucleoside reverse transcriptase inhibitors (NNRTIs) and protease inhibitors (PIs) are major substrates as well as both inhibitors and inducers of the cytochrome P450 (CYP) enzyme system.20 Introduction of low-dose ritonavir to “boost” bioavailability of most PIs has further increased risk for clinically significant drug interactions;21 ritonavir is an extremely potent inhibitor of CYP 3A4 and 2D6, both of which metabolize nearly 70 % of all medications that undergo CYP450 metabolism.22,23 PIs and NNRTIs can also affect activity of P-glycoprotein, a ubiquitous transport protein that prevents accumulation of toxins.24
Although polypharmacy and its impact on drug–drug interactions has been well-described in various studies from the general population,13,14,25–33 there are limited data among the aging population of HIV-infected adults.2,34–36 Therefore, we sought to examine the potential impact of polypharmacy on the risk of drug–drug interactions between ARVs and other medications (ARV/non-ARV interactions) in a US cohort of HIV-infected adults seen in the outpatient setting. In particular, we aimed to characterize the extent of polypharmacy, determine the types of medication classes prescribed and rate of prescribed ARV/non-ARV combinations with the potential for clinically significant interactions among persons of different ages, and identify risk factors for such exposures.
METHODS
The HIV Outpatient Study (HOPS)
The HOPS is an ongoing prospective observational cohort study of HIV-infected adults that has accrued data longitudinally since 1993. In this cross-sectional analysis, we included data from eight clinics (university-based, public, and private) participating in the HOPS after January 1, 2006, located in the following six cities: Chicago, IL; Denver, CO; Stony Brook, NY; Philadelphia, PA; Tampa, FL; and Washington, DC. Patient data, including demographic and social characteristics, symptoms, diagnoses, prescribed medications (including dose and duration), and laboratory values are abstracted from medical charts and entered by trained staff into a single database. These data are reviewed for quality and analyzed centrally. Data quality assurance measures include supervisory reviews of randomly selected charts to ascertain accuracy and completeness of abstracted data, and centralized checks of data files to resolve discrepancies in diagnosis and treatment start and stop dates, and in diagnosis codes versus descriptive text field information. Annually, the institutional review boards of the Centers for Disease Control and Prevention (Atlanta, GA), Cerner Corporation (Vienna, VA), and each local site have reviewed and approved the HOPS protocol and consents. The study protocol conforms to the guidelines of the US Department of Health and Human Services (DHHS) for the protection of human participants in research. The present analysis is based on the HOPS data set updated as of June 30, 2011.
Study Population
We performed cross-sectional analyses of patients who were classified as active (i.e., having attended at least two medical visits) in the HOPS from January 1, 2006 to December 31, 2010. For our analysis, active patients were selected who had a CD4+ T-lymphocyte (CD4) count and HIV RNA viral load (VL) recorded within 6 months before or during the 5-year study period, and who had taken ARV therapy for > 2 weeks during the study period. Observation period started on January 1, 2006 or the first HOPS visit thereafter (referred to as the “baseline” date). It extended to the earlier of death date, last HOPS visit plus six months or December 31, 2010, allowing for up to 5 years of observation for each patient.
Outcome Variables
We used the comprehensive University of Liverpool HIV drug interactions database (www.hiv-druginteractions.org), which aggregates published findings chiefly from European and North American studies to classify a medication as contraindicated or as having moderate or high evidence of interaction with a concurrently prescribed ARV. Non-ARV drug classes and categories were adapted from this database. We considered only combinations of prescribed ARV medications with prescribed non-ARV medication (termed from here on “ARV/non-ARV combinations”) where both drugs had been prescribed concomitantly for > 1 day.
Independent Variables: Definitions for Analysis
Values for baseline characteristics were values assessed during the preceding 6 months that were closest to the baseline date. The presence or absence of a diagnosis at baseline was based on diagnoses made before the baseline date and within 6 months thereafter. Insurance status was classified as private, public or none (i.e., uninsured). Illicit substances included amphetamines, cocaine, and heroin. “Poly-substance abuse” and “alcohol abuse” were determined from documentation of such in the medical chart notes. The category “other mental illness” included psychosis, schizophrenia, bipolar disorder, mania, or chronic mental illness. In addition to Hodgkin lymphoma and leukemia, cancers at the following anatomic sites were defined as non-AIDS-defining: skin, liver, lung, bone, brain (excluding primary CNS lymphoma), breast, ovarian, prostate, testes, thyroid gland, esophagus, kidney, pancreas, stomach, small bowel, colon, and anorectum. Although polypharmacy is typically defined as concomitant use of five or more medications,37 in the case of HIV-infected patients who are frequently prescribed three or more medications (ARVs) to treat one condition (HIV infection), we also describe total and non-ARV medication burden so that alternative cut-offs for characterizing polypharmacy could be considered.
Statistical Analyses
We assessed the percentage of patients within the 5-year study period who were prescribed ≥ 1 ARV/non-ARV combination that was contraindicated or had moderate or high evidence of interaction. We compared patient characteristics between groups using chi-square tests for categorical variables and Wilcoxon or Kruskal-Wallis tests for continuous variables. We used Yates corrected chi-square tests to compare percentages of patients prescribed specific medication classes and frequencies of ARV/non-ARV combinations with clinically significant interactions among patients < 50 vs. ≥ 50 years old at the midpoint of observation for each patient. We used multivariable logistic regression to obtain adjusted odds ratios (aORs) with associated 95 % confidence intervals (CIs) and assess factors independently associated with having been prescribed ARV/non-ARV combinations that were either contraindicated or had moderate or high evidence of interaction. In all modeling, variables with a univariate significance level (p value) < 0.05 were initially included in multivariable analyses. We constructed final multivariable models using backward manual selection procedures, retaining only those variables for which the significance level was < 0.05. Descriptive data summaries, and univariate and multivariable logistic regression analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). Yates corrected chi-square tests were performed using StatCalc (EpiInfo 2002 revision 2; Centers for Disease Control and Prevention, Atlanta, GA).
RESULTS
Patient Baseline Characteristics
Among the 4,145 active patients with at least two visits in the HOPS anytime from January 1, 2006 to December 31, 2010, we excluded 335 for the following reasons (applied hierarchically): 268 were prescribed antiretroviral therapy for < 2 weeks during follow-up, 46 had no recorded CD4 count, and 21 had no recorded HIV VL.
Demographic and clinical characteristics of the 3,810 patients in our analytic cohort stratified by age at baseline are shown in Table 1. Among these patients (median age, 44 years), 1,189 (31 %) were < 40 years old, 1,615 (42 %) were 40–49 years old, and 1,006 (26 %) were ≥ 50 years old. Overall, 3,001 (79 %) were male, 1,800 (47 %) were non-white race or Hispanic ethnicity and 2,158 (57 %) had private insurance. The median baseline CD4 count was 432 cells/μL (interquartile range [IQR], 255–640 cells/μL) and 2,169 (57 %) patients had a prior AIDS diagnosis.
Table 1.
Demographic and Clinical Characteristics of the 3,810 Patients at Baseline, Stratified by Age, the HIV Outpatient Study, 2006–2010
| Baseline Characteristic* | Age < 40 years (n = 1,189) | Age 40–49 years (n = 1,615) | Age ≥ 50 years (n = 1,006) | P value† |
|---|---|---|---|---|
| Age, years: median (IQR‡) | 34 (29–37) | 44 (42–47) | 54 (52–58) | < 0.001 |
| Male sex, n (%) | 861 (72.4) | 1,304 (80.7) | 836 (83.1) | < 0.001 |
| Race and ethnicity, n (%) | < 0.001 | |||
| White, non-Hispanic | 513 (43.2) | 901 (55.8) | 596 (59.2) | |
| Black, non-Hispanic | 453 (38.1) | 475 (29.4) | 286 (28.4) | |
| Hispanic | 174 (14.6) | 181 (11.2) | 97 (9.6) | |
| Other | 49 (4.1) | 58 (3.6) | 27 (2.7) | |
| Year of HOPS entry, n (%) | < 0.001 | |||
| 2005 or earlier | 724 (60.9) | 1,234 (76.4) | 805 (80.0) | |
| 2006 or later | 465 (39.1) | 381 (23.6) | 201 (20.0) | |
| Years observed, median (IQR) | 4.3 (2.3–5.0) | 5.0 (2.8–5.0) | 5.0 (2.6–5.0) | < 0.001 |
| Total person-years of observation | 4,308 | 6,306 | 3,895 | 0.19 |
| Primary insurance type, n (%) | 0.05 | |||
| Private | 666 (56.0) | 942 (58.4) | 550 (54.7) | |
| Public | 408 (34.3) | 544 (33.7) | 383 (38.1) | |
| None | 115 (9.7) | 127 (7.9) | 73 (7.3) | |
| HIV risk, n (%) | < 0.001 | |||
| MSM | 691 (58.1) | 959 (59.4) | 567 (56.4) | |
| Heterosexual | 372 (31.3) | 397 (24.6) | 233 (23.2) | |
| IDU | 40 (3.4) | 142 (8.8) | 131 (13.0) | |
| Other/unknown | 86 (7.2) | 117 (7.2) | 75 (7.5) | |
| Prior AIDS diagnosis, n (%) | 538 (45.3) | 978 (60.6) | 653 (64.9) | < 0.001 |
| Current/prior tobacco use, n (%) | 461 (38.8) | 690 (42.7) | 446 (44.3) | 0.02 |
| Illicit substance§ or alcohol abuse, n (%) | 77 (6.5) | 156 (9.7) | 78 (7.8) | 0.01 |
| Depression, n (%) | 399 (33.6) | 633 (39.2) | 366 (36.4) | 0.01 |
| Anxiety, n (%) | 155 (13.0) | 265 (16.4) | 167 (16.6) | 0.02 |
| Insomnia, n (%) | 162 (13.6) | 258 (16.0) | 186 (18.5) | 0.008 |
| Other mental illness‖, n (%) | 79 (6.6) | 122 (7.6) | 59 (5.9) | 0.24 |
| Cancer¶, n (%) | 26 (2.2) | 85 (5.3) | 104 (10.3) | < 0.001 |
| Dyslipidemia, n (%) | 702 (59.0) | 1,246 (77.2) | 820 (81.5) | < 0.001 |
| Hypertension, n (%) | 212 (17.8) | 489 (30.3) | 513 (51.0) | < 0.001 |
| Cardiovascular disease, n (%) | 78 (6.6) | 172 (10.7) | 218 (21.7) | < 0.001 |
| Diabetes, n (%) | 83 (7.0) | 193 (12.0) | 249 (24.8) | < 0.001 |
| Hepatitis B or C, n (%) | 113 (9.5) | 321 (19.9) | 250 (24.9) | < 0.001 |
| Nadir CD4+ cell count < 200 cells/mm3, n (%) | 515 (43.3) | 860 (53.3) | 545 (54.2) | < 0.001 |
| Nadir CD4+ cell count, cells/mm3, median (IQR) | 236 (74–351) | 181 (59–320) | 182 (67–325) | < 0.001 |
| CD4+ cell count, cells/mm3, median (IQR) | 419 (243–603) | 432 (253–655) | 444 (270–661) | 0.01 |
| CD4+ cell count category, cells/mm3 | 0.03 | |||
| < 200 | 241 (20.3) | 304 (18.8) | 168 (16.7) | |
| 200–349 | 219 (18.4) | 321 (19.9) | 202 (20.1) | |
| 350–499 | 279 (23.5) | 315 (19.5) | 203 (20.2) | |
| 500+ | 450 (37.9) | 675 (41.8) | 432 (43.0) | |
| HIV RNA viral load, log10 copies/mL, median (IQR) | 2.6 (1.4–4.5) | 1.5 (1.4–4.0) | 1.4 (1.4–2.8) | < 0.001 |
| Viral load < 400 copies/mL, n (%) | 593 (49.9) | 1,029 (63.7) | 741 (73.7) | < 0.001 |
| Daily number of non-ARV prescription medications prescribed, median (IQR) | 2 (1–4) | 3 (1–6) | 4 (2–7) | < 0.001 |
| Daily number of ARV medications prescribed, median (IQR) | 3 (0–3) | 3 (1–3) | 3 (3–3) | < 0.001 |
| Total daily number of medications prescribed, median (IQR) | 4 (2–6) | 5 (3–8) | 7 (4–10) | < 0.001 |
| ARV treatment status | < 0.001 | |||
| ARV-naïve, n (%) | 321 (27.0) | 222 (13.8) | 114 (11.3) | |
| ARV-experienced, currently not prescribed ARVs, n (%) | 130 (10.9) | 161 (10.0) | 78 (7.8) | |
| ARV-experienced, currently prescribed ARVs, n (%) | 738 (62.1) | 1,232 (76.3) | 814 (80.9) | |
| Time prescribed ARVs during follow-up, n (%) | < 0.001 | |||
| < 75 % | 179 (15.1) | 208 (12.9) | 76 (7.6) | |
| 75–99 % | 286 (24.2) | 557 (34.6) | 345 (34.3) | |
| 100 % | 719 (60.7) | 845 (52.5) | 584 (58.1) | |
| ARV regimen type, n (%) | 0.17 | |||
| PI-containing | 369 (31.0) | 619 (38.3) | 411 (40.9) | |
| NNRTI-containing | 306 (25.7) | 468 (29.0) | 301 (29.9) | |
| PI and NNRTI-containing | 24 (2.0) | 61 (3.8) | 49 (4.9) | |
| Triple NRTI | 22 (1.9) | 48 (3.0) | 30 (3.0) | |
| Non-HAART# | 11 (0.9) | 26 (1.6) | 19 (1.9) | |
| Integrase inhibitor | 6 (0.5) | 5 (0.3) | 2 (0.2) | |
| Fusion inhibitor | 0 (0.0) | 3 (0.2) | 1 (0.1) | |
| Other ARV agent** | 0 (0.0) | 2 (0.1) | 1 (0.1) |
*Characteristics and diagnoses were applied as follows: insurance status at closest visit to baseline date; any diagnoses up to 6 months after baseline date; nadir CD4 at any time before or during follow-up period; CD4 cell count at closest visit to baseline date; viral load at closest visit to baseline date; ARV experience up to and including beginning of baseline date; daily number of medications taken at closest visit to baseline date
†Comparisons made across three age groups
‡ IQR interquartile range, MSM men who have sex with men, IDU injection drug use, ARV antiretroviral, PI protease inhibitor, NNRTI non-nucleoside reverse transcriptase inhibitor, NRTI nucleoside/nucleotide reverse transcriptase inhibitor, HAART highly active antiretroviral therapy
§Illicit substances includes the following entries: amphetamines, cocaine, heroin, poly-substance abuse
‖Other mental illness includes the following diagnosis entries: psychosis/schizophrenia, bipolar disorder, mania, chronic mental illness
¶Cancers are non-AIDS-defining: Hodgkin, skin, liver, lung, bone, brain, breast, ovarian, prostate, testicular, anal/rectal, colon, thyroid, esophageal, renal, pancreatic, leukemia, stomach/GI, other
#HAART defined as three or more ARVs from two different classes
**Two dual NRTI regimens, one not specified
As expected, the percentage of patients diagnosed with dyslipidemia, hypertension, cardiovascular disease, diabetes, and non-AIDS-defining cancers increased with age (Table 1). Greater percentages of older persons compared with younger persons also had injection drug use as their risk factor for HIV transmission (3.4 % < 40 years old, 8.8 % 40–49 years old, and 13 % ≥ 50 years old), were co-infected with hepatitis B or C virus (9.5 % < 40 years old, 20 % 40–49 years old, and 25 % ≥ 50 years old), and were current or former tobacco smokers (39 % < 40 years old, 43 % 40–49 years old, and 44 % ≥ 50 years old). Older patients had a higher median daily non-ARV medication burden: two (IQR 1–4) for age < 40 years, three (IQR 1–6) for age 40–49 years, and four (IQR 2–7) for age 50 years (P <0.001).
Frequency of Prescriptions of ARV/non-ARV Combinations with Potential for Clinically Significant Interactions
Among the 3,810 patients analyzed during the 5-year observation period, 267 (7 %) were prescribed at least one contraindicated ARV/non-ARV combination. Average duration of prescribed contraindicated combinations per year of observation was 82 days. In addition, 1,267 (33 %) patients were prescribed at least one ARV/non-ARV combination with moderate or high evidence of interaction. Average duration of prescribed interacting combinations per year of observation was 71 days.
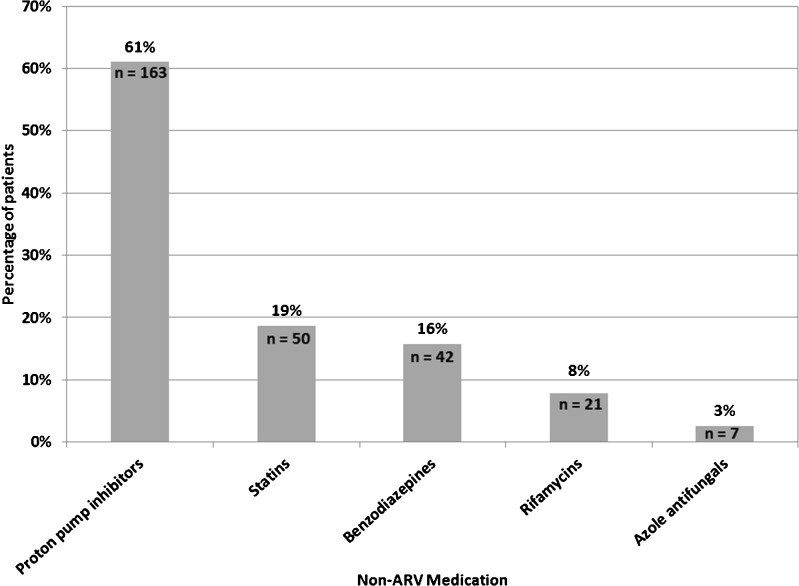
Among the 267 patients prescribed one or more contraindicated ARV/non-ARV combinations, 163 (61 %) were prescribed a proton pump inhibitor (PPI) together with either atazanavir or nelfinavir, 50 (19 %) were prescribed a contraindicated statin (simvastatin or lovastatin) together with a PI, and 42 (16 %) were prescribed a contraindicated benzodiazepine (alprazolam, triazolam, diazepam, clorazepate, and flurazepam) together with a PI (Fig. 1).
Figure 1.
Non-ARVs prescribed among patients prescribed contraindicated ARV/non-ARV combinations (N = 267)*, by class. *Some patients were prescribed more than one contraindicated non-ARV medication together with an ARV, or different contraindicated combinations at different times during the observation.
Among the 1,267 patients prescribed at least one ARV/non-ARV combination with moderate or high evidence of interaction, the most commonly prescribed medications were H2 antagonists and PPIs together with PIs (25 %), erectile dysfunction agents together with PIs and NNRTIs (22 %), and the antidepressants, bupropion, sertraline, and paroxetine together with PIs and NNRTIs and lithium together with atazanavir (19 %).
Factors Associated with Prescription of ARV/non-ARV Combinations with Potential for Clinically Significant Interactions
In multivariable logistic regression analyses, baseline variables independently associated with increased odds of being prescribed a contraindicated ARV/non-ARV combination included older age, anxiety, dyslipidemia, higher daily non-ARV medication burden, and having been prescribed a PI (Table 2). Baseline variables independently associated with higher odds of being prescribed an ARV/non-ARV combination with moderate or high evidence of interaction (but not contraindicated) are also shown in this table. Higher nadir CD4 cell count was associated with lower odds of having been prescribed an ARV/non-ARV combination with moderate or high evidence of interaction (Table 2).
Table 2.
Univariate and Multivariable Logistic Regression of Factors Associated with Prescription of ARV/non-ARV Combinations, the HIV Outpatient Study, 2006–2010 (N = 3,810)
| Baseline Characteristic | Contraindicated ARV/non-ARV combinations | ARV/non-ARV combinations with moderate or high evidence of interaction, but not contraindicated | ||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| OR (95 % CI) | OR (95 % CI) | OR (95 % CI) | OR (95 % CI) | |
| Age per 10 years | 1.49 (1.31–1.69) | 1.17 (1.01–1.35) | 1.36 (1.26–1.45) | 1.13 (1.04–1.23) |
| Non-Hispanic white race/ethnicity | 1.59 (1.23–2.06) | – | 0.98 (0.85–1.12) | – |
| Year of HOPS entry ≤ 2005 | 2.74 (1.90–3.96) | – | 1.84 (1.57–2.17) | 1.26 (1.04–1.52) |
| Private insurance | 0.86 (0.67–1.10) | – | 0.72 (0.63–0.82) | – |
| MSM HIV risk | 1.14 (0.88–1.47) | – | 0.80 (0.69–0.91) | – |
| Prior AIDS diagnosis | 1.40 (1.08–1.82) | – | 1.83 (1.59–2.10) | – |
| Current or prior smoker | 1.39 (1.08–1.78) | – | 1.43 (1.25–1.64) | 1.16 (1.00–1.35) |
| Illicit substance* or alcohol abuse | 0.61 (0.35–1.05) | – | 1.93 (1.53–2.44) | 1.46 (1.13–1.88) |
| Depression | 1.62 (1.26–2.08) | – | 2.03 (1.77–2.33) | 1.47 (1.26–1.71) |
| Anxiety | 2.46 (1.86–3.26) | 1.78 (1.32–2.40) | 1.68 (1.40–2.01) | – |
| Other mental illness† | 1.18 (0.74–1.88) | – | 1.47 (1.13–1.89) | – |
| Cancer | 1.23 (0.75–2.02) | – | 1.36 (1.03–1.81) | – |
| Dyslipidemia | 3.57 (2.38–5.35) | 1.96 (1.28–2.99) | 1.88 (1.60–2.21) | 1.24 (1.03–1.49) |
| Hypertension | 2.02 (1.57–2.59) | – | 1.82 (1.58–2.09) | – |
| Cardiovascular disease | 1.82 (1.32–2.50) | – | 1.81 (1.49–2.21) | – |
| Diabetes | 2.06 (1.53–2.78) | – | 1.57 (1.30–1.90) | – |
| Hepatitis B or C | 1.26 (0.93–1.71) | – | 1.60 (1.35–1.89) | – |
| CD4+ cell count per 100 cells (cells/mm3) | 1.02 (0.98–1.06) | – | 0.97 (0.95–0.99) | – |
| Nadir CD4+ cell count per 100 cells (cells/mm3) | 0.91 (0.85–0.98) | – | 0.85 (0.81–0.88) | 0.94 (0.90–0.98) |
| HIV RNA viral load, log10 copies/mL | 0.86 (0.79–0.95) | – | 1.02 (0.97–1.06) | 1.12 (1.07–1.19) |
| Daily non-ARV medication burden | 1.18 (1.15–1.21) | 1.13 (1.10–1.17) | 1.19 (1.17–1.21) | 1.13 (1.11–1.16) |
| Prescription of a PI-containing ARV regimen | 3.12 (2.40–4.05) | 2.10 (1.59–2.76) | 2.17 (1.90–2.49) | 1.54 (1.32–1.80) |
*Illicit substances includes the following entries: amphetamines, cocaine, heroin, poly-substance abuse
†Other mental illness includes the following diagnosis entries: psychosis/schizophrenia, bipolar disorder, mania, chronic mental illness
Frequency of Prescribed Total and Non-ARV Medications and Differences in Prescribed Specific Medication Classes Stratified by Age
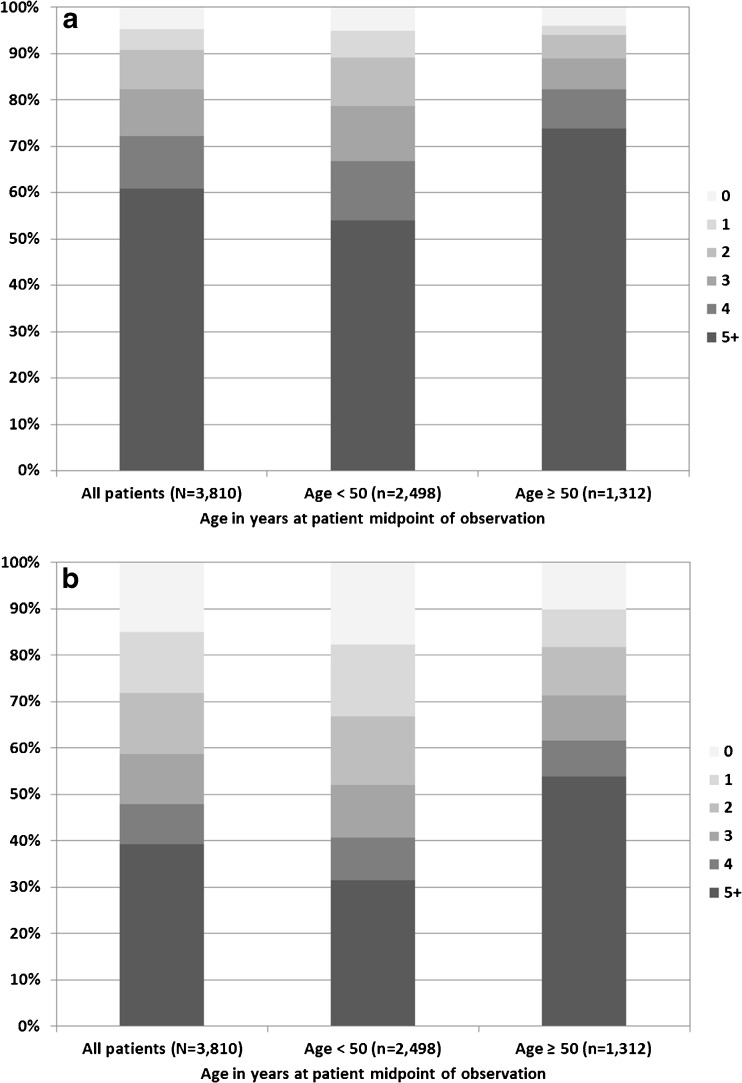
Figures 2a and 2b show the total number of medications and the number of non-ARV medications, respectively, prescribed at midpoint of observation for each patient, stratified by age. Of 3,810 patients analyzed (median age 46 years), 2,319 (61 %) patients were prescribed ≥ 5 medications (including ARVs): 969 (74 %) of 1,312 patients ≥ 50 years old (median age 55 years) compared with 1,350 (54 %) of 2,498 patients < 50 years (median age 42 years) (Fig. 2a). Overall, 1,494 (39 %) patients were prescribed ≥ 5 non-ARV medications: 706 (54 %) of 1,312 patients ≥ 50 years old compared with 788 (32 %) of 2,498 patients < 50 years (Fig. 2b).
Figure 2.
a Number of total medications (including ARVs) prescribed, overall and by age group, the HIV Outpatient Study, 2006–2010 (N = 3,810). b Number of non-ARV medications prescribed, overall and by age group, the HIV Outpatient Study, 2006–2010 (N = 3,810).
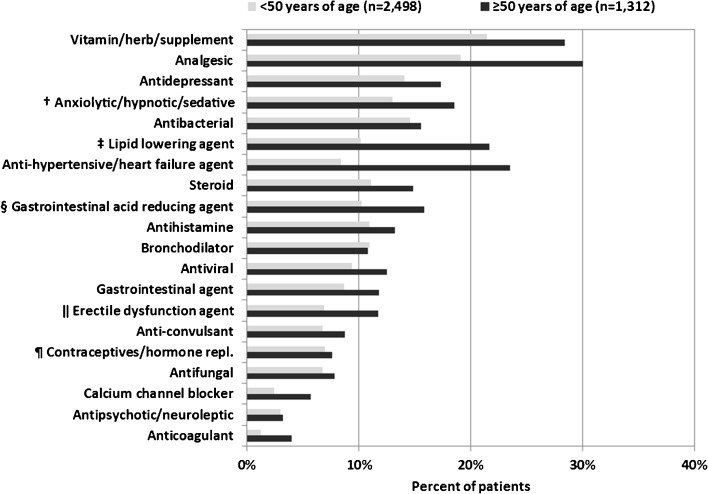
Figure 3 shows the percentage of patients prescribed specific medication classes at the midpoint of observation for each patient, stratified by age at the time of classification. During the 5-year period, compared with patients < 50 years, older patients were more likely to have been prescribed an ARV/non-ARV combination that was contraindicated (2.86 versus 1.98 per 100 person-years, unadjusted OR 1.44, 95 % CI 1.14–1.82) or had moderate or high evidence of interaction (15.6 vs. 12.1 per 100 person-years, unadjusted OR 1.29, 95 % CI 1.15–1.44).
Figure 3.
Prescribed non-ARV medications by class and age, the HIV Outpatient Study, 2006–2010 (N = 3,810)* . *Age group and medication use defined/determined at midpoint of patient observation. †Benzodiazepines ‡Statins §Proton Pump Inhibitors ‖Among males ¶Among females
DISCUSSION
Polypharmacy has been shown to increase the risk of adverse drug reactions and geriatric syndromes such as cognitive impairment, urinary incontinence, and falls.38 In this cross-sectional analysis spanning a 5-year period, we found that a greater number of daily non-ARV medication can also increase the risk of ARV/non-ARV combinations with potential for clinically significant interactions for HIV-infected persons, particularly those ≥ 50 years old. With increasing age, comorbidities such as cardiovascular disease, hyperlipidemia, diabetes, non-AIDS-defining cancers, and declines in renal and hepatic function become highly prevalent among HIV-infected patients.16–19 A Southern Alberta Cohort Study examining the total daily pill burden (TDPB) among HIV-infected patients over a period of 20 years found that there was a higher TDPB among older patients due to increases in non-ARV drugs required for managing comorbidities.2 Consistent with this study, a greater proportion of older patients in the HOPS were prescribed ≥ 5 non-ARV medications as well as ≥ 5 total medications (including ARVs) compared with those < 50 years old. In addition, patients ≥ 50 years old were more likely to have been prescribed an ARV/non-ARV combination that was contraindicated or had moderate or high evidence of interaction.
Among this aging, HIV-infected cohort (median age, 44 years), 7 % of patients were prescribed at least one ARV/non-ARV combination that was contraindicated and 33 % were prescribed a combination that had moderate or high evidence of interaction. Prescription of contraindicated ARV/non-ARV combinations predominantly involved the use of PPIs, statins (simvastatin and lovastatin) and benzodiazepines, agents frequently prescribed in this cohort and commonly used for the treatment of comorbidities among older patients. For example, 73 % of patients in our study had a baseline diagnosis of dyslipidemia, for which statins are first-line pharmacological agents.39 Co-administration of simvastatin and lovastatin with protease inhibitors may significantly increase serum levels of the statin, resulting in potential myopathy, including rhabdomyolysis.40,41
In contrast to the results of our study, the Swiss HIV Cohort Study found that only 2 % of their patients had been prescribed contraindicated ARV/non-ARV combinations, which largely involved midazolam,35 while 59 % had been prescribed ARV/non-ARV combinations that were not contraindicated, yet had interactions requiring potential dose adjustment and/or close monitoring. The differences between our observations and those of the Swiss HIV Cohort Study scientists may be explained at least in part by their use of a customized version of the Liverpool database, use of different definitions for important but not contraindicated interactions, and their review of all interactions by two pharmacists.
According to the US DHHS guidelines on the use of antiretroviral agents in adults and adolescents, providers should thoroughly review patients’ current medications in order to design an ARV regimen that minimizes undesirable drug interactions35,42 and utilize medication therapy management (MTM) services for patients with complex medical histories. ARV regimen modification, dose adjustment, and substitution of certain non-ARV or ARV drugs may be required in some patients. Significant drug interactions with different ARV agents and suggested recommendations on contraindications, dose modifications, and alternative agents can be found in the DHHS guidelines as well as online drug interactions databases, such as the University of Liverpool HIV drug interactions database. The addition of MTM services (CPT codes 99605-99607 for reimbursement) that include pharmacists can help ensure that a thorough evaluation for potential drug interactions takes place, especially for patients prescribed multiple co-medications with their ARV regimens.43 Finally, electronic medical record systems that provide information on the clinical relevance of drug–drug interactions, availability of therapeutic alternatives, and monitoring parameters or management options may decrease the risk of toxicities or therapeutic failure.44
We acknowledge the challenges providers face following these recommendations when caring for patients who frequently experience multiple chronic comorbidities.7,35 Our objective is to focus attention on the real burden of polypharmacy and clinically significant drug interactions among the HIV population. There are many circumstances in which co-prescribing of medications with the potential for drug interactions may be unavoidable, especially if patients have limited therapeutic options for treatment of their HIV infection or comorbidities. Drug interactions are not always clinically significant and providers may have been well aware of these interactions and monitored patients closely or made dosage adjustments.
There are several limitations to our study. First, we analyzed medications that were prescribed concurrently but cannot ascertain if they were actually taken by the patient as prescribed. Second, recommendations on ARV/non-ARV combinations according to the University of Liverpool database may differ from US labeling or guidelines, since the University of Liverpool database considers recommendations from both US and European labeling. Therefore, estimates of prescription of contraindicated ARV/non-ARV combinations in our cohort may be slightly lower if based solely on US labeling or guidelines. Third, in evaluating the medication burden, we did not systematically collect and could not account for use of over-the-counter drugs or herbal medications, some of which have significant potential for interaction with ARVs. Fourth, we also did not examine interactions between multiple non-ARV agents that patients may be prescribed, only interactions between ARVs and non-ARVs; therefore, our estimates may be conservative in terms of the total burden of drug–drug interactions. Finally, we did not examine the contribution of the many different ARV/non-ARV interactions to major clinical outcomes, such as virologic failure, end-organ injury or treatment discontinuation, because our medical record-based observational study of patients in routine HIV care is not well powered or adequately controlled for this purpose.
In conclusion, a substantial minority of patients in our large multi-site US HIV cohort was prescribed ARV/non-ARV combinations with the potential for clinically significant interactions, and the risk for exposure to such combinations increased with age. Reasons for this finding include the overall increasing use of co-medications and the high prevalence of comorbidities among older patients for which treatments often interact with ARVs. Providers should thoroughly review and adjust patients’ current medications in order to minimize the potential drug interactions with their ARV regimens and consider medical therapy management monitoring for patients with multiple comorbidities.
Acknowledgements
Disclaimer
The findings and conclusions of this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Funders
The Centers for Disease Control and Prevention (CDC) supported the HOPS data collection through a contract with the Cerner Corporation. The co-authors from the CDC participated in the design of the study and the analysis and interpretation of the data and approved the final version of the manuscript.
Prior presentations
This manuscript was presented at the XIX International AIDS Conference in Washington DC, USA on July 24th, 2012.
Conflict of Interest
The authors state that they have no financial disclosures or conflicts of interest to report.
REFERENCES
- 1.Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41(2):194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 2.Krentz HB, Cosman I, Lee K, Ming JM, Gill MJ. Pill burden in HIV infection: 20 years of experience. Antivir Ther. 2012;17(5):833–40. doi: 10.3851/IMP2076. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362(9377):22–9. doi: 10.1016/S0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 4.Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 5.Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet. 2010;376(9734):49–62. doi: 10.1016/S0140-6736(10)60676-9. [DOI] [PubMed] [Google Scholar]
- 6.CDC 2012: Centers for Disease Control and Prevention. HIV Surveillance Report, 2010; vol. 22. http://www.cdc.gov/hiv/topics/surveillance/resources/reports/. Published March 2012. Accessed June 5, 2012.
- 7.Brooks JT, Buchacz K, Gebo KA, Mermin J. HIV Infection and Older Americans: The Public Health Perspective. 2012;102:1516–26. doi: 10.2105/AJPH.2012.300844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jyrkka J, Vartiainen L, Hartikainen S, Sulkava R, Enlund H. Increasing use of medicines in elderly persons: a five-year follow-up of the Kuopio 75 + Study. Eur J Clin Pharmacol. 2006;62(2):151–8. doi: 10.1007/s00228-005-0079-6. [DOI] [PubMed] [Google Scholar]
- 9.Linjakumpu T, Hartikainen S, Klaukka T, Veijola J, Kivelä SL, Isoaho R. Use of medications and polypharmacy are increasing among the elderly. J Clin Epidemiol. 2002;55(8):809–17. doi: 10.1016/S0895-4356(02)00411-0. [DOI] [PubMed] [Google Scholar]
- 10.Barat I, Andreasen F, Damsgaard EM. Drug therapy in the elderly: what doctors believe and patients actually do. Br J Clin Pharmacol. 2001;51(6):615–22. doi: 10.1046/j.0306-5251.2001.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franceschi M, Scarcelli C, Niro V, et al. Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: a prospective study of 1,756 patients. Drug Saf. 2008;31(6):545–56. doi: 10.2165/00002018-200831060-00009. [DOI] [PubMed] [Google Scholar]
- 12.Gnjidic D, Le Couteur DG, Kouladjian L, Hilmer SN. Deprescribing trials: methods to reduce polypharmacy and the impact on prescribing and clinical outcomes. Clin Geriatr Med. 2012;28(2):237–53. doi: 10.1016/j.cger.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Lin CF, Wang CY, Bai CH. Polypharmacy, aging and potential drug-drug interactions in outpatients in Taiwan: a retrospective computerized screening study. Drugs Aging. 2011;28(3):219–25. doi: 10.2165/11586870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Secoli SR, Figueras A, Lebrão ML, de Lima FD, Santos JL. Risk of potential drug-drug interactions among Brazilian elderly: a population-based, cross-sectional study. Drugs Aging. 2010;27(9):759–70. doi: 10.2165/11538460-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Tamura BK, Bell CL, Inaba M, Masaki KH. Outcomes of polypharmacy in nursing home residents. Clin Geriatr Med. 2012;28(2):217–36. doi: 10.1016/j.cger.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Buchacz K, Baker RK, Palella FJ, Jr., et al. Disparities in prevalence of key chronic diseases by gender and race/ethnicity among antiretroviral-treated HIV-infected adults in the US. Antivir Ther. 2013;18(1):65–75. [DOI] [PubMed]
- 17.Hasse B, Ledergerber B, Furrer H, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53(11):1130–9. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 18.Kilbourne A, Justice A, Rabeneck L, Rodriquez-Barradas M, Weissman S. General medical and psychiatric comorbidity among HIV-infected veterans in the post-HAART era. J Clin Epi. 2001;54(12):S22–8. doi: 10.1016/S0895-4356(01)00443-7. [DOI] [PubMed] [Google Scholar]
- 19.Nachega JB, Hsu AJ, Uthman OA, Spinewine A, Pham PA. Antiretroviral therapy adherence and drug-drug interactions in the aging HIV population. AIDS. 2012;26(Suppl 1):S39–53. doi: 10.1097/QAD.0b013e32835584ea. [DOI] [PubMed] [Google Scholar]
- 20.Rainey PM. HIV drug interactions: the good, the bad, and the other. Ther Drug Monit. 2002;24(1):26–31. doi: 10.1097/00007691-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Josephson F. Drug-drug interactions in the treatment of HIV infection: focus on pharmacokinetic enhancement through CYP3A inhibition. J Intern Med. 2010;268(6):530–9. doi: 10.1111/j.1365-2796.2010.02301.x. [DOI] [PubMed] [Google Scholar]
- 22.Dresser GK, Bailey DG. A basic conceptual and practical overview of interactions with highly prescribed drugs. Can J Clin Pharmacol. 2002;9(4):191–8. [PubMed] [Google Scholar]
- 23.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Griffin L, Annaert P, Brouwer KL. Influence of drug transport proteins on the pharmacokinetics and drug interactions of HIV protease inhibitors. J Pharm Sci. 2011;100(9):3636–54. doi: 10.1002/jps.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooney D, Pascuzzi K. Polypharmacy in the elderly: focus on drug interactions and adherence in hypertension. Clin Geriatr Med. 2009;25(2):221–33. doi: 10.1016/j.cger.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Haider SI, Johnell K, Thorslund M, Fastbom J. Trends in polypharmacy and potential drug-drug interactions across educational groups in elderly patients in Sweden for the period 1992–2002. Int J Clin Pharmacol Ther. 2007;45(12):643–53. doi: 10.5414/CPP45643. [DOI] [PubMed] [Google Scholar]
- 27.Hohl CM, Dankoff J, Colacone A, Afilalo M. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med. 2001;38(6):666–71. doi: 10.1067/mem.2001.119456. [DOI] [PubMed] [Google Scholar]
- 28.Hovstadius B, Hovstadius K, Astrand B, Petersson G. Increasing polypharmacy - an individual-based study of the Swedish population 2005-2008. BMC Clin Pharmacol. 2010;10:16. doi: 10.1186/1472-6904-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibrahim IA, Kang E, Dansky KH. Polypharmacy and possible drug-drug interactions among diabetic patients receiving home health care services. Home Health Care Serv Q. 2005;24(1–2):87–99. doi: 10.1300/J027v24n01_07. [DOI] [PubMed] [Google Scholar]
- 30.Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30(10):911–8. doi: 10.2165/00002018-200730100-00009. [DOI] [PubMed] [Google Scholar]
- 31.Nobili A, Licata G, Salerno F, et al. Polypharmacy, length of hospital stay, and in-hospital mortality among elderly patients in internal medicine wards. The REPOSI study. Eur J Clin Pharmacol. 2011;67(5):507–19. doi: 10.1007/s00228-010-0977-0. [DOI] [PubMed] [Google Scholar]
- 32.Rosholm JU, Bjerrum L, Hallas J, Worm J, Gram LF. Polypharmacy and the risk of drug-drug interactions among Danish elderly. A prescription database study. Dan Med Bull. 1998;45(2):210–3. [PubMed] [Google Scholar]
- 33.Slabaugh SL, Maio V, Templin M, Abouzaid S. Prevalence and risk of polypharmacy among the elderly in an outpatient setting: a retrospective cohort study in the Emilia-Romagna region, Italy. Drugs Aging. 2010;27(12):1019–28. doi: 10.2165/11584990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Gianotti N, Galli L, Bocchiola B, et al. Number of daily pills, dosing schedule, self-reported adherence and health status in 2010: a large cross-sectional study of HIV-infected patients on antiretroviral therapy. HIV Med. 2013;14(3):153–60. [DOI] [PubMed]
- 35.Marzolini C, Elzi L, Gibbons S, et al. Prevalence of comedications and effect of potential drug-drug interactions in the Swiss HIV Cohort Study. Antivir Ther. 2010;15(3):413–23. doi: 10.3851/IMP1540. [DOI] [PubMed] [Google Scholar]
- 36.Marzolini C, Back D, Weber R, et al. Ageing with HIV: medication use and risk for potential drug-drug interactions. J Antimicrob Chemother. 2011;66(9):2107–11. doi: 10.1093/jac/dkr248. [DOI] [PubMed] [Google Scholar]
- 37.Viktil KK, Blix HS, Moger TA, Reikvam A. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol. 2007;63(2):187–95. doi: 10.1111/j.1365-2125.2006.02744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007;5(4):345–51. doi: 10.1016/j.amjopharm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 39.National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143–3421. [PubMed]
- 40.Hsyu PH, Schultz-Smith MD, Lillibridge JH, Lewis RH, Kerr BM. Pharmacokinetic interactions between nelfinavir and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors atorvastatin and simvastatin. Antimicrob Agents Chemother. 2001;45(12):3445–50. doi: 10.1128/AAC.45.12.3445-3450.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams D, Feely J. Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin Pharmacokinet. 2002;41(5):343–70. doi: 10.2165/00003088-200241050-00003. [DOI] [PubMed] [Google Scholar]
- 42.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed February 27, 2013.
- 43.Kauffman Y, Nair V, Herist K, Thomas V, Weidle PJ. HIV medication therapy management services in community pharmacies. J Am Pharm Assoc. 2012;52(6):e287–91. doi: 10.1331/JAPhA.2012.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baysari MT, Westborrk J, Braithwaite J, et al. The role of computerized decision support in reducing errors in selecting medicines for prescription. Drug Saf. 2011;34(4):289–98. doi: 10.2165/11588200-000000000-00000. [DOI] [PubMed] [Google Scholar]